Abstract
Astragalin is a flavonoid existed in several edible and medicinal plants and was recorded to have multiple biological and pharmacological significances. This work aimed to assess the possible protective effect of astragalin administration against oxidative tension, acute inflammation and histopathological deformations in a mouse paw edema model induced following intra sub-plantar injection of carrageenan. Thirty-six male Swiss mice were divided into four groups: control, carrageenan, astragalin (75 mg/kg) + carrageenan, and indomethacin (10 mg/kg) + carrageenan. Astragalin administration for five consecutive days to carrageenan injected mice showed a significant reduction in the development of paw in a time dependent effect, inhibited lipoperoxidation by-product, malondialdehyde and increased superoxide dismutase and catalase activities. Astragalin was found also to suppress the inflammatory signaling in the inflamed tissue as exhibited by the decreased myeloperoxidase activity along with the decreased protein and transcriptional level of pro-inflammatory cytokines including tumor necrosis factor-alpha, interleukin-1 beta and interleukin-6. Moreover, inducible nitric oxide synthase and cyclooxygenase-2 expressions and their products (nitric oxide and prostaglandin E2) were downregulated. Additionally, astragalin decreased monocyte chemoattractant protein-1 and nuclear factor kappa B expression in the inflamed paw tissue. The recorded findings provide evidences for the potential application of astragalin as a plant-derived remedy for the treatment of acute inflammation due to its promising antioxidant and anti-inflammatory activities along with its ameliorative impact against the histopathological changes in the paw tissue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammation is a defense immune response developed in order to encounter the deleterious effects of pathogens, xenobiotics, irradiation, and even against the damaged cells [1]. Acute inflammation includes removing and/or neutralizing internal or external stimuli and further initiating the healing process to restore normal tissue homeostasis [2]. However, excessive acute inflammatory responses may develop to chronic which is implicated in the pathogenesis of different chronic inflammatory disorders such as cancers, neurodegenerative and cardiovascular disorders [3]. Several microcirculatory events were found to be associated with the development of inflammatory responses including alterations in the vascular permeability, leukocytes infiltration coupled with excessive release of pro-inflammatory mediators resulting in the redness, swelling, hyperthermia and pain at the injured site [1, 4]. Previous studies demonstrated that the development of inflammation is closely related to the excessive production reactive oxygen species (ROS), depletion of the cellular antioxidant system and increased peroxidation of membrane lipids, which regulates the redox status in the injured tissue [5, 6]. Currently prescribed anti-inflammatory drugs including non-steroidal anti-inflammatory drugs are able to treat several inflammatory diseases, however, their application have been correlated with serious health problems such as hepato-renal injury and gastrointestinal complications [7]. Therefore, developing novel anti-inflammatory therapy with minimum side effects and maximum efficiency is necessary [8].
Carrageenan is a seaweed polysaccharide used to induce a classical murine model of paw edema with the advantage of high constancy and short duration with apparent manifestations to evaluate the potential anti-inflammatory activity of natural compounds [9]. Carrageenan is known to enhance biphasic inflammatory cascades: the early phase which starts after one hour includes the oversecretion of histamine, leukotriene, kinin, and cyclooxygenase overactivity, while the late one is accompanied by leukocytes recruitment and overproduction of prostaglandins and bradykinin in the inflamed tissue [10]. Due to their enriched bioactive chemical entities, natural products represent the main source for the development of therapeutic agents. Flavonoids are essential constituents in our foods and they are associated several medicinal activities [11]. Astragalin or kaempferol-3-β-D-glucoside is a naturally existing flavonoid in different plants. Astragalin displays numerous biological and pharmacological activities such as antioxidant [12], hepatoprotective [13], anticancer [14], anti-diabetic [15], neuroprotective [16], and cardioprotective activity [17]. Previous studies exhibited the anti-inflammatory properties of astragalin [18]. Astragalin was found to deactivate myeloperoxidase and downregulate the pro-inflammatory cytokines including interleukin-1 β (IL-1β), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) in mastitis and lung injury model induced by lipopolysaccharide (LPS). The anti-inflammatory of astragalin is proceeding via downregulation of nuclear factor kappa-B (NF-κB) signaling pathway [19]. In addition, astragalin inhibited the development of inflammatory responses in different experimental models through halting levels of prostaglandin E2 (PGE2), MAPK, NF-κB expression. Hence, the current study was designed to explore the possible antioxidant and anti-inflammatory activities of astragalin against carrageenan-mediated paw edema in mice through investigating the level and expression of different inflammatory mediators and cytokines in the inflamed paw tissue.
