Abstract
Protocatechuic acid (PCA) is a major metabolite of anthocyanins. It has numerous pharmacological effects, including anti-inflammatory, antioxidant, and antitumoral activities. In the present study, we investigated the in vivo protective effect of PCA on acute lung injury (ALI) induced by lipolysaccharide (LPS) in mice. We treated mice with PCA 1 h before the intratracheal (i.n.) administration of LPS. The pulmonary injury severity was evaluated 6 h after LPS administration. We found that pretreatment with a 30 mg/kg of PCA markedly attenuated the LPS-induced histological alterations in the lung. In addition, PCA inhibited the production of several inflammatory cytokines, including tumor necrosis factor alpha (TNF-α), interleukin-1 beta (IL-1β), and IL-6, at 6 h in the bronchoalveolar lavage fluid (BALF) after LPS challenge. Furthermore, PCA significantly reduced the number of total cells, neutrophils, and macrophages in the BALF, and it significantly decreased the wet/dry weight (W/D) ratio of lungs and the protein concentration in the BALF. Additionally, Western blotting showed that PCA efficiently blunted nuclear factor-kappa B (NF-κB) activation by inhibiting the degradation and phosphorylation of IκBα, as well as the translocation of p65 from cytoplasm to the nucleus. In conclusion, these results indicate that PCA was highly effective in inhibiting acute lung injury (ALI) and may be a promising potential therapeutic reagent for ALI treatment. PCA may utilize the NF-κB pathway to attenuate the nonspecific pulmonary inflammation induced by LPS administration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Acute lung injury (ALI) and its more severe form, acute respiratory distress syndrome (ARDS), are characterized by an acute inflammation process in the airspaces and lung parenchyma [1]. Inflammatory response that leads to organ dysfunction and failure tends to be the major problem after injury in many clinical conditions, such as sepsis, severe burns, acute pancreatitis, hemorrhagic shock, and trauma. In these conditions, multiple organ dysfunction syndrome leads to organ failure; in addition, mortality becomes high, potentially exceeding 50% [1–4]. Several animal models have been developed to study the pathophysiological mechanisms involved in ALI. In particular, in vivo intratracheal administration of LPS has gained wide acceptance as a clinically relevant model of severe lung injury [5, 6]. LPS is a component of the Gram-negative bacterial cell wall, which could induce a disturbance in the immune and inflammatory responses [7]. In mice with ALI, LPS administration has been shown to injure epithelial cell layers, induce epithelial cell apoptosis, and lead to the release of proinflammatory cytokines, chemotactic factors, and reactive oxygen species, which cause the aggregation of neutrophilic leukocytes and, ultimately, lung tissue injury [8, 9]. Several candidate therapies have been applied to reduce lung injury, but the specific therapies used to prevent or reverse severe pulmonary injury remain inadequate [10, 11]. Thus, the present study was designed to determine whether PCA could prevent ALI induced by LPS administration in BALB/c mice.
Protocatechuic acid (PCA) is a major benzoic acid derivative found in vegetables, nuts, brown rice, fruits, and herbal medicines, and it also has a strong antioxidative effect [12]. The PCA used in this study comes from the leaves of ilex chinenses. Numerous pharmacological effects, such as antioxidative, antibacterial, and antitumor promotion activities, have been attributed to PCA [13–16]. It has been shown that PCA could inhibit hepatic damage by LPS administration [17]. In addition, Min et al. [18] have reported that PCA could inhibit LPS-stimulated tumor necrosis factor alpha (TNF-α) and interleukin-1 beta (IL-1β) secretion in RAW264.7 cells via the activation of the nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinase pathways. However, there are no published reports about the anti-inflammatory effects of PCA on LPS-induced ALI. In the present study, we sought to investigate whether an intraperitoneal (i.p.) injection of PCA could protect against non-specific pulmonary inflammation in mice. Our results could help to develop PCA as a potential treatment for ALI and meanwhile lay the foundation for the use of PCA as a clinical anti-infective drug.
