Abstract
The 26 December 2004 tsunami covered significant portion of a coastal zone with a blanket of potentially contaminated sediments. In this report are presented results on mercury concentrations in sediments deposited by the tsunami in a coastal zone of Thailand. Since the total mercury concentrations are insufficient to assess mercury mobility and bioavailability in sediment, its fractionation was applied. Sediments were sampled within 50 days after the event and analyzed by sequential extraction method. The procedure of sequential extraction involved five subsequent stages performed with solutions of chloroform, deionized water, 0.5 M HCl, 0.2 M NaOH, and aqua regia. The mean concentration of total mercury in sediments was 119 ± 50 ng g−1 dry mass (range 66–230). The fractionation revealed that mercury is mainly bound to the least bioavailable sulphides 75 ± 6% (range 62–86), organomercury compounds 14 ± 7% (range 4–26), and humic matter 9 ± 7% (range 1–27). The lowest contributions bring fractions of water-soluble mercury 0.8 ± 1.0% (range 0.1–3.6) and acid soluble mercury 0.9 ± 0.5% (range 0.2–2.1). Although, the total mercury content is similar in a reference sample and in the tsunami sediments, the highly toxic organomercury fraction contribution is higher in the latter. The results were compared with chemical and sedimentological properties of the sediments but no significant correlations were obtained between them.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The large tsunami which was generated by an earthquake on the 26 December 2004 affected most of countries around the Indian Ocean. It was the first wave of such a dimension in this region during the human written history. The estimated tsunami death toll was about 300,000 coastal zone inhabitants. However, the casualties and physical destruction on land are only the most immediate impacts. Large erosion and sedimentation associated with translation of huge amount of sea water on land which introduces salt into surface and ground waters have caused extensive environmental damage. Some of the early findings of post-tsunami studies documented catastrophic modification of the run-up behavior of the tsunami due to human development (Liu et al. 2005), protective role of the coastal vegetation (Danielsen et al. 2005; Kathiresan and Rajendran 2005), and reaction of beach meiofauna communities (Altaff et al. 2005; Kotwicki and Szczuciński 2006). UNEP report (2005) presented overview of the post-tsunami environmental assessment underlying that one of the important long-term consequences is related to water and soil contamination.
Soils in the coastal zones impacted by tsunami were touched in several ways. They were covered with seawater and most of them also by at least few cm thick layer of salinated sediments, usually of marine origin. Only in Thailand about 20,300 ha of land were covered by seawater during that event (UNEP 2005). Tsunami belongs to natural disasters which may be prominent mechanisms of hazardous material releases (Young et al. 2004). In consequence, the soils in the coastal zone may contain some pollutants released due to damages of waste disposal storages, factories, fuel stations, etc. Some of the environmental effects of the tsunami may be similar to that of coastal floodings generated by storms, typhoons, and hurricanes.
The recent tsunami sediments deposited in coastal zone of Thailand are 8 cm thick on average and cover most of the tsunami-inundated land (Szczuciński et al. unpublished). The sediments were studied for bioavailable contaminants by Szczuciński et al. (2005). It was found that they contain significantly elevated amounts of salts (Na, K, Ca, Mg, Cl, SO4) in water-soluble fraction, and of Cd, Cu, Zn, Pb, and As in bioavailable fraction in relation to a reference sample. The origin of these contaminants is probably complex. Major ions in the water-soluble fraction of sediments (K, Na, Ca, Mg, Cl, and SO4) were found to be highly correlated to each other and were delivered in dissolved form with sea water. Heavy metals (Cu, Pb, Zn) were also correlated to salt content and probably were tied to them in the sediments, however, their source is rather litho or anthropogenic. Arsenic had elevated concentrations in restricted areas—related to mining activity, and was probably of lithogenic origin. Szczuciński et al. (2005) concluded that the bioavailable metals and metalloids might reach toxic levels in food chain causing serious environmental hazard. It is particularly possible in land depressions left after mining activity, which were filled for longer periods of time with salty water after the tsunami.
In the above-mentioned study also total content of mercury in tsunami sediments was determined. This metal is of particular interest because of its high toxicity, stability of its chemical species, and its ability for bioaccumulation. However, the total element’s concentration is of low importance if its bioavailability, transformations and migration in the environment are considered. Organomercury species such as methylmercury are at least one order of magnitude more mobile than inorganic mercury species, and thus are more toxic and more easily bioaccumulated. Soluble inorganic mercury species such as mercury chloride are more susceptible to transport by natural process than other inorganic mercury species and serve as the substrate for mercury methylation process. Mercury sulphide is chemically stable in the sediment and soil and thus are the least toxic (Stein et al. 1996; Jackson 1998a, b; Ullrich et al. 2001; Boszke et al. 2002, 2003).
To assess the mobility and bioavailability of mercury, a development of adequate methods is required. Sequential extraction of solid-state matrices by solutions of increasing strength of complexation is applied (Tessier et al. 1979; Calmano 1983; Hall et al. 1996; Biester and Scholz 1997; Lechler et al. 1997; Wallschlager et al. 1998a, b; Biester et al. 2002; Sladek et al. 2002; Bloom et al. 2003). This approach is based on an assumption that a given solution causes extraction of a certain fraction of mercury species. The fraction includes a number of different but similar chemical species as none of the solutions is selective. Determination of mobility of trace metals including mercury, on the basis of the sequential extraction procedures arouses much controversy because there are no natural reference materials to permit determination of the method’s accuracy. However, an advantage of the technique is determination of mercury species at relatively low concentrations (Biