Abstract
Anti-inflammatory and analgesic activity of protocatechuic acid (PCA), a natural product, was evaluated in different rat models (viz., carrageenan-induced paw oedema, cotton pellet-induced granuloma and Freund’s adjuvant arthritis) of inflammation and chemical and heat induced mouse models of pain. Treatment with PCA inhibited significantly different biological parameters like hind paw oedema, granuloma exudates formation and arthritis index in carrageenan oedema, cotton pellet granuloma and Freund’s adjuvant arthritis, respectively. The biochemical changes viz., glutathione, superoxide dismutase, catalase, lipid peroxidation and NO in oedematous or in liver tissues and serum alanine aminotransferase and lactic dehydrogenase occurred during different types of inflammation were either significantly restored or inhibited with PCA pretreatment. Present experimental findings demonstrate promising anti-inflammatory and analgesic activity of PCA which is comparable with that of standard drugs used.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Protocatechuic acid (PCA), a polyphenolic compound, shown to inhibit cyclooxygenase-2, nitric oxide synthase (in vitro) in the expression of cyclo-oxygenase, myeloperoxidase, as well as nitrite and nitrate levels in CCl4-induced hepatic damage (Hsu et al. 2009).
The hepatoprotective activity of PCA against tert-butyl hydroperoxide (t-BHP) induced liver injury has been attributed to its antioxidant and anti-inflammatory property, mainly by blocking stress signal transduction (Liu et al. 2002).
PCA treatment significantly lowered serum marker enzymes and liver antioxidants of diabetic rats in inflammatory conditions. In addition, it also reduces plasma C-reactive proteins and von Willebrand factor levels, interleukin-6, tumor necrosis factor-α, and monocyte chemoattractant protein-1 levels in heart and kidney (Lin et al. 2009). Based on their experimental findings, Lin et al. 2009 suggested that PCA was able to ameliorate complications in metabolic disorders through its beneficial effects like triglyceride-lowering, anticoagulatory, antioxidative and anti-inflammatory activities.
Tyrosinase-derived reactive quinone intermediate(s) of PCA was shown to bind nucleophilic residues of proteins and sulfhydryl group including oxygen radical-generating leukocytes (Yoshimasa and Koji 2001). Free radicals are involved in different diseases and health conditions, including coronary heart disease, inflammation, stroke, diabetes mellitus, rheumatic disease, liver disorders, renal failure, and cancer and aging (Lee et al. 2009).
Keeping the above information in mind, the present study was undertaken to evaluate anti-inflammatory, anti-arthritic, and analgesic activities of PCA in rat and mouse models, respectively. Further, an attempt was made to understand the underlying mechanism(s) of anti-inflammatory activity of PCA.
Materials and methods
Animals
Albino mice (25–30 g; 4–5 weeks) and Wistar rats (160–200 g; 12–14 weeks) of either sex procured from Serum Institute of India, Pune were used for the present study. Rats and mice were maintained in animal house having conditions; temperature (24 ± 2°C), relative humidity (44–56%) and light–dark cycle (12 h/12 h). Animals were provided with standard diet and water ad libitum. The food was withdrawn 14 h before the start of the experiment. Animal handling and experimental procedures were performed according to animal ethics guidelines provided by Committee for the purpose of Control and Supervision of Experiments on Animals (CPCSEA) and the experimental protocol (DYPIPSR/IAEC/09-10/P-12) was approved by the Institutional Animal Ethics Committee during the meeting held on 25 February 2010. The animal house registration number with government of India is 198/99/CPCSEA.
Drugs
Lambda carrageenan (S.D., Fine Chemicals, India), pentazocine (Aventis Pharmaceuticals, India), protocatechuic acid and complete Freund’s adjuvant (Sigma–Aldrich, St Louis, MO, USA), diclofenac sodium (J.B. Chemicals and Pharmaceuticals, India), aspirin (German Remedies, India), and readymade reagent kits (Biolab Diagnostics Pvt, Ltd, Boisar, India) were used for various biochemical assays.
Preparation of drug solution
PCA and diclofenac sodium were prepared as a suspension in 0.5% sodium carboxymethylcellulose (CMC). Adequate care was taken to minimize the auto-oxidation of PCA by preparing freshly, prior to its administration.