MATERIALS AND METHODS
Animals
BALB/c mice (male, 8–12-week-old, 18–20 g each) were purchased from the Center of Experimental Animals of Baiqiuen Medical College of Jilin University (Jilin, China). All animals were housed in microisolator cages and received food and water ad libitum, with the laboratory temperature of 24 ± 1°C and relative humidity of 40–80%. Before experimentation, mice were housed for a minimum of 2–3 days to adapt them to the environment. All animal experiments were performed in accordance with the guide for the Care and Use of Laboratory Animals published by the US National Institute of Health.
Reagents
Protocatechuic acid was purchased from National Institutes for Food and Drug Control (Beijing, China, purity >98%). LPS (Escherichia coli O127: B8) was purchased from Sigma Co. Dexamethasone (DEX). Sodium phosphate injection (no. H41020055) was purchased from Changle Pharmaceutical Co (Xinxiang, Henan, China). Mouse TNF-α, IL-6, and IL-1β enzyme-linked immunosorbent assay (ELISA) kits were purchased from Biolegend, Inc. (San Diego, CA, USA). MPO determination kits were provided by Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu Province, China). Rabbit mAb IκBα, p65, and mouse mAb p-IκBα were purchased from Cell Signaling Technology Inc (Beverly, MA, USA). Horseradish peroxidase (HRP)-conjugated goat anti-rabbit, goat-mouse antibodies (GE Healthcare, Buckinghamshire, UK). Nuclear and cytoplasmic protein extraction kit and cell lysis buffer for Western and IP were purchased from Beyotime Institute of Biotechnology, China. All other chemicals were of reagent grade.
LPS-induced ALI Model in Mice
All mice were randomly divided into four groups: the control group, LPS group, protocatechuic acid (PCA) + LPS group, and dexamethasone (DEX) + LPS group. Before inducing acute lung inflammation, protocatechuic acid (30 mg/kg) was given with an intraperitoneal (i.p.) injection, while dexamethasone (0.5 mg/kg) was intraperitoneal injected as a positive control. Control and LPS mice were given an equal volume of distilled water instead of protocatechuic acid or DEX. One hour later, mice were slightly anesthetized with an inhalation of diethyl ether. Then, 10 μg of LPS was instilled intranasally (i.n.) in 50 μl PBS to induce lung injury. Control mice were given a 50-μL PBS i.n. instillation without LPS.
Bronchoalveolar Lavage and Cell Counting
At 6 hours after LPS challenge, mice were sacrificed by diethyl ether. BALF was obtained by intratracheal instillation through lavaging the lungs three times with 0.5 mL of sterile PBS (7.2), the fluid recovery rate was 87 ± 2%. After immediate centrifuging (4°C, 3000 rpm) each BALF sample for 10 min, cell-free supernatant was obtained and stored at −80°C for further subsequent analysis of protein and cytokine levels. Remaining cell pellets were resuspended in PBS for total cells, neutrophils, and macrophage counts using hemacytometer and cytospins by staining with the Wright–Giemsa staining method.
Cytokine Assays
TNF-α, IL-1β, and IL-6 in supernatant of BALF were measured by ELISA kits according to the instructions recommended by the manufactures (BioLegend, Inc. Camino Santa Fe, Suite E, San Diego, CA, USA). The optical density (OD) of the microplate was read at 450 nm. The levels of TNF-α, IL-1β, and IL-6 were expressed as picto- or nanogram per milliliter of BALF based on the appropriate standard curve.
Protein Analysis
Protein concentrations in the supernatant of the BALF were quantified using the BCA protein assay kit to evaluate vascular permeability in the airways of mice.
Lung Wet/Dry Weight Ratio
After intratracheal instillation of LPS for 6 h, mice were euthanized; the lungs were excised by blunt dissection. The lungs were blotted dry, weighed to obtain the “wet” weight, and then placed in an oven at 80°C for 48 h to acquire the “dry” weight. The ratio of the wet lung to the dry lung was calculated to assess tissue edema.
Myeloperoxidase Activity in Lung
Myeloperoxidase (MPO) activity in lung was determined using test kits purchased from Nanjing jiancheng Bioengineering Institute (China); every procedure was according to the instructions. Six hours after LPS treatment, mice under diethyl ether anesthesia were killed, the right lungs were excised. One hundred milligrams of lung tissue were homogenized and fluidized in extraction buffer to obtain 5% of homogenate. The sample including 0.9 mL homogenate and 0.1 mL of reaction buffer was heated to 37°C in water bath for 15 min; then, the enzymatic activity was determined by measuring the changes in absorbance at 460 nm using a 96-well plate reader.
