Abstract
Feasible countermeasures to mitigate mercury (Hg) accumulation and its deleterious effects on crops are urgently needed worldwide. Selenium (Se) fertilizer application is a cost-effective strategy to reduce Hg concentrations, promote agro-environmental sustainability and food safety, and decrease the public health risk posed by Hg-contaminated soils and its accumulation in food crops. This holistic review focuses on the processes and detoxification mechanisms of Hg in whole soil–plant systems after Se application. The reduction of Hg bioavailability in soil, the formation of inert HgSe or/and HgSe-containing proteinaceous complexes in the rhizosphere and/or roots, and the reduction of plant root uptake and translocation of Hg in plant after Se application are systemically discussed. In addition, the positive responses in plant physiological and biochemical processes to Se application under Hg stress are presented to show the possible mechanisms for protecting the plant. However, application of high levels Se showed synergistic toxic effect with Hg and inhibited plant growth. The effectiveness of Se application methods, rates, and species on Hg detoxification is compared. This review provides a good approach for plant production in Hg-contaminated areas to meet food security demands and reduce the public health risk.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mercury (Hg) is the most dangerous heavy metal (HM) because of its high toxicity to living organisms even at low concentrations. It is ranked third among the 87 hazardous substances by Agency for Toxic Substances and Disease Registry (ATSDR 2017) and has become a public concern since the recognition of Minamata disease in 1956 (Gallego et al. 2012; Ren et al. 2014). The global amount of Hg mass accumulated in soils was assumed to be in the range of 250–1000 Gg (Obrist et al. 2018). The world distribution of known sites contaminated with Hg from active and previous Hg gold and silver mining and processing, non-ferrous metal smelters, chlor-alkali plants, and factories that used or may have used Hg as a catalyst to produce acetaldehyde, polyvinylchloride, and vinyl acetate (Chen et al. 2012a) is presented in Fig. 1 (Map by Kocman et al. 2013). Mercury enters agricultural soils through anthropogenic activities, such as smelting, metalliferous mining, coal burning, pesticide, fertilizer, sludge application, and sewage irrigation (Saunders et al. 2010; Meunier et al. 2011).
Global distribution of contaminated sites with Hg: primary Hg mining (a), chlor-alkali plants (b), large-scale precious metal mining (c), non-ferrous metals processing (d), artisanal and small-scale gold mining (e), and other industrial sites (f) (Map by Kocman et al. 2013).
Mercury ions are easily taken up by plant roots and rapidly transported to edible plant parts (Ren et al. 2014). The accumulation of Hg in plants can result in disorders in biochemical and physiological processes (Patra and Sharma 2000; Benavides et al. 2005), such as blocking essential functional groups in biomolecules, displacing essential metal ions from biomolecules in photosynthetic pigments, reducing photosynthetic rates, and negatively affecting plant nutrient uptake and homeostasis, which lead to the inhibition of root and shoot growth and yield production (Wang and Greger 2004; Patra et al. 2004). After accumulating in plants, Hg is readily biomagnified in the food chain and can threaten human health and the ecological environment (Templeton and Liu 2010; Han et al. 2015). Therefore, feasible countermeasures for the remediation of Hg-contaminated farmlands are urgently necessary to reduce toxic Hg concentrations, promote agro-environmental sustainability and food safety, and reduce the public health risk posed by Hg-contaminated soils.
Over the past several decades, techniques such as soil washing (Makino et al. 2008), low-temperature thermal desorption (Qiu et al. 2014), and phytoremediation (Belimov et al. 2005) have been applied to the treatment of Hg-polluted soils. Although soil washing can remove soluble and exchangeable Hg from heavily polluted soils, it can also remove essential soil elements (Wang et al. 2020). The high costs and soil disturbance associated with soil washing also need to be considered, as well as the costs of thermal treatment and its effects on soil properties. Phytoremediation, an inexpensive and facile approach for soil remediation, involves the selection of capable plant species to degrade, extract, contain, or sequester a soil contaminant through physical, chemical, and biological processes (Burd et al. 2000). However, this method can result in plant death from exposure to high contaminant concentration. Therefore, identifying other approaches that can maintain a plant species, while reducing plant Hg accumulation is highly desired.
Selenium (Se) is an essential micronutrient for humans and animals; it is predominantly obtained by consumption of cereals, vegetables, meat, and fish (Rayman 2000). Recently, the application of Se fertilizers to reduce Hg has gained considerable attention as a cost-effective strategy for mitigating Hg accumulation and the deleterious effects of Hg on plants (Feng et al. 2013b; Wang et al. 2016a). Many previous studies found that the application of exogenous Se could reduce the accumulation of Hg in plants cultivated in flooded soils (Zhang et al. 2012; Wan et al. 2016), unflooded soils (Shanker et al. 1995; Tran et al. 2018a), or in hydroponic systems (Mounicou et al. 2006; Lin et al. 2012). In addition, some studies also confirmed that Se application could alleviate Hg-induced oxidative stress by regulating the metabolism of reactive oxygen species (ROS), such as superoxide anions (O2−), hydroxyl radicals (OH−), and hydrogen peroxides (H2O2). Moreover, the resultant processes may scavenge excess oxygen free radicals, decrease lipid peroxidation (LPO), enhance the activity of antioxidant enzymes, and prevent the inhibition of photosynthesis (Pandey and Gupta 2015; Wu et al. 2016). Therefore, Se application at appropriate dosages could stimulate plant growth and counteract the diverse environmental stresses caused by Hg contamination (Kumar et al. 2012; Malik et al. 2012).
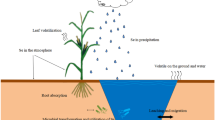
This review explored available information on the mechanisms underlying Hg detoxification in soil–plant systems through the application of exogenous Se. The transformation of Hg speciation and bioavailability in soils, the uptake from the soil and translocation, the transformation of Hg within plants, and the physiological and biochemical responses of the plant after Se application are discussed to confirm the possible mechanisms (Fig. 2). In addition, the effects of Se species, rates, and application methods under Hg stress are also discussed.
Reduction of Hg bioavailability in soil after Se application
The protective effect of Se against Hg toxicity was first noted by Pařízek and Oštádalová (1967) over 50 years ago in rats; most of the early studies were in mammals. Later, there were many studies that demonstrated that Se application could reduce the toxicity of many heavy metals, including Hg, Cd, and Pb, through reduction of HMs accumulation by plants (Mukherjee and Sharma 1988; Shanker et al. 1996a; Thangavel et al. 1999). The protective effect involved the binding of Se to Hg, thereby acting as a “tonic” that sequestered Hg in a form that no longer harmed important biomolecules. To understand how Se protects against Hg toxicity, it is necessary to understand the interaction processes between Hg and Se in the soil.
Immobilization of Hg in soil after Se application
The speciation of Hg and Se in soil
The speciation of Hg in soil
The most common forms of Hg in soils include elemental Hg (Hg0), mercuric mercury (Hg2+), mercuric sulfide (HgS), and methyl Hg (CH3Hg+) (Clarkson and Magos 2006; Yang et al. 2008). Hg2+ is the dominant and highly soluble Hg species under the highly oxidizing conditions of unflooded soils (Fernandez-Martinez et al. 2015). Mercury is reduced in the soil environment, as follows:
Hg0 ⇆ Hg22+ ⇆ Hg2+ ⇆ (CH3)Hg ⇆ (CH3)2Hg (Shanker et al. 1996b; McNear et al. 2012)
Bacterial merB (organomercurial lyase) facilitates the protonolysis of organic-Hg to Hg2+, whereas bacterial merA (mercuric ion reductase) transforms Hg2+ to Hg0 (Ruiz and Daniell 2009).
Mercuric chloride and mercuric hydroxide are likely to be reduced to Hg0 as follows:
Hg2+ + Cl2 and Hg2+ + [OH]2 into Hg0) (Shanker et al. 1996b; McNear et al. 2012)
The speciation of Se in soil
Selenium exists in different forms in the soil, including selenate (SeO42−), selenite (SeO32−), elemental Se (Se0), and selenide (Se2−) (Zhang et al. 2014). Se0 and Se2− have poor mobility (Tolu et al. 2011). SeO32− and SeO42− are both highly available for plant uptake, whereas SeO32− is less available than SeO42− due to its strong adsorption onto soil particles (Nakamaru and Altansuvd 2014). Long periods of overlying water cause low pH values and anoxic conditions in flooded paddy soil (Rothenberg and Feng 2012). Under anoxic conditions, SeO42− can be reduced to SeO32− and then rapidly transformed into Se0 and even to Se2− or organic Se by sulfate-reducing bacteria (SRB) as follows:
SeO42− → SeO32− → Se0 → Se2− (Yang et al. 2008; Li et al. 2014a)
Immobilization processes of Hg in soil
Immobilization of Hg in soil via HgS complex formation
The anoxic conditions or highly oxidizing conditions of rhizospheres enhance microbial activity, decrease pH, and promote the release of carbon-rich root exudates that can facilitate the formation of sulfides (S2−) (Jia et al. 2015). In addition, Hg2+ ion is a class B metal ion with a strong affinity for ligands with soft donor atoms (Rayner-Canham and Overton 2010). At typical concentrations in soil, Hg2+ tends to form stable complexes with OH−, Cl−, and S containing functional groups of organic ligands (Powell et al. 2004). In addition, Barnett et al. (1997) postulated that Hg can form HgS upon binding with –SH groups of organic matter that exists at a higher redox potential than S2−. The affinity of Hg2+ for S2− results in the formation of mercuric sulfide precipitation (HgS) low solubility complex, as follows:
Hg2+ + S2− → HgS (Boszke et al. 2006; Jonsson et al. 2012)
Immobilization of Hg in soil via inert HgSe complex formation
Selenium often occurs as an isomorphous substituent of sulfur (S) in sulfide crystal lattices. In addition, S and Se have the same atomic structure, the same charge (S2− and Se2−), and similar atomic radii and ionic radii (S: 0.184 nm, Se: 0.191 nm); thus, Se can easily be incorporated into the crystalline lattices of S (Zhang 2014b). Therefore, S2− can be replaced by Se2− to form inert mercuric selenide (HgSe) precipitates or an isomorphous series of HgS–HgSe (in cinnabar ore), because the binding affinity of Se2− with Hg (logK 1045) is one million times greater than that of S with Hg (logK 1039) (Syversen and Kaur 2012; Zhang et al. 2014). Moreover, the solubility product constants of HgSe precipitates (Ksp∼10−58–10−65) are drastically lower than those of HgS precipitates (Ksp ∼10−52) (Björnberg et al. 1988). When Se and Hg coexist in soil under appropriate conditions, Hg can first thermodynamically react with Se to form an inert, highly stable HgSe precipitate.
Se may thermodynamically react with Hg2+/Hg0 to form an insoluble HgSe complex in the rhizosphere (Yang et al. 2008; McNear et al. 2012), as presented in the following chemical equations:
Hg0 + Se0 → HgSe
and/or Hg2+ + Se2− → HgSe
Immobilization of Hg in soil via organo-HgSe complex processes
Besides inert HgSe complex, organic HgSe complexes are also found in soil. When paddy soil is supplemented with Se, Se may displace S in the R-SH, R-SSH, and R-SS-R groups to form more stable chemicals, such as R-SeH, R-SeSeH, and R-SeSe-R (Khan and Wang 2009). Simultaneously, Hg binds to non-R-SH, R-SSH, and R-SS-R and may be released and readsorbed by strong Se functional groups (Laurier et al. 2003; Shoham-Frider et al. 2007), thereby forming strong complexes with Se-organic ligands, which are more inert and stable and less available to microbes and plants. Xu et al. (2019) further suggested that HgSe in soil may contain HgSe, CH3HgSe−, and (CH3Hg)2Se, as well as HgSeR, RSHgSeR, CH3Hg-SeR, and CH3Hg-SeSR, which play dominant roles in soil Hg levels. However, this finding needs to be verified further.
Promotion of Hg immobilization in soil
Wang et al. (2016b) demonstrated that Se2− can react with Hg2+ under anoxic and suboxic conditions and form HgSe complexes, despite sulfate input in paddy soil. They also found by transmission electron microscopy and energy-dispersive X-ray spectroscopy that the molar ratios of Hg:Se and Hg:S were 1 in nanoparticles. However, another study showed that Hg LIII-edge synchrotron radiation X-ray absorption near-edge structure (XANES) spectrum exhibited that the typical spectral feature was HgSe instead of α-HgS (Wang et al. 2016a). Furthermore, Zhang et al. (2012) found that Se contents were positively correlated (P < 0.01) with Hg contents in flooded soil due to the formation of HgSe complexes in the rhizosphere. Other studies reported that application of SeO32−- or SeO42−- to dryland soil promoted the formation of HgSe precipitate in the rhizospheres of radish (Raphanus sativus L.) (Shanker et al. 1996b), tomato (Solanum lycopersicum L.) (Shanker et al. 1996a), or pak choi (Brassica rapa L. var. chinensis) (Tran et al. 2018a). In addition, HgSe compounds may react further with dissolved organic matter in the rhizosphere to form high molecular weight HgSe complexes (Plant et al. 2003; Chiasson-Gould et al. 2014).
Transformation of Hg into immobile Hg speciation in soil after Se application
The formation of sufficiently stable insoluble HgSe bonds in soils after Se application may limit the amount of bioavailable Hg2+ in the soil rhizosphere through the transformation of Hg species.
The fractions of Hg in soil
Mercury exist in different speciations in soil, and Hg fractions were arranged following the sequential mobile levels with toxicity decreasing in that order, such as mobile fractions, semi-mobile fractions, and non-mobile fractions (Han et al. 2003; Fernandez-Martinez et al. 2005). The mobile Hg fraction (water-soluble and exchangeable Hg) represents less than 2% of total soil Hg, but this fraction contains the most available Hg, including oxidized inorganic (Hg2+-mercuric and Hg22+-mercurous) and oxidized organic (CH3Hg+-methyl mercury and C2H5Hg+-ethyl mercury) (Boening 2000; Li et al. 2009). The semi-mobile fractions force Hg to strongly bind to sites in natural organic matter, iron and manganese oxides, humic acid, fulvic acid, and amino acids, thereby forming thermodynamically stable complexes (Han and Banin 2000; Zhong and Wang 2009). The non-mobile fractions, which include the combination of Hg0, HgS, and HgSe, have lower bioavailability because of their very low solubility, thereby leading to less toxicity (Boszke et al. 2002; Covelli et al. 2009).
Transformation of Hg into less mobile fractions in soil
Chemical and biological reactions can change Hg speciation and binding to different chemical species in the soil addition of Se, which in turn changes the solubility and bioavailability of Hg-bound chemicals (Reis et al. 2010; Xu et al. 2017). The results of our recent study on pak choi under dryland cultivation conditions demonstrated that the application of SeO32− and SeO42− in soil with concentration from 0.5 to 2.5 mg/kg reduced Hg bioavailability and plant uptake (with reduce Hg 10.7–77.7% in root and 5.8–59.2% in shoot) by enhancing Hg binding in soils via changes in soil Hg fractions (Tran et al. 2018a). This transformation was accompanied by a large increase in the proportions of residual Hg fractions (as HgSe) and a dramatic reduction in the proportions of water-soluble Hg fraction (Tran et al. 2018a), as shown in Fig. 3.
