Abstract
Thymoquinone (TQ) is an active constituent in Nigella sativa (black cumin) and is extensively reported for its distinguished antioxidant and anti-inflammatory bioactivities. Despite the local protective response of acute inflammation, it contributes to the development of various disease conditions such as cell death, organ damage, or carcinogenesis. Hence, in this study, the effects of orally administered TQ (50 mg/kg and 100 mg/kg) for 14 days against edema development, oxidative stress, and inflammation were investigated in paw edema induced by carrageenan in mice. Indomethacin (10 mg/kg) was used as a reference drug. The results revealed that TQ reduced the paw edema volume in a time-dependent manner, attenuated acetic acid-provoked writhing movements, and reduced xylene-triggered ear edema. Hematological findings revealed marked normalization of altered counts of WBCs, and platelets. Furthermore, paw tissue levels of malondialdehyde and nitric oxide showed marked decreases together with increases in nuclear factor erythroid 2-related factor 2, glutathione, superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase after TQ administration. Additionally, TQ decreased pro-inflammatory mediators, such as interleukin-1 beta, tumor necrosis factor-alpha, interleukin-6, monocyte chemoattractant protein-1, C-reactive protein, myeloperoxidase, and nuclear factor kappa-B in the inflamed paw tissue. Moreover, appreciable decreases were recorded in cyclooxygenase-2 and its product prostaglandin E2 and the immune reaction of tumor necrosis factor-alpha in TQ-treated mice. Histopathological findings further validated the potential antiedematous, anti-inflammatory power of TQ in inflamed tissues. Conclusively, the results encourage the potent application of TQ to subside acute inflammatory events because of its striking antioxidant and anti-inflammatory properties in inflamed paw tissue.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Inflammation is an ordered immune response to counteract tissue injury that can be triggered by noxious stimuli such as microbes, toxicants, irradiation, or even against the damaged cells (Alblihed 2020; Cordaro et al. 2020). The acute inflammatory reaction is accompanied by several microcirculatory events, including changes in the vascular permeability, excess leukocytic infiltration, and extra-production of pro-inflammatory mediators with subsequent redness, swelling, hyperthermia, and pain (Su et al. 2019; Zhang et al. 2020). These cellular and molecular sequences are considered as guarding mechanisms of tissues against the initial injury as well as restoring their homeostasis (Zhang et al. 2020). However, an uncontrolled inflammatory response may lead to chronic inflammation that contributes to the development of various chronic disorders such as cancers, neurodegeneration, autoimmune, cardiac, and aging disorders (Alblihed 2020; Pop et al. 2020).
During the inflammatory process, the phagocytes produce an excess amount of reactive oxygen species (ROS) (Mitrea et al. 2020). Owing to their instability, ROS tend to steal electrons from other cellular molecules that consequently trigger oxidative stress, depletion of the antioxidant system, and lipid peroxidation (Li et al. 2021; Pop et al. 2020). Furthermore, ROS activates the transcription factor, nuclear factor kappa-B (NF-κB), which controls and maintains the inflammatory condition by stimulating the release of pro-inflammatory cytokines (El-Shitany and Eid 2019b). Cyclooxygenase-2 (COX-2) enzyme has a principal role in the production of prostaglandins in inflamed tissue (Almeer et al. 2019). Based on the major contribution of ROS in initiating inflammatory processes, their neutralization by antioxidants stands as a potent inhibitor for tissue inflammation and its consequences (Zhang et al. 2020).
Inflammation and edematous conditions are currently treated by synthetic anti-inflammatory agents that are unfortunately associated with various adverse effects (Abdel-Lateff et al. 2020, Akhtar and Shabbir 2019). For instance, nonsteroidal anti-inflammatory drugs like indomethacin and ibuprofen are associated with gastrointestinal tract disturbances such as vomiting, bleeding, gastric ulcer, diarrhea, and increased risk of renal tubular necrosis and myocardial infarction (Alblihed 2020). Additionally, corticosteroids have established serious health conditions such as hypertension, compromised immunity, diabetes, and osteoporosis (Abdel-Lateff et al. 2020). In this regard, alternative anti-inflammatory therapy with maximum efficacy and minimum adverse effects is urgently needed (El-Dershaby et al. 2022; Zhang et al. 2020). The public and scientific community have recently accepted natural products due to their safety profile and abundant bioactive chemical ingredients (Majdalawieh and Fayyad 2015).
Nigella sativa (black cumin) is a member of the Ranunculaceae family and is used extensively to treat cardiovascular disorders, gastrointestinal diseases, respiratory diseases, hypertension, dyslipidemia, diabetes, and various types of cancer (Al Aboud et al. 2021; Pop et al. 2020). Its bioactive components explain its beneficial effects (Pop et al. 2020). Among them, thymoquinone (TQ) is an aromatic ketone derived from the seed oil extract of this plant (Al Aboud et al. 2021). TQ possesses various cytoprotective properties like antioxidant, anti-inflammatory, and anti-tumor activities (Al-Brakati et al. 2019; Lei et al. 2012). Several studies have shown that TQ effectively alleviated inflammatory conditions in experimental models of osteoarthritis (Wang et al. 2015), acute pancreatitis (Dur et al. 2016), otitis media (Gülmez et al. 2017b), corrosive oesophagitis (Karaca et al. 2017), and bronchial asthma (Su et al. 2016). Concerning the dermato-pharmacological effects, TQ was efficient against skin fungal and bacterial infections as well as skin tumors (Al Jabre 2005; Ivankovic et al. 2006; Kundu et al. 2013). Kundu et al. (2013) reported that TQ inhibits epidermal inflammation induced by phorbol ester via attenuation of NF-κB and COX-2 in addition to enhancement of cytoprotective enzymes expression in mice skin. Moreover, topically applied TQ for four weeks significantly relieved the signs of allergy or hypersensitivity in hand eczema, similar to the effect of betamethasone (Yousefi et al. 2013).
As well known, carrageenan is widely used for the induction of acute inflammation by stimulating cell infiltration, mainly neutrophils, and producing inflammatory mediators such as myeloperoxidase and cytokines (Zhang et al. 2020). The Inhibition of carrageenan-mediated inflammatory response has been shown to be highly predictive of anti-inflammatory drug activity in human inflammatory diseases (Morris 2003). Furthermore, oxidative damage and lipid peroxidation are implicated in the progress of inflammatory responses.
With this background in mind, we investigated the anti-inflammatory efficacy of TQ using the xylene-induced ear edema test, the antinociceptive activity test, and carrageenan-induced paw edema in mice via examination of the oxidative stress markers and inflammatory mediators in the inflamed skin.
Material and methods
Chemicals and reagents
TQ (CAS number: 490–91-5), indomethacin (CAS number: 53–86-1), and carrageenan (CAS number: 9064–57-7) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Acetic acid and xylene were obtained from El-Gomhouria Co. For Trading Drugs, Chemicals & Medical Supplies. All other reagents were of high analytical grade.