Histopathological Evaluation
The lungs were harvested at 6 h after the injection of LPS. Then, the lungs tissue was fixed with 10% neutral formalin for 24 h, imbedded in paraffin and sliced. Then, the paraffin sections were stained with hematoxylin and eosin (H&E).
Evaluation of lung edema and inflammatory cell infiltration was observed under the light microscopy.
Western Blot Analysis
Tissues were harvested at 6 h after LPS administration and frozen in liquid nitrogen immediately until homogenization. Proteins were extracted from the lungs using a nuclear and cytoplasmic protein extraction kit. Whole cell lysates was performed using a cell lysis buffer for Western and IP according to the manufacturer’s protocol. Protein concentrations were determined by BCA protein assay kit, and equal amounts of protein were loaded per well on a 10% sodium dodecyl sulfate polyacrylamide gel. Subsequently, proteins were transferred onto polyvinylidene difluoride membrane. The blots were then washed in Tris–Tween-buffered saline [TTBS, 20 mM Tris–HCl buffer, pH 7.6, containing 137 mM NaCl and 0.05% (vol ol) Tween 20], blocked overnight with 5% (wt ol) nonfat dry milk, and probed according to the method described by Towbin et al. Incubation with primary antibodies IκBα, p-IκBα, and p65 (Beverly, MA, USA), in diluent buffer [5% BSA (wt ol) and 0.1% Tween 20 in TBS] was performed overnight at 4°C (1:1,000 dilution). Membrane was then washed three times for 5 min each with TTBS. The primary antibody was probed with HRP-conjugated goat anti-rabbit (1:7,000) and HRP-conjugated goat anti-mouse (1:7000) for 1 h, washed three times in TTBS and processed with supersignal west pico chemiluminescent substrate. The β-actin Western blot was performed as an internal control of protein loading.
Statistical Analysis
All values are expressed in the form of mean ± SEM. Differences between mean values of normally distributed data were assessed with one-way ANOVA (Dunnett’s t test) and two-tailed Student’s t test. Statistical significance was accepted at p < 0.05 or p < 0.05.
RESULTS
Effects of Protocatechuic Acid on Inflammatory Cell Count in BALF of LPS-Induced ALI Mice
After intratracheal instillation of LPS for 6 h, the number of total cells, neutrophils, and macrophages significantly increased compared with the control group (p < 0.01). Protocatechuic acid (30 mg/kg) and DEX (0.5 mg/kg) significantly decreased the number of total cells (p < 0.05), neutrophils (p < 0.05 or p < 0.01), and macrophage (p < 0.05) compared to those in the LPS group (Fig. 1).
Effects of protocatechuic acid on the production of inflammatory cytokines TNF-α, IL-1β, and IL-6 in the BALF of LPS-induced ALI mice. Mice were given an intraperitoneal injection of protocatechuic acid (30 mg/kg) 1 h prior to an i.n. administration of LPS. BALF was collected at 6 h following LPS challenge to analyze the inflammatory cytokines TNF-α (a), IL-1β (b), and IL-6 (c). The values presented are mean ± SEM (n = 6 in each group). # p < 0.05, ## p < 0.01 vs. control group, *p < 0.05, **p < 0.01 vs. LPS group.
Effect of Protocatechuic Acid on TNF-α, IL-1ß, and IL-6 Production in LPS-Induced ALI Mice
The effect of protocatechuic acid on TNF-α, IL-1β, and IL-6 production was analyzed at 6 h after LPS challenge by ELISA. As shown in Fig. 2, TNF-α, IL-6, and IL-1β levels of LPS-vehicle mice in the BALF were significantly increased compared to those in control group (p < 0.01 or p < 0.05). Protocatechuic acid and DEX significantly reduced TNF-α (p < 0.05), IL-6 (p < 0.05 or p < 0.01), and IL-1β (p < 0.05) production compared to those in the LPS group.