Under flooded soil conditions, Wang et al. (2014) reported that SeO32− application at low dose (1 μg/g) and high dose (5 μg/g) reduced Hg concentrations in water-soluble fractions, thereby reducing Hg bioavailability. Tang et al. (2017) also indicated that Hg2+ levels in soil solution are significantly reduced (P > 0.05) during rice (Oryza sativa L.) growth with soil SeO32− and SeO42− application 3.0 and 6.0 mg/kg–1. Xu et al. (2019) demonstrated that the water-soluble fraction and human stomach acid soluble fraction were reduced with addition Se concentrations of 20−500 mg/kg under anoxic conditions, because HSe− and Se2− can react with bioavailable Hg in these mobile fractions to form a stable and insoluble Hg-Se complex in the rhizosphere or on the root surface of rice plants. Moreover, Se may displace S in the ReSH, R-SSH, and R-SS-R groups to form more stable chemical forms, such as ReSeH, R-SeSeH, and R-SeSe-R. As a result, humic acid fractions are converted into strong-complexed fractions (Xu et al. 2019). Humic acid fraction is composed of Hg bound to the non-RSH functional groups in humic acid, which can be readily released. The strong-complexed fraction includes elemental Hg, Hg bound up to organo-sulfurs, Hg-Ag amalgams, and Fe/Mn oxides (Shoham-Frider et al. 2007), as shown in Fig. 3.
The water-soluble Hg fraction is the most mobile and bioavailable fraction and can be easily transported by natural processes and absorbed by plants (Issaro et al. 2009). The reduced bioavailability of Hg in soils after Se application is reflected by the increase in IR value (reduced partition index), which is used to describe the relative binding strength and fractional redistribution of Hg in soils (Tran et al. 2018a).
Prevention of methyl Hg production in soil after Se application
Despite numerous studies on the interaction between Hg2+ and Se in soil, the mechanisms by which CH3Hg+ interacts with Se are not well understood. Selenium can serve as a mediator to prevent CH3Hg+ production through the formation of Hg–Se complexes, thereby decreasing the amount of available Hg2+ to methylating bacteria.
Methyl Hg processes in soil
Under reducing conditions that occur in many permanently or periodically flooded soils, Hg may be biogeochemically transformed into organo-Hg forms, of which CH3Hg+ is the most prevalent form (Boszke et al. 2006; Kerin et al. 2006; Frohne et al. 2012). CH3Hg+ is also the most toxic Hg species because of its high mobility and bioavailability (Boening 2000; Li et al. 2009). The most mobile Hg fractions are the most susceptible to Hg methylation, whereas the direct conversion of insoluble HgS species to CH3Hg+ in anaerobic soils is insignificant (Boszke et al. 2002; Covelli et al. 2009; Gray et al. 2015). The potential mechanism for Hg2+ uptake by methylating microorganisms is the energy-dependent uptake of Hg2+ by active transport (Zhang et al. 2010; Thomas et al. 2018). SRB is considered the primary methylator of Hg2+, whereas Fe-reducing bacteria also methylate Hg (Rothenberg et al. 2014; Wang et al. 2016a).
Prevention of methyl Hg processes in soil
Limiting the amount of bioavailable Hg2+ decreased or at least considerably prevented the production CH3Hg+ in the soil rhizosphere. Wang et al. (2014) reported that SeO32− application restricts the amount of bioavailable Hg2+ in paddy soils by decreasing microbial CH3Hg+ production (reduce 13−44% CH3Hg+ concentration in soil), which is primarily mediated by SRB (Yang et al. 2008; Truong et al. 2014), and suppresses Hg methylation, and reduces CH3Hg+ concentrations in soil (Wang et al. 2014; Zhang 2014a). Wang et al. (2016a) suggested that CH3Hg+–Se antagonism in soil results in reduction of soil CH3Hg+ levels under anoxic or suboxic conditions (CH3Hg+ levels reduced 10−87% in low-Se soil with addition Se concentrations of 0.5−6.0 mg/kg; and CH3Hg+ levels decreased 13−46% in high-Se soil with addition Se concentrations of 0.5−2.0 mg/kg). In addition, CH3Hg+–Se antagonism may be predominantly governed by microbial processes, specifically by strains of SRB. Soil CH3Hg+ concentrations were consistently lower after Se treatments under anoxic and suboxic conditions independent of sulfate input (Wang et al. 2016b), as shown in Fig. 3.
In addition, Se may also directly affect the microbes that regulate Hg methylation. Hg and Se co-exposure reportedly decreases the growth of SRB in comparison with Hg exposure alone (Truong et al. 2013). Selenium addition also enhanced the demethylation and evaporation of CH3Hg+ (Khan and Wang 2010; Dang et al. 2019), leading to the reduction in soil CH3Hg+ production.
Reduction of Hg availability on the interface of soil–plant root after Se application
Besides decreases of Hg bioavailability in soil after Se application, decline of Hg availability on the interface of soil–plant root also was identified by directly tracking inert HgSe or/and HgSe-containing proteinaceous complexes in the roots. These complexes reduced Hg accumulation in plants by inhibiting Hg uptake and transport. In addition, the restriction of Hg access into the root of plants, due to the promotion of the formation of Fe plaques outside plant roots after Se application, may also be important for reducing the accumulation of Hg in roots and shoots.
Reduction of Hg availability by formation of insoluble HgSe precipitate in root
Formation of insoluble HgSe precipitate in root
Formation of inert insoluble HgSe precipitate
The formation of HgSe insoluble complexes within plants cannot be completely ruled out, although HgSe insoluble precipitate likely dominates in the soil. Hypothetical pathways for Hg uptake in plants involve cellular entry through ionic channels and competition with the closest chemical relatives of essential metals for Hg2+ transporters (Blazka and Shaikh 1992; Clemens 2006). Hg2+ and CH3Hg+ are the principal chemical forms of Hg taken up by roots from the soil (Clemens 2013), and Hg2+ accumulates in roots (Meng et al. 2014; Zhao et al. 2014). Selenium is primarily taken up from the soil by plants as SeO42− or SeO32− (Zhu et al. 2009). After absorption by the plant root, SeO42− is reduced to SeO32−, reacts with glutathione (GSH), and is reduced to Se2− in the rhizosphere (Zhu et al. 2009; Han et al. 2015).
The combination of Se2− with Hg2+ forms the HgSe complexes in roots, as follows:
Hg0 + Se0 → HgSe
and/or Hg2+ + Se2− → HgSe, which may drastically increase the accumulation of Hg in roots (Zhang et al. 2012; Li et al. 2015).
Under flooded soil conditions, over 90% of Hg was restricted to rice roots after SeO32− application of 0.01−0.5 μg/mL in Hg-contaminated soil, and 27.8% of Hg was present as the HgSe complex (Li et al. 2015). Zhao et al. (2013) analyzed the speciation of Hg (with Hg L3-edge XANES) in garlic (Allium sativum L.) tissues under hydroponic solution conditions, and they concluded that the direct binding of Se and Hg as HgSe only occurs in roots (<10%) and bulbs (<1%). Zhang et al. (2012) reported that the molar ratio of Hg:Se in the roots was approximately 1:1, which was not found in the aerial shoots. Zhao et al. (2014) suggested that rice exposure to both Se and Hg may lead to the formation of a HgSe complex in rice roots that is easily absorbed, as indicated by the significant correlation between Se and Hg in rice roots. Synchrotron radiation X-ray fluorescence (SRXRF) technique revealed that Se and Hg is concentrated in the epidermis and pericycle of rice roots (Zhao et al. 2014), as shown in Fig. 4.
However, Zhou et al. (2013) demonstrated that Hg concentration in rice shoots decreased by approximately 50%, whereas the transfer coefficient of Hg from roots to shoots did not drastically change after SeO32− amendment of 14.6−100 g/L. These results indicated that an insoluble HgSe complex formed in the rhizosphere and not in the root. Therefore, the presence of insoluble HgSe and/or proteinaceous complexes in plant roots is still unknown and requires further study.
Formation of Se- and Hg-containing proteinaceous complex
In addition to the inert insoluble HgSe precipitate in the roots, a high molecular weight Se- and Hg-containing proteinaceous complex also forms in the root extract of plants under hydroponic conditions (Afton and Caruso 2009; McNear et al. 2012).
When taken up by plant cells, Hg2+ exhibits high affinity and can react intensely with the sulfhydryl (–SH) groups of proteins in the root cell walls (Carrasco-Gil et al. 2011; Azevedo and Rodriguez 2012). SeO32− could replace S in essential S metabolites (Cys and Met) by physicochemical similarity and be converted quickly to SeCys and SeMet (Aborode et al. 2016; Bluemlein et al. 2009). Then, SeCys and SeMet can be incorporated into selenoenzymes and selenoproteins by replacing Cys and Met (Montesbayon et al. 2002; Navarro-Alarcon and Cabrera-Vique 2008).
In biological systems, selenols can readily replace thiols in amino acids because of the chemical resemblance of selenols to thiols, thereby leading to the complexation of Hg2+ and CH3Hg+ with selenol-containing biomolecules. Compared with thiols, binding between Hg and selenols was stronger. Therefore, Hg2+ and CH3Hg+ complexes with selenols were more stable than their thiol analogs, thereby showing Hg–Se antagonism, resulting in the effective reduction of Hg2+ and CH3Hg+ in plant with the addition of Se into the soil (Wang et al. 2014; Zhang et al. 2012).
Size exclusion chromatography and proteolysis revealed that water-soluble Hg was localized in the roots in association with Se in the form of a high molecular weight entity, which was difficult to be translocated and metabolized. Yathavakilla and Caruso (2007) found that water-soluble Hg associated with Se and formed a high molecular weight (>600 kDa) proteinaceous complex in the roots of soybean (Glycine max L.) grown in soil containing both Hg and Se. Mounicou et al. (2006) found a high molecular weight (>70 kDa) compound containing Se and Hg in the root extract of Indian mustard (Brassica juncea L. Czern.) grown in hydroponics. This compound was associated with either a polysaccharide or a protein (Mounicou et al. 2006). Afton and Caruso (2009) identified a possible Se–Hg association in a plant-root protein in green onion (Allium fistulosum L.) grown in perlite media by applying size exclusion and capillary-reversed phase chromatography coupled with inductively coupled plasma mass spectrometry (ICPMS). McNear et al. (2012) used capillary-reversed phase chromatography coupled with ICPMS, μ-XANES, and micro-synchrotron X-ray fluorescence and found that Hg may bind to –SH groups of the cell wall or plasma membrane proteins in green onion roots and may react with reduced Se2− to form a HgSe–BSS complex. However, Se2− reacted with an abundant amount of free Hg2+ to form a solid HgSe precipitate outside the root in the perlite media. HgSe–BSS comprised a Hg2+ and Se2− core to which GSH was appended via a Se–S or Hg–S bond (McNear et al. 2012). Compared with Hg-containing proteins with small molecular weights, the formation of Hg-Se-containing proteins with high molecular weights can more effectively inhibit the translocation of CH3Hg+ to the aboveground parts of rice plants (Fig. 4).
Wang et al. (2016a) also proposed that a CH3Hg+–Se interaction can exist within rice roots through the formation of CH3Hg+–Se complexes, when CH3Hg+ distribution in roots was enhanced under the SeO32− and SeO42− fertilization. They concluded that CH3Hg+–Se antagonism within plants was likely sufficient to induce such a reduction (Wang et al. 2016a).
Reduction of Hg uptake by plant root by formation of insoluble HgSe precipitate
The reduction in the Hg bioavailability in the rhizosphere can drastically inhibit Hg2+ uptake from soil by roots. Tang et al. (2017) reported that Hg2+ concentrations in rice roots decreased by 22−48% after 3.0 and 6.0 mg/kg SeO32− and SeO42− application to flooded soil. Zhao et al. (2014) also speculated that Se (after SeO32− application of 1 and 5 mg/kg) inhibits Hg2+ uptake through a substantial decrease of Hg2+ concentrations in rice tissues. Previous pot experiments found that SeO32− and SeO42− application of 0.5−6.0 μg/mL decreased root Hg concentrations by approximately 90% in tomato (Shanker et al. 1996a) and > 90% in radish (Shanker et al. 1996b) and by approximately 80% in pak choi with SeO32− and SeO42− application of 0.5−2.5 mg/kg under dryland cultivation conditions (Tran et al. 2018a). Under hydroponic conditions, the formation of insoluble HgSe complexes in the rhizosphere resulted in the reduction of root Hg accumulation. Hg2+ accumulation in rice roots decreased by 10.3−53.0% at Hg concentration of 100 μg/L with SeO32− application of 14.6−100 g/L (Zhou et al. 2013) or at high Hg exposure (1 and 10 mg/L) with SeO32− application of 1.0−10 mg/L (Zhao et al. 2014).
Reduction of Hg availability to root by promotion of the formation of Fe plaques after Se application
The reduction in Hg bioavailability in the rhizosphere under flooded conditions can also be explained by another hypothesis; the promotion of Fe plaques on root surfaces after Se application may sequester Hg through adsorption and/or coprecipitation.
Formation of Fe plaques on root surfaces
Iron plaque is a layer of crystalline or amorphous Fe (hydr)oxides formed through the reaction of oxygen and soluble reductive Fe2+ (Fu et al. 2018). Flooded soils present a strongly reducing environment, in which SO42−, Fe3+, and Mn4+ can be reduced to S2−, Fe2+, and Mn2+ by S2−, thereby promoting the formation of Fe plaques on root surfaces (Murase and Kimura 1997). Previous studies showed that wetland plants with more soluble reductive Fe2+ in the medium can readily form thicker Fe plaques (Cheng et al. 2014), and Fe plaque formation can contribute to less metal accumulation in the roots of plants (Wang et al. 2011; Sebastian and Prasad 2016). Iron plaques on root surfaces can sequester Hg through adsorption and/or coprecipitation and reduce the amount of bioavailable Hg in the rhizosphere.