Experimental animals and ethics statement
Male Swiss mice, 20–25 g in weight and 8–10 weeks in age, were obtained from the animal house of the VACSERA (Cairo, Egypt). They were housed in ordinary cages at 12 h light/dark cycle, 25 ± 1 ℃ temperatures, and fed on rodent feed with free access to water. The animal care procedures are in agreement with the National Institutes of Health (NIH) Guidelines for the Care and Use of Laboratory Animals 8th edition and the Institutional Animal Ethics Committee guidelines for Laboratory Animal Care at Zoology Department, Faculty of Science, Helwan University (Approval Number: HU2020/Z/RKA820-01).
Xylene-induced ear edema test
For assessment of the anti-inflammatory action of TQ, a xylene-induced ear edema test was done following the method of Wang et al. (2014). In brief, mice received the TQ and indomethacin prior to xylene application. After 1 h, 20 μl of pure xylene/rat was applied to the inner aspect of the right ear. One hour following the xylene administration, all mice were killed, and both ears were sampled and weighed. The ear swelling was assessed depending on the difference in weight of both ears.
Antinociceptive activity of TQ
An acetic acid-induced writhing test was performed to estimate the pain-relieving efficacy of TQ (Dey et al. 2010). One hour prior to acetic acid (0.5 ml of 0.6% aqueous solution, intraperitoneal injection), all mice were treated with TQ and indomethacin. After that, mice were housed in separate cages. The writhing represents the contraction of the abdominal muscle for each mouse. After acetic acid injection for 20 min, these movements were counted for 10 min. Percent of inhibition was measured according to this equation:
The percent of inhibition = [(Wc − Wt) × 100] / Wc.
where Wc is the number of writhes in control mice and Wt is the number of writhes in treated mice.
Induction of paw edema by carrageenan injection
After being adapted for two weeks under the lab conditions, mice were allocated into five groups as follows.
The first group (control): Mice were orally administered normal saline (4 ml/kg) by oral gavage, simultaneously with the drug’s administration to the other groups.
The second group (carrageenan): Mice were injected with carrageenan (100 μl of 1%) subcutaneously in the subplantar tissues of the left hind paw of each animal (Zhang et al. 2020).
The third and fourth groups were orally administered TQ at doses of 50 mg/kg and 100 mg/kg body weight (Mansour et al. 2002), and the fifth group (indomethacin) received 10 mg/kg/day of indomethacin as a reference drug according to Mondal et al. (2019).
All groups received their treatments for seven consecutive days. Indomethacin and TQ were administered orally to the mice for seven days prior to carrageenan injection. A Vernier caliper instantly estimated the paw volume (LETICA Scientific Instruments, Barcelona, Spain) before and after carrageenan injection at 2 h, 4 h, 6 h, and 8 h. The obtained results were displayed as the difference in the paw volume (mL) in comparison with the right rear paw of the same animal. At 8 h, mice were sacrificed, and blood was collected from the retroorbital sinus. Paw skin samples were sampled and divided into two parts. The first part was immediately homogenized to obtain 50% (w/v) homogenate in an ice-cold medium containing 50 mM Tris–HCl (pH 7.4) and centrifuged at 500 × g for 10 min at 4 ℃. The supernatant was used for the biochemical analysis, whereas the second part was used for the histopathological examination.
Counting of white blood cells and platelets
Blood samples were collected in test tubes containing EDTA as an anticoagulant for counting white blood cells (WBCs) using a hemocytometer according to the method described by Coles (1986).
Estimation of C-reactive protein level
C-reactive protein (CRP) serum level was estimated colorimetrically using DiaSys Diagnostic kit (Germany).
Evaluation of antioxidant enzymatic activities in paw tissue
Superoxide dismutase (SOD) and catalase (CAT) activities in paw tissue were estimated by the difference in the color intensity at 539 nm and 240 nm, according to Fisher et al. (2003) and Aebi (1984), correspondingly. Glutathione peroxidase (GPx) and glutathione reductase (GR) were measured according to the method explained by Paglia and Valentine (1967) and Factor et al. (1998), respectively. Furthermore, glutathione (GSH) levels were determined by the reduction of Ellman’s reagent to yield a yellow compound. The amount of reduced chromogen indicates GSH content, and its absorbance was estimated at 405 nm (Ellman 1959). Malondialdehyde (MDA) in the paw tissue was estimated using thiobarbituric acid reactive substances at 535 nm (Ohkawa et al. 1979).
Estimation of cytosolic and nuclear Nrf2 activity in paw tissue
Briefly, tissue samples were incubated in 1 ml hypotonic solution containing 0.1% tergitol for 3 min. Next, tissue samples were homogenized. The solution was centrifuged to pellet nuclei (1000 × g, 5 min). The supernatant (cytoplasmic fraction) was re-centrifuged (15,000 × g, 3 min) to pellet debris.
Cytokines and inflammatory mediators in paw tissue
ELISA kits (CUSABIO Life Sciences, Wuhan, China) were utilized for the measurement of levels of interleukin-1 beta (IL-1β; cat. no: CSB-E04621m), tumor necrosis factor-alpha (TNF-α; cat. no: CSB-E04741m), interleukin-6 (IL-6; cat. no: CSB-E04640r), COX-2 (cat. no: CSB-E12910m), prostaglandin E2 (PGE2; cat. no: CSBPA040059), NF-κB (cat. no: CSB-E13148r), and monocyte chemoattractant protein-1 (MCP-1; cat. no: CSB-E07430m) according to the manufacturer’s information.
Myeloperoxidase activity and nitric oxide levels in paw tissue
Myeloperoxidase (MPO) activity was analyzed following the protocol of Bradley et al. (1982) with slight modifications. The paw tissue homogenate underwent three freeze-thawing cycles and centrifugation at 15,000 × g for 10 min at 4 ℃. Next, MPO activity was evaluated by adding 200 μL of the paw supernatant to 2.8 mL of 50 mM phosphate buffer (pH 6.0) and 1 mL of 1.67 mM o-dianisidine hydrochloride containing 0.0005% (v/v) H2O2. The MPO activity was calculated based on the change in the absorbance at 450 nm and presented as U/mg protein. Furthermore, levels of nitric oxide (NO) were measured as stated by Green et al. (1982) by adding the Griess reagent and sulfanilamide for 10 min in the dark at 30 ℃, and the absorbance of the bright reddish-purple azo dye was assessed at 540 nm.
Hematoxylin and eosin staining
Paw tissue specimens were fixed in 10% neutral buffered formalin for 24 h, dehydrated, and molten in paraplast. The resulting blocks were cut into 4–5 μm thick sections and stained with hematoxylin and eosin. The microscopic examination was performed under a Nikon microscope (Eclipse E200-LED, Tokyo, Japan).
Immunohistochemistry procedures
Sections from paw tissues were incubated in 10% H2O2 for 30 min to eliminate endogenous peroxidase activity and blocked for 1 h with 10% normal goat serum at room temperature. Sections were further incubated with primary anti-TNF-α antibodies (Abcam, Cambridge, MA; 1/1000) for 24 h at 4 ℃. Antibody detection was performed using the Histostain-Plus Bulk kit (Invitrogen) against rabbit IgG, and finally, 3,3’-diaminobenzidine was used for visualization. A Nikon microscope was used to capture photomicrographs at 400 × magnification (Eclipse E200-LED, Tokyo, Japan). TNF-α expression was estimated by counting each rat's TNF-α ( +) cells in random sections.