Materials and methods
Chemicals
Astragalin (CAS number: 480-10-4, 97% purity) and carrageenan (CAS number: 9000-07-1) were supplied from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA), and all other used chemicals were of analytical grade.

Experimental protocol and model induction
Thirty-six male Swiss mice (20–25 g, 6–8 weeks) were sourced from the animal facility of King Fahd for medical research, King Abdul Aziz University, Jeddah, Saudi Arabia. Mice were placed into four equal groups:
-
Control (Ctrl) group: mice were treated with normal saline (0.9% NaCl) for five consecutive days.
-
Carrageenan (Cgn) group: mice were injected with 0.1 mL of 1% w/v carrageenan suspended in 0.9% NaCl into the sub-plantar tissues of the left hind paw.
-
Astragalin and carrageenan (Astr + Cgn) group: mice were gavaged with astragalin (75 mg/kg) for five days based on Soromou et al. [20] before the induction of paw edema.
-
Indomethacin and carrageenan (Imc + Cgn) group: mice were administered with indomethacin (10 mg/kg) for five consecutive days according to Gafarzadeh et al. [21] before the induction of paw edema.
Both astragalin and indomethacin (an anti-inflammatory reference drug) were administered after being dissolved in normal saline. The used dose of astragalin was applied after a preliminary investigation using 25, 50 and 75 mg/kg. Interestingly, 75 mg/kg was found to inhibit significantly the development of paw edema following carrageenan injection as compared with the lower doses, which was also in line with a previous study [20]. The thickness of the paw tissue before and after carrageenan application (at 2, 4, 6, and 8 h) was determined. The recorded measurements were figured as the difference in the paw volume (mL) and were referenced to the right hind paw of the same animal. At 8 h, mice were decapitated and paw tissue samples were isolated and separated into two parts. One sample was homogenized immediately to yield 50% (w/v) homogenate in ice-cold medium containing 50 mM Tris–HCl (pH 7.4) and centrifuged at 500×g for 10 min at 4 °C. The developed paw supernatant was employed for the determination of biochemical assays, while the second sample was used to examine the histopathological changes and molecular studies.
Estimation of pro-inflammatory cytokines and other inflammatory mediators in the inflamed tissue
Concentration of tumor necrosis factor alpha (TNF-α, Cat. No: CSB-E04741m), interlukin-1 beta (IL-1β, Cat. No: CSB-E04621m), interlukin-6 (IL-6, Cat. No: CSB-E04627m), cyclooxygenase-2 (COX-2, Cat. No: CSB-E12910m), prostaglandin E2 (PGE2, Cat. No: CSB-PA040059), and monocyte chemoattractant protein-1 (MCP-1, Cat. No: CSB-E07430m) were measured in paw skin supernatant by ELISA kits sourced from CUSABIO Life Sciences, Wuhan, China following the manufacturer’s procedures.
Myeloperoxidase (MPO) activity
MPO activity was determined in the inflamed tissue based on the modified procedures demonstrated by Bradley et al. [22]. After three freeze–thaw cycles of the homogenate and centrifugation at 15,000×g for 10 min at 4 °C, level of MPO activity was assessed by adding 200 μL of the paw skin supernatant with 2.8 mL of 50 mM phosphate buffer (pH 6.0) and 1 mL of 1.67 mM o-dianisidine hydrochloride containing 0.0005% (v/v) H2O2. The change in the absorbance at 450 nm was noticed, and MPO activity was presented as U/mg protein.
Measurement of oxidative stress index in the injured skin tissue
Nitric oxide (NO) concentration in paw supernatant was quantified based on the protocol described by Green et al. [23], by adding the Griess reagent (a mixture of naphthylene diamine dihydrochloride (0.1%) and sulfanilamide [1% in 5% H3PO4]) for 10 min in dark at 30 °C, and the absorbance of the developed bright reddish-purple azo dye was measured at 540 nm. Lipoperoxidation level in the paw supernatant was determined using 1 mL of 0.67% thiobarbituric acid and 1 mL of 10% trichloroacetic acid in a boiling water bath for 30 min. Thiobarbituric acid reactive substances were measured by absorbance at 535 nm and presented in term of malondialdehyde (MDA) [24]. Activity of superoxide dismutase (SOD) was explored based on the described protocol by Nishikimi et al. [25]. Catalase (CAT) activity was determined according to the described method by Aebi [26].
Quantitative Real-time PCR
Total RNA from the injured tissue was isolated, and first strand cDNA was developed according to the manufacturer’s protocol. The mRNA expression of iNOS in the paw tissue was detected using real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR) technique using an Applied Biosystems 7500 Instrument. The thermal conditions for qRT-PCR were denaturated initially at 94 °C for 2 min, followed by 40 cycles of 94 °C for 30 s and 60 °C for 30 s, and a final extension at 72 °C for 10 min. After PCR amplification, the ΔCt from three repeated experiments was determined by subtracting the Ct value of the standard gene, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) from that of each sample (Ct). The applied primers sequences for GAPDH was 5′-CCCTTAAGAGGGATGCTGCC-3′ (forward) and 5′-ACTGTGCCGTTGAATTTGCC-3′ (reverse), and for iNOS was 5′-GCGCTCTAGTGAAGCAAAGC-3′ (forward) and 5′-GCACATCAAAGCGGCCATAG-3′ (reverse).
Histopathological and immunohistochemical analysis
A piece from the injected paw skin with carrageenan was fixed overnight in 4% neutral formaldehyde. Skin samples were then paraffinized, sectioned (4–5 μm), and further stained with hematoxylin and eosin (H&E) to evaluate the histopathological deformations in all experimented groups. To study the immunoreactivity of NF-kB in the injured tissue, purified primary antibodies with avidin–biotin-peroxidase (ABC) and peroxidase substrate (Pierce™ Peroxidase IHC Detection Kit, Thermo Fisher Scientific, CA, USA) were employed. Briefly, skin sections were treated with 0.3% H2O2 to deactivate the endogenous peroxidase. Sections were incubated with primary antibody for 24 h at 4 °C, and then with biotinylated rabbit anti-mouse secondary antibody (Dako system kit) and avidin–biotin complex (ABC) reagents for 1 h at 30 °C in a humidified room. Finally, the skin sections were counterstained with hematoxylin, dehydrated, and mounted using Aquatex fluid (Merck KGaA, Darmstadt, Germany).
Statistical analyses
The recoded results are illustrated as the mean ± standard deviation (SD). By using the statistical package SPSS, version 17.0, one-way analysis of variance (ANOVA) followed by Post Hoc Duncan’s test were employed to evaluate the difference between control and treated groups. A p-value ˂ 0.05 was considered statistically significant.
Results
Impact of astragalin on the development of paw edema in response to carrageenan injection
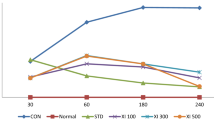
As shown in Fig. 1, sub-plantar injection of carrageenan was found to develop edema in the left hind paw skin one hour later, and the thickness of injured tissue increased in a time dependent manner with the maximum effect recorded after 8 h. However, astragalin and indomethacin (anti-inflammatory reference drug) treated groups at doses of 75 and 10 mg/kg, respectively reduced significantly (p < 0.05) the volume of the developed edema as compared to the model mice. These observations reflect the ability of astragalin to attenuate the vascular alterations following carrageenan injection.
Impact of astragalin (Astr, 75 mg/kg) or indomethacin (Icm, 10 mg/kg) on paw edema thickness in carrageenan (Cgn)-mediated paw edema in mice. Findings are figured as mean ± SD (n = 7); p < 0.05 shows statistical significance; #&$Significant alterations against control and carrageenan injected mice, respectively
Impact of astragalin on the histological deformations associated with carrageenan injection
In addition to the observed redness and swelling of the paw tissue upon carrageenan injection, histopathological examination exhibited epithelial hyperplasia, severe infiltration of leukocytes, and sub epidermal edema. These features of acute inflammation were mostly inhibited following astragalin administration. Likewise, the anti-inflammatory activity evoked by astragalin was similar to that applied by indomethacin treatment (Fig. 2a).
Impact of astragalin (Astr, 75 mg/kg) or indomethacin (Icm, 10 mg/kg) on a histological changes in paw skin after carrageenan (Cgn) injection in mice. White arrow: sub epidermal edema, red arrow: acute inflammation. b Immunoreactivity of NF-κB the inflamed skin tissue of different treated groups, 400×. Scale Bar = 100 Μm. (Color figure online)
Impact of astragalin on the oxidative stress markers in the edematous tissue following carrageenan injection
Carrageenan application was found to disturb the redox status in the paw tissue as confirmed by the significantly elevated (p < 0.05) lipid peroxidation and its byproduct, MDA (Fig. 3a) coupled with depletion of the antioxidants including SOD (Fig. 3b) and CAT (Fig. 3c) with respect to the normal mice. On contrast, astragalin and indomethacin administration showed similar findings and reduced the increased lipoperoxidation and enhanced the antioxidant capacity when compared to the model group; reflecting the antioxidant properties of astragalin associated with the development of acute inflammation in response to carrageenan injection in the paw tissue.
Impact of astragalin (Astr, 75 mg/kg) or indomethacin (Icm, 10 mg/kg) on a MDA, b SOD, c CAT, d mRNA expression of iNOS and e NO levels in carrageenan (Cgn)-mediated paw edema in mice. Results are expressed as mean ± SD (n = 7). p < 0.05 shows significant significance; #&$ significant alterations against control and carrageenan injected mice, respectively. PCR results were performed in triplicate using Gapdh as a housekeeping gene
Impact of astragalin on the activity of iNOS and NO level in the edematous tissue following carrageenan injection
As shown in Fig. 3d, e, the mRNA expression of iNOS was found to be increased markedly (p < 0.05) associated with the elevation of NO in carrageenan-induced paw edema. On the other hand, treatment with astragalin and indomethacin attenuated significantly the upregulation of iNOS expression and decreased the elevated NO level as compared to carrageenan injected mice. Indomethacin treated animals showed better improvement in the expression of iNOS and NO level as compared to the model group.
Impact of astragalin on the levels of COX-2, PGE2, MCP-1 and MPO in the edematous tissue following carrageenan injection
To understand the causes of swelling and redness of paw tissue following carrageenan injection, the level of PGE2 and its precursor, COX-2 activity were assessed. Carrageenan injection enhanced significantly (p < 0.05) the activity of COX-2 and its product, PGE2 as compared to the control values. Remarkably, both Astragalin and indomethacin deactivated significantly (p < 0.05) COX-2 and subsequently decreased PGE2 level in the inflamed foci as compared to carrageenan treated mice (Fig. 4a, b).
Impact of astragalin (Astr, 75 mg/kg) or indomethacin (Icm, 10 mg/kg) on the levels of a COX-2, b PGE2, c MCP-1 and d MPO in the inflamed skin tissue after carrageenan (Cgn) injection. Results are expressed as mean ± SD (n = 7). p < 0.05 shows significant significance; #&$Significant alterations against control and carrageenan injected mice, respectively
To elucidate the migration and infiltration of leukocytes into the inflamed tissue, levels of MCP-1 (Fig. 4c) and MPO (Fig. 4d) were determined in the injured tissue. In comparison to the normal mice, carrageenan-challenged mice showed a significant raise (p < 0.05) in the level of MPO and MCP-1 in the damaged paw tissue. Interestingly, astragalin and indomethacin administration to the model group decreased significantly (p < 0.05) the elevated MCP-1 and MPO as compared to carrageenan injected group.
Impact of astragalin on the level of the pro-inflammatory cytokines and NF-κB in the edematous tissue following carrageenan injection
In order to evaluate the inflammatory response in carrageenan-injected mice, levels of pro-inflammatory cytokines were estimated in the inflamed tissue. A significant elevation (p < 0.05) in the levels of TNF-α (Fig. 5a), IL-1β (Fig. 5b) and IL-6 (Fig. 5c) was observed following carrageenan injection as compared to the control mice. Meanwhile, astragalin administered mice alone showed a non-significant change in the examined pro-inflammatory cytokines. However, astragalin and indomethacin administration to mice injected with carrageenan decreased significantly (p < 0.05) the elevated inflammatory cytokines with respect to the model group; reflecting the ability of astragalin to inhibit the development of acute inflammatory response upon carrageenan application.
Impact of astragalin (Astr, 75 mg/kg) or indomethacin (Icm, 10 mg/kg) on the protein levels of a TNF-α, b IL-1β, c IL-6 and d NF-κB in carrageenan (Cgn)-mediated paw edema in mice. Results are expressed as mean ± SD (n = 7). p < 0.05 shows significant significance; #&$Significant alterations against control and carrageenan injected mice, respectively
In order to clarify the molecular mechanism implicated in the anti-inflammatory properties of astragalin, immunoreactivity and level of NF-κB (Figs. 2b and 5d) were investigated following carrageenan injection. NF-κB regulates and controls the expression and activity of different inflammatory molecules. The recorded data showed a significant elevation (p < 0.05) in the level of NF-κB and expression in the injured paw tissue after carrageenan application as compared to the control untreated mice. Notably, astragalin and indomethacin were able to decrease the level and expression of this transcriptional factor significantly upon carrageenan intoxication.
Discussion
Long term intake of the anti-inflammatory medications including non steroid anti-inflammatory drugs were found to be coupled with numerous health problems such as gastric ulcer, renal and cardiac deficits. Hence, it's mandatory to find alternative safe and effective anti-inflammatory drug derived from natural resources. Here, we evaluated the antioxidant and anti-inflammatory activities of astragalin in paw edema murine model induced by carrageenan. Carrageenan-mediated paw edema is an accepted experimental model employed to assess the novel anti-inflammatory compounds. In the present work, carrageenan injection triggered oxidative tension in the paw skin as indicated by the raised lipoperoxidation in the form of MDA formation and the declined SOD and CAT activities. Previous reports demonstrated the development of oxidative and nitrosative damages upon carrageenan application as confirmed by the excessive formation of ROS and reactive nitrogen species (RNS) and their cytotoxic active derivatives [27]. Among the produced ROS, hydroxyl and hydroperoxyl radicals which mostly attack membrane lipids and enhance its peroxidation [28]. MDA elevation reflects the incidence of oxidative damage and subsequently inflammatory responses [29]. SOD is a metalloenzyme used to catalyze the dismutaion of superoxide radicals into less active hydrogen peroxide and molecular oxygen. Meanwhile, CAT degrades the formed hydrogen peroxide by SOD into water and molecular oxygen [30]. The overproduced ROS during the inflammatory responses was reported to exhaust and deactivate antioxidant enzymes including thiol-containing proteins, SOD and CAT which represents the major cytoprotective defense barrier against internal or external stimuli [31].
Remarkably, astragalin administration in carrageenan injected mice elicited a decrease in lipid peroxidation by-product and increased SOD and CAT activities; reflecting its antioxidant capacity and its ability to scavenge ROS generated after carrageenan injection. Indeed, astragalin-containing medicinal plants have promising protective impact against the development of oxidative stress [32]. Karna et al. [12] showed that astragalin administration in combination with monotropein and spiraeoside was found to quench ROS and RNS in varicocelized rats along with inhibiting lipid peroxidation and enhancing activities of antioxidant enzymes. Additionally, astragalin prevented testicular dysfunction associated with diabetes via restoring the balance between oxidants and antioxidants in the testicular tissue [33]. Moreover, astragalin inhibited lung injury following lipopolysaccharide via upregulation of nuclear factor erythroid-2-related factor 2 and heme oxygenase-1 pathway [34].
In association with the intensive inflammatory reaction, carrageenan application causes microcirculatory alterations resulting in the formation of edema and increased its thickness in a time dependent effect [35], which has been confirmed in the current investigation. On the other hand, astragalin administration decreased distinctly the volume of the developed edematous tissue.
Furthermore, earlier studies demonstrated that excessive ROS generation following carrageenan injection triggers the progression of inflammation through activation of immune cells and release of different pro-inflammatory mediators resulting in tissue injury. In the current study, carrageenan injected mice exhibited overproduction of pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α) and increased levels of MCP-1 and MPO activity along with elevated COX-2 and iNOS activities and their products (PGE2 and NO), as reported in previous studies [6, 27]. At the inflamed foci, activated macrophages, monocytes, fibrocytes and endothelial cells produce excessively different inflammatory mediators. Cross-communication between the pro-inflammatory cytokines through direct or indirect pathways has been reported during inflammatory response, which has been also linked with the development of oxidative stress. It has been demonstrated that TNF-α and pro-inflammatory interleukins are mainly released simultaneously to potentiate pro-inflammatory vascular and cellular reactions in response to infections. The over secreted TNF-α and IL-β was found to enhance production of IL-6. The elevation in these pro-inflammatory cytokines has been attributed to the activation of NF-κB which further activate their mRNA expression [36]. At high concentration, TNF-α and IL-1β activates the expression of endothelial cell adhesion molecules and enhances the activity of several inflammatory molecules including COX-2 and further PGE2 synthesis [37, 38]. Prostaglandins regulate numerous biological functions like blood pressure, digestive system integrity, immune response and fertility. Alteration in prostaglandins metabolism has been linked with the genesis of several pathological conditions [39, 40]. PGE2 when produced in high levels is significantly coupled with the induction of typical characters of acute inflammation including redness, swelling and pain [41]. Accumulative evidences demonstrated a positive correlation between the increased pro-inflammatory cytokines and iNOS upregulation and subsequent NO production [42]. Moreover, these soluble mediators were found to enhance production of acute phase protein and tissue damage such as in rheumatoid arthritis [37]. The obtained high PGE2 in the current study may be due to the increased activity of COX-2 following carrageenan injection.