Effects of protocatechuic acid on the number of total cells, neutrophils, and macrophages in the BALF of LPS-induced ALI mice. Mice were given an intraperitoneal injection of protocatechuic acid (30 mg/kg) 1 h prior to an i.n. administration of LPS. BALF was collected at 6 h after LPS administration to measure the number of total cells (a), neutrophils (b), and macrophage (c). The values presented are the mean ± SEM (n = 4–6 in each group). # p < 0.05, ## p < 0.01 vs. control group, *p < 0.05, **p < 0.01 vs. LPS group.
Effects of Protocatechuic Acid on LPS-Induced Lung W/D Ratio and Protein Concentration in BALF
Six hours later LPS administration, the lung W/D ratio (Fig. 3a) and total protein concentration (Fig. 3b) in the BALF were evaluated. LPS instilled for 6 h caused a significant increase in both lung W/D ratio (p < 0.01) and the total protein concentration (p < 0.05) in BALF compared to the control group. PCA and DEX significantly decreased the lung W/D ratio (p < 0.05) and the total protein concentration (p < 0.05) in the BALF compared to those in the LPS group.
Effects of protocatechuic acid on the lung W/D ratio and total concentration in the BALF of LPS-induced ALI mice. Mice were given an intraperitoneal injection of protocatechuic acid (30 mg/kg) 1 h prior to an i.n. administration of LPS. The lung W/D ratio (a) and total protein concentration in BALF (b) were determined at 6 h after LPS given. The values presented are the mean ± SEM (n = 4–6 in each group). # p < 0.05, ## p < 0.01 vs. control group, *p < 0.05, **p < 0.01 vs. LPS group.
Effects of PCA on MPO Activity on Lung Tissues from Mice with LPS-Induced ALI
PMNs are the major components of inflammatory and immunological reactions in injured lungs. MPO activity increase reflects PMN accumulation in the lung. Thus, MPO plays an important role in the development of LPS-induced ALI. As shown in Fig. 4, 6 h after LPS was given, MPO activity (p < 0.01) had significantly increased in the LPS group compared to the control group. Intraperitoneal injection of PCA and DEX significantly reduced MPO activity (p < 0.01) compared with the LPS group.
Effects of protocatechuic acid on MPO activity in lungs of LPS-induced ALI. Mice were given an intraperitoneal injection of protocatechuic acid (30 mg/kg) 1 h prior to an i.n. administration of LPS. MPO activity was determined at 6 h after LPS administration. The values presented are the mean ± SEM (n = 4–6 in each group). # p < 0.05, ## p < 0.01 vs. control group, *p < 0.05, **p < 0.01 vs. LPS group.
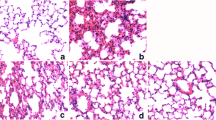
Effects of PCA on LPS-Mediated Lung Histopathological Changes
Lungs, which were harvested at 6 h after LPS injection, were subjected to H&E staining. As shown in Fig. 5, lung tissues from the control showed a normal structure and no histopathological changes under a light microscope. In LPS group, the lung showed significant pathological changes, such as inflammatory cells infiltration, alveolar wall thickening, alveolar hemorrhage, and even lung tissues destruction. In contrast, PCA and DEX were found to decrease many of the symptoms of ALI.
Effects of protocatechuic acid on histopathological changes in lung tissues in LPS-induced ALI mice (a–d magnification, ×200; A1, B1, C1, and D1 magnification, ×400). Mice were given an intraperitoneal injection of protocatechuic acid (30 mg/kg) 1 h prior to an i.n. administration of LPS. Lungs (n = 3) from each experimental group were processed for histological evaluation at 6 h after LPS challenge. Lungs were prepared for H&E staining. a and A1 control group; b and B1 LPS group; c and C1 protocatehuic acid group; d and D1 DEX group.
Effect of PCA on NF-κB Activation and IκBα Phosphorylation and Degradation in ALI Mice Induced by LPS
To evaluate the effect of PCA on NF-κB activation in a mice ALI model, we homogenized the lung tissue to obtain total, nuclear, and cytoplasmic proteins. In our study, Fig. 6 shows that LPS-induced IκBα degradation was significantly blocked by pretreatment with PCA, which was related to IκBα phosphorylation. LPS-treated mice also showed decrease in cytoplasm but increase in nuclear extracts of p65 subunit of NF-κB.