Statistical analysis
All data were expressed as the mean ± standard error (SE) after being analyzed by one-way analysis of variance (ANOVA) and post hoc Duncan’s multiple range test. Statistically significant differences were considered when p values were less than 0.05.
Results
The anti-inflammatory effect of TQ on ear edema in mice
The anti-inflammatory impact of TQ against xylene-induced ear edema is depicted in Fig. 1. The carrageenan-challenged group had significant (P < 0.05) inhibition in edema formation induced by xylene related to the control group. In contrast, TQ dose-dependently subsided xylene-induced edema in relation to the model group. Indomethacin at the dose of 10 mg/kg also caused a marked decrease in edema formation (P < 0.05) related to control. These findings showed the anti-oedematous and anti-inflammatory effect of TQ against edema formation by xylene in mice.
Effect of thymoquinone (TQ, 50 mg/kg and 100 mg/kg) or indomethacin (10 mg/kg) on ear edema volume in carrageenan-injected mice. Data are represented as mean ± SE (n = 7); #: p < 0.05 indicates a significant change versus the control group; $: p < 0.05 indicates a significant change versus the carrageenan-injected group
Antinociceptive effect of TQ on writhing movements in mice
The effect of TQ on the pain sensation indicated by the number of writhes in mice is shown in Fig. 2. Notably, substantial increases (P < 0.05) were detected in the number of writhes after injection of carrageenan compared to those of the control group. However, TQ at doses of 50 and 100 mg/kg induced a noteworthy antinociceptive effect (P < 0.05) following carrageenan injection. The reduction was dose-dependent, and the highest impact on the writhing count was observed at 100 mg/kg. Remarkably, the reference drug, indomethacin (10 mg/kg), declined the number of writhes noticeably related to the model group. These results revealed that the pre-treatment of TQ markedly relieved the pain, as shown by the decreased number of writhes in mice.
Effect of thymoquinone (TQ, 50 mg/kg and 100 mg/kg) or indomethacin (10 mg/kg) on writhing movements in carrageenan-injected mice. Data are represented as mean ± SE (n = 7); #: p < 0:05 indicates a significant change versus the control group; $: p < 0:05 indicates a significant change versus the carrageenan-injected group
The anti-oedematous activity of TQ in carrageenan-injected mice
As displayed in Fig. 3, subplanter injection of carrageenan developed marked edema in the rear paw skin after 1 h, reaching its maximum after 8 h. However, mice treated with either TQ or indomethacin for five days before carrageenan injection showed a notable decrease in (P < 0.05) the volume of paw edema in a time-dependent manner after 2 h, 4 h, and 8 h when compared to the model group. Remarkably, treatment of the carrageenan-injected group with the higher dose of TQ (100 mg/kg) evoked the maximum inhibition after 8 h that achieved a comparable anti-oedematous activity with that of 10 mg/kg/day of indomethacin. The results reflect the anti-oedematous action of TQ on the developed edema by attenuating the vascular variations following carrageenan injection.
Effect of thymoquinone (TQ, 50 mg/kg and 100 mg/kg) or indomethacin (10 mg/kg) on paw edema volume in carrageenan-injected mice. Data are represented as mean ± SE (n = 7); #: p < 0.05 indicates a significant change versus the control group; $: p < 0.05 indicates a significant change versus the carrageenan-injected group
Effect of TQ on CRP levels, WBCs, and platelets count in carrageenan-injected mice
Significant (P < 0.05) increases were recorded in levels of CRP, WBCs, and platelet count after carrageenan injection compared to the control group. Adversely, the level of CRP, WBCs, and platelet counts of indomethacin and TQ-treated mice recorded a significant decrease from those of the model group, as displayed in Fig. 4.
Effect of thymoquinone (TQ, 50 mg/kg and 100 mg/kg) or indomethacin (10 mg/kg) on CRP levels, WBCs, and platelet count in carrageenan-injected mice. Data are represented as mean ± SE (n = 7); #: p < 0.05 indicates a significant change versus the control group; $: p < 0.05 indicates a significant change versus the carrageenan-injected group
Effect of TQ on oxidative stress biomarkers in paw tissue in carrageenan-injected mice.
Considering the implication of oxidant free radicals in the pathogenesis of inflammatory reactions and the ability of dietary antioxidants in scavenging or neutralizing free radicals, the oxidative stress biomarkers were examined in paw tissue (Fig. 5). Significant depletion (P < 0.05) was detected in the content of GSH associated with notable inhibitions (P < 0.05) of the enzymatic activities of SOD, CAT, GPx, and GR in the carrageenan group in comparison with the control group. Also, carrageenan injection induced noteworthy increments in the levels of MDA in paw tissue in relation to the control group. In contrast, pre-treatment of TQ or indomethacin before carrageenan injection significantly boosted (P < 0.05) the activities of antioxidant enzymes and GSH levels (P < 0.05) if compared with the model group. It is noteworthy that the pre-treatment of carrageenan-injected mice with TQ at the dose of 100 mg/kg efficiently restored the GSH and antioxidant enzymes in paw tissue to be near the normal control values. Also, the MDA levels in paw tissue of mice that were treated with the high dose of TQ evoked non-significant change in the control group.
Effect of thymoquinone ((TQ, 50 mg/kg and 100 mg/kg) or indomethacin (10 mg/kg) on oxidative stress markers in carrageenan-injected mice. Data are represented as mean ± SE (n = 7); #: p < 0.05 indicates a significant change versus the control group; $: p < 0.05 indicates a significant change versus the carrageenan-injected group
In order to better understand the antioxidant potency of TQ against carrageenan-induced oxidative injury in paw tissue, the levels of nuclear and cytoplasmic Nrf2 were assessed in all tested groups (Fig. 6). In comparison with the controls, carrageenan injection provoked significant increases (P < 0.05) in the cytoplasmic Nrf2 together with decreases (P < 0.05) in its nuclear levels in the paw tissue of treated mice. In contrast, the pre-treatment of the model group with TQ reversed notably the carrageenan-induced alterations in both cytoplasmic and nuclear Nrf2 (P < 0.05) in relation to the model group. Furthermore, administration of indomethacin-induced marked increase (P < 0.05) in the nuclear Nrf2 without any significant changes in the cytoplasmic one compared to the model group.
Effect of thymoquinone (TQ, 50 mg/kg and 100 mg/kg) or indomethacin (10 mg/kg) on the cytoplasmic and nuclear levels of Nrf-2 in carrageenan-injected mice. Data are represented as mean ± SE (n = 7); #: p < 0.05 indicates a significant change versus the control group; $: p < 0.05 indicates a significant change versus the carrageenan-injected group
Effect of TQ on pro-inflammatory cytokines and NF-κB in paw tissue of carrageenan-injected mice
The modulating effect of TQ on the levels of inflammatory mediators in the inflamed paw tissues after carrageenan injection is shown in Fig. 7. In comparison with the control group, the model group’s tissue levels of IL-1β, TNF-α, and IL-6 were meaningfully augmented (P < 0.05). Meanwhile, the administration of indomethacin or TQ at 50 mg/kg and 100 mg/kg before carrageenan injection notably subsided (P < 0.05) the inflammation via lessening these indices in comparison with the carrageenan-injected group. In addition, immunohistochemical examination showed that carrageenan injection increased the expression of TNF-α as compared to its expression in the control group. At the same time, the administration of either indomethacin or TQ lessened its expression in the paw tissue as compared to the model group (Fig. 8).