Anti-inflammatory activity evaluation
Carrageenan-induced paw oedema
Rats were divided into five groups (6 rats/group). The rats of group I, II, and III were administered with different doses of PCA 25, 50, and 100 mg/kg orally, respectively. The rats from group IV and group V received diclofenac sodium (15 mg/kg) and 0.5% CMC (1 ml/100 gm), respectively daily orally for 8 days. Rats from all the groups received the drugs 1 h prior to subcutaneous injection of 1% (w/v) carrageenan (prepared in 0.9% NaCl) into the sub-plantar region of right hind paw (Winter et al. 1962). The hind paw volume was measured by volume displacement method using plethysmometer (UGO Basile 7140, Italy) by immersing the paw till the level of lateral malleolus , at various time intervals (0, 1, 3, and 6 h) after carrageenan injection.
Results were expressed as percentage inhibition of oedema formation by comparing with the CMC-treated control group. The average values between the treated animals and control group was calculated for each time interval and analyzed statistically. The percentage inhibition of oedema formation was calculated using the formula,
where V c is the mean of paw oedema in control group and V t is the mean of paw oedema in test group.
The various biochemical alterations in serum viz., lysosomal enzymes (ALT, LDH), non enzymatic antioxidants, glutathione (GSH), enzymatic antioxidants, superoxide dismutase (SOD), lipid peroxides (LPO) and reactive free radicals, NO (nitric oxide) were also determined in oedematous tissue at 6 h after carrageenan injection. Animals were killed under mild ether anaesthesia, blood was withdrawn directly by cardiac puncture and serum was separated by centrifugation (Remi, USA) at 2,500 rpm below 30°C for 30 min. Serum ALT (Reitmen and Frankel 1957) and LDH (Amdor et al. 1963) were determined using readymade reagent kits.
Oedematous tissue of hind paw of each animal was separated under controlled temperature (4–8°C), washed and then homogenized in phosphate buffer (pH 7.4) using homogenizer (Remi). Homogenate was used for various biochemical estimation viz., nitric oxide (NO) (Gaur et al. 2009; Green et al. 1982), LPO (Slater and Sawyer 1972), and other antioxidants viz., GSH (Ellaman 1959), SOD (Mishra and Fridovich 1972), and catalase (Aebi 1974; Colowick et al. 1984) were assayed in supernatant after centrifuging the homogenate for 10 min at 5,000 rpm using cold centrifuge.
Cotton pellet-induced granuloma
Rats were divided into five groups (6/group). The rats from group I, II, and III were administered with different doses of PCA (25, 50, and 100 mg/kg) orally, respectively. The rats from group IV and V were administered with diclofenac sodium (15 mg/kg) and 0.5% CMC (1 ml/100 gm), respectively daily orally for 8 days. On the 1st day, after 1 h of drug administration, the rats were anaesthetized with ether anaesthesia and four sterilised cotton pellets (10 ± 1 mg) were inserted on either side (2 on each side) of ventral region by making small subcutaneous incision. The incisions were sutured by sterile catgut (Winter and Porter 1957). On the 9th day, 24 h after the last dose, the rats were anaesthetized with ether anaesthesia and blood was withdrawn directly from the heart, serum was separated by the procedure described earlier and serum ALT, LDH activity were assayed by using readymade reagent kits. Cotton pellets was carefully removed and freed from extraneous tissues. Each pellet was then dried at 60°C in an oven for 6 h or until constant weight was obtained. The net dry weight, i.e. after subtracting the initial weight of the cotton pellet, was determined. The liver of each animal was removed rapidly, washed, cut into small pieces and homogenized in phosphate buffer (pH 7.4) using homogenizer (Remi). Homogenate was used for the determination of NO and LPO by the methods described earlier. Glutathione, SOD, and catalase levels were assayed in supernatant by the methods described earlier.