Effect of protocatechuic acid on the activation of NF-κB and IκBα phosphorylation and degradation in lungs of ALI mice. Mice were given an intraperitoneal injection of protocatechuic acid (30 mg/kg) 1 h prior to an i.n. administration of LPS. Six hours later, total nuclear and cytoplasm extracts from the lung tissues were subjected to Western blotting. β-Actin was used as an internal control. Experiments were repeated three times and results were obtained. The values presented are the mean ± SEM. # p < 0.05, ## p < 0.01 vs. control group, *p < 0.05, **p < 0.01 vs. LPS group.
DISCUSSION
LPS, the main component of the outer membranes of Gram-negative bacteria, plays a key role in producing inflammatory responses [19]. LPS can enter the bloodstream and elicit inflammatory responses that may lead to shock and, ultimately, death [20]. It indicates that LPS might be the most important antigen that leads to the development of ALI. The etiologies and mechanisms of ALI have been extensively investigated in various experimental models. One widely used ALI murine model utilizes the intratracheal administration of LPS [21]. LPS-induced ALI was characterized by the release of a variety of pro-inflammatory cytokines that orchestrate different inflammatory responses via interactions between LPS and a number of immune cells [22]. The development of an ALI model by way of i.n. LPS instillation is well suited for preliminary pharmacological studies of new drugs or other therapeutic agents as i.n. instillation of LPS into mice could develop a controlled ALI without causing systemic inflammation and multi-organ failure [23]. Steroidal agents, glucocorticoids, have been clinically used as anti-inflammatory drugs in the treatment of ARDS and ALI. Therefore, DEX could be applied as the positive control to evaluate the anti-inflammatory efficiency of PCA in LPS-induced ALI. In the present study, for the first time, we explored the effect of PCA on LPS-induced ALI in mice. The results showed that pretreatment with PCA could attenuate lung injury resulting from LPS and can inhibit the W/D ratio, pro-inflammatory cytokine production, inflammatory cell migration into the lung, and protein leakage, as well as inhibit the degradation of IκBα and NF-κB activation. The data presented here demonstrate that PCA exerts potent anti-inflammatory effects in mice during ALI- induced by LPS.
Edema is a representative symptom of inflammation not only in systemic inflammation but also in local inflammation. To quantify the magnitude of pulmonary edema, we determined the W/D ratio of sampled lung tissue. Our experiments found that PCA might significantly inhibit the lung edema, as was shown by the W/D ratio in PCA group being significantly lower than that of the LPS group.
LPS administration is known to induce the production of several inflammatory and chemotactic cytokines. TNF-α, IL-1β, and IL-6 are characterized cytokines involved in the inflammatory process of ALI. These cytokines, as well as other proinflammatory compounds, initiate, amplify, and perpetuate the inflammatory response in ALI and ARDS. TNF-α and IL-1β not only amplify the inflammatory cascade and cause inflammatory injury but also recruit neutrophils into the lung [24]. High levels of TNF-α and IL-6 in the BALF have been noted in patients with ALI and ARDS, and the persistent elevation of pro-inflammatory cytokines in humans with ALI or sepsis has also been associated with more severe outcomes [25]. TNF-α is the earliest and primary endogenous mediator of the process of an inflammatory reaction. TNF-α may play a role in the initiation or progression of multiple organ failure in endotoxic shock, and it has been shown to be a particularly important mediator of ALI [26]. TNF-α, as a pro-inflammatory factor, is the first multifunctional cytokine produced after trauma or infection and is released mainly from LPS-stimulated monocytes and macrophages, which both damage vascular endothelial cells, increase their permeability, and induce alveolar epithelial cells to produce other cellular factors and chemotactic factors. TNF-α can also influence the initiation and development of ALI by affecting cell apoptosis [27]. IL-1β plays a key role in the progression of ALI, and it can inhibit fluid transport across the distal lung epithelium [28] to cause surfactant abnormalities [29] and to increase protein permeability across the alveolar-capillary barrier [30]. In this experiment, we found that when pretreated with PCA, the expression of TNF-α, IL-1β, and IL-6 in mice was significantly decreased. These results indicate that the protective effects of PCA on ALI induced by LPS administration may be related to the compound’s inhibition of inflammatory factors.