Reduction of Hg uptake by plant root via promotion of the formation of Fe plaques
The addition of Se enhanced the development of Fe plaque of root, which hindered both Hg2+ and CH3Hg+ uptake (Li et al. 2014b; Zhou et al. 2014). Specifically, Se2− exhibited enhanced reducing ability and increased Fe2+ and Mn2+ concentrations in the soil solution by reducing high-valence Fe and Mn in the soil (Huang et al. 2019). These changes dramatically enhanced the Fe content of Fe plaque on root surfaces and likely blocked the entry of Hg2+ into root tissues because Hg enters root cells under the mediation of essential element transporters (Zhou et al. 2017). In addition, Fe oxide and Fe plaques on roots had a high affinity for SeO32−, thereby reducing the probability of contact between Fe bacteria and Fe(OH)3 (Zhou and Shi 2007). This phenomenon possibly further blocked the dissolution of Fe(OH)3 in Fe plaques to Fe2+ by the action of Fe bacteria (Qu et al. 2003) and increased root surface areas (Ding et al. 2014). Therefore, Se addition can promote amount of Fe plaque on the root surface, thereby acting as a natural barrier that blocked Hg uptake in plant root. For example, the adsorption capacity of Hg on Fe plaque of rice roots surface increased by 1.42 times with Se application, which markedly restricted the translocation of Hg from root to the shoot under hydroponics condition (Zhou and Li 2019), as shown in Fig. 3.
Reduction of Hg translocation within plant after Se application
The reduction of Hg translocation from root to aerial part of plant after Se application was due to the conversion of labile Hg species to insoluble HgSe and/or proteinaceous complexes in the rhizosphere and/or roots. These complexes reduced Hg2+ bioavailability in soil, suppressed Hg methylation in the rhizosphere, and decreased Hg accumulation by the plant root and shoot. However, insoluble HgSe and/or proteinaceous complexes were not detected in stem and leaf extracts. The reduction in Hg bioavailability in aerial part of plant after Se application can also be explained by transformation of Hg into less toxicity speciation and sequestration of Hg in the vacuoles of root cell.
Restrict HgSe and/or proteinaceous complexes in roots of plant
A high molecular weight Se- and Hg-containing proteinaceous complex was not detected in stem and leaf extracts. Mounicou et al. (2006) and Afton and Caruso (2009) suggested that the interaction between Hg and Se is primarily restricted to plant roots. The interaction of root-bound Hg and Se resulted in the production of a putative high molecular weight proteinaceous complex that was not metabolized or translocated to plant shoots, leaves, or fruits (Mounicou et al. 2006; Afton and Caruso 2009). In line with this finding, Zhang et al. (2012) reported a 1:1 molar ratio of Hg:Se in rice roots with none bound in the aerial shoots, indicating that a HgSe insoluble complex formed in the roots (Zhang et al. 2012). Tang et al. (2017) also found that Hg uptake was reduced only after soil SeO32− and SeO42− application but not after foliar application (Table 1).
Reduction Hg translocation within plant by insoluble HgSe and/or proteinaceous complexes in the root
The conversion of labile Hg species to insoluble HgSe and/or proteinaceous complexes in the root may act as an effective barrier for the translocation of Hg from the root to the aboveground tissues. Under hydroponic conditions, the reduction of Hg accumulation in plant stems and leaves after SeO32− application of 1−5 mg/L (Mounicou et al. 2006) or in plant leaves after SeO32− application of 30 mg/L (Afton and Caruso 2009) resulted in the formation of HgSe complex, which was unavailable to plants because of its high stability. The upward translocation of Hg through the root vessel to the leaf tip may be obstructed after SeO32− and SeO42− application by using μ-SRXRF, thereby resulting in the absence of Hg2+ in garlic leaves (Zhao et al. 2013) or in rice stalks and leaves (Zhao et al. 2014).
In addition, the reduction of Hg uptake in the soil–root systems may also be the cause of the inhibition of Hg translocation to the aerial parts of the plant. Zhou et al. (2013) found that Hg concentration in rice shoots decreased by approximately 50% after SeO32− application of 14.6−100 g/L in hydroponics, whereas the translocation factor (TFs) of Hg from the roots to shoots did not drastically change. Zhang et al. (2012) found a consistent reduction in the translocation of Hg2+ to aerial shoots of rice (i.e., stem, leaf, husk, and grain) with increasing Se levels under flooded soil conditions. They also found that Se concentrations in soil had significant negative correlations with the TFs of Hg2+ in different aerial shoots (Zhang et al. 2012). Tang et al. (2017) also reported that the concentrations of Hg2+ in straw and brown rice tissues were reduced by 15–58% and 26–74% by soil-applied SeO32− and SeO42− of 3.0 and 6.0 mg/kg, respectively. Rice grains exhibited the lowest Hg accumulation (decreased by 30%) when 0.5 μg SeO32− mL−1 was applied (Li et al. 2015). Similar results were also reported in another research; Hg levels were reduced by 90% in tomato (Shanker et al. 1996a), 90% in radish (Shanker et al. 1996b), and 60% in pak choi (Tran et al. 2018a) after SeO32− was applied to upland soil (Table 1).
However, increasing shoot Hg concentration was observed under SeO42− and Hg co-exposure; shoot Hg concentration was threefold greater at the highest Se and Hg co-exposure treatment levels in our previous study (Tran et al. 2018a). The relatively high shoot Hg accumulation in plants may be due to the presence of Hg0 in the soil (Kocman et al. 2004), which may have volatilized from the soil and condensed on the leaf surface or enter inner leaf tissues through stomata openings (Patra and Sharma 2000; Martínez-Trinidad et al. 2013).
Reduction of Hg translocation within plant by transformation of Hg into less toxic speciation
Mercury phytotoxicity can be mitigated by changes in the localization patterns and speciation of Hg in plant tissues treated with exogenous Se; Hg species are transformed into low-toxicity species through the reduction of the Hg–protein complex. Zhao et al. (2013) suggested that the percentage of high-toxicity Hg–S binding species, i.e., Hg(GSH)2, decreased, whereas that of low-toxicity Hg–S binding species, i.e., Hg(Met)2, increased in garlic tissues treated with SeO32− and SeO42−. Selenium can compete with Hg in binding with –SH groups, such as the thiol groups of Cys in membrane proteins (Feng et al. 2013a). A SeO32− can enter the root and be quickly converted into organic forms or other biomolecules (de Souza et al. 1998; Zhu et al. 2009). As a result, Hg phytotoxicity was reduced, because GSH is a tripeptide that comprises glutamic acid, Cys, and glycine; these substances protect cells from oxidative stress by binding with oxidizing agents (Patty et al. 2009), as shown in Fig. 4.
Reduction of Hg translocation within plant by sequestration of Hg in the vacuoles of root cell
Huang et al. (2017) proposed another hypothesis for the reduction in HM translocation to the aboveground tissues of plants, as follows: Se decreases HM transport from the roots to the shoots by changing HM speciation and distribution in the root. Selenium application increased GSH and phytochelatin (PC) synthesis in plant tissues through the transformation of SeO32− into organic Se (SeCys or SeMet) (Han et al. 2015; Abd-Allah et al. 2016). After the chelation of Hg2+ and GSH and PC in the cytoplasm of root cells, Hg–PCs or Hg–GS complexes were sequestered in vacuoles via the mediation of ATP-binding cassette (ABC) transporters (Park et al. 2012; Sharma et al. 2016). Thus, the increase of GSH and PC concentrations after Se application led to the reduction in Hg mobility in the root (Park et al. 2012; Sharma et al. 2016). Moreover, Krupp et al. (2009) identified Hg2+-PC, but no CH3Hg+ PC complexed in the rice roots, suggesting that the binding to PCs may inhibit the translocation of Hg2+ from rice roots to stems, but not CH3Hg+ (Fig. 4).
In addition, the amendment of Se can enhance the development of apoplastic barriers in the root endodermis and exodermis, which can mediate the uptake of Hg through the apoplastic pathway or reduce the activity of membrane transporters and thereby reduce the uptake of Hg by roots (Meyer et al. 2009; Wang et al. 2014). Selenium addition also decreased the absorption of Hg into root cells through the symplastic pathway, because Se induced a lower activity of membrane transporters (Wang et al. 2014). The recent study also demonstrated that the main detoxification mechanism for plants in Hg-contaminated soil is the sequestration of Hg into inactive compartments, such as the epidermis, the vacuole, and the cuticle (Geng et al. 2019).
Reduction of oxidative stress induced by Hg in plants after Se application
The possible mechanisms of Hg phytotoxicity can be induced by change of the permeability of the cell membrane, Hg’s high affinity to react with the –SH groups, Hg’s affinity to react with phosphate groups and active groups of ADP or ATP, the replacement of essential ions metalloproteins, and Hg’s ability to disrupt functions involving critical or nonprotected proteins (Patra and Sharma 2000; Patra et al. 2004). Mercury inhibits the activity of plasma membrane–localized aquaporins, which are water channel proteins that enhance water permeation, thereby causing a physical obstruction to the water flow and reducing plant water uptake and transpiration rate (Sas-Nowosielska et al. 2008; Clemens 2013). The substitution of the central atom of chlorophyll (Chl) and magnesium (Mg) by Hg in vivo prevents photosynthetic light harvesting in the affected Chl molecules, resulting in a breakdown of photosynthesis (Patra et al. 2004; Tangahu et al. 2011). The strong interaction with –SH groups disrupts the stability of the group, resulting in the overproduction of ROS and free radicals (Patra et al. 2004; Clemens 2013), triggering oxidative stress (Shiyab et al. 2009), modifying nucleic acids, oxidizing proteins, and inducing LPO (Cho and Park 2000; Moreno-Jimenez et al. 2009). These interactions influence the antioxidant defense system (Israr and Sahi 2006) by interfering with the modulation of the nonenzymatic antioxidants and the enzymatic antioxidants (Sparks 2005; Ortega-Villasante et al. 2005; Israr et al. 2006). Both organic and inorganic Hg accumulation in root may block the entry or binding of ions, such as potassium, magnesium, and manganese, ion carriers, thereby reducing the uptake and transport of some mineral nutrients and inducing nutrient deficiency (Boening 2000). Mercury can bind with DNA, thus causing damage to chromosomes and inducing genotoxicity (Sharma et al. 1990; Cenkci et al. 2009).
The application of appropriate levels of Se significantly balances ROS production by increasing the activity of enzymatic and nonenzymatic antioxidant systems, reducing the amount of lipid peroxidation products, and increasing the concentrations of photosynthetic pigments and essential elements and the level of DNA methylation. However, Se application in excess concentration is toxic to plants and may trigger oxidative stress and reduce crop yields (Hartikainen 2005; Kolbert et al. 2016) (Fig. 5).
Balance ROS production after Se application
The stability of –SH groups is disrupted through their strong interactions with Hg (Patra et al. 2004; Clemens 2013). The disruption of –SH group stability results in ROS and free radical overproduction, which triggers oxidative stress (Shiyab et al. 2009). Accordingly, Hg stress interferes with the modulation of nonenzymatic antioxidants, such as GSH, PCs, ascorbic acid (AsA), proline, carotenoids (Cars), and α-tocopherol, and enzymatic antioxidants, such as superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), glutathione reductase (GR), peroxidase (POD), glutathione peroxidase (GSH-Px), and nitrate reductase (NR) (Sparks 2005; Ortega-Villasante et al. 2005; Israr et al. 2006). Moreover Hg stress induces LPO (Cho and Park 2000; Moreno-Jimenez et al. 2009), as shown in Fig. 6.
Scavenging ROS species overproduction
The enhanced production of ROS is the precursor of oxidative stress and cell damage (Shahid et al. 2014a; Shahid et al. 2014b; Natasha et al. 2018), and O2− and H2O2 are the two most important ROS species in plants under metal stress (Shahid et al. 2013). In plants, O2− can first be catalyzed to H2O2 by SOD with remarkably high reaction rates and then is further degraded into H2O by CAT and APX. APX utilizes ascorbate as a specific electron donor to reduce H2O2 to H2O. A high H2O2 content exerts toxic effects on plants by inducing electrolyte leakage, plasmolysis, and membrane damage (Singh et al. 2018). Mercury exposure can induce reactive oxygen species production and lead to oxidative damage to biological macromolecules.
The ameliorative effects of Se on metal-induced oxidative stress responses may be partly attributed to the improvement of ROS scavenging capability and the change in membrane physicochemical characteristics, such as O2− and H2O2. Under hydroponic conditions, the pretreating rice with SeO32− decreased metal-induced growth inhibition, recovered root cell viability, and dramatically depressed O2− and H2O2 accumulation in rice tissues (Lin et al. 2012). Huang et al. (2019) also showed that the addition of SeO32− greatly reduced H2O2 concentrations in rice tissues (roots and shoots) under two different water management regimes, i.e., flooded and unflooded.
Increasing enzymatic antioxidants
The Hg-induced generation of ROS triggers the activation of components of the antioxidative defense system of plants. Plants contain various types of enzymatic antioxidants to respond to oxidative stress, such as SOD, APX, CAT, GR, GSH-Px, POD, and NR. SOD can protect plant cells from harmful peroxidation reactions (Zhao et al. 2019) and is the first line of intercellular defense against ROS because it catalyzes O2−. CAT and APX are involved in H2O2 detoxification and its conversion to nonphytotoxic H2O and O2 (Alscher et al. 2002; Dinakar et al. 2008). GSH-Px plays an important role in maintaining the cellular antioxidant to pro-oxidant ratio by scavenging H2O2 with the help of GSH (Feng et al. 2013a). NR catalyzes the first step in nitrate assimilation and enhances nutrient metabolism (Beauvais-Flück et al. 2018). GR is crucial for maintaining optimal GSH levels, which is required for the synthesis of PCs, for the function of the GSH–AsA cycle, and as a reductant in numerous biochemical reactions (Pawlik-Skowronska et al. 2007).
The formation of Hg–Se precipitates may equilibrate ROS production and scavenging by restricting Hg2+ to the roots (Liu et al. 2015; Wang et al. 2015), thereby limiting the association between Hg2+ and –SH groups and enhancing shoot GSH translocation (Patra and Sharma 2000). Therefore, Se supplementation can improve the efficiency of antioxidant defense systems and protect the plant against oxidative stress under Hg contamination. The dramatic increase in the activities of antioxidant enzymes, such as SOD, CAT, POD, and GSH-Px in pak choi shoots (Tran et al. 2018b) or NR and POD in common bean leaves (Phaseolus vulgaris L.) (Shrivastava et al. 2016), was observed after SeO32− application. Moreover, Se markedly increased the efficiency of the GSH–AsA cycle, which is involved in modulating the concentrations of GSH and AsA and the activities of GR and DHAR in Chinese cabbage (Brassica rapa subsp. pekinensis) tissues (Wu et al. 2016). Huang et al. (2019) also suggested that SeO32− application increased SOD, CAT, APX, GSH-Px, and GR activities in rice grown in flooded and unflooded soil (Fig. 6).