Freund’s adjuvant-induced arthritis
Rats were divided into five groups (12/group). The rats from group I, II, and III were administered daily with different doses of PCA (25, 50, and 100 mg/kg), group IV received diclofenac sodium (15 mg/kg, orally) on alternate day for 14 days and group V received 0.5% CMC (0.1 ml/10 gm) orally daily for 14 days. Arthritis was induced by injecting 0.1 ml complete Freund’s adjuvant into the plantar region of the left hind paws subcutaneously (s.c.) prior to drug treatment. The changes in paw volume were measured on various days (1, 3, 6, 9, 12, 15, 18, and 21st) by using plethysmometer (UGO Basile 7140, Italy). (Newbould 1965; Kaneria et al. 2007) The arthritic index was determined using various biological parameters like hind paw oedema, grip function strength, motility of ankle joints and pain threshold by the method described by Naik et al. 1979. On 13th and 21st day, the arthritic index was empirically scored on the scale (0–4) for the various parameters mentioned above, and subsequently six rats were killed under mild ether anaesthesia. The liver of each animal was removed rapidly washed, cut into small pieces and homogenized in phosphate buffer (pH 7.4) using homogenizer (Remi). Homogenate was used for the determination of NO and LPO by methods described earlier. Glutathione, SOD and catalase levels were assayed in supernatant by the methods described earlier.
Determination of arthritic index
Freund’s adjuvant rats were tested for determining the arthritic index using relevant biological parameters on 13th and 21st day.
Parameters for arthritic index determination | Score |
Hind paw oedema-mild-(1), moderate-(2) and marked-(4) | 0–4 |
Motility of joints by moving ankle joints for four times, squeak response for each ankle joint movement was counted for pain threshold as:- Four squeaks-(1), two squeaks-(2), one squeaks-(3), or no squeaks-(4). | 0–4 |
Grip function strength measured by keeping the rats on an inclined plane (wire mesh) and scored on the basis of:-Sliding down immediately-(1), sliding down slowly-(2) or maintaining by holding to wire mesh for more than 3 min-(4) | 0–4 |
Analgesic activity evaluation
Acetic acid-induced writhing
Mice were administered intraperitoneally (i.p.) with 0.1 ml/10 g of 0.3% (v/v) acetic acid. The mice were then observed for writhing movements (stretching of hind limbs and bending of trunk) for 30 min (Witkin et al. 1961). Mice exhibiting writhing movements were selected. Forty-eight hours later, the selected mice were randomly divided into five groups (6/group).
The mice from group I, II, and III were administered with different doses of PCA (35, 70, and 140 mg/kg), mice from group IV and V received diclofenac sodium (15 mg/kg) and 0.5% CMC (0.1 ml/10 gm), orally, respectively, 1 h prior to acetic acid injection (0.1 ml/10 g of 3% (v/v) intraperitoneally. The numbers of writhing episodes were counted for 30 min following acetic acid injection.
Hot plate method
Mice were divided into five groups (6/group). The mice from group I, II, and III were administered with PCA (35, 70, and 140 mg/kg), group IV and V received pentazocine (10 mg/kg, i.p.) and 0.5% CMC (0.1 ml/10 gm), orally, respectively. Fifteen minutes after the test drug administration, each mouse was individually placed on hot plate, maintained at 55 ± 5°C (Zhang et al. 2008). The reaction time to heat stimulus in the form of paw licking or jumping was taken as an end point of pain threshold (Zhang et al. 2008). The cut off time was maintained at 20 s in order to avoid damage to paws. Analgesic activity was tested at 15, 30, 45, and 60 min after the administration of drugs.
Results
Carrageenan-induced paw oedema
In carrageenan paw oedema, PCA treatment (50 and 100 mg/kg) and diclofenac sodium (15 mg/kg) reduced oedema formation significantly at 3rd and 6th h (Table 1).
Pretreatment with PCA and diclofenac sodium inhibited serum ALT and LDH (Table 3) and oedematous LPO and NO significantly in carrageenan oedema (Table 2). Similarly, antioxidants, SOD, catalase, and GSH in oedematous tissue were significantly elevated by PCA and diclofenac sodium treatment (Table 2).
Cotton pellet-induced granuloma
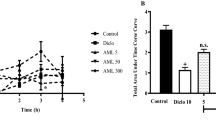
In cotton pellet-induced granuloma, rats treated with PCA and diclofenac sodium orally inhibited the exudates and granular tissue formations, and the inhibitory effect was found to be dose dependent (Fig. 1). The serum lysosomal enzymes, ALT, LDH and liver LPO, NO levels were significantly inhibited during cotton pellet granuloma, by PCA and diclofenac sodium treatment (Fig. 1). The liver antioxidants, SOD, catalase, and GSH levels of cotton pellet granuloma rats were significantly elevated with PCA treatment. The effects of PCA on anti-inflammatory activity and antioxidant parameters were found to be dose dependant (Table 3). The effects of diclofenac sodium (15 mg/kg) on both biological and biochemical changes in cotton pellet granuloma were comparable with that of PCA (100 mg/kg) treated group (Fig. 2).