Effect of thymoquinone (TQ, 50 mg/kg and 100 mg/kg) or indomethacin (10 mg/kg) on inflammatory cytokines and NF-κB in carrageenan-injected mice. Data are represented as mean ± SE (n = 7); #: p < 0.05 indicates a significant change versus the control group; $: p < 0.05 indicates a significant change versus the carrageenan-injected group
To elucidate the implicated mechanisms in the anti-inflammatory activity of TQ, levels of NF-κB were assessed following carrageenan injection in mice. As known, NF-κB has a vital role in the regulation and control of the expression of inflammatory mediators. Our results showed a marked increase (P < 0.05) in the level of NF-κB in the inflamed paw tissue in the model group relative to the control mice. Remarkably, TQ and indomethacin notably lessened (P < 0.05) the level of this critical transcriptional factor upon carrageenan application in mice. Moreover, TQ at doses of 100 mg/kg was able to reduce the levels of NF-κB in paw tissues close to those in the control group, reflecting its ability to suppress the development of acute inflammatory reactions following carrageenan application.
Effect of TQ on the levels of NO, COX‑2, and PGE2 in paw tissue following carrageenan injection in mice
COX-2 has a vital role during inflammatory events by modulating the production of PGE2 from the arachidonic acid cascade. Compared to the control group, our results demonstrate noteworthy increases (P < 0.05) in the levels of NO, COX-2, and PGE2 after carrageenan injection. Adversely, pre-treatment with indomethacin and TQ at doses of 50 mg/kg and 100 mg/kg lessened (P < 0.05) the levels of these indices in paw tissues when compared with the model group. These results indicate that TQ induced remarkable deactivation of COX-2 with a subsequent decrease in its product, PGE2, in the inflamed paw tissue related to the carrageenan-treated group (Fig. 9).
Effect of thymoquinone (TQ, 50 mg/kg and 100 mg/kg) or indomethacin (10 mg/kg) on the levels of NO, COX-2, and PGE-2 in carrageenan-injected mice. Data are represented as mean ± SE (n = 7); #: p < 0.05 indicates a significant change versus the control group; $: p < 0.05 indicates a significant change versus the carrageenan-injected group
Effect of TQ on the levels of MCP-1 and MPO in paw tissues after carrageenan injection in mice
For assessment of leukocytic migration and infiltration in the inflamed paw tissue, levels of MCP-1 and MPO were measured (Fig. 10). Compared to the normal mice, intraplantar injection of carrageenan triggered the activity (P < 0.05) of MPO and increased (P < 0.05) the level of MCP-1 when compared with the control group. Interestingly, pre-treatment of carrageenan-challenged mice with TQ or indomethacin-induced significant reduction (P < 0.05) in MPO activity and MCP-1 content in paw tissue compared to the model group. These findings signify the anti-inflammatory effect of TQ that is mediated by inhibition of neutrophil migration and infiltration at the inflamed foci.
Effect of thymoquinone (TQ, 50 mg/kg and 100 mg/kg) or indomethacin (10 mg/kg) on the levels of MCP-1 and MPO in carrageenan-injected mice. Data are represented as mean ± SE (n = 7); #: p < 0.05 indicates a significant change versus the control group; $: p < 0.05 indicates a significant change versus the carrageenan-injected group
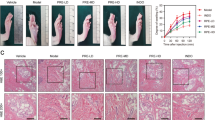
Histological changes in inflamed paw tissue in response to TQ treatment
As presented in Fig. 11, histopathological examination of paw tissue of carrageenan-exposed mice exhibited epithelial hyperplasia, infiltration of inflammatory cells, and subepidermal edema. These inflammatory signs were markedly decreased following both doses of TQ.
Discussion
Inflammation studies are currently one of the principal hubs of scientific research worldwide. Looking for novel substances for alleviating inflammatory ailments is still a subject of intense interest. Considering the associated adverse effects with the presently used anti-inflammatory medications, improving drugs from natural compounds is the product strategy for treating inflammatory responses (Cordaro et al. 2020; Zhang et al. 2020).
For a preliminary screening and understanding of the anti-oedematous and anti-inflammatory effects of TQ, the model of xylene-induced ear edema and inflammation was utilized. In agreement with previous reports (Akhtar and Shabbir 2019, Cui et al. 2020; Liu et al. 2020), the application of xylene on the ear edema in our study resulted in excess release of inflammatory mediators, inflammatory cell infiltration, local vasodilatation, capillary permeability, and edema. In contrast, pre-treated mice with TQ displayed a significant decrease in ear edema, indicating the suppressive effect of TQ on capillary permeability, edema development, and inflammation in mice ears. Demirel et al. (2018) reported that topically applied TQ resulted in a marked decrease in tissue edema in acute otitis externa in rats.
Pain is an unpleasant sensation associated with inflammation and generated by PGE2, histamine, serotonin, bradykinin, and inflammatory cytokines like TNF-α, IL-1β, IL-6, and IL-8 in the peritoneal fluid (Liu et al. 2020). Acetic acid-triggered abdominal writhing was established to assess the peripheral antinociceptive effect of TQ. Injection of acetic acid induces tissue damage and the release of inflammatory mediators such as histamine, bradykinin, and PG that, in turn, arouse nociceptors (Karim et al. 2019; Paradee et al. 2021). This process involves the activation of COX that catalyzes the conversion of arachidonic acid to PGE2 at the peritoneal receptors (Moharram et al. 2021). However, TQ antagonized acetic acid-induced shriveling in the abdominal muscles that advocates its peripheral antinociceptive effect via inhibiting inflammatory mediators and the PG pathway. It was formerly stated that administration of TQ notably relieved the formalin-induced pain in rats, and the antinociception was mediated through the NO/cGMP/KATP channel pathway (Parvardeh et al. 2018). Also, N. sativa fixed oil, including TQ, possesses appreciable anti-inflammatory and analgesic effects (Mahboubi et al. 2018). Amin et al. (2014) found that TQ markedly alleviated the neuropathic pain resulting from chronic constriction of the sciatic nerve in rats.
Carrageenan-mediated-paw edema is a well-known experimental model for the acute inflammatory response and screening of the anti-inflammatory potential of natural compounds and synthetic chemicals (Liu et al. 2020). In this model, carrageenan injection in the mice’s rear paws resulted in intense inflammatory events with a biphasic phenomenon (Mehrzadi et al. 2021). The early phase (0 to 1 h post-injection) is characterized by over secretion of serotonin, bradykinin, and histamine. These mediators initiate tissue edema by encouraging the local blood flow and increasing capillary permeability (Zahra et al. 2020). The delayed phase (after 1 h and peaked after 8 h) is associated with leukocytic migration and prostaglandin (Zhang et al. 2020). As previously reported (Mehrzadi et al. 2021; Zahra et al. 2020), our study revealed a visible increase in the paw volume after intraplantar injection of carrageenan compared to that of the control untreated paws. However, pre-treatment of mice with TQ in doses of 50 mg/kg and 100 mg/kg significantly decreased the edema volume of the paws injected with carrageenan. Likewise, indomethacin exhibited a significant anti-edema effect and reduced paw volume of the induced paws. These results imply the potent anti-inflammatory effect of TQ that is probably because of the inhibition of mediators of inflammation.