In ALI, neutrophils are the predominant inflammatory cells. LPS directly stimulates the neutrophils to migrate into the lungs; here, the activated neutrophils begin to release oxidants, proteases, and other inflammatory mediators that lead to lung injury [31]. In addition to neutrophils, macrophages are known to play a crucial role in endotoxin-induced ALI [32]. In this study, we found that the number of total cells, neutrophils, and macrophages was markedly decreased in the PCA group. MPO is expressed in neutrophils and is generally associated with the killing of bacteria and oxidative tissue injury, and oxidative stress and the subsequent production of free radicals may aggravate inflammation. These effects suggest that oxidative stress may be an important mechanism of corticosteroid resistance and that oxidative stress is increased in most severe inflammatory diseases, including ALI and ARDS. The results from our current study showed that pretreatment with PCA significantly improved LPS-induced ALI in mice and was associated with a reduction in MPO activity. In addition, the lung histological examination also demonstrated that PCA had a significantly anti-inflammatory activity during LPS-induced ALI.
The NF-κB transcription factor plays a critical role in a number of different cellular processes. NF-κB is normally sequestered in the cytoplasm by a family of inhibitory proteins known as IκBs. A wide variety of stimuli, which have been extensively studied in the previous two decades, can cause the phosphorylation of IκBα, a process that is followed by the protein’s ubiquitination and subsequent degradation. The loss of IκBα results in the release of free NF-κB unit p65, which translocate from the cytoplasm to the nucleus, where p65 bands to the cis-acting κB-enhancer element of the target gene and activates the expression of pro-inflammatory mediator [33]. The translocation of NF-κB p65 from cytoplasm to the nucleus is often taken as an indication of NF-κB activation and is related to the cellular response to the oxidant or to the inflammatory and acute immune response [34]. The results from the present study provide novel in vivo evidence showing that PCA inhibits the degradation of IκBα and prevents the translocation of NF-κB into the nucleus of the lung. Activation of NF-κB appears to play a central role in the development of pulmonary inflammation and acute lung injury [35]. Studies have shown an association between NF-κB and the expression of cytokines and chemokines [36, 37]. These findings are supported by a study of patients with ARDS, which showed enhanced NF-κB activation [38]. Our results demonstrated that LPS-induced NF-κB activation in lung tissue was inhibited by PCA pretreatment. Therefore, it might be suggested that the potent anti-inflammatory effects of PCA may involve the IκBα activation. Although the cellular targets and molecular mechanisms leading to IκBα activation by PCA remain to be elucidated, our study has shed some light on this issue.
In summary, our study shows that PCA has a protective effect against LPS-induced ALI. Pretreatment with PCA-attenuated pulmonary histological changes, lung edema, and MPO activity reduced the inflammatory cell infiltration into lung tissue and inhibited the release of inflammatory cytokines into the BALF. These data strongly suggest that PCA has potent anti-inflammatory activity and may represent a novel strategy for the modulation of inflammatory response. Furthermore, we found that PCA inhibited the NF-κB activation via degradation of IκBα and phosphorylation. Although PCA exerted an anti-inflammatory effect in our study, further and more comprehensive studies are still required before the full clinical application of this drug can be realized.
REFERENCES
Goodman, R.B., J. Pugin, J.S. Lee, and M.A. Matthay. 2003. Cytokine-mediated inflammation in acute lung injury. Cytokine & Growth Factor Reviews 14: 523–535.
Cohen, J. 2002. The immunopathogenesis of sepsis. Nature 420: 885–891.
Angus, D.C., and R.S. Wax. 2001. Epidemiology of sepsis: an update. Critical Care Medicine 29: S109–S116.
Brun-Buisson, C. 2000. The epidemiology of the systemic inflammatory response. Intensive Care Medicine 26: S64–S74.
Sato, K., M.B. Kadiiska, A.J. Ghio, et al. 2002. In vivo lipid-derived free radical formation by NADPH oxidase in acute lung injury induced by lipopolysaccharide: a model for ARDS. The FASEB Journal 16: 1713–1720.