Freund’s adjuvant-induced arthritis
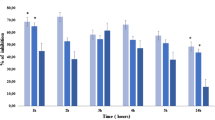
Treatment with PCA (50 and 100 mg/kg, p.o.) and diclofenac sodium (15 mg/kg, p.o.) significantly reduced the paw swelling in adjuvant arthritic rats from 3rd to 21st day (Fig. 3). The arthritic index was also improved with the treatment of PCA and was comparable with that of diclofenac sodium (15 mg/kg) treated group (Table 4).
Treatment with PCA (50 and 100 mg/kg) and diclofenac sodium (15 mg/kg) to arthritic rats significantly decreased LPO and NO and increased antioxidants, SOD, catalase and GSH in liver on both 13th and 21st day (Table 7). However, the maximum effect on these biochemical changes with PCA treatment was observed on 21st day. The anti-arthritic activity of PCA (100 mg/kg) was found to be equivalent to that of diclofenac sodium (15 mg/kg) (Tables 5, 6, 8).
Analgesic activity evaluation
Acetic acid-induced writhing
PCA treatment elicits a dose-dependent inhibition of acetic acid-induced writhing movements in mice. However, diclofenac sodium (15 mg/kg) treatment showed a greater reduction of writhing movements than PCA (140 mg/kg) (Fig. 4).
Hot plate method
In hot plate method, the onset of analgesic effect of PCA (140 mg/kg) was observed at 30 min and persisted up to 60 min after its administration, whereas the onset of analgesic effect of pentazocine (10 mg/kg) was observed at 15 min and lasted up to 60 min. The pentazocine (10 mg/kg, i.p.) showed a better analgesic activity in terms of onset and duration of effect.
Statistical analysis
The results are expressed as mean ± SEM. The results of different experiments were subjected to statistical analysis using one way ANOVA followed by Dunnett’s multiple comparison test for control group. The p value <0.05 was considered to be significant.
Discussion
Inflammation is divided into three phases: (1) acute inflammation, (2) the immune system response and (3) chronic inflammation (Boominathan et al. 2004). Carrageenan is known for its classic biphasic effect; first phase is mediated by the release of histamine and serotonin during the 1st h and release of kinins up to 2.5 h, while the second phase is mediated by the release of prostaglandins from 2.5 to 6 h (DiRosa et al. 1971). It has been reported that the second phase is found to be sensitive to most of the clinically effective anti-inflammatory drugs (Crunkhon and Peacocks 1971; Winter et al. 1962). Keeping such documented reports in mind, the inhibition of carrageenan oedema at 3rd and 6th h by PCA can be attributed to decreased availability of prostaglandins, either due to its inhibition of synthesis or release. However, such presumption needs further confirmation by determining cyclo-oxygenase activity under the effect of PCA either in in vitro or in vivo models.
Cotton pellet granuloma is a chronic inflammatory model, in which dry weight of the cotton pellet represents the formation of granulomatous tissue (Winter and Porter 1957; Thangam and Dhananjayan 2003), and is largely due to proliferation of macrophages, neutrophils, fibroblasts, and multiplication of small blood vessels (Swingle 1974; Bhattacharya et al. 1992) including angiogenesis, nitric oxide synthesis and kinins. During angiogenesis, inflammatory cells are recruited to the inflammation site and also supplies nutrients and oxygen for the formation of granuloma tissue (Ghosh 2005). It is presumed that PCA treatment was able to inhibit granuloma formation either by suppressing or interfering vital events like angiogenesis and/or other mediators.
Documented evidence indicates that lysosomal cathepsin enzymes play an important role in the genesis of acute as well as chronic inflammation (Weissmann 1967; Kaneria et al. 2007; Panda and Naik 2008). Most of the anti-inflammatory drugs were found to inhibit either release of lysosomal enzymes or stabilize lysosome membrane (Kalyanpur et al. 1968; Anderson et al. 1971; Naik et al. 1979). The elevation of serum marker enzymes (ALT and LDH) can be correlated directly to the degree of disruption of lysosomes by the noxious inflammatory agent (Drent et al. 1996; Naik and Panda 2007) and lysosome membrane stabilization effect of PCA might be resulted in the inhibition of the elevated lysosomal enzymes.