NO is a biological mediator performs that regulates the homeostasis of several physiological processes. During pathological circumstances and at high NO concentration, NO-derives radicals including peroxynitrite is produced in large amount and causes severe cellular impairments such as DNA oxidation and lipoperoxidation resulting in tissue injury and inflammation [43]. The increased NO level in the current study in the paw tissue may be due to the overactivation of iNOS activity which represents the rate-limiting enzyme in NO formation. Beside, TNF-α provokes NO production by stimulating iNOS and boosts the responses of neutrophils to inflammatory stimuli [44]. Earlier reports stated that iNOS upregulation and high NO have profound impact on COX2 activity and PGE2 formation [45]. Wu [46] showed that elevated NO level stimulates PGE2 synthesis through increasing COX-2 half-life by producing free radicals and suppressing COX-2 autoinactivation. Additionally, NO-derived radical (peroxynitrite) enhances lipoperoxidation which trigger the release of arachidonic acid from plasma membrane resulting in COX-2 activation and PGE2 production accordingly [47]. MPO is heme containing protein secreted mainly from neutrophils and widely used as oxidative stress and inflammatory marker [48]. In combination with hydrogen peroxide and halides, MPO generates hypochlorous acid, a powerful oxidant and is implicated in oxygen-dependent microbicidal activity of phagocytes. Excessive production of MPO-derived pro-oxidants has been associated with tissue injury in acute and chronic inflammation [49]. MCP-1 is a chemokine that control migration and infiltration of leukocytes at the inflamed foci [50]. It has been reporting that the elevated MCP-1 level following carrageenan application in acute pleural inflammation model; suggesting the infiltration of innate immune cells at inflamed tissue [51].
It is widely established that the regulation of inflammation is a complex process coupled with multiple pathways including NF-κB signaling pathway. NF-κB is a transcriptional factor located in the cytoplasm bound to IκBs. During pathological conditions, NF-κB detached from IκBs and translocates into nucleus in order to control the secretion of inflammatory mediators [52]. Therefore, accumulative evidences attributed the elevation in level and expression of the examined inflammatory cytokines including TNF-α, IL-1β, IL-6, iNOS, NO, COX-2, PGE2, MPO and MCP-1 to the activation of NF-κB in the inflamed paw tissue, which may be due to overproduction of ROS follow carrageenan injection [8, 53, 54].
The suppression of pro-inflammatory cytokines is essential target to regulate and control the progression of acute and chronic inflammatory response. In the current investigation, astragalin supplementation to carrageenan treated mice showed potent anti-inflammatory properties through preventing the overproduction of the examined inflammatory molecules in the inflamed paw skin. Astragalin was found to decrease IL-1β-mediated increase in COX-2 and iNOS activities along with their products, namely PGE2 and NO in human osteoarthritis chondrocyte [55]. Authors elucidated this anti-inflammatory effect to the ability of astragalin to deactivate NF-B and MAPK signaling. In another study, Han et al. [33] showed that astragalin protected the testicular tissue following streptozotocin exposure through suppressing iNOS activity, NO and TNF-α levels along with improving the antioxidant status. Additionally, astragalin blocked the immigration and infiltration of leukocytes as confirmed by the deactivation of MPO and attenuation of TNF-α, IL-1β, IL-6 and their mediator, NF-κB in mastitis model induced by lipopolysaccharide [19]. Due to its potent anti-inflammatory properties, astragalin has been suggested to treat allergic inflammation and airway thickening induced by ovalbumin through inhibiting MCP-1 and α-SMA [56].
Conclusion
The obtained findings demonstrated that astragalin administration in carrageenan-induced paw edema improved the antioxidant status and elicited anti-inflammatory activity in the inflamed tissue through inhibiting the release of inflammatory cytokines including TNF-α, IL-1β, IL-6, and deactivating COX-2 and iNOS activities and their products (PGE2 and NO) along with inhibiting the activity of MPO and preventing the infiltration of the inflammatory cells at the inflamed tissue. Astragalin also inhibited the oxidative challenge associated with the developed acute inflammation. The recorded antioxidant and anti-inflammatory effects could be due to the ability of astragalin to decrease NF-κB p65 level and quenching ROS.