In accordance with former studies, our results revealed notable increases in WBCs count and platelet levels in carrageenan-injected mice in comparison with the control (Akhtar and Shabbir 2019, Shabbir et al. 2018). These results indicated the activation of the immune system against tissue injury caused by foreign agents (Akhtar and Shabbir 2019). Treatment with TQ normalized WBCs and platelets’ levels, reflecting its potential immunomodulatory effect. Our results are in harmony with earlier reports (Gülmez et al. 2017a; Nemmar et al. 2011), which revealed the normalization of disordered hematological markers by TQ in experimental models of otitis media and arthritis in rats.
CRP, a sensitive acute-phase protein, is a key inflammatory mediator secreted by hepatic tissue after being stimulated with cytokines such as IL-1, IL-6, and TNF-α (Hamsa and Kuttan 2011). It indicates the extent of tissue inflammation, and its increase is a predictive risk factor for the occurrence of organ damage (Jisha et al. 2019). In this study, carrageenan injection increased the CRP level in paw tissue, indicating a robust inflammatory response of the injured tissue, which agrees with former findings (Jisha et al. 2019; Zhang et al. 2020). On the contrary, the level of CRP displayed a notable decline in TQ-treated groups, which explains its potent anti-inflammatory property. Treatment of arthritic rats with TQ significantly decreased the levels of CRP in an experimental model of rheumatoid arthritis (Arjumand et al. 2019).
In inflammatory conditions, excess ROS is the culprit of cellular and organ damage (El-Shitany and Eid 2019b, Mehrzadi et al. 2021). In agreement with former authors (Alblihed 2020; Mehrzadi et al. 2021), marked increments were noticed in the levels of MDA accompanied by notable decreases in GSH levels and SOD, CAT, GR, and GPx activities in the paw tissues of carrageenan-injected mice. In addition, carrageenan injection resulted in notable increases in the levels of Nrf2 in the cytoplasm with decreases in the nucleus in the paw tissue of the model group. RT-PCR analysis that was performed by El-shitany and Eid revealed that carrageenan injection significantly decreased the Nrf2 expression of mRNA (El-Shitany and Eid 2019a). Remarkably, the treatment of mice with both TQ and indomethacin before carrageenan counteracted ROS overproduction and lessened the oxidative deleterious effects of carrageenan in mice paw tissue. These results are in accordance with former reports (Al Aboud et al. 2021; Dera et al. 2020; Dur et al. 2016; Kundu et al. 2013). Because of its potent radical scavenging ability, TQ alleviates the cellular oxidative stress and lessens the consequent lipid peroxidation (Al Aboud et al. 2021). Supporting former studies (Dera et al. 2020), the antioxidant efficacy of TQ may refer to the elevation of Nrf2 levels and its nuclear translocation along with blockage of Keap1 and induction of HO-1 expression. Our finding revealed that TQ at both tested doses decreased the cytoplasmic Nrf2 and increased its nuclear levels in the paw tissue. Normally, Nrf2 is attached to Keap1 in the cytosol, but under stress conditions of excess ROS production, it moves to the nucleus and binds to the antioxidant response element (ARE), and initiates the activation of cytoprotective enzymes (Albarakati et al. 2020; El-Khadragy et al. 2021; Yuan et al. 2020). It was reported that TQ treatment abrogated lung inflammation in a rat model of lung fibrosis through enhancement of the Nrf2/HO-1 signaling pathway (Ahmad et al. 2020). Modulation of Nrf2 signaling by TQ administration was also reported in different animal models (Amin et al. 2021; Hamdan et al. 2019; Sabir et al. 2022).
Furthermore, excessive ROS generation is strongly related to the progression of inflammation following carrageenan injection by activating immune cells and secretion of pro-inflammatory mediators (Alblihed 2020). In our study, carrageenan-injected mice displayed noteworthy elevations in levels of pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) in paw tissue compared with control mice. This agrees with former studies (Alblihed 2020; Cui et al. 2020; Mehrzadi et al. 2021). These inflammatory cytokines are produced by macrophages and monocytes, fibroblasts, and endothelial cells in situations of inflammatory reactions and cellular stress (Zhang et al. 2020). TNF-α, a pro-inflammatory cytokine, is involved in regulating the secretion of other inflammatory mediators (Liu et al. 2020; Mehrzadi et al. 2021). Also, IL-1β is a pro-inflammatory cytokine that enhances both local and systemic immune reactions (Mehrzadi et al. 2021). Furthermore, IL-1β and TNF-α trigger the expression of various enzymes, such as COX-2, with subsequent production of prostaglandins as PGE-2 (Alblihed 2020; Zhang et al. 2020). COX-2/PGE-2 signaling pathway plays a vital role in various inflammatory diseases (Alblihed 2020). In the present study, intraplantar injection of carrageenan induced the production of inflammatory mediators such as COX-2 and PGE-2, as reported in former studies (El-Shitany and Eid 2019b, Mitrea et al. 2020; Zhang et al. 2020). In contrast, TQ treatment notably attenuated all these inflammatory mediators in paw tissue, indicating its potent anti-inflammatory effect, which is in harmony with previous authors (Kundu et al. 2013; Su et al. 2016; Wang et al. 2015). TQ was reported to decrease the protein and gene expression of COX-2 in mice skin exposed to phorbol ester (Kundu et al. 2013). The reduction of these pro-inflammatory cytokines represents a key target to control and regulate the development of acute and chronic inflammation (Alblihed 2020).
It has been reported that both IL-1β and TNF-α trigger the production of inducible nitric oxide synthase (iNOS), resulting in the development of inflammatory responses via the production of NO (Alblihed 2020; Mehrzadi et al. 2021). Our results revealed high NO levels in paw tissue injected with carrageenan, similar to other reports (El-Shitany and Eid 2019b, Zhang et al. 2020). NO is an intercellular molecule that maintains the tissue homeostasis of numerous physiological functions (Alblihed 2020). However, high NO levels under pathological circumstances liberate peroxynitrite radicals that result in severe cellular damage such as oxidation of DNA and peroxidation of lipid molecules with subsequent tissue oxidative injury and inflammatory events (Zhang et al. 2020). Besides, earlier reports stated that high NO enhances the liberation of arachidonic acid from the cellular membrane, which activates COX-2 and PGE2 formation (Alblihed 2020; Zhang et al. 2020). Furthermore, elevated NO levels increase the COX-2 half-life via the production of free radicals and inhibition of COX-2 auto inactivation (Alblihed 2020). Similar to previous reports (Saghir et al. 2019; Wang et al. 2015), TQ pre-treatment decreased the NO levels in inflamed tissue, which is assumed by the regulation of inflammatory cytokines and the iNOS signaling pathway.