In the present experiments, PCA treatment significantly decreased the levels of NO and LPO and increased GSH, catalase, and SOD in carrageenan oedematous tissue. Nitric oxide, a pleotropic short-lived free radical, was increased in oedematous tissue, and known to initiates cellular injury (Nagy et al. 2007). Superoxide dismutase is reported to be therapeutically useful in protecting tissue injury (ischemia, inflammation, hypoxia, etc.) from reactive oxygen species and superoxide anions (McCord 1992). Similarly, pleiotropic roles of GSH include (1) the maintenance of cells in a reduced state and (2) indirect participation in the protection of cells against oxidative stress (Halici et al. 2007).
Adjuvant arthritis animal model is very similar to human rheumatoid arthritis, resembles to pathological and serological changes, including the inflammatory mediators (Gao et al. 2008). In adjuvant-induced arthritis model, rat develops chronic swelling and pain in multiple joints with release of cytokines from inflammatory cells, culminating in erosion of cartilage and bone destruction causing severe disability (Malik et al. 2010). In this study, arthritic rats treated chronically with PCA, reduced paw oedema, improved motility of joints, pain threshold and grip strength, which is a clear indication of improvement in arthritic condition (see “Determination of arthritic index”).
In arthritic condition, accumulated granulocytes and macrophages in the oedematous tissue produces large amounts of free radicals, superoxide anions, and hydrogen peroxides (Halley and Cheeseman 1993). Formation of high levels of free radicals causes depletion of antioxidant enzymes which initiates tissue damage during arthritis (Campo et al. 2003; Pathak et al. 2010).
Biochemical findings demonstrate that NO and non-enzymatic antioxidant (GSH) and putative enzymatic antioxidants (SOD and catalase) in oedematous and liver tissue during acute and chronic inflammation were significantly elevated with PCA treatment, respectively. Thus, our experimental findings with PCA suggest its ability to ameliorate the oxidative stress during inflammatory (acute and chronic) conditions mainly through: (1) decreased lipid peroxidation, preventing harmful free radical formation and oxidative chain reactions and (2) down regulation of NO formation by scavenging/neutralizing free radicals and also activating antioxidant enzymes (Brouet and Ohishima 1995; Mortellini et al. 2000; Naik et al. 2011). Hence, it is believed that the antioxidant activity of PCA might be participating either directly or indirectly in the maintenance of membrane integrity, which might help to prevent the elevation of serum marker enzymes during inflammation or proliferation process.
Administration of acetic acid causes irritation and visceral pain, reflected in writhing movements (Vyklicky et al. 1979. Pre-treatment with PCA (70 and 140 mg/kg) significantly reduced acetic acid-induced writhing movements, thereby suggesting its analgesic activity. The pain induced by hot plate method is supraspinally mediated pain; hence, it is used to screen centrally acting analgesics (Eddy and Leimback 1953). Treatment with PCA prolonged the reaction time to heat stimulus in mice, clearly indicating its analgesic activity, which may be mediated through central pain receptors.
In conclusion, the PCA treatment showed promising anti-inflammatory activity in both acute and chronic rat models of inflammation. The anti-inflammatory activity of PCA can be attributed to its antioxidant and membrane stabilizing property. PCA also elicits significant analgesic activity in mice, which is beneficial as pain is an integral component of inflammation.