References
Chen L et al (2018) Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 9(6):7204
Nathan C, Ding A (2010) Nonresolving inflammation. Cell 140(6):871–882
Ahmed AU (2011) An overview of inflammation: mechanism and consequences. Front Biol 6(4):274
Laveti D et al (2013) Anti-inflammatory treatments for chronic diseases: a review. Inflamm Allergy-Drug Targets. 12(5):349–361
Makni S et al (2019) Emex spinosa (L.) Campd ethyl acetate fractions effects on inflammation and oxidative stress markers in carrageenan induced paw oedema in mice. J Ethnopharmacol 234:216–224
Ou Z et al (2019) Anti-inflammatory effect and potential mechanism of betulinic acid on lambda-carrageenan-induced paw edema in mice. Biomed Pharmacother 118:109347
Harirforoosh S, Asghar W, Jamali F (2013) Adverse effects of nonsteroidal antiinflammatory drugs: an update of gastrointestinal, cardiovascular and renal complications. J Pharm Pharmaceut Sci 16(5):821–847
Zhang H et al (2020) Diallyl Disulfide suppresses inflammatory and oxidative machineries following carrageenan injection-induced paw Edema in Mice. Mediat Inflamm 2020:8508906
Bao Y et al (2018) Therapeutic effects of Smilax glabra and Bolbostemma paniculatum on rheumatoid arthritis using a rat paw edema model. Biomed Pharmacother 108:309–315
Vinegar R et al. (1987) Pathway to carrageenan-induced inflammation in the hind limb of the rat. In: Federation proceedings.
Abdelfattah MS et al (2020) Rutin and selenium co-administration reverse 3-nitropropionic acid-induced neurochemical and molecular impairments in a mouse model of huntington's disease. Neurotox Res 37(1):77–92
Karna KK et al (2019) The ameliorative effect of monotropein, astragalin, and spiraeoside on oxidative stress, endoplasmic reticulum stress, and mitochondrial signaling pathway in varicocelized rats. BMC Complement Altern Med 19(1):333
Abdelhafez OH et al (2018) Hepatoprotective potential of Malvaviscus arboreus against carbon tetrachloride-induced liver injury in rats. PLoS ONE 13(8):e0202362
Chen M et al (2017) Astragalin-induced cell death is caspase-dependent and enhances the susceptibility of lung cancer cells to tumor necrosis factor by inhibiting the NF-small ka CyrillicB pathway. Oncotarget 8(16):26941–26958
Rey D et al (2019) Astragalin augments basal calcium influx and insulin secretion in rat pancreatic islets. Cell Calcium 80:56–62
Yan L, Zhou QH (2012) Study on neuroprotective effects of astragalan in rats with ischemic brain injury and its mechanisms. Zhongguo Ying Yong Sheng Li Xue Za Zhi 28(4):373–377
Qu D et al (2016) Cardioprotective effects of Astragalin against myocardial ischemia/reperfusion injury in isolated rat heart. Oxid Med Cell Longev 2016:8194690
Jia Q et al (2019) Astragalin suppresses inflammatory responses and bone destruction in mice with collagen-induced arthritis and in human fibroblast-like Synoviocytes. Front Pharmacol 10:94
Li F et al (2013) Astragalin suppresses inflammatory responses via down-regulation of NF-kappaB signaling pathway in lipopolysaccharide-induced mastitis in a murine model. Int Immunopharmacol 17(2):478–482
Soromou LW et al (2012) Astragalin attenuates lipopolysaccharide-induced inflammatory responses by down-regulating NF-κB signaling pathway. Biochem Biophys Res Commun 419(2):256–261
Ghafarzadeh S et al (2019) Crocin exerts improving effects on indomethacin-induced small intestinal ulcer by antioxidant, anti-inflammatory and anti-apoptotic mechanisms. Vet Res Forum 10(4):277–284
Bradley PP et al (1982) Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol 78(3):206–209
Green LC et al (1982) Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 126(1):131–138
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358
Nishikimi M, Appaji N, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 46(2):849–854
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Zouari Bouassida K. et al. (2018) Effects of Juniperus phoenicea hydroalcoholic extract on inflammatory mediators and oxidative stress markers in carrageenan-induced paw oedema in mice. Biomed Res Int.
Ayala A, Muñoz MF, Argüelles S (2014) Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev, 2014.