Regulation of inflammatory reaction is a complex process related to several pathways, such as the NF-κB signaling pathway that controls the expression of various pro-inflammatory mediators (El-Shitany and Eid 2019b). In accordance with former studies (El-Shitany and Eid 2019b, Liu et al. 2020; Mitrea et al. 2020), this study found a significant increment in paw NF-κB levels in the carrageenan group compared to the control. During pathological conditions, NF-κB translocates into the nucleus to control the release of different inflammatory mediators (Cui et al. 2020). Accordingly, the elevations in levels of inflammatory cytokines such as TNF-α, IL-1β, IL-6, NO, COX-2, and PGE2 are attributed to the activated NF-κB in the inflamed paw tissue following carrageenan injection (Alblihed 2020; Cui et al. 2020; Zhang et al. 2020). This explains the anti-inflammatory ability of TQ, as evidenced by low levels of NF-κB in TQ-treated mice. Former authors stated the ability of TQ to subside tissue inflammatory response by deactivating NF-κB and MAPKs signaling (Arjumand et al. 2019; Dera et al. 2020; Wang et al. 2015). Also, TQ was reported to lessen the nuclear translocation and the DNA binding of NF-κB by hindering the phosphorylation and consequent degradation of IkB-α in mice skin exposed to phorbol ester (Kundu et al. 2013).
Acute inflammation is characterized by adhesion and infiltration of white blood cells, particularly neutrophils. MPO is a protein secreted from neutrophils and is a reliable index to assess the degree of neutrophil infiltration (Abdel-Lateff et al. 2020). Its reaction with H2O2 and halides results in hypochlorous acid that possesses a strong oxidant with microbicidal activities (Alblihed 2020). However, a high MPO level indicates tissue injury in acute inflammatory response (Cui et al. 2020). In harmony with former reports (Abdel-Lateff et al. 2020; Cui et al. 2020; Zhang et al. 2020), this study revealed marked increases in MPO activity and MCP-1 levels in paw edema tissues. This suggests that carrageenan resulted in infiltration of neutrophils in inflammatory sites, as confirmed by histological alterations. MCP-1 is a vital chemokine that controls the immigration and infiltration of monocytes/macrophages at the inflamed foci (Zhang et al. 2020). Elevated MCP-1 levels were recorded following carrageenan injection in a model of acute pleurisy, demonstrating the infiltration of leukocytes in the inflamed tissues (Lansley et al. 2017). In this study, mice that received TQ displayed low levels of MPO and MCP-1 in inflamed paw tissues, and this is similar to former reports (Al Aboud et al. 2021; Karaca et al. 2017). TQ administration decreased the levels of hepatic MCP-1 in sepsis injury in BALB/c mice and MCP-1 levels of murine microglia cells treated with lipopolysaccharide (Taka et al. 2015; Wang et al. 2019). These findings further demonstrate the TQ protection against inflammation through blocking the migration and infiltration of immune cells in inflamed tissue as validated by the inhibition of MPO and downregulation of pro-inflammatory cytokines including MCP-1.
Conclusion
The current study collectively validated that TQ administration exerted remarkable inhibitory activity on the carrageenan-induced paw edema and consequently associated pain in mice. TQ could enhance cellular antioxidant activity significantly and lessen pro-inflammatory cytokine secretion. The anti-inflammatory characteristic of TQ is mediated by inhibition of COX-2 and NF-κB. Moreover, pre-treatment with TQ also provoked marked attenuation of ear edema, further confirming its potency in acute inflammatory models. Taken together, our results demonstrated that TQ possesses interesting anti-inflammatory, anti-oxidative, and analgesic activities that will be of interest for further investigation.
Data availability
All relevant data are within the paper.
References
Abdel-Lateff A, Alarif WM, Algandaby MM, Alburae NA, Abdel-Naim AB (2020) Euryops arabicus displays anti-inflammatory activities in experimental models. J Ethnopharmacol 247:112278
Aebi H (1984) [13] Catalase in vitro. Methods Enzymol 105:121–126
Ahmad A, Alkharfy KM, Jan BL, Ahad A, Ansari MA, Al-Jenoobi FI, Raish M (2020) Thymoquinone treatment modulates the Nrf2/HO-1 signaling pathway and abrogates the inflammatory response in an animal model of lung fibrosis. Exp Lung Res 46:53–63
Akhtar G, Shabbir A (2019) Urginea indica attenuated rheumatoid arthritis and inflammatory paw edema in diverse animal models of acute and chronic inflammation. J Ethnopharmacol 238:111864
Al Aboud D, Baty RS, Alsharif KF, Hassan KE, Zhery AS, Habotta OA, Elmahallawy EK, Amin HK, Moneim AEA, Kassab RB (2021) Protective efficacy of thymoquinone or ebselen separately against arsenic-induced hepatotoxicity in rat. Environ Sci Pollut Res 28:6195–6206
Al Jabre S (2005) In vitro antifungal activity of thymoquinone against Scopulariopsis brevicaulis. Arab J Pharm Sci 3:27–33
Albarakati AJA, Baty RS, Aljoudi AM, Habotta OA, Elmahallawy EK, Kassab RB, Abdel Moneim AE (2020) Luteolin protects against lead acetate-induced nephrotoxicity through antioxidant, anti-inflammatory, anti-apoptotic, and Nrf2/HO-1 signaling pathways. Mol Biol Rep 47:2591–2603
Alblihed MA (2020) Astragalin attenuates oxidative stress and acute inflammatory responses in carrageenan-induced paw edema in mice. Mol Biol Rep 47:6611–6620
Al-Brakati A, Kassab R, Lokman M, Elmahallawy E, Amin H, Abdel Moneim A (2019) Role of thymoquinone and ebselen in the prevention of sodium arsenite–induced nephrotoxicity in female rats. Hum Exp Toxicol 38:482–493
Almeer RS, Hammad SF, Leheta OF, Abdel Moneim AE, Amin HK (2019) Anti-inflammatory and anti-hyperuricemic functions of two synthetic hybrid drugs with dual biological active sites. Int J Mol Sci 20:5635
Amin B, Taheri MM, Hosseinzadeh H (2014) Effects of intraperitoneal thymoquinone on chronic neuropathic pain in rats. Planta Med 80:1269–1277
Amin N, Du X, Chen S, Ren Q, Hussien AB, Botchway BOA, Hu Z, Fang M (2021) Therapeutic impact of thymoquninone to alleviate ischemic brain injury via Nrf2/HO-1 pathway. Expert Opin Ther Targets 25:597–612
Arjumand S, Shahzad M, Shabbir A, Yousaf MZ (2019) Thymoquinone attenuates rheumatoid arthritis by downregulating TLR2, TLR4, TNF-α, IL-1, and NFκB expression levels. Biomed Pharmacother 111:958–963