References
Aebi H (1974) Catalase. In: Hu Bergmeyer Inc. Verlag (ed) Methods of enzymatic analysis. Chemic Academic press Inc., Verlag, pp 673–685
Amdor E, Darfman LE, Wacker WEC (1963) Serum lactate dehydrogenase activity on analytical assessment of current assays. Clin Chem 9:391–399
Anderson AJ, Bocklehurst WE, Wills AL (1971) Evidence for the role of lysosomes in the formation of prostaglandins during carrageenan induced inflammation in rats. Pharmacol Res Comm 3:13–19
Bhattacharya S, Pal S, Nag Choudhari AK (1992) Pharmacological studies of the anti-inflammatory profile of Mikania cordata (Burm) B.L. Robinson root extract in rodents. Phytother Res 6:255–260
Boominathan V, Parimaladevi B, Mandal SC, Ghoshal SK (2004) Anti-inflammatory evaluation of Ionidium suffruticosam Ging. in rats. J Ethnopharmacol 91:367–370
Brouet I, Ohishima H (1995) Curcumin an anti-tumor promoter and anti-inflammatory agent, inhibits induction of nitric oxide synthase in activated macrophages. Biochem Biophys Res Commun 206:533–540
Campo GM, Avenoso A, Campo S, Ferlazzo AM, Altavilla D, Calatroni A (2003) Efficacy of treatment with glycosaminoglycans on experimental collagen-induced arthritis in rats. Arthritis Res Ther 5(3):122–131
Colowick SP, Kaplan NO, Packer L (1984) Methods in enzymology, vol 105. Academic Press, London, pp 121–125
Crunkhon P, Peacocks SER (1971) Mediators of the inflammation induced in the rat paw by carrageenan. Br J Pharmacol 42:392–402
DiRosa M, Firoud JP, Willoughby DA (1971) Studies of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. J Pathol 104:15–59
Drent M, Cobben NA, Henderson RF, Wouters EFM, Van Dieijien-visser M (1996) Usefulness of lactate dehydrogenase and its isoenzymes as indicators of lung damage or inflammation. Eur Res J 9:1736–1742
Eddy ND, Leimback D (1953) Synthetic analgesics. II. Dithyienylbutenyl-amines and dithyienylbutylamines. J Pharmacol Exp Ther 3:544–547
Ellaman GL (1959) Tissue sulfhydryl group. Arch Biochem Biophys 82:70–77
Gao Q, Shan J, Di L, Jiang L, Xu H (2008) Therapeutic effects of daphnetin on adjuvant-induced arthritic rats. J Ethnopharmacol 120:259–263
Gaur V, Aggarwal A, Kumar A (2009) Protective effect of naringin against ischemic reperfusion cerebral injury: possible neurobehavioral, biochemical and cellular alterations in rat brain. Eur J Pharmacol 616:147–154
Ghosh MN (2005) Some common evaluation techniques. Fundamentals of experimental pharmacology, 3rd edn. Hilton & Company, India, pp 175–190
Green LC, Wagner DA, Glagowski J (1982) Analysis of nitrate, nitrite and (15 N) nitrate in biological fluids. Anal Biochem 126:131–138
Halici Z, Dengiz G, Odabasoglu F, Suleyman H, Cadirci E, Halici M (2007) Amiodarone has anti-inflammatory and anti-oxidative properties: an experimental study in rats with carrageenan-induced paw oedema. Eur J Pharmacol 566:215–221
Halley AE, Cheeseman KH (1993) Measuring free radical reactions in vivo. B Med Bull 49:494–505
Hsu CC, Hsu CL, Tsai SE, Fu TY, Yen GC (2009) Protective effect of Millettia reticulate Benth against CCl(4)-induced hepatic damage and inflammatory action in rats. J Med Food 12(4):821–828
Kalyanpur SG, Pohujani SM, Naik SR, Sheth UK (1968) Study of biochemical effects of anti-inflammatory drugs in carrageenan-induced oedema and cotton pellet granuloma. Biochem Pharmacol 17:797–803
Kaneria MS, Naik SR, Kohali RK (2007) Anti-inflammatory, antiarthritic and analgesic activity of a herbal formulation. Ind J Exp Biol 45:278–284
Lee KH, Kim AJ, Choi EM (2009) Antioxidant and anti-inflammatory activity of pine pollen extract in vitro. J Phytother Res 23:41–48
Lin CY, Huang CS, Huang CY, Yin MC (2009) Anticoagulatory, anti-inflammatory and antioxidative effects of protocatechuic acid in diabetic mice. J Agric Food Chem 57(15):6661–6667
Liu CL, Wang JM, Chu CY, Cheng MT, Tseng TH (2002) In vivo protective effect of protocatechuic acid on ter-butyl hydroperoxide induced rat hepatotoxicity. J Food Chem Toxicol 40:635–641