Sangeetha Lakshmi B et al (2018) Changes in the inflammatory and oxidative stress markers during a single hemodialysis session in patients with chronic kidney disease. Ren Fail 40(1):534–540
Ighodaro O, Akinloye O (2018) First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex J Med 54(4):287–293
Yin M et al (2019) Oleuropein suppresses oxidative, inflammatory, and apoptotic responses following glycerol-induced acute kidney injury in rats. Life Sci 232:116634
Cho I-H et al (2014) Astragalin inhibits airway eotaxin-1 induction and epithelial apoptosis through modulating oxidative stress-responsive MAPK signaling. BMC Pulm Med 14(1):122
Han X-X et al (2019) Protective effects of Astragalin on spermatogenesis in streptozotocin-induced diabetes in male mice by improving antioxidant activity and inhibiting inflammation. Biomed Pharmacother 110:561–570
Zheng D et al (2019) Astragalin reduces lipopolysaccharide-induced acute lung injury in rats via induction of heme oxygenase-1. Arch Pharmacal Res 42(8):704–711
Almeer RS et al (2019) Anti-inflammatory and anti-hyperuricemic functions of two synthetic hybrid drugs with dual biological active sites. Int J Mol Sci 20(22):5635
Tanaka T, Narazaki M, Kishimoto T (2014) IL-6 in inflammation, immunity, and disease. Cold Spring Harbor Perspect Biol 6(10):a016295
Mateen S et al (2016) Understanding the role of cytokines in the pathogenesis of rheumatoid arthritis. Clin Chim Acta 455:161–171
Alsousi A, Siddiqui S, Igwe O (2017) Cytokine-mediated differential regulation of cyclooxygenase-2, high mobility group box 1 protein and matrix metalloproteinase-9 expression in fibroblast-like synovial cells. J Clin Exp Pharmacol 7:4
Legler DF et al (2010) Prostaglandin E2 at new glance: novel insights in functional diversity offer therapeutic chances. Int J Biochem Cell Biol 42(2):198–201
Funk CD (2001) Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 294(5548):1871–1875
Ricciotti E, FitzGerald GA (2011) Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol 31(5):986–1000
Soufli I et al (2016) Overview of cytokines and nitric oxide involvement in immuno-pathogenesis of inflammatory bowel diseases. World J Gastrointest Pharmacol Ther 7(3):353–360
Al-Megrin WA et al (2020) Coenzyme Q10 activates the antioxidant machinery and inhibits the inflammatory and apoptotic cascades against lead acetate-induced renal injury in rats. Front Physiol 11:64
Halici Z et al (2007) Amiodarone has anti-inflammatory and anti-oxidative properties: an experimental study in rats with carrageenan-induced paw edema. Eur J Pharmacol 566(1–3):215–221
Mansouri MT et al (2015) A study of the mechanisms underlying the anti-inflammatory effect of ellagic acid in carrageenan-induced paw edema in rats. Indian J Pharmacol 47(3):292
Wu KK (1995) Inducible cyclooxygenase and nitric oxide synthase. In: Advances in pharmacology. Elsevier. pp 179–207.
Davidge ST et al (1995) Nitric oxide produced by endothelial cells increases production of eicosanoids through activation of prostaglandin H synthase. Circ Res 77(2):274–283
Dkhil MA et al (2018) Ziziphus spina-christi (L.) leaf extract alleviates myocardial and renal dysfunction associated with sepsis in mice. Biomed Pharmacother 102:64–75
Van der Veen BS, de Winther MP, Heeringa P (2009) Myeloperoxidase: molecular mechanisms of action and their relevance to human health and disease. Antioxid Redox Signal 11(11):2899–2937
Deshmane SL et al (2009) Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res 29(6):313–326
Lansley SM, Cheah HM, Lee YC (2017) Role of MCP-1 in pleural effusion development in a carrageenan-induced murine model of pleurisy. Respirology 22(4):758–763
Al-Brakati et al (2019) The protective efficacy of soursop fruit extract against hepatic injury associated with acetaminophen exposure is mediated through antioxidant, anti-inflammatory, and anti-apoptotic activities. Environ Sci Pollut Res Int 26(13):13539–13550
Vargas-Ruiz R et al (2020) Effect of phenolic compounds from Oenothera rosea on the kaolin-carrageenan induced arthritis model in mice. J Ethnopharmacol 253:112711
Pei H et al (2020) Alkaloids from black pepper (Piper nigrum L.) exhibit anti-inflammatory activity in murine macrophages by inhibiting activation of NF-kappaB pathway. J Agric Food Chem. 68(8):2406–2417
Ma Z et al (2015) Astragalin inhibits IL-1β-induced inflammatory mediators production in human osteoarthritis chondrocyte by inhibiting NF-κB and MAPK activation. Int Immunopharmacol 25(1):83–87
Kim YH et al (2017) Astragalin inhibits allergic inflammation and airway thickening in ovalbumin-challenged mice. J Agric Food Chem 65(4):836–845
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no conflict of interest.
Ethical Approval
The experimental design and the employed animals were approved by the Research Ethics Committee, Taif University (Application No.: 41-00151) in accordance with the National Institutes of Health (NIH) Guidelines for the Care and Use of Laboratory Animals 8th edition.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alblihed, M.A. Astragalin attenuates oxidative stress and acute inflammatory responses in carrageenan-induced paw edema in mice. Mol Biol Rep 47, 6611–6620 (2020). https://doi.org/10.1007/s11033-020-05712-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05712-z