Bradley PP, Priebat DA, Christensen RD, Rothstein G (1982) Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Investig Dermatol 78:206–209
Coles E (1986) A text book of veterinary clinical pathology. 4-Ed WB Sounders Company Philadelphia. London, Toronto, p 15–40
Cordaro M, Siracusa R, Fusco R, D’Amico R, Peritore AF, Gugliandolo E, Genovese T, Scuto M, Crupi R, Mandalari G (2020) Cashew (Anacardium occidentale L.) nuts counteract oxidative stress and inflammation in an acute experimental model of Carrageenan-induced Paw edema. Antioxidants 9:660
Cui L, Zhu W, Yang Z, Song X, Xu C, Cui Z, Xiang L (2020) Evidence of anti-inflammatory activity of Schizandrin A in animal models of acute inflammation. Naunyn-Schmiedeberg’s archives of pharmacology 393(11):2221–2229
Demirel H, Arlı C, Özgür T, İnci M, Dokuyucu R (2018) The role of topical thymoquinone in the treatment of acute otitis externa; an experimental study in rats. J Int Adv Otol 14:285–289
Dera A, Rajagopalan P, Ahmed I, Alfhili M, Alsughayyir J, Chandramoorthy HC (2020) Thymoquinone attenuates IgE-mediated allergic response via pi3k-Akt-NFκB pathway and upregulation of the Nrf2-HO1 axis. J Food Biochem 44:e13216
Dey YN, De S, Ghosh AK (2010) Evaluation of analgesic activity of methanolic extract of Amorphophallus paeoniifolius tuber by tail flick and acetic acid-induced writhing response method. Int J Pharm Biosci 1:662–668
Dur A, Kose H, Kocyigit A, Kocaman O, Ismayilova M, Sonmez FC (2016) The anti-inflammatory and antioxidant effects of thymoquinone on ceruleine induced acute pancreatitis in rats. Bratisl Lek Listy 117:614–618
El-Dershaby NH, El-Hawash SA, Kassab SE, Dabees HG, Abdel Moneim AE, Abdel Wahab IA, Abd-Alhaseeb MM, El-Miligy MM (2022) Rational design of biodegradable sulphonamide candidates treating septicaemia by synergistic dual inhibition of COX-2/PGE2 axis and DHPS enzyme. J Enzyme Inhib Med Chem 37:1737–1751
El-Khadragy MF, Al-Megrin WA, Alomar S, Alkhuriji AF, Metwally DM, Mahgoub S, Amin HK, Habotta OA, Abdel Moneim AE, Albeltagy RS (2021) Chlorogenic acid abates male reproductive dysfunction in arsenic-exposed mice via attenuation of testicular oxido-inflammatory stress and apoptotic responses. Chem Biol Interact 333:109333
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
El-Shitany NA, Eid BG (2019) Icariin modulates carrageenan-induced acute inflammation through HO-1/Nrf2 and NF-kB signaling pathways. Biomed Pharmacother 120:109567
El-Shitany NA, Eid BG (2019) Icariin modulates carrageenan-induced acute inflammation through HO-1/Nrf2 and NF-kB signaling pathways. Biomed Pharmacother 120:109567
Factor VM, Kiss A, Woitach JT, Wirth PJ, Thorgeirsson SS (1998) Disruption of redox homeostasis in the transforming growth factor-alpha/c-myc transgenic mouse model of accelerated hepatocarcinogenesis. J Biol Chem 273:15846–15853
Fisher AE, Maxwell SC, Naughton DP (2003) Catalase and superoxide dismutase mimics for the treatment of inflammatory diseases. Inorg Chem Commun 6:1205–1208
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem 126:131–138
Gülmez M, Okuyucu Ş, Dokuyucu R, Gökçe H (2017) The effect of caffeic acid phenethyl ester and thymoquinone on otitis media with effusion in rats. Int J Pediatr Otorhinolaryngol 96:94–99
Hamdan AM, Al-Gayyar MM, Shams MEE, Alshaman US, Prabahar K, Bagalagel A, Diri R, Noor AO, Almasri D (2019) Thymoquinone therapy remediates elevated brain tissue inflammatory mediators induced by chronic administration of food preservatives. Sci Rep 9:7026
Hamsa T, Kuttan G (2011) Evaluation of the anti-inflammatory and anti-tumor effect of Ipomoea obscura (L) and its mode of action through the inhibition of pro inflammatory cytokines, nitric oxide and COX-2. Inflammation 34:171–183
Ivankovic S, Stojkovic R, Jukic M, Milos M (2006) The antitumor activity of thymoquinone and thymohydroquinone in vitro and in vivo. Exp Oncol 28(3):220–224
Jisha N, Vysakh A, Vijeesh V, Latha MS (2019) Anti-inflammatory efficacy of methanolic extract of Muntingia calabura L. leaves in Carrageenan induced paw edema model. Pathophysiology 26:323–330
Karaca G, Aydin O, Pehlivanli F, Altunkaya C, Uzun H, Güler O (2017) Effectiveness of thymoquinone, zeolite, and platelet-rich plasma in model of corrosive oesophagitis induced in rats. Ann Surg Treat Res 92:396
Karim N, Khan I, Khan W, Khan I, Khan A, Halim SA, Khan H, Hussain J, Al-Harrasi A (2019) Anti-nociceptive and anti-inflammatory activities of asparacosin A involve selective cyclooxygenase 2 and inflammatory cytokines inhibition: an in-vitro, in-vivo, and in-silico approach. Front Immunol 10:581
Kundu JK, Liu L, Shin J-W, Surh Y-J (2013) Thymoquinone inhibits phorbol ester-induced activation of NF-κB and expression of COX-2, and induces expression of cytoprotective enzymes in mouse skin in vivo. Biochem Biophys Res Commun 438:721–727
Lansley SM, Cheah HM, Lee YG (2017) Role of MCP-1 in pleural effusion development in a carrageenan-induced murine model of pleurisy. Respirology 22:758–763
Lei X, Liu M, Yang Z, Ji M, Guo X, Dong W (2012) Thymoquinone prevents and ameliorates dextran sulfate sodium-induced colitis in mice. Dig Dis Sci 57:2296–2303
Li Z, Liu Y, Wang F, Gao Z, Elhefny MA, Habotta OA, Moneim AEA, Kassab RB (2021) Neuroprotective effects of protocatechuic acid on sodium arsenate induced toxicity in mice: role of oxidative stress, inflammation, and apoptosis. Chem Biol Interact 337:109392
Liu N, Zhang G-X, Niu Y-T, Wang Q, Zheng J, Yang J-M, Sun T, Niu J-G, Yu J-Q (2020) Anti-inflammatory and analgesic activities of indigo through regulating the IKKβ/IκB/NF-κB pathway in mice. Food Funct 11:8537–8546
Mahboubi M, Mohammad Taghizadeh Kashani L, Mahboubi M (2018) Nigella sativa fixed oil as alternative treatment in management of pain in arthritis rheumatoid. Phytomedicine 46:69–77
Majdalawieh AF, Fayyad MW (2015) Immunomodulatory and anti-inflammatory action of Nigella sativa and thymoquinone: a comprehensive review. Int Immunopharmacol 28:295–304
Mansour MA, Nagi MN, El-Khatib AS, Al-Bekairi AM (2002) Effects of thymoquinone on antioxidant enzyme activities, lipid peroxidation and DT-diaphorase in different tissues of mice: a possible mechanism of action. Cell Biochem Funct 20:143–151
Mehrzadi S, Khalili H, Fatemi I, Malayeri A, Siahpoosh A, Goudarzi M (2021) Zingerone mitigates carrageenan-induced inflammation through antioxidant and anti-inflammatory activities. Inflammation 44:186–193