Malik JK, Manvi FV, Nanjware BR, Dwivedi DK, Purohit P, Chouhan S (2010) Anti-arthritic activity of leaves of Gymnema sylvestre R.Br. leaves in rats. Scholar Res Library 2(1):336–341
McCord JM (1992) Superoxide production and human disease. In: Jesaitis A, Dratz E (eds) Molecular basis of oxidative damage by leukocytes. CRC, Boca Raton, pp 225–239
Mishra HP, Fridovich I (1972) Role of superoxide anion in auto-oxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Mortellini R, Foresti R, Bassi R, Green CJ (2000) Curcumin, an antioxidant and anti-inflammatory agent induces hemeoxygenase-1 and protects endothelial cells against oxidative stress. Free Radic Biol Med 28:1303–1312
Nagy G, Clark JM, Buzas EI, Gorman CL, Cope AP (2007) Nitric oxide, chronic inflammation and autoimmunity. Immunol Lett 111:1–5
Naik SR, Panda VS (2007) Antioxidant and hepatoprotective effects of Ginkgo biloba phytosomes in carbon tetrachloride-induced liver injury in rodents. Liver Int 27(3):393
Naik SR, Sattar PB, Sheth UK (1979) Studies on two new derivatives of N-aralkyl-o-ethoxybenzamides: part-III-pharmacological and biochemical studies on their anti-arthritic activity in rat. Ind J Exp Biol 17:1353–1356
Naik SR, Thakare VN, Patil SR (2011) Protective effect of curcumin on experimentally induced inflammation, hepatotoxicity and cardiotoxicity in rats: evidence of its antioxidant property. Exp Toxicol Pathol 63:419–431
Newbould BB (1965) Suppression of adjuvant-induced arthritis in rats with 2-butoxycarbonylmethylene-4-oxothiazolidine. Br J Pharmacol Chemother 24(3):632–640
Panda VS, Naik SR (2008) Cardioprotective activity of Ginkgo biloba Phytosomes in isoproterenol-induced myocardial necrosis in rats: a biochemical and histoarchitectural evaluation. Exp Toxicol Pathol 64(4–5):397–404
Pathak NL, Patel NJ, Kasture SB, Jivani NP, Bhalodia YS, Malavia SV (2010) Free radical scavenging activity of Albizia lebbeck methanolic extract in arthritic rats. Int J Pharma Res Dev 1(12):1–8
Reitmen S, Frankel S (1957) Colorimetric method for determination of glutamic oxaloacetate and glutamic pyruvic transaminase. Am J Clin Pathol 28:56–63
Slater TF, Sawyer BC (1972) The stimulatory effect of carbon tetrachloride and other halogenoalkanes or peroxidative reactions in rat liver fractions in vitro. Biochem J 123:805–814
Swingle KF (1974) Anti-inflammatory agents, chemistry and pharmacology, vol 2. Academic Press, New York, pp 33–47
Thangam C, Dhananjayan R (2003) Anti-inflammatory potential of the seeds of Carum copticum Linn. Ind J Pharmacol 35:388–391
Vyklicky L (1979) The techniques for the study of pain in animals. In: Bonica JJ, Liebeskind LC, Albe-Fessard FD (eds) Advances in pain research and therapy, vol 3. Raven Press, New York, pp 727–745
Weissmann G (1967) The role of lysosomes in inflammation and disease. Ann Rev Med 18:97–112
Winter CA, Porter CC (1957) Effect of alteration in side chain on anti-inflammatory and liver glycogen activities of hydrocortisones esters. J Am Pharm Assoc Sci Edu 46:515–519
Winter CA, Risley EA, Nuss GW (1962) Carrageenan induced oedema in the hind paw of the rat as an assay for anti-inflammatory drug. Proc Soc Exp Biol Med 111:544–547
Witkin LB, Heubner F, Gardi F, Okeefe E, Seppitaletta S, Plummer AJ (1961) Pharmacology of 2-amino-indane hydrochloride (su-8629): a potent non-narcotic analgesic. J Pharmacol Exp Ther 133(3):400–408
Yoshimasa N, Koji THO (2001) A Catechol antioxidant protocatechuic acid potentiates inflammatory leukocyte-derived oxidative stress in mouse skin via a tyrosinase bioactivation pathway. Free Rad Biol Med 30:967–978
Zhang G, Xiao-Dong Huang X, Wang H, Leung A, Chan C, David WF, Yu Z (2008) Anti-inflammatory and analgesic effects of the ethanol extract of Rosa multiflora Thunb. Hips. J Ethnopharmacol 118:290–294
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lende, A.B., Kshirsagar, A.D., Deshpande, A.D. et al. Anti-inflammatory and analgesic activity of protocatechuic acid in rats and mice. Inflammopharmacol 19, 255–263 (2011). https://doi.org/10.1007/s10787-011-0086-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-011-0086-4