Increasing nonenzymatic antioxidants
Glutathione and phytochelatin
Glutathione (GSH) is one of the most important antioxidants in plants that efficiently plays a role in counteracting the adverse effects of heavy metals (Gratao et al. 2005; Asgher et al. 2017). Under metal stress conditions, GSH is converted to a GSSG, disulfide bridge; the increase of the ratio of GSH/GSSG is an indicator of oxidative stress (Jozefczak et al. 2012; Hernandez et al. 2015). The balance between GSH and GSSG is a central component in maintaining the redox state of the cell (Sharma et al. 2012). GSH functions as an indicator of oxidative stress and can react directly with ROS. This reaction promotes the regeneration of AsA, which also plays a crucial role in protecting cells against oxidative stress in plants (Aravind and Prasad 2005). PCs are the oligomers of GSH, produced by the enzyme PC synthase. PCs are a group of novel heavy metal-binding polypeptides (Cobbett 2000) that act as chelators and are important for heavy metal detoxification in plants. They belong to a family of cysteine-rich polypeptides that are produced in plants under Hg stress (Yadav 2010).
The SeO32− ion is reduced to Se2− by GSH and subsequently chelated by PC (Cui et al. 2008; Bluemlein et al. 2009). The formation of the intermolecular Se–S bond between Se–cysteinyl-serine and GSH indicates that Se2− can bind to –SH groups present in GSH and PCs and reduce the number of free –SH groups necessary for Hg detoxification. Limiting the association between Hg2+ and –SH groups and enhancing shoot GSH translocation (Patra and Sharma 2000) results in direct chelation of PCs with Hg ions to reduce toxicity through the synthesis of metal-binding peptides of GSH (Mishra et al. 2006; Jozefczak et al. 2012). This response may directly degrade ROS-like OH− and reduce the translocation of Hg in the plants. The reactions are as follows (Bluemlein et al. 2009):
4GSH + SeO32−+ 2H+ → GSSG + GS–Se–GS + 3H2O,
GS–Se–GS + 2H+ → 2GSH + Se–PC2
In addition, GSH content drastically increases the synthesis of several important substances, such as GSH-Px, which protects cells by reducing and counterbalancing intracellular peroxide levels (Han et al. 2015). Under hydroponic conditions, Han et al. (2015) found that increasing SeO32− levels enhanced the GSH and AsA contents in leaves of flue-cured tobacco (Nicotiana tabacum L.). The addition of SeO42− increased GSH concentrations in Chinese brake fern (Pteris vittata L.) fronds, and this effect intensified with prolonged exposure period to Se (Srivastava et al. 2009) (Fig. 6).
Ascorbic acid
Ascorbic acid (AsA) is an important antioxidant that maintains the GSH pool in a plant system and acts as a substrate in the AsA–GSH cycle. AO and APX can catalyze the oxidation of AsA to dehydroascorbate (DHA) (Ohkawa et al. 1989). The hydrolyzed DHA is recycled to AsA, and the reaction is catalyzed by dehydroascorbate reductase (DHAR), which also involves the conversion of GSSG to GSH (Hossain et al. 2010; Yin et al. 2010), thereby regulating the redox state of the cell (Chen and Gallie 2004). GR and DHAR play important roles in keeping the metabolic balance between GSH and AsA contents in the GSH-AsA cycle (Sharma and Dietz 2009).
The response of AsA to Hg accumulation in plants has been observed in many plants in different studies. Cui et al. (2014) demonstrated that alfalfa (Medicago sativa L.) exposed to Hg-induced oxidative stress enhanced concentrations of AsA to mitigate the oxidative stress and reestablish the redox homeostasis. Kováčik et al. (2017) studied the effect of AsA on the Hg-induced oxidative damage in green algae (Coccomyxa subellipsoidea). Singh et al. (2018) treated rice plants treated with 10 μM SeO42− and increased the AsA content by 14.7% compared to those not treated with Se (Fig. 6).
Proline
Proline is an antioxidant amino acid used in protein biosynthesis. Proline tends to accumulate in the cytosol of the plants under metal stress (Matysik et al. 2002; Aslam et al. 2017). Proline can scavenge free oxygen radicals (Alia et al. 2001) by promoting GSH synthesis in plant cells (Pavlikova et al. 2007). Thus, proline is considered a ROS scavenger under Hg stress conditions. In addition, an increased level of proline in plants enhances the production of glutamate kinase and may increase the glutamic acid level due to the synthesis of GSH and PCs in the plant cell (Pavlikova et al. 2007). This process leads to further chelation with metals and reduces metal toxicity by vacuolar sequestration (Chandrakar et al. 2016).
Thus, exogenous applied SeO32− reduced proline accumulation in pak choi shoots through GSH regulation under Hg stress (Tran et al. 2018b). Moreover, high proline accumulation is an important adaptive mechanism for plants under HM stress, because proline acts as an osmolyte and reduces osmotic potential (Pandey and Gupta 2015). The supplementation of SeO32− through seed priming reduced the total phenolic content of rice seedlings (Moulick et al. 2016). Phenolics have a well-known protective role in plants, and the synthesis and accumulation of proline in plant tissues increases under various biotic and abiotic stresses (Khaliq et al. 2015), as shown in Fig. 6.
Carotenoids
Carotenoids (Cars) are plant pigments that function as nonenzymatic antioxidants (Strzalka et al. 2003). Cars play an important role in the protection of chlorophyll pigments under stress conditions. Carotenoids are produced in response to metal stresses (Hale et al. 2001) and increase the antioxidant response of plants to protect regular physiological status against biotic or abiotic stresses (Neill et al. 2002). The role of Cars during Hg stress seems to be limited since their content decreased with increasing Hg concentration (Baek et al. 2012). However, an increase in Car concentrations is reflective of the beneficial effect of Se supplementation on HM stress in cucumber (Cucumis sativus L.) (Hawrylak-Nowak et al. 2014) or tomato (Alyemeni et al. 2018), indicating that Se plays a protective role in Chl and ROS elimination (Han et al. 2012) (Fig. 6).
α-Tocopherol
Tocopherol nonenzymatic antioxidants are also known as vitamin E. Naturally, there are four types of tocopherols (α, β, γ, and δ-tocopherol), which differ in the position of their methyl group (Li et al. 2012). α-Tocopherol is the main form of tocopherol in the green organs of plants (Munné-Bosch and Alegre 2002). It is a membrane-associated nonenzymatic antioxidant that helps in the scavenging of single oxygen and lipid peroxidases (Stahl and Sies 2003). Narang et al. (2008) determined the antioxidant response of α-tocopherols to Hg-induced oxidative stress. The concentration of α-tocopherol increased after 10 days of SeO32− treatment in broccoli leaves (Brassica oleracea L. var. italica) of metal-enriched plants (Pedrero et al. 2008) (Fig. 6).
Decreasing lipid peroxidation
Free radicals and H2O2 are widely reported to cause damage to the lipid bilayer, which mostly results in lipid peroxidation (LPO) (Shahid et al. 2017; Abbas et al. 2018). Malondialdehyde (MDA) is an index of LPO that rapidly increases when membrane lipids are damaged under ROS overproduction (Mishra et al. 2011; Sharma et al. 2012). The production of MDA to protect lipid membranes against Hg stress has been well documented (Alfanie et al. 2015; Cabrita et al. 2019). Lipid peroxidation and loss of membrane integrity increased linearly with increasing accumulation of Hg in the leaf tissues of Hg-stressed plants (Ansari et al. 2009; Chen and Yang 2012; Cui et al. 2014).
The ameliorative effects of Se on Hg-induced oxidative stress responses may be partly attributed to the improvement of the scavenging capability of ROS, the decrease in LPO, and the change in membrane physicochemical characteristics, such as O2−, H2O2, and MDA levels. Tran et al. (2018b) demonstrated that MDA content decreased in pak choi shoots after SeO32− application in unflooded soil (Fig. 5).
Increase in photosynthetic pigment content after Se application
Photosynthesis is a key metabolic process of autotrophs that is sensitive to toxic metals. In plants during photosynthesis, Hg ions may substitute for other essential metal ions and thereby disturb the photosynthetic electron transport chain (Patra et al. 2004; Azevedo and Rodriguez 2012). The substitution of Mg as the central atom of Chl with Hg in vivo prevents photosynthetic light collection in affected Chl molecules and results in the breakdown of photosynthesis (Patra et al. 2004; Tangahu et al. 2011).
Restricting Hg uptake and translocation within plants through Se application may prevent Hg2+ from replacing metal ions (Mg2+), which ultimately balances the photosynthetic electron transport chain and increases photosynthesis rates. Thus, Se helps maintain the integrity of membrane systems in chloroplasts (Vinit-Dunand et al. 2002; Patra et al. 2004; Azevedo and Rodriguez 2012). The SPAD values (represent for Chl content) increased in pak choi leaves after SeO32− application in unflooded soil because of the amelioration of Chl deficiency under Hg stress (Tran et al. 2018b). Similarly, Mozafariyan et al. (2014) showed that Chl a and Chl b concentrations significantly increased in Cd-exposed peppers (Capsicum annuum L.) after SeO32− application (Fig. 5).
Reduction of genotoxic effects after Se application
Mercury is considered genotoxin. Most of the DNA damage caused by Hg stems from ROS formation or by its interaction with the proteins associated with DNA replication systems (Kültz 2005; Angelé-Martínez et al. 2017). These ROS have the potential to interact and damage the purine and pyrimidine bases of the DNA strand, which may lead to strand breakage (Fracasso et al. 2002; Sallmyr et al. 2008). The dramatic change in genomic template stability values suggested that the presence of SeO42− effectively reduced the toxic effect of HMs on the DNA of rice seedlings grown in solution (Pandey and Gupta 2015). Specifically, the reduction in genomic template stability indicated that DNA repair and replication were effective in the presence of low levels of DNA alteration (Pandey and Gupta 2015). Selenium addition induced methylenetetrahydrofolate reductase, which was repressed in rice roots subjected to Se and CH3Hg+ co-exposure, suggesting that Se supplementation alleviated the effect on DNA damage and DNA synthesis induced by Hg treatment (Li et al. 2018).
Moreover, the accumulation of free radicals produced from methylation stress and the direct attack of DNA cytosine by methyl radicals increased DNA methylation level in leaves (Parra et al. 2001). Selenium supplementation to hydroponically grown plant under HM stress protected ramie tissues from abnormal methylation by reducing the level of DNA methylation (Wang et al. 2014). The protective role of Se against changes in DNA methylation patterns may be attributed to the removal of ROS and/or the elimination of HMs from enzymes (Fig. 5).
Reduction of the toxicity of Hg to plant proteins after Se application
The cellular toxicity of Hg2+ ions is considered to be associated with its binding with –SH groups in functional proteins because Hg2+ ions have high affinity for S ligands (Chen et al. 2012b). Hg2+ or CH3Hg+ exposure can form Hg-binding proteins (15–25 kDa) in rice roots (Li et al. 2016). Similar to other heavy metals, Hg interacts with plant proteins (Sheng Zhou et al. 2009; Krishna Sahu et al. 2012). The ability of Hg to change cell membrane permeability with its high affinity for –SH groups, replace essential ion metalloproteins, and disrupt functions involving critical or unprotected proteins, phosphate groups, and active ADP or ATP groups can cause protein precipitation (Patra and Sharma 2000; Patra et al. 2004). The abundance of 49 proteins changed significantly in the roots of Hg-stressed knotgrass (Paspalum distichum L.); 32 proteins were up-regulated, and 17 were down-regulated (Ding et al. 2019).
The reduced disturbance of functional proteins in roots with Se treatment is an important mechanism for the protective effects of Se against Hg. Selenium can regulate the expression of proteins associated with stress response, sulfur and GSH metabolism, DNA replication and the cell cycle, and energy and carbohydrates, suggesting that these proteins participate in the protective effects of Se on Hg toxicity (Li et al. 2018). Considering the thermodynamically higher stability of Hgsenols than Hg-thiols, the formation of Hg-Se complexes in rice roots can prevent the binding of Hg to functional proteins (Feng et al. 2013a). High molecular weight proteinaceous complexes in the rhizosphere are formed under Se addition (Mounicou et al. 2006; Yathavakilla and Caruso 2007) (Fig. 5).
Sun et al. (2016) applied two-dimensional gel electrophoresis (2-DE) coupled with mass spectrometry to perform proteomic analysis and found that the expression of 21 of the 26 identified HM-associated proteins in cucumber tissues increased after the addition of SeO32−. Selenium can also effectively influence ATPase synthesis by maintaining membrane lipid integrity, modulating pH and Ca2+ homeostasis, and competing with HMs for entrance to root cells via ion channels. Thus, the addition of SeO32− sharply alleviates HM toxicity in rice tissues by increasing root H+-ATPase and Ca2+-ATPase activities (Lin et al. 2012). Li et al. (2018) also found that Se addition induced the formation of additional Hg-containing proteins in the range of 55–70 kDa (high molecular weight) but decreased the Hg content of functional proteins of 15–25 kDa (small molecular weight), and protected the proteins and enzymes from Hg destruction (Fig. 5).
Toxic effects of Se application with excess concentration to plants
Selenium doses need to be specifically monitored given the narrow range between deficiency and toxicity in plants. Others have applied high levels Se and showed synergistic toxic effect with Hg and inhibited plant growth as a pro-oxidant (Han et al. 2013; Zhao et al. 2013; Feng et al. 2013a).
Under hydroponic conditions, the study of Han et al. (2015) reported that the addition of Se (5 mg/L) decreased concentration of HM but decreased the fresh weights of leaves and roots in flue-cured tobacco and increased the MDA content (Han et al. 2015). In the roots of faba bean (Vicia faba L.) exposed to 50 μM Pb, the addition of a higher level of Se (6 μM) greatly enhanced the O2− level and decreased the cell viability and total –SH content (Mroczek-Zdyrska and Wójcik 2011). High doses of Se (>2 mg/L) exerted toxic effects on growth of spinach (Spinacia oleracea L.) plants because of its interaction with different nutrients (Saffaryazdi et al. 2012). In ryegrass (Lolium perenne L.), 1 mg/kg SeO42− added to soil was believed to be marginally toxic (Hartikainen et al. 2000), which is similar to the Se level considered to be toxic to paddy rice in a hydroponic system (0.8 mg/L SeO32−) (Feng et al. 2013a). Under unflooded soil conditions, a significant growth improvement of pak choi was only found at low Se treatment (1.0 mg/kg) because of the synergistic toxic effect of Se with Hg when applied at a high Se rate (2.5 mg/kg) in our previous study (Tran et al. 2018b). In addition, excess Se (≥11.1 mg/kg) also inhibited the growth of flue-cured tobacco (Han et al. 2013).