Harrod, K.S., A.D. Mounday, J.A. Whitsett, et al. 2000. Adenoviral E3-14.7K protein in LPS-induced lung inflammation. American Journal of Physiology. Lung Cellular and Molecular Physiology 278: 631–639.
Mayeux, P.R. 1997. Pathobiology of lipopolysaccharide. Journal of Toxicology and Environmental Health 51: 415–435.
Rochelle, L.G., B.M. Fischer, and K.B. Adler. 1998. Concurrent production of reactive oxygen and nitrogen species by airway epithelial cells in vitro. Free Radical Biology & Medicine 24: 863–868.
Rahman, I. 2002. Oxidative stress, transcription factors and chromatin remodeling in lung inflammation. Biochemical Pharmacology 64: 935–942.
Fengyi Wan, and Michael J. Lenardo. 2010. The nuclear signaling of NF-κB: current knowledge, new insights, and future perspectives. Cell Research 20: 24-33.
Anzueto, A. 2002. Exogenous surfactant in acute respiratory distress syndrome: more is better. European Respiratory Journal 19: 787–789.
Lin, W.L., Y.J. Hsieh, F.P. Chou, C.J. Wang, M.T. Cheng, and T.H. Tseng. 2003. Hibiscus protocatechuic acid inhibits lipopolysaccharide-induced rat hepatic damage. Archives of Toxicology 77: 42–47.
Shi, G.F., L.J. An, B. Jiang, S. Guan, and Y.M. Bao. 2006. Alpinia protocatechuic acid protects against oxidative damage in vitro and reduces oxidative stress in vivo. Neuroscience Letters 403: 206–210.
Stagos, D., G. Kazantzoglou, D. Theofanidou, G. Kakalopoulou, P. Magiatis, S. Mitaku, and D. Kouretas. 2006. Activity of grape extracts from Greek varieties of Vitis vinifera against mutagenicity induced by bleomycin and hydrogen peroxide in Salmonella typhimurium strain TA102. Mutation Research 609: 165–175.
Liu, W.H., C.C. Hsu, and M.C. Yin. 2008. In vitro anti-helicobacter pyloriactivity of diallyl sulphides and protocatechuic acid. Phytotherapy Research 22: 53–57.
Tseng, T.H., J.D. Hsu, M.H. Lo, F.P. Chou, C.L. Huang, C.Y. Chu, and C.J. Wang. 1998. Inhibitory effect of hibiscus protocatechuic acid on tumor promotion in mouse skin. Cancer Letters 126: 199–207.
Lin, W.L., Y.J. Hsieh, F.P. Chou, C.J. Wang, M.T. Cheng, and T.H. Tseng. 2003. Archives of Toxicology 77: 42–47.
Min, S.W., S.N. Ryu, and D.H. Kim. 2010. Anti-inflammatory effects of black rice, cyanidin-3-O-β-D-glycoside, and its metabolites, cyanidin and protocatechuic acid. International Immunopharmacology 10: 959–966.
Saluk-Juszczak, J., and B. Wachowicz. 2005. The proinflammatory activity of lipopolysaccharide. Postepy Biochemii 51: 280–287.
Hudson, L.D., J.A. Milberg, D. Anardi, et al. 1995. Clinical risks for development of the acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine 151: 293–301.
Karmpaliotis, D., I. Kosmidou, E.P. Ingenito, et al. 2002. Angiogenic growth factors in the pathophysiology of a murine model of acute lung injury. American Journal of Physiology. Lung Cellular and Molecular Physiology 283: 585–595.
Faffe, D.S., V.R. Siedl, P.S. Chagas, et al. 2000. Respiratory effects of lipopolysaccharide-induced inflammatory lung injury in mice. European Respiratory Journal 15: 85–91.
Szarka, R.J., N. Wang, L. Gordon, P.N. Nation, and R.H. Smith. 1997. A murine model of pulmonary damage induced by lipopolysaccharide via intranasal instillation. Journal of Immunological Methods 202: 49–57.