Mitrea D, Malkey R, Florian T, Filip A, Clichici S, Bidian C, Moldovan R, Hoteiuc O, Toader A, Baldea I (2020) Daily oral administration of chlorogenic acid prevents the experimental carrageenan-induced oxidative stress. J Physiol Pharmacol 71:55–65
Moharram FA, Nagy MM, El Dib RA, El-Tantawy MM, El Hossary GG, El-Hosari DG (2021) Pharmacological activity and flavonoids constituents of Artemisia judaica L aerial parts. J Ethnopharmacol 270:113777
Mondal A, Maity TK, Bishayee A (2019) Analgesic and anti-inflammatory activities of quercetin-3-methoxy-4′-glucosyl-7-glucoside isolated from Indian medicinal plant Melothria heterophylla. Medicines 6:59
Morris CJ (2003) Carrageenan-induced paw edema in the rat and mouse. Inflammation protocols 225:115–121
Nemmar A, Al-Salam S, Zia S, Marzouqi F, Al-Dhaheri A, Subramaniyan D, Dhanasekaran S, Yasin J, Ali BH, Kazzam EE (2011) Contrasting actions of diesel exhaust particles on the pulmonary and cardiovascular systems and the effects of thymoquinone. Br J Pharmacol 164:1871–1882
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Paradee N, Koonyosying P, Kusirisin W, Janthip R, Kanjanapothi D, Pattanapanyasat K, Srichairatanakool S (2021) Analgesic, anti-inflammatory and anti-ulcer properties of Thai Perilla frutescence fruit oil in animals. Biosci Rep 41(1):BSR20203166
Parvardeh S, Sabetkasaei M, Moghimi M, Masoudi A, Ghafghazi S, Mahboobifard F (2018) Role of L-arginine/NO/cGMP/K(ATP) channel signaling pathway in the central and peripheral antinociceptive effect of thymoquinone in rats. Iran J Basic Med Sci 21:625–633
Pop RM, Sabin O, Suciu Ș, Vesa SC, Socaci SA, Chedea VS, Bocsan IC, Buzoianu AD (2020) Nigella sativa’s anti-inflammatory and antioxidative effects in experimental inflammation. Antioxidants 9:921
Sabir S, Saleem U, Akash MSH, Qasim M, Chauhdary Z (2022) Thymoquinone induces Nrf2 mediated adaptive homeostasis: implication for mercuric chloride-induced nephrotoxicity. ACS Omega 7:7370–7379
Saghir SAM, Al-Gabri NA, Khafaga AF, El-Shaer NH, Alhumaidh KA, Elsadek MF, Ahmed BM, Alkhawtani DM, Abd El-Hack ME (2019) Thymoquinone-PLGA-PVA nanoparticles ameliorate bleomycin-induced pulmonary fibrosis in rats via regulation of inflammatory cytokines and iNOS signaling. Animals 9(11):951
Shabbir A, Batool SA, Basheer MI, Shahzad M, Sultana K, Tareen RB, Iqbal J, SaeedUl H (2018) Ziziphora clinopodioides ameliorated rheumatoid arthritis and inflammatory paw edema in different models of acute and chronic inflammation. Biomed Pharmacother 97:1710–1721
Su X, Ren Y, Yu N, Kong L, Kang J (2016) Thymoquinone inhibits inflammation, neoangiogenesis and vascular remodeling in asthma mice. Int Immunopharmacol 38:70–80
Su Y, Xiong S, Lan H, Xu L, Wei X (2019) Molecular mechanism underlying anti-inflammatory activities of lirioresinol B dimethyl ether through suppression of NF-κB and MAPK signaling in in vitro and in vivo models. Int Immunopharmacol 73:321–332
Taka E, Mazzio EA, Goodman CB, Redmon N, Flores-Rozas H, Reams R, Darling-Reed S, Soliman KF (2015) Anti-inflammatory effects of thymoquinone in activated BV-2 microglial cells. J Neuroimmunol 286:5–12
Wang Y, Chen P, Tang C, Wang Y, Li Y, Zhang H (2014) Antinociceptive and anti-inflammatory activities of extract and two isolated flavonoids of Carthamus tinctorius L. J Ethnopharmacol 151:944–950
Wang D, Qiao J, Zhao X, Chen T, Guan D (2015) Thymoquinone inhibits IL-1β-induced inflammation in human osteoarthritis chondrocytes by suppressing NF-κB and MAPKs signaling pathway. Inflammation 38:2235–2241
Wang F, Lei X, Zhao Y, Yu Q, Li Q, Zhao H, Pei Z (2019) Protective role of thymoquinone in sepsis-induced liver injury in BALB/c mice. Exp Ther Med 18:1985–1992
Yousefi M, Barikbin B, Kamalinejad M, Abolhasani E, Ebadi A, Younespour S, Manouchehrian M, Hejazi S (2013) Comparison of therapeutic effect of topical Nigella with Betamethasone and Eucerin in hand eczema. J Eur Acad Dermatol Venereol 27:1498–1504
Yuan X, Fu Z, Ji P, Guo L, Al-Ghamdy AO, Alkandiri A, Habotta OA, Abdel Moneim AE, Kassab RB (2020) Selenium nanoparticles pre-treatment reverse behavioral, oxidative damage, neuronal loss and neurochemical alterations in pentylenetetrazole-induced epileptic seizures in mice. Int J Nanomed 15:6339–6353
Zahra Z, Khan MR, Shah SA, Maryam S, Majid M, Younis T, Sajid M (2020) Vincetoxicum arnottianum ameliorate inflammation by suppressing oxidative stress and pro-inflammatory mediators in rat. J Ethnopharmacol 252:112565
Zhang H, Shang C, Tian Z, Amin HK, Kassab RB, Abdel Moneim AE, Zhang Y (2020) Diallyl disulfide suppresses inflammatory and oxidative machineries following carrageenan injection-induced paw edema in mice. Mediators of inflamm 15:8508906
Funding
The authors thank the Taif University Researchers Supporting Program (Project number: TURSP-2020/151), Taif University, Saudi Arabia, for supporting the current study.
Author information
Authors and Affiliations
Contributions
Conceptualization and supervision: R.B.K. Animal treatments, molecular and biochemical investigations: O.A.H., R.B.K., A.A.A.O. A.T., and M.A. Histological methodology and investigation: O.A. and M.S.L. Data analysis, software, data curation, and visualization: K.F.A., A.A., H.K.A., S.M.D., and R.A.E. Writing – reviewing and editing manuscript: R.A.E., H.H.A.H., and N.D. Resources: H.A.A., F.A. H.H.A.H., and N.D. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The animal care procedures are in agreement with the National Institutes of Health (NIH) Guidelines for the Care and Use of Laboratory Animals 8th edition and the Institutional Animal Ethics Committee guidelines for Laboratory Animal Care at Zoology Department, Faculty of Science, Helwan University (Approval Number: HU2020/Z/RKA820-01).
Consent to participate
Not applicable.
Consent for publication
Consented.
Conflict of interest
The author declares no competing interest.
Additional information
Responsible Editor: Lotfi Aleya
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hijazy, H.H.A., Dahran, N., Althagafi, H.A. et al. Thymoquinone counteracts oxidative and inflammatory machinery in carrageenan-induced murine paw edema model. Environ Sci Pollut Res 30, 16597–16611 (2023). https://doi.org/10.1007/s11356-022-23343-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-23343-8