When Se was added in soil at an excessive rate, inorganic Se strongly transformed into organic species led some vital substances (for example GSH) might not satisfy the metabolism demands, caused GSH deficient (Han et al. 2015). An imbalance in the levels of GSH by excessive Se gives rise to ROS production because GSH are not sufficient to quench ROS and result in a ROS burst. Thus, oxidative stress appeared and plant growth was inhibited (Hartikainen et al. 2000; de la Luz Mora et al. 2008; Feng et al. 2013a). In addition, the toxicity of Se is thought to be due to its chemical similarity to S, leading to the non-specific replacement of S by Se in proteins and other S compounds (Cheng et al. 2016). The photosynthetic performance may be decreased by Se replacing S amino acids in photosynthetic proteins (Freeman et al. 2010). Specifically, higher exogenous Se levels caused an inhibition in the chlorophyll contents in lettuce (Lactuca sativa L.) (Abbas 2013; Abbas 2012; Chen et al. 2005; Xue et al. 2001). Besides, high Se concentrations may also be incorporated as SeCys and SeMet into selenoenzymes and selenoproteins, which replace Cys and Met and induce Se toxicity in plants (Montesbayon et al. 2002; Navarro-Alarcon and Cabrera-Vique 2008). Moreover, Hawrylak-Nowak (2008) suggested that reduction in maize (Zea mays L.) plant biomass at higher Se concentration might have been a result due to the accumulation of phosphorus in the shoot tissues.
Effects of different Se application approaches on Hg detoxification
Selenium application has been demonstrated to reduce the accumulation of Hg in plants. However, its mechanism is clarified by exploring the potential effects of Se species (SeO32− and SeO42−), Se doses, and Se application methods (soil or foliar application) on Hg2+–Se and CH3Hg+–Se antagonistic interactions in soil–plant systems (Fig. 2).
Se species
Earlier studies have investigated Se accumulation in plants by treating plant growth media or soil with SeO32− and SeO42−, which are the main Se species taken up by plants (Ellis and Salt 2003). Both forms of Se can limit the absorption and bioaccumulation of Hg in plants (Hu et al. 2014; Tang et al. 2017; Huang et al. 2018). The Se species SeO32− and SeO42− were equally effective in reducing the Hg content in radish and tomato plants cultivated in upland soil (Shanker et al. 1996b; Shanker et al. 1996a) and in reducing Hg accumulation in garlic in hydroponic culture (Zhao et al. 2013). Moreover, SeO32− and SeO42− were equally effective in reducing the CH3Hg+ concentrations of high-Se and low-Se paddy soils (Wang et al. 2016a) or inhibiting sulfate-mediated CH3Hg+ production regardless of sulfate input (Wang et al. 2016b). Tang et al. (2017) demonstrated that the inhibitory effects of Se application on Hg2+ bioaccumulation in rice depended on Se doses rather than on the Se species (SeO32− and SeO42−), given that SeO42− is rapidly transformed to SeO32− under flooded conditions (Wang et al. 2016a; Wang et al. 2016b; Tang et al. 2017). The conversion of SeO32− and SeO42− to other Se species (e.g., Se0 and Se2−) under anoxic conditions (Martin et al. 2011; Li et al. 2014a; Wang et al. 2016a) may account for the similar abilities of SeO32− and SeO42− to reduce CH3Hg+ concentrations.
Our recent study demonstrated that SeO32− application can reduce the concentrations of Hg in pak choi roots more than SeO42− for upland soil (Tran et al. 2018a). Selenite treatments significantly decreased the proportion of Hg in pak choi shoots, whereas Hg accumulation notably increased in shoots of pak choi when treated with SeO42−. These different phenomena can be ascribed to the differences in Hg tolerance among various plant species and different experimental conditions. Thus, these results helped identify the Se species that can be useful for Se amendment in future studies.
In addition, Se may have different effects on the accumulation and translocation of Hg species (Hg2+ and CH3Hg+) in plant tissues. The inhibitory effect of Se on Hg2+ uptake rather than the direct effect of Se on CH3Hg+ substantially decreased Hg2+ concentrations and negligibly decreased root CH3Hg+ concentrations in rice tissues (Zhao et al. 2014). These results were consistent with those obtained by Zhang et al. (2012), who suggested that increasing soil Se concentrations can inhibit the absorption of Hg2+ in rice roots but not that of CH3Hg+. Soil Se levels were negatively correlated with the TFs of Hg2+ that mediate Hg uptake from the soil to the root, which were positively correlated with the TFs of CH3Hg+ (Zhang et al. 2012).
Se doses
Selenium dose is more important than Se speciation in controlling Hg accumulation in plant. The results of our study showed that the SeO32− or SeO42− application can inhibit the absorption and bioaccumulation of Hg in pak choi grown in dryland soil. Notably, this inhibition may only significantly occur when SeO32− or SeO42− application rate is at an appropriate level (2.5 mg/kg) (Tran et al. 2018a). Under flooded conditions, Hg2+ levels of rice root decreased significantly by 36 to 48% under 6.0 mg/kg SeO32− or SeO42− treatment, respectively, which was higher compared with the decrease of ~22% for 3.0 mg/kg SeO32− or SeO42− (Tang et al. 2017). Feng et al. (2009) also found that the inhibition and stimulation effects of SeO32− on the essential elements depended on the Se dosage applied. Low SeO32− dosages significantly decreased essential element contents in Chinese brake fern, whereas high dosages enhanced the uptake of essential elements (Feng et al. 2009). Zhao et al. (2013) also found that a high amount of SeO32− or SeO42− (100 mg/L) treatment significantly increased Hg concentrations in the roots of garlic. In addition, high concentrations of SeO32− or SeO42− also inhibited garlic growth due to the phytotoxicity induced by Se. High Se application (Se > 5 μg/mL) did not reduce Hg accumulation in rice plants (Li et al. 2019).
In addition, Se also showed a dose-dependent effect on the formation of Fe plaques; only low doses of Se (≤ 1.0 mg/kg) promoted Fe plaque formation (Chang et al. 2013). Thus, the mechanisms underlying Hg detoxification in soil–plant systems after Se application resulted in the reduction of the accumulation of Hg in plants and improvement of the growth of plants. Li et al. (2015) demonstrated that the treatment with the appropriate level of Se (0.5 μg/mL in this study) is an efficient way to reduce Hg accumulation in rice and increase rice yield and quality.
Se application methods
The Hg–Se interactions were found in the rhizosphere (i.e., soil or rice root) instead of in the aboveground tissues (Wang et al. 2016a; Tang et al. 2017), which is probably be the reason for the reduced Hg bioaccumulation following Se application. Therefore, the two Se applications (to soil and leaves) differed distinctly in terms of their effects on Hg accumulation in plants. Soil Se application significantly reduced Hg accumulation in most cases, whereas foliar Se application had insignificant effects (Wang et al. 2016a). Specifically, the accumulation of CH3Hg+ in rice grains was largely inhibited (7–73%) after soil SeO32− and SeO42− application of 0.5–6.0 mg/kg, whereas no significant changes were found after foliar application (30 and 80 g/ha). Similarly, Tang et al. (2017) reported that Hg2+ concentrations in rice roots decreased after soil SeO32− and SeO42− application but not after foliar application, indicating that soil application could evidently reduce tissue Hg2+ concentrations by 0–48% in root, 15–58% in straw, and 26–74% in brown rice, although both applications resulted in comparable Se accumulation in aboveground tissues. In addition, foliar application of Se also has no effect on Hg accumulation in grape berries (Vitis vinifera L.), while the heavy metal content of Pb, Cr, Cd, As and Ni in grape berries was reduced under Se fertilizer treatments reduced compared to the control (Zhu et al. 2017).
Conclusion and future perspectives
The applications of Se at the appropriate rate can serve as potential strategy for Hg detoxification in soil and plant tissues and can reduce the public health risk. This review showed that the mechanisms for Hg detoxification by Se application included the following:
-
(1)
Selenium application reduced the bioavailability of Hg in soil through the transformation of Hg into an immobile speciation and suppression of Hg methylation.
-
(2)
Selenium application led the formation of inert HgSe or/and HgSe-containing proteinaceous complexes in the rhizosphere and/or roots results in immobilization of Hg on the interface of soil–plant root.
-
(3)
Prevention of plant root uptake and translocation of Hg by increasing Fe plaques on root surfaces and sequestering Hg into the vacuoles of root cells.
-
(4)
Reduction of plant oxidative stress under Hg stress by activating antioxidant systems, increasing photosynthetic pigment concentrations, decreasing lipid peroxidation products, alleviating the effect on DNA damage, and reducing protein participation.
-
(5)
However, application of high rate Se showed synergistic toxic effect with Hg and inhibit the plant growth.
Besides the Hg bioavailability and the changes of Hg speciation in soil, Hg uptake, and transport within plants, more research is needed on the mechanisms associated with the detoxification of Hg by application of Se better. In addition, application methods need to be studied to understand the following:
-
(1)
Selenium bioavailability and speciation transformation in soil and the combined capacity of Se and Hg to form stable HgSe complexes need to be clarified.
-
(2)
The changes in Se speciation in plants must also be clarified given their effects on competitive adsorption or chelation between Se and Hg.
-
(3)
Selenium absorption and transport within plants and the physiological and biochemical mechanisms underlying these processes need to be examined.
Availability of data and materials
Not applicable
References
Abbas SM (2012) Effects of low temperature and selenium application on growth and the physiological changes in sorghum seedlings. J Stress Physiol Biochem 8:268–286
Abbas SM (2013) Low levels of selenium application attenuate low temperature stress in sorghum [Sorghum bicolor (L.) Moench.] seedlings. Pak J Bot 45:1597–1604
Abbas G, Murtaza B, Bibi I, Shahid M, Niazi NK, Khan MI, Amjad M, Hussain M, Natasha (2018) Arsenic uptake, toxicity, detoxification, and speciation in plants: physiological, biochemical, and molecular aspects. Int J Environ Res Public Health 15(1):59
Abd-Allah EF, Abeer H, Alqarawi AA (2016) Mitigation of cadmium induced stress in tomato (Solanum lycopersicum L.) by selenium. Pak J Bot 48:953–961
Aborode FA, Raab A, Voigt M, Costa LM, Krupp EM, Feldmann J (2016) The importance of glutathione and phytochelatins on the selenite and arsenate detoxification in Arabidopsis thaliana. J Environ Sci 49:150–161
Afton SE, Caruso JA (2009) The effect of Se antagonism on the metabolic fate of Hg in Allium fistulosum. J Anal At Spectrom 24:759–766
Alfanie I, Muhyi R, Suhartono E (2015) Effect of heavy metal on malondialdehyde and advanced oxidation protein products concentration: a focus on arsenic, cadmium, and mercury. J Medical Bioengineering 4(1)
Alia A, Mohanty P, Matysik J (2001) Effect of proline on the production of singlet oxygen. Amino Acids 21:195–200
Alscher RG, Erturk N, Heath LS (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 53:1331–1341
Alyemeni MN, Ahanger MA, Wijaya L, Alam P, Bhardwaj R, Ahmad P (2018) Selenium mitigates cadmium-induced oxidative stress in tomato (Solanum lycopersicum L.) plants by modulating chlorophyll fluorescence, osmolyte accumulation, and antioxidant system. Protoplasma 255:985–986
Angelé-Martínez C, Nguyen KVT, Ameer FS, Anker JN, Brumaghim JL (2017) Reactive oxygen species generation by copper (II) oxide nanoparticles determined by DNA damage assays and EPR spectroscopy. Nanotoxicology 11:278–288
Ansari MKA, Ahmad A, Umar S, Iqbal M (2009) Mercury-induced changes in growth variables and antioxidative enzyme activities in Indian mustard. J Plant Interact 4:131–136
Aravind P, Prasad MNV (2005) Modulation of cadmium-induced oxidative stress in Ceratophyllum demersum by zinc involves ascorbate-glutathione cycle and glutathione metabolism. Plant Physiol Biochem 43:107–116
Asgher M, Per TS, Anjum S, Khan MIR, Masood A, Verma S, Khan NA, Asgher M, Per TS, Anjum S, Khan MIR, Masood A, Verma S, Khan NA (2017) Contribution of glutathione in heavy metal stress tolerance in plants. Reactive oxygen species and antioxidant systems in plants: role and regulation under abiotic stress Springer 297–313.
Aslam M, Saeed MS, Sattar S, Sajad S, Sajjad M, Adnan M, Iqbal M, Sharif MT (2017) Specific role of proline against heavy metals toxicity in plants. Int J Pure App Biosci 5:27–34
ATSDR (2017) Substance priority list agency for toxic substances and disease registry
Azevedo R, Rodriguez E (2012) Phytotoxicity of mercury in plants: a review. Aust J Bot 2012:1–6
Baek S, Han T, Ahn SK, Kang H, Cho MR, Lee SC, Im KH (2012) Effects of heavy metals on plant growths and pigment contents in Arabidopsis thaliana. Plant Pathol J 28(4):446–452
Barnett MO, Harris LA, Turner RR, Stevenson RJ, Henson TJ, Melton RC, Hoffman DP (1997) Formation of mercuric sulfide in soil. Environ Sci Technol 31:3037–3043
Beauvais-Flück R, Slaveykova VI, Cosio C (2018) Molecular effects of inorganic and methyl mercury in aquatic primary producers: comparing impact to a macrophyte and a green microalga in controlled conditions. Geosciences 8:393
Belimov AA, Hontzeas N, Safronova VI, Demchinskaya SV, Piluzza G, Bullitta S, Glick BR (2005) Cadmium-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard (Brassica juncea L. Czern.). Soil Biol Biochem 37:241–250
Benavides MP, Gallego SM, Tomaro ML (2005) Cadmium toxicity in plants. Braz J Plant Physiol 17:21–34
Björnberg A, Håkanson L, Lundbergh K (1988) A theory on the mechanisms regulating the bioavailability of mercury in natural waters. Environ Pollut 49:53–61
Blazka ME, Shaikh ZA (1992) Cadmium and mercury accumulation in rat hepatocytes: interactions with other metal ions. Toxicol Appl Pharmacol 113:118–125
Bluemlein K, Klimm E, Raab A, Feldmann J (2009) Selenite enhances arsenate toxicity in Thunbergia alata. Environ Chem 6:486–494
Boening DW (2000) Ecological effects, transport, and fate of mercury: a general review. Chemosphere 40:1335–1351
Boszke L, Glosinska G, Siepak J (2002) Some aspects of speciation of mercury in a water environment. Pol J Environ Stud 11(4):285–298
Boszke L, Kowalski A, Szczuciński W, Rachlewicz G, Lorenc S, Siepak J (2006) Assessment of mercury mobility and bioavailability by fractionation method in sediments from coastal zone inundated by the 26 December 2004 tsunami in Thailand. Environ Geol 51:527–536
Burd GI, Dixon DG, Glick BR (2000) Plant growth-promoting bacteria that decrease heavy metal toxicity in plants. Can J Microbiol 46:237–245
Cabrita MT, Duarte B, Cesário R, Mendes R, Hintelmann H, Eckey K, Dimock B, Cacador I, Canario J (2019) Mercury mobility and effects in the salt-marsh plant Halimione portulacoides: uptake, transport, and toxicity and tolerance mechanisms. Sci Total Environ 650:111–120
Carrasco-Gil S, Alvarez-Fernandez A, Sobrino-Plata J, Millan R, Carpena-Ruiz RO, Leduc DL, Andrews JC, Abadia J, Hernandez LE (2011) Complexation of Hg with phytochelatins is important for plant Hg tolerance. Plant Cell Environ 34:778–791
Cenkci S, Yildiz M, Cigerci IH, Konuk M, Bozdag A (2009) Toxic chemicals-induced genotoxicity detected by random amplified polymorphic DNA (RAPD) in bean (Phaseolus vulgaris L.) seedlings. Chemosphere 76:900–906
Chandrakar V, Naithani SC, Keshavkant S (2016) Arsenic-induced metabolic disturbances and their mitigation mechanisms in crop plants: a review. Biologia 71:367–377
Chang H, Zhou XB, Wang WH, Zhou YX, Dai WC, Zhang CM, Yu SH (2013) Effects of selenium application in soil on formation of iron plaque outside roots and cadmium uptake by rice plants. Adv Mater Res 750–752:1573–1576
Chen Z, Gallie DR (2004) The ascorbic acid redox state controls guard cell signaling and stomatal movement. Plant Cell 16:1143–1162
Chen J, Yang ZM (2012) Mercury toxicity, molecular response and tolerance in higher plants. Biometals 25:847–857
Chen TF, Zheng WJ, Luo Y, Yang F, Bai Y, Tu F (2005) Effects of selenium stress on photosynthetic pigment contents and growth of Chlorella vulgaris. J Plant Physiol Mol Biol 31:369–373
Chen CY, Driscoll CT, Lambert KF, Mason RP, Rardin LR, Schmitt CV, Serrell NS, Sunderland EM (2012a) Sources to seafood: mercury pollution in the marine environment. Maine Sea Grant Publications 64
Chen YA, Chi WC, Huang TL, Lin CY, Nguyen TTQ, Hsiung YC, Chia LC, Huang HJ (2012b) Mercury-induced biochemical and proteomic changes in rice roots. Plant Physiol Biochem 55:23–32
Cheng H, Wang M, Wong MH, Ye ZH (2014) Does radial oxygen loss and iron plaque formation on roots alter Cd and Pb uptake and distribution in rice plant tissues? Plant Soil 375:137–148
Cheng B, Lian HF, Liu YY, Yu XH, Sun YL, Sun XD, Shi QH, Liu SQ (2016) Effects of selenium and sulfur on antioxidants and physiological parameters of garlic plants during senescence. J Integr Agric 15(3):566–572
Chiasson-Gould SA, Blais JM, Poulain AJ (2014) Dissolved organic matter kinetically controls mercury bioavailability to bacteria. Environ Sci Technol 48:3153–3161
Cho UH, Park JO (2000) Mercury-induced oxidative stress in tomato seedlings. Plant Sci 156:1–9
Clarkson TW, Magos L (2006) The toxicology of mercury and its chemical compounds. Crit Rev Toxicol 36:609–662
Clemens S (2006) Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 88:1707–1719
Clemens S (2013) Mercury in plants. Mercury exacerbation of alzheimer’s disease
Cobbett CS (2000) Phytochelatins and their roles in heavy metal detoxification. Plant Physiol 123:825–832
Covelli S, Acquavita A, Piani R, Predonzani S, De Vittor C (2009) Recent contamination of mercury in an estuarine environment (Marano lagoon, Northern Adriatic, Italy). Estuar Coast Shelf S 82:273–284
Cui SY, Jin H, Kim SJ, Kumar AP, Lee YI (2008) Interaction of glutathione and sodium selenite in vitro investigated by electrospray ionization tandem mass spectrometry. J Biochem 143:685–693
Cui WT, Fang P, Zhu KK, Mao Y, Gao CY, Xie YJ, Wang J, Shen WB (2014) Hydrogen-rich water confers plant tolerance to mercury toxicity in alfalfa seedlings. Ecotoxicol Environ Saf 105:103–111
Dang F, Li ZZ, Zhong H (2019) Methylmercury and selenium interactions: mechanisms and implications for soil remediation. Crit Rev Environ Sci Technol 49(19):1737–1768
de la Luz MM, Pinilla L, Rosas A, Cartes P (2008) Selenium uptake and its influence on the antioxidative system of white clover as affected by lime and phosphorus fertilization. Plant Soil 303:139–149
de Souza MP, Pilonsmits EA, Lytle CM, Hwang S, Tai J, Honma TS, Yeh L, Terry N (1998) Rate-limiting steps in selenium assimilation and volatilization by indian mustard. Plant Physiol 117(4):1487–1494
Dinakar N, Nagajyothi PC, Suresh S, Udaykiran Y, Damodharam T (2008) Phytotoxicity of cadmium on protein, proline and antioxidant enzyme activities in growing Arachis hypogaea L. seedlings. J Environ Sci 20:199–206
Ding YZ, Feng RW, Wang RG, Guo JK, Zheng XQ (2014) A dual effect of Se on Cd toxicity: evidence from plant growth, root morphology and responses of the antioxidative systems of paddy rice. Plant Soil 375:289–301
Ding W, Zhang J, Wu SC, Zhang S, Christie P, Liang P (2019) Responses of the grass Paspalum distichum L. to Hg stress: a proteomic study. Ecotoxicol Environ Saf 183:109549
Ellis DR, Salt DE (2003) Plants, selenium and human health. Curr Opin Plant Biol 6:273–279
Feng RW, Wei CY, Tu SX, Wu FC (2009) Effects of Se on the uptake of essential elements in Pteris vittata L. Plant Soil 325:123–132
Feng RW, Wei CY, Tu SX (2013a) The roles of selenium in protecting plants against abiotic stresses. Environ Exp Bot 87:58–68
Feng RW, Wei CY, Tu SX, Ding YZ, Song ZG (2013b) A dual role of Se on Cd toxicity: evidences from the uptake of Cd and some essential elements and the growth responses in paddy rice. Biol Trace Elem Res 151:113–121
Fernandez-Martinez R, Loredo J, Ordonez A, Rucandio MI (2005) Distribution and mobility of mercury in soils from an old mining area in Mieres, Asturias (Spain). Sci Total Environ 346:200–212
Fernandez-Martinez R, Larios R, Gomez-Pinilla I, Gomez-Mancebo B, Lopez-Andres S, Loredo J, Ordonez A, Rucandio I (2015) Mercury accumulation and speciation in plants and soils from abandoned cinnabar mines. Geoderma 253:30–38
Fracasso ME, Perbellini L, Soldà S, Talamini G, Franceschetti P (2002) Lead induced DNA strand breaks in lymphocytes of exposed workers: role of reactive oxygen species and protein kinase C. Mutat. Res./Genet. Toxicol Environ Mutagen 515:159–169
Freeman JL, Tamaoki M, Stushnoff C, Quinn CF, Cappa JJ, Devonshire J, Fakra SC, Marcus MA, McGrath SP, Hoewyk DV, Pilon-Smits EAH (2010) Molecular mechanisms of selenium tolerance and hyperaccumulation in Stanleya pinnata. Plant Physiol 153(4):1630–1652
Frohne T, Rinklebe J, Langer U, Du Laing G, Mothes S, Wennrich R (2012) Biogeochemical factors affecting mercury methylation rate in two contaminated floodplain soils. Biogeosciences 9:493–507
Fu YQ, Yang XJ, Shen H (2018) Root iron plaque alleviates cadmium toxicity to rice (Oryza sativa) seedlings. Ecotoxicol Environ Saf 161:534–541
Gallego SM, Pena LB, Barcia RA, Azpilicueta CE, Lannone MF, Rosales EP, Zawoznik MS, Groppa MD, Benavides MP (2012) Unravelling cadmium toxicity and tolerance in plants: Insight into regulatory mechanisms. Environ Exp Bot 83:33–46
Geng N, Wu YC, Zhang M, Tsang DCW, Rinklebe J, Xia YF, Lu DB, Zhu LF, Palansooriya KN, Kim K, Ok YS (2019) Bioaccumulation of potentially toxic elements by submerged plants and biofilms: A critical review. Environ Int 131:105015
Gratao PL, Polle A, Lea PJ, Azevedo RA (2005) Making the life of heavy metal-stressed plants a little easier. Funct Plant Biol 32:481–494
Gray JE, Theodorakos PM, Fey DL, Krabbenhoft DP (2015) Mercury concentrations and distribution in soil, water, mine waste leachates, and air in and around mercury mines in the Big Bend region. Environ Geochem Health 37:35–48
Hale KL, McGrath SP, Lombi E, Stack SM, Terry N, Pickering IJ, George GN, Pilon-Smits EAH (2001) Molybdenum sequestration in Brassica species. A role for anthocyanins? Plant Physiol 126:1391–1402
Han FX, Banin A (2000) Long-term transformations of cadmium, cobalt, copper, nickel, zinc, vanadium, manganese, and iron in arid-zone soils under saturated condition. Commun Soil Sci Plan 31:943–957
Han Y, Kingston HM, Boylan HM, Rahman GMM, Shah S, Richter RC, Link DD, Bhandari S (2003) Speciation of mercury in soil and sediment by selective solvent and acid extraction. Anal Bioanal Chem 375:428–436
Han RM, Zhang JP, Skibsted LH (2012) Reaction dynamics of flavonoids and carotenoids as antioxidants. Molecules 17:2140–2160
Han D, Li XH, Xiong SL, Tu SX, Chen ZG, Li JP, Xie ZJ (2013) Selenium uptake, speciation and stressed response of Nicotiana tabacum L. Environ Exp Bot 95:6–14
Han D, Xiong SL, Tu SX, Liu JC, Chen C (2015) Interactive effects of selenium and arsenic on growth, antioxidant system, arsenic and selenium species of Nicotiana tabacum L. Environ Exp Bot 117:12–19
Hartikainen H (2005) Biogeochemistry of selenium and its impact on food chain quality and human health. J Trace Elem Med Biol 18(4):309–318
Hartikainen H, Xue TL, Piironen V (2000) Selenium as an anti-oxidant and pro-oxidant in ryegrass. Plant Soil 225:193–200
Hawrylak-Nowak B (2008) Effect of selenium on selected macronutrients in maize plants. J Elem 13:513–519
Hawrylak-Nowak B, Dresler S, Wojcik M (2014) Selenium affects physiological parameters and phytochelatins accumulation in cucumber (Cucumis sativus L.) plants grown under cadmium exposure. Sci Hortic 172:10–18
Hernandez LE, Sobrino-Plata J, Montero-Palmero MB, Carrasco-Gil S, Flores-Cáceres ML, OrtegabVillasante C, Escobar C (2015) Contribution of glutathione to the control of cellular redox homeostasis under toxic metal and metalloid stress. J Exp Bot 66:2901–2911
Hossain MA, Hasanuzzaman M, Fujita M (2010) Up-regulation of antioxidant and glyoxalase systems by exogenous glycinebetaine and proline in mung bean confer tolerance to cadmium stress. Physiol Mol Biol Plants 16:259–272
Hu Y, Duan GL, Huang YZ, Liu YX, Sun GX (2014) Interactive effects of different inorganic As and Se species on their uptake and translocation by rice (Oryza sativa L.) seedlings. Environ Sci Pollut R 21:3955–3962
Huang GX, Ding CF, Guo FY, Li XG, Zhang TL, Wang XX (2017) Underlying mechanisms and effects of hydrated lime and selenium application on cadmium uptake by rice (Oryza sativa L.) seedlings. Environ Sci Pollut Res 24:18926–18935
Huang QQ, Xu YM, Liu YY, Qin X, Huang R, Liang XF (2018) Selenium application alters soil cadmium bioavailability and reduces its accumulation in rice grown in Cd-contaminated soil. Environ Sci Pollut Res 25:31175–31182
Huang QQ, Liu YY, Qin X, Zhao LJ, Liang XF, Xu YM (2019) Selenite mitigates cadmium-induced oxidative stress and affects Cd uptake in rice seedlings under different water management systems. Ecotoxicol Environ Saf 168:486–494
Israr M, Sahi S (2006) Antioxidative responses to mercury in the cell cultures of Sesbania drummondii. Plant Physiol Biochem 44(10):590–595
Israr M, Sahi S, Datta R, Sarkar D (2006) Bioaccumulation and physiological effects of mercury in Sesbania drummondii. Chemosphere 65:591–598
Issaro N, Abi-Ghanem C, Bermond A (2009) Fractionation studies of mercury in soils and sediments: a review of the chemical reagents used for mercury extraction. Anal Chim Acta 631:1–12
Jia Y, Bao P, Zhu YG (2015) Arsenic bioavailability to rice plant in paddy soil: influence of microbial sulfate reduction. J Soils Sediments 15:1960–1967
Jonsson S, Skyllberg U, Nilsson MB, Westlund PO, Shchukarev A, Lundberg E, Björn E (2012) Mercury methylation rates for geochemically relevant HgII species in sediments. Environ Sci Technol 46:11653–11659
Jozefczak M, Remans T, Vangronsveld J, Cuypers A (2012) Glutathione is a key player in metal-induced oxidative stress defenses. Int J Mol Sci 13:3145–3175
Kerin EJ, Gilmour CC, Roden E, Suzuki MT, Coates JD, Mason RP (2006) Mercury methylation by dissimilatory iron-reducing bacteria. Appl Environ Microbiol 72:7919–7921
Khaliq A, Aslam F, Matloob A, Hussain S, Geng M, Wahid A, Rehman HU (2015) Seed priming with selenium: consequences for emergence, seedling growth, and biochemical attributes of rice. Biol Trace Elem Res 166:236–244
Khan MAK, Wang F (2009) Mercury-selenium compounds and their toxicological signifificance: toward a molecular understanding of the mercury-selenium antagonism. Environ Toxicol Chem 28(8):1567–1577
Khan MAK, Wang F (2010) Chemical demethylation of methylmercury by selenamino acids. Chem Res Toxicol 23(7):1202–1206
Kocman D, Horvat M, Kotnik J (2004) Mercury fractionation in contaminated soils from the Idrija mercury mine region. J Environ Monit 6(8):696–703
Kocman D, Horvat M, Pirrone N, Cinnirella S (2013) Contribution of contaminated sites to the global mercury budget. Environ Res 125:160–170
Kolbert Z, Lehotai N, Molnár Á, Feigl G (2016) “The roots” of selenium toxicity: a new concept. Plant Signal Behav 11:1241935
Kováčik J, Rotková G, Bujdoš M, Babula P, Peterková V, Matúš P (2017) Ascorbic acid protects Coccomyxa subellipsoidea against metal toxicity through modulation of ROS/NO balance and metal uptake. J Hazard Mater 339:200–207
Krishna Sahu G, Upadhyay S, Bhusan Sahoo B (2012) Mercury induced phytotoxicity and oxidative stress in wheat (Triticum aestivum L.) plants. Physiol Mol Biol Plants 18(1):21–31
Krupp EM, Mestrot A, Wielgus J, Meharg AA, Feldman J (2009) The molecular form of mercury in biota: identifification of novel mercury peptide complexes in plants. Chem Commun 2009:4257–4259
Kültz D (2005) Molecular and evolutionary basis of the cellular stress response. Annu Rev Physiol 67:225–257
Kumar M, Bijo AJ, Baghel RS, Reddy CRK, Jha B (2012) Selenium and spermine alleviates cadmium induced toxicity in the red seaweed Gracilaria dura by regulating antioxidant system and DNA methylation. Plant Physiol Biochem 51:129–138
Laurier F, Cossa D, Gonzalez JL, Breviere E, Sarazin G (2003) Mercury trans formations and exchanges in a high turbidity estuary: the role of organic matter and amorphous oxyhydroxides. Geochem Cosmochim Acta 67(18):3329–3345
Li YH, Yang LS, Ji YF, Sun HF, Wang WY (2009) Quantification and fractionation of mercury in soils from the Chatian mercury mining deposit, southwestern China. Environ Geochem Health 31:617–628
Li Z, Keasling JD, Niyogi KK (2012) Overlapping photoprotective function of vitamin E and carotenoids in Chlamydomonas. Plant Physiol 158:313–323
Li DB, Cheng YY, Wu C, Li WW, Li N, Yang ZC, Tong ZH, Yu HQ (2014a) Selenite reduction by Shewanella oneidensis MR-1 is mediated by fumarate reductase in periplasm. Sci Rep 4.