Matthay, M.A., and G.A. Zimmerman. 2005. Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. American Journal of Respiratory Cell and Molecular Biology 33: 319–327.
Minamino, T., and I. Komuro. 2006. Regeneration of the endothelium as a novel therapeutic strategy for acute lung injury. The Journal of Clinical Investigation 116: 2316–2319.
Zhang, H.Q., H.D. Wang, D.X. Lu, R.B. Qi, Y.P. Wang, Y.X. Yan, and Y.M. Fu. 2008. Berber- ine inhibits cytosolic phospholipase A2 and protects against LPS-induced lung injury and lethality independent of the alpha2-adrenergic receptor in mice. Shock 29: 617–622.
Marsh, C.B., and M.D. Weavers. 1996. The response to gram-negative pathogenesis of sepsis. Factors that modulate bacterial infection. Clinics in Chest Medicine 17: 183–197.
Roux, J., H. Kawakatsu, B. Gartland, M. Pespeni, D. Sheppard, M.A. Matthay, et al. 2005. Interleukin-1beta decreases expression of the epithelial sodium channel alpha-subunit in alveolar epithelial cells via a p38 MAPK-dependent signaling pathway. Journal of Biological Chemistry 280: 18579–18589.
Hybertson, B.M., Y.M. Lee, H.G. Cho, O.J. Cho, and J.E. Repine. 2000. Alveolar type II cell abnormalities and peroxide formation in lungs of rats given IL-1 intratracheally. Inflammation 24: 289–303.
Lee, Y.M., B.M. Hybertson, H.G. Cho, L.S. Terada, O. Cho, A.J. Repine, et al. 2000. Platelet- activating factor contributes to acute lung leak in rats given interleukin-1 intratracheally. American Journal of Physiology. Lung Cellular and Molecular Physiology 279: 75–80.
Cepkova, M., and M.A. Matthay. 2006. Pharmacotherapy of acute lung injury and the acute respiratory distress syndrome. Journal of Intensive Care Medicine 21: 119–143.
Sone, Y., V.B. Serikov, and N.C. Staub Sr. 1999. Intravascular macrophage depletion attenuates endotoxin lung injury in anesthetized sheep. Journal of Applied Physiology 87: 1354–1359.
Hawiger, J. 2001. Innate immunity and inflammation: a transcriptional paradigm. Immunologic Research 23: 99–109.
Emmanuelle, D., M. Céline, O. Stéphanie, L. Marie-Josèphe, and B.-F. Michelle. 2005. A role for PKCζ in the LPS-induced translocation NF-κB p65 subunit in cultured myometrial cells. Biochimie 87: 513–521.
Fan, J., R.D. Ye, and A.B. Malik. 2001. Transcriptional mechanisms of acute lung injury. American Journal of Physiology. Lung Cellular and Molecular Physiology 281: L1037–L1050.
Blackwell, T.S., and J.W. Christman. 1997. The role of nuclear factor-κB in cytokine gene regulation. American Journal of Respiratory Cell and Molecular Biology 17: 3–9.
Manning, A.M., F.P. Bell, C.L. Rosenbloom, J.G. Chosay, C.A. Simmons, J.L. Northrup, R.J. Shebuski, C.J. Dunn, and D.C. Anderson. 1995. NF-κB is activated during acute inflammation in vivo in association with elevated endothelial cell adhesion molecule gene expression and leukocyte recruitment. Journal of Inflammation 45: 283–296.
Schwartz, M.D., E.E. Moore, F.A. Moore, R. Shenkar, P. Moine, J.B. Haenel, and E. Abraham. 1996. Nuclear factor-kB is activated in alveolar macrophages from patients with acute respiratory distress syndrome. Critical Care Medicine 24: 1285–1292.
ACKNOWLEDGMENTS
This work was supported by the National Nature Science Foundation of China (no. 31072168).
Author information
Authors and Affiliations
Corresponding authors
Additional information
M. Wei and X. Chu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wei, M., Chu, X., Jiang, L. et al. Protocatechuic Acid Attenuates Lipolysaccharide-Induced Acute Lung Injury. Inflammation 35, 1169–1178 (2012). https://doi.org/10.1007/s10753-011-9425-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-011-9425-2