Li Y, Zhao J, Gao Y, Li Y, Li B, Zhao Y, Chai Z (2014b) Effects of iron plaque and selenium on the absorption and translocation of inorganic mercury and methylmercury in rice (Oryza sativa L.). Asian J Ecotoxicol 9(5):972–977
Li YF, Zhao JT, Li YY, Li HJ, Zhang JF, Li B, Gao YX, Chen CY, Luo MY, Huang R, Li J (2015) The concentration of selenium matters: a field study on mercury accumulation in rice by selenite treatment in qingzhen, Guizhou, China. Plant Soil 391:195–205
Li YY, Zhao JT, Li YF, Xu XH, Zhang BW, Liu YJ, Cui LW, Li B, Gao YX, Chai ZF (2016) Comparative metalloproteomic approaches for the investigation proteins involved in the toxicity of inorganic and organic forms of mercury in rice (Oryza sativa L.) roots. Metallomics 8(7):663–671
Li YY, Li H, Li YF, Zhao JT, Guo JX, Wang R, Li B, Zhang ZY, Gao YX (2018) Evidence for molecular antagonistic mechanism between mercury and selenium in rice (Oryza sativa L.): a combined study using 1, 2-dimensional electrophoresis and SR-XRF techniques. J Trace Elem Med Biol 50:435–440
Li YY, Hua WJ, Zhao JT, Chen CM, Wang W, Li B, Li YF (2019) Selenium decreases methylmercury and increases nutritional elements in rice growing in mercury-contaminated farmland. Ecotox Environ Safe 182:109447
Lin L, Zhou WH, Dai HX, Cao FB, Zhang GP, Wu FB (2012) Selenium reduces cadmium uptake and mitigates cadmium toxicity in rice. J Hazard Mater 235:343–351
Liu WX, Shang SH, Feng X, Zhang GP, Wu FB (2015) Modulation of exogenous selenium in cadmium-induced changes in antioxidative metabolism, cadmium uptake, and photosynthetic performance in the 2 tobacco genotypes differing in cadmium tolerance. Environ Toxicol Chem 34:92–99
Makino T, Takano H, Kamiya T, Itou T, Sekiya N, Inahara M, Sakurai Y (2008) Restoration of cadmium-contaminated paddy soils by washing with ferric chloride: Cd extraction mechanism and bench-scale verification. Chemosphere 70:1035–1043
Malik JA, Goel S, Kaur N, Sharma S, Singh I, Nayyar H (2012) Selenium antagonises the toxic effects of arsenic on mungbean (Phaseolus aureus Roxb.) plants by restricting its uptake and enhancing the antioxidative and detoxification mechanisms. Environ Exp Bot 77:242–248
Martin AJ, Simpson S, Fawcett S, Wiramanaden CIE, Pickering IJ, Belzile N, Chen YW, London J, Wallschlager D (2011) Biogeochemical mechanisms of selenium exchange between water and sediments in two contrasting lentic environments. Environ Sci Technol 45:2605–2612
Martínez-Trinidad S, Hernández Silva G, Martínez Reyes J, Solorio Munguía G, Solís Valdez S, Ramírez Islas ME, García Martínez R (2013) Total mercury in terrestrial systems (air-soil-plant-water) at the mining region of San Joaquín, Queretaro, Mexico. Geofis Int 52:43–58
Matysik J, Alia BB, Mohanty P (2002) Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr Sci 82(5):525–532
McNear DH, Afton SE, Caruso JA (2012) Exploring the structural basis for selenium/mercury antagonism in Allium fistulosum. Metallomics 4:267–276
Meng B, Feng XB, Qiu GL, Anderson CWN, Wang JX, Zhao L (2014) Localization and speciation of mercury in brown rice with implications for Pan-Asian public health. Environ Sci Technol 48:7974–7981
Meunier L, Koch I, Reimer KJ (2011) Effect of particle size on arsenic bioaccessibility in gold mine tailings of Nova Scotia. Sci Total Environ 409:2233–2243
Meyer CJ, Jame L, Seago JL Jr, Peterson C (2009) Environmental effects on the maturation of the endodermis and multiseriate exodermis of Iris germanica roots. Ann Bot London 103(5):687–702
Mishra S, Srivastava S, Tripathi R, Govindarajan R, Kuriakose S, Prasad M (2006) Phytochelatin synthesis and response of antioxidants during cadmium stress in Bacopa monnieri L. Plant Physiol Biochem 44:25–37
Mishra S, Jha AB, Dubey RS (2011) Arsenite treatment induces oxidative stress, upregulates antioxidant system, and causes phytochelatin synthesis in rice seedlings. Protoplasma 248:565–577
Montesbayon M, Leduc DL, Terry N, Caruso JA (2002) Selenium speciation in wild-type and genetically modified Se accumulating plants with HPLC separation and ICP-MS/ES-MS detection. J Anal Atom Spectrom 17:872–879
Moreno-Jimenez E, Esteban E, Carpena-Ruiz RO, Penalosa JM (2009) Arsenic- and mercury-induced phytotoxicity in the Mediterranean shrubs Pistacia lentiscus and Tamarix gallica grown in hydroponic culture. Ecotoxicol Environ Saf 72:1781–1789
Moulick D, Ghosh D, Santra SC (2016) Evaluation of effectiveness of seed priming with selenium in rice during germination under arsenic stress. Plant Physiol Biochem 109:571–578
Mounicou S, Shah M, Meija J, Caruso JA, Vonderheide AP, Shann J (2006) Localization and speciation of selenium and mercury in Brassica juncea-implications for Se-Hg antagonism. J Anal At Spectrom 21:404–412
Mozafariyan M, Shekari L, Hawrylak-Nowak B, Kamelmanesh MM (2014) Protective role of selenium on pepper exposed to cadmium stress during reproductive stage. Biol Trace Elem Res 160:97–107
Mroczek-Zdyrska M, Wójcik M (2011) The influence of selenium on root growth and oxidative stress induced by lead in Vicia faba L. minor plants. Biol Trace Elem Res 147:320–328
Mukherjee A, Sharma A (1988) Effects of cadmium and selenium on cell division and chromosomal aberrations in Allium sativum L. Water Air Soil Pollut 37:433–438
Munné-Bosch S, Alegre L (2002) The function of tocopherols and tocotrienols in plants. Crit Rev Plant Sci 21(1):31–57
Murase J, Kimura M (1997) Anaerobic reoxidation of Mn2+, Fe2+, S0 and S2- in submerged paddy soils. Biol Fertil Soils 25:302–306
Nakamaru YM, Altansuvd J (2014) Speciation and bioavailability of selenium and antimony in non-flooded and wetland soils: a review. Chemosphere 111:366–371
Narang U, Bhardwaj R, Thukral A, Gard S (2008) Mercury-induced lipid peroxidation and changes in antioxidants in Eichhornia crassipes (Mart.) Solms. Plant Stress 2(1):70–74
Natasha SM, Niazi NK, Khalid S, Murtaza B, Bibi I (2018) A critical review of selenium biogeochemical behavior in soil-plant system with an inference to human health. Environ Pollut 234:915–934
Navarro-Alarcon M, Cabrera-Vique C (2008) Selenium in food and the human body: a review. Sci Total Environ 400:115–141
Neill SO, Gould KS, Kilmartin PA, Mitchell KA, Markham KR (2002) Antioxidant activities of red versus green leaves in Elatostema rugosum. Plant Cell Environ 25:539–547
Obrist D, Kirk JL, Zhang L, Sunderland EM, Jiskra M, Selin NE (2018) A review of global environmental mercury processes in response to human and natural perturbations: changes of emissions, climate, and land use. Ambio 47(2):116–140
Ohkawa J, Okada N, Shinmyo A, Takno M (1989) Primary structure of cucumber (Cucumis sativus) ascorbate oxidase deduced from cDNA sequence: homology with blue copper proteins and tissue-specific expression. Proc Natl Acad Sci USA 86:1239–1243
Ortega-Villasante C, Rellan-Alvarez R, Del Campo FF, Carpena-Ruiz RO, Hernandez LE (2005) Cellular damage induced by cadmium and mercury in Medicago sativa. J Exp Bot 56:2239–2251
Pandey C, Gupta M (2015) Selenium and auxin mitigates arsenic stress in rice (Oryza sativa L.) by combining the role of stress indicators, modulators and genotoxicity assay. J Hazard Mater 287:384–391
Pařízek J, Oštádalová I (1967) The protective effect of small amounts of selenite in sublimate intoxication. Experiential 23(2):142–143
Park J, Song WY, Ko D, Eom Y, Hansen TH, Schiller M, Lee TG, Martinoia E, Lee Y (2012) The phytochelatin transporters AtABCC1 and AtABCC2 mediate tolerance to cadmium and mercury. Plant J 69:278–288
Parra R, Pastor MT, Perez-Paya E, Amo-Marco JB (2001) Effect of in vitro shoot multiplication and somatic embryogenesis on 5-methylcytosine content in DNA of Myrtus communis L. Plant Growth Regul 33:131–136
Patra M, Sharma A (2000) Mercury toxicity in plants. Bot Rev 66:379–422
Patra M, Bhowmik N, Bandopadhyay B, Sharma A (2004) Comparison of mercury, lead and arsenic with respect to genotoxic effects on plant systems and the development of genetic tolerance. Environ Exp Bot 52:199–223
Patty C, Barnett B, Mooney B, Kahn A, Levy S, Liu Y, Pianetta P, Andrews JC (2009) Using X-ray microscopy and Hg L3 XANES to study Hg binding in the rhizosphere of spartina cordgrass. Environ Sci Technol 43:7397–7402
Pavlikova D, Pavlik M, Staszkova L, Tlustos P, Szakova J, Balik J (2007) The effect of potentially toxic elements and sewage sludge on the activity of regulatory enzyme glutamate kinase. Plant Soil Environ 53:201–206
Pawlik-Skowronska B, Pirszel J, Brown MT (2007) Concentrations of phytochelatins and glutathione found in natural assemblages of seaweeds depend on species and metal concentrations of the habitat. Aquat Toxicol 83:190–199
Pedrero Z, Madrid Y, Hartikainen H, Camara C (2008) Protective effect of selenium in broccoli (Brassica oleracea) plants subjected to cadmium exposure. J Agric Food Chem 56:266–271
Plant JA, Kinniburgh DG, Smedley PL, Fordyce FM, Klinck BA (2003) Arsenic and selenium. In: Treatise on geochemistry. Elsevier, London, UK, pp 17–66
Powell KJ, Brown PL, Byrne RH, Gajda T, Hefter G, Sjöberg S, Wanner H (2004) Chemical speciation of Hg(II) with environmental inorganic ligands. Aust J Chem 57:993–1000
Qiu R, Zhang JF, Dong ZQ, Feng H, Lai L (2014) Low-temperature thermal desorption of farmland soil contaminated by mercury. Environ Sci Technol 37:48–52 (in Chinese)
Qu D, Zhang YP, Schnell S, Conrad R (2003) Reduction of iron oxides and its effect on microbial processes in anaerobic paddy soil. Acta Pedol Sin 40:858–863 (in Chinese)
Rayman MP (2000) The importance of selenium to human health. Lancet 356:233–241
Rayner-Canham G, Overton T (2010) Descriptive inorganic chemistry, 5th edn. W.H. Freeman and Company, New York
Reis AT, Rodrigues SM, Davidson CM, Pereira E, Duarte AC (2010) Extractability and mobility of mercury from agricultural soils surrounding industrial and mining contaminated areas. Chemosphere 81(11):1369–1377
Ren JH, Sun HH, Wang SF, Luo J, Ma LNQ (2014) Interactive effects of mercury and arsenic on their uptake, speciation and toxicity in rice seedling. Chemosphere 117:737–744
Rothenberg SE, Feng X (2012) Mercury cycling in a flflooded rice paddy. J Geophys Res 117(G03003)
Rothenberg SE, Windham-Myers L, Creswell JE (2014) Rice methylmercury exposure and mitigation: a comprehensive review. Environ Res 133:407–423
Ruiz ON, Daniell H (2009) Genetic engineering to enhance mercury phytoremediation. Curr Opin Biotechnol 20:213–219
Saffaryazdi A, Lahouti M, Ganjeali A, Bayat H (2012) Impact of selenium supplementation on growth and selenium accumulation on spinach (Spinacia oleracea L.) plants. Not Sci Biol 4:95–100
Sallmyr A, Fan J, Rassool FV (2008) Genomic instability in myeloid malignancies: increased reactive oxygen species (ROS), DNA double strand breaks (DSBs) and error-prone repair. Cancer Lett 270:1–9
Sas-Nowosielska A, Galimska-Stypa R, Kucharski R, Zielonka U, Malkowski E, Gray L (2008) Remediation aspect of microbial changes of plant rhizosphere in mercury contaminated soil. Environ Monit Assess 137:101–109
Saunders JR, Knopper LD, Koch I, Reimer KJ (2010) Arsenic transformations and biomarkers in meadow voles (Microtus pennsylvanicus) living on an abandoned gold mine site in Montague, Nova Scotia, Canada. Sci Total Environ 408:829–835
Sebastian A, Prasad MN (2016) Iron plaque decreases cadmium accumulation in Oryza sativa L. and serves as a source of iron. Plant Biol 18:1008–1015
Shahid M, Ferrand E, Schreck E, Dumat C (2013) Behavior and impact of zirconium in the soil–plant system: plant uptake and phytotoxicity. Rev Environ Contam Toxicol 221:107–127
Shahid M, Dumat C, Pourrut B, Silvestre J, Laplanche C, Pinelli E (2014a) Influence of EDTA and citric acid on lead-induced oxidative stress to Vicia faba roots. J Soils Sediments 14:835–843
Shahid M, Pourrut B, Dumat C, Nadeem M, Aslam M, Pinelli E (2014b) Heavy-metal-induced reactive oxygen species: phytotoxicity and physicochemical changes in plants. Rev Environ Contam Toxicol 232:1–44
Shahid M, Dumat C, Khalid S, Schreck E, Xiong T, Niazi NK (2017) Foliar heavy metal uptake, toxicity and detoxification in plants: a comparison of foliar and root metal uptake. J Hazard Mater 325:36–58
Shanker K, Mishra S, Srivastava S, Srivastava R, Dass S, Prakash S, Srivastava MM (1995) Effect of selenite and selenate on plant uptake of cadmium by kidney bean with reference to Cd-Se interaction. Chem Speciat Bioavailab 7:97–100
Shanker K, Mishra S, Srivastava S, Srivastava R, Daas S, Prakash S, Srivastava MM (1996a) Effect of selenite and selenate on plant uptake and translocation of mercury by tomato (Lycopersicum esculentum). Plant Soil 183:233–238
Shanker K, Mishra S, Srivastava S, Srivastava R, Dass S, Prakash S, Srivastava MM (1996b) Study of mercury-selenium (Hg-Se) interactions and their impact on Hg uptake by the radish (Raphanus sativus) plant. Food Chem Toxicol 34:883–886
Sharma SS, Dietz KJ (2009) The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci 14:43–50
Sharma A, Talukder G, Sharma A (1990) Advances in cell and chromosome research. Aspect Publications Ltd, West Yorkshire, pp 197–213
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. Aust J Bot 2012:1–26
Sharma SS, Dietz KJ, Mimura T (2016) Vacuolar compartmentalization as indispensable component of heavy metal detoxification in plants. Plant Cell Environ 39:1112–1126
Sheng Zhou Z, Guo K, Elbaz A, Yang ZM (2009) Salicylic acid alleviates mercury toxicity by preventing oxidative stress in roots of Medicago sativa. Environ Exp Bot 65:27–34
Shiyab S, Chen J, Han FXX, Monts DL, Matta FB, Gu MM, Su Y, Masad MA (2009) Mercury-induced oxidative stress in Indian mustard (Brassica juncea L.). Environ Toxicol 24:462–471
Shoham-Frider E, Shelef G, Kress N (2007) Mercury speciation in sediments at a municipal sewage sludge marine disposal site. Mar Environ Res 64(5):601–615
Shrivastava S, Shrivastav A, Sharma J (2016) Co-exposure effects of selenium and mercury on phaseolus vulgaris excised leaves segment by enhancing the NR, anti-oxidative enzyme activity and detoxification mechanisms. Adv Tech Biol Med 4:1–5
Singh R, Upadhyay AK, Singh DP (2018) Regulation of oxidative stress and mineral nutrient status by selenium in arsenic treated crop plant Oryza sativa. Ecotox Environ Safe 148:105–113
Sparks DL (2005) Toxic metals in the environment: the role of surfaces. Elements 1:193–197
Srivastava M, Ma LQ, Rathinasabapathi B, Srivastava P (2009) Effects of selenium on arsenic uptake in arsenic hyperaccumulator Pteris vittata L. Bioresour Technol 100:1115–1121
Stahl W, Sies H (2003) Antioxidant activity of carotenoids. Mol Asp Med 24:345–351
Strzalka K, Kostecka-Guga A, Latowski D (2003) Carotenoids and environmental stress in plants: significance of carotenoid-mediated modulation of membrane physical properties. Russ J Plant Physl 50:168–172
Sun HY, Dai HX, Wang XY, Wang GH (2016) Physiological and proteomic analysis of selenium-mediated tolerance to Cd stress in cucumber (Cucumis sativus L.). Ecotox Environ Safe 113:114–126
Syversen T, Kaur P (2012) The toxicology of mercury and its compounds. J Trace Elem Med Biol 26:215–226
Tang WL, Dang F, Evans D, Zhong H, Xiao L (2017) Understanding reduced inorganic mercury accumulation in rice following selenium application: selenium application routes, speciation and doses. Chemosphere 169:369–376
Tangahu BV, Abdullah SRS, Basri H, Idris M, Anuar N, Mukhlisin M (2011) A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int J Chem Eng 2011:1–31
Templeton DM, Liu Y (2010) Multiple roles of cadmium in cell death and survival. Chem Biol Interact 188:267–275
Thangavel P, Sulthana AS, Subburam V (1999) Interactive effects of selenium and mercury on the restoration potential of leaves of the medicinal plant, Portulaca oleracea Linn. Sci Total Environ 243:1–8
Thomas SA, Rodby KE, Roth EW, Wu J, Gaillard JF (2018) Spectroscopic and microscopic evidence of biomediated HgS species formation from Hg(II)–cysteine complexes: implications for Hg(II) bioavailability. Environ Sci Technol 52
Tolu J, Le Hecho I, Bueno M, Thiry Y, Potin-Gautier M (2011) Selenium speciation analysis at trace level in soils. Anal Chim Acta 684(1–2):126–133
Tran TAT, Dinh QT, Cui ZW, Huang J, Wang D, Wei TJ, Liang DL, Sun X, Ning P (2018a) Comparing the influence of selenite (Se4+) and selenate (Se6+) on the inhibition of the mercury (Hg) phytotoxicity to pak choi. Ecotoxicol Environ Saf 147:897–904
Tran TAT, Zhou F, Yang WX, Wang MK, Dinh QT, Wang D, Liang DL (2018b) Detoxification of mercury in soil by selenite and related mechanisms. Ecotoxicol Environ Saf 159:77–84
Truong HYT, Chen YW, Belzile N (2013) Effffect of sulfifide, selenite and mercuric mercury on the growth and methylation capacity of the sulfate reducing bacterium Desulfovibrio desulfuricans. Sci Total Environ 449:373–384
Truong HYT, Chen YW, Saleh M, Nehzati S, George GN, Pickering IJ, Belzile N (2014) Proteomics of Desulfovibrio desulfuricans and X-ray absorption spectroscopy to investigate mercury methylation in the presence of selenium. Metallomics 6:465–475
Vinit-Dunand F, Epron D, Alaoui-Sosse B, Badot PM (2002) Effects of copper on growth and on photosynthesis of mature and expanding leaves in cucumber plants. Plant Sci 163:53–58
Wan YN, Yu Y, Wang Q, Qiao YH, Li HF (2016) Cadmium uptake dynamics and translocation in rice seedling: Influence of different forms of selenium. Ecotox Environ Safe 133:127–134
Wang YD, Greger M (2004) Clonal differences in mercury tolerance, accumulation, and distribution in willow. J Environ Qual 33:1779–1785
Wang MY, Chen AK, Wong MH, Qiu RL, Cheng H, Ye ZH (2011) Cadmium accumulation in and tolerance of rice (Oryza sativa L.) varieties with different rates of radial oxygen loss. Environ Pollut 159:1730–1736
Wang X, Tam NFY, Fu S, Ametkhan A, Ouyang Y, Ye ZH (2014) Selenium addition alters mercury uptake, bioavailability in the rhizosphere and root anatomy of rice (Oryza sativa). Ann Bot-London 114:271–278
Wang NB, Zhao J, He XY, Sun HY, Zhang GP, Wu FB (2015) Comparative proteomic analysis of drought tolerance in the two contrasting Tibetan wild genotypes and cultivated genotype. BMC Genomics 16:432
Wang YJ, Dang F, Evans RD, Zhong H, Zhao JT, Zhou DM (2016a) Mechanistic understanding of MeHg-Se antagonism in soil-rice systems: the key role of antagonism in soil. Sci Rep-UK 6:19477
Wang YJ, Dang F, Zhao JT, Zhong H (2016b) Selenium inhibits sulfate-mediated methylmercury production in rice paddy soil. Environ Pollut 213:232–239
Wang ZZ, Wang HB, Wang HJ, Li QC, Li Y (2020) Effect of soil washing on heavy metal removal and soil quality: A two-sided coin. Ecotoxicol Environ Safe 960(25):00
Wu ZC, Wang FH, Liu S, Du YQ, Li FR, Du RY, Wen D, Zhao J (2016) Comparative responses to silicon and selenium in relation to cadmium uptake, compartmentation in roots, and xylem transport in flowering Chinese cabbage (Brassica campestris L. ssp chinensis var. utilis) under cadmium stress. Environ Exp Bot 131:173–180
Xu X, Meng B, Zhang C, Feng X, Gu C, Guo J, Bishop K, Xu Z, Zhang S, Qiu G (2017) The local impact of a coal-fifired power plant on inorganic mercury and methyl-mercury distribution in rice (Oryza sativa L.). Environ Pollut 223:11
Xu XH, Yan M, Liang LC, Lu QH, Han JL, Liu L, Feng XB, Guo JY, Wang YJ, Qiu GL (2019) Impacts of selenium supplementation on soil mercury speciation, and inorganic mercury and methylmercury uptake in rice (Oryza sativa L.). Environ Pollut 249:647–654
Xue T, Hartikainen H, Piironen V (2001) Antioxidative and growth-promoting effect of selenium on senescing lettuce. Plant Soil 237:55–61
Yadav SK (2010) Heavy metals toxicity in plants: an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S Afr J Bot 76:167–179
Yang DY, Chen YW, Gunn JM, Belzile N (2008) Selenium and mercury in organisms: Interactions and mechanisms. Environ Rev 16:71–92
Yathavakilla SKV, Caruso JA (2007) A study of Se-Hg antagonism in Glycine max (soybean) roots by size exclusion and reversed phase HPLC-ICPMS. Anal Bioanal Chem 389:715–723
Yin L, Wang S, Eltayeb AE, Uddin MI, Yamamoto Y, Tsuji W, Takeuchi Y, Tanaka K (2010) Overexpression of dehydroascorbate reductase, but not monodehydroascorbate reductase, confers tolerance to aluminum stress in transgenic tobacco. Planta 231:609–621
Zhang H (2014a) Interactions of mercury and selenium in soil-rice system. In: Impacts of selenium on the biogeochemical cycles of mercury in terrestrial ecosystems in mercury mining areas. Springer, Berlin Heidelberg, pp 135–149
Zhang H (2014b) Advances in research on the mechanisms of selenium–mercury interactions and health risk assessment. In: Impacts of selenium on the biogeochemical cycles of mercury in terrestrial ecosystems in mercury mining areas. Springer, Berlin Heidelberg, pp 17–34
Zhang H, Feng XB, Larssen T, Shang L, Li P (2010) Bioaccumulation of methylmercury versus inorganic mercury in rice (Oryza sativa L.) grain. Environ Sci Technol 44:4499–4504
Zhang H, Feng X, Zhu J, Sapkota A, Meng B, Yao H, Qin H, Larssen T (2012) Selenium in soil inhibits mercury uptake and translocation in Rice (Oryza sativa L.). Environ Sci Technol 46:10040–10046
Zhang H, Feng XB, Jiang CX, Li QH, Liu Y, Gu CH, Shang LH, Li P, Lin Y, Larssen T (2014) Understanding the paradox of selenium contamination in mercury mining areas: high soil content and low accumulation in rice. Environ Pollut 188:27–36
Zhao JT, Gao YX, Li YF, Hu Y, Peng XM, Dong YX, Li B, Chen CY, Chai ZF (2013) Selenium inhibits the phytotoxicity of mercury in garlic (Allium sativum). Environ Res 125:75–81
Zhao J, Li Y, Li Y, Gao Y, Li B, Hu Y, Zhao Y, Chai Z (2014) Selenium modulates mercury uptake and distribution in rice (Oryza sativa L.), in correlation with mercury species and exposure level. Metallomics 6:1951–1957
Zhao AQ, Gao LY, Chen BQ, Feng L (2019) Phytoremediation potential of Miscanthus sinensis for mercury-polluted sites and its impacts on soil microbial community. Environ Sci Pollut Res 26:34818–34829
Zhong H, Wang WX (2009) The role of sorption and bacteria in mercury partitioning and bioavailability in artificial sediments. Environ Pollut 157:981–986
Zhou XB, Li YY (2019) Effect of iron plaque and selenium on mercury uptake and translocation in rice seedlings grown in solution culture. Environ Sci Pollut Res 26(14):13795–13803
Zhou XB, Shi WM (2007) Effect of iron plaque outside roots on selenium uptake and translocation by iron deficient rice (Oryza sativa L.). Pedosphere 17:580–570
Zhou XB, Wang WH, Yu SH, Zhou YX (2013) Interactive effects of selenium and mercury on their uptake by rice sedlings. Res J Appl Sci Eng Technol 5:4733–4739
Zhou XB, Shu-Hui YU, Wang WH, Chang H, Zhou YX (2014) Effects of application of selenium in soil on the formation of root surface iron plaque and mercury uptake by rice plants. J Southwest Univ 36(1):91–95
Zhou XB, Gao AX, Lai F, Zhang CM, Xu WH (2017) The role of selenium in soil: effect on the uptake and translocation of arsenic in rice (Oryza sativa L.). Int J Agric Biol 19:1227–1234
Zhu YG, Pilon-Smits EAH, Zhao FJ, Williams PN, Meharg AA (2009) Selenium in higher plants: understanding mechanisms for biofortification and phytoremediation. Trends Plant Sci 14:436–442
Zhu SM, Liang YL, Zhao FJ, Gao DK, An XJ, Kong FC (2017) Spraying foliar selenium fertilizer on quality of table grape (Vitis vinifera L.) from different source varieties. Sci Hortic 218:87–94
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 41171379 and 41571454, to D.L. Liang).
Author information
Authors and Affiliations
Contributions
Thi Anh Thu Tran synthesized document for the section “Reduction of Hg bioavailability in soil” and wrote the whole manuscript; Quang Toan Dinh synthesized document for the section “Reduction of Hg availability on the interface of soil–plant root”; Fei Zhou and Hui Zhai synthesized document for the section “Reduction of Hg uptake and translocation within plant”; Mingyue Xue and Zekun Du synthesized document for the section “Reduction of oxidative stress induced by Hg in plants”; Gary S Bañuelos revised and commented on the manuscript; Dongli Liang developed article ideas, revised, and commented on the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Elena Maestri
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Se application reduced Hg soil bioavailability via transformation to immobile species.
• Se application in soil led to formation HgSe complexes in rhizosphere and/or roots.
• Se application prevented to root uptake and translocation of Hg to aerial parts.
• Se application positively affected physiological and biochemical processes of plants.
• Se doses only significantly given the narrow range between deficiency and toxicity.
Rights and permissions
About this article
Cite this article
Tran, T.A.T., Dinh, Q.T., Zhou, F. et al. Mechanisms underlying mercury detoxification in soil–plant systems after selenium application: a review. Environ Sci Pollut Res 28, 46852–46876 (2021). https://doi.org/10.1007/s11356-021-15048-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-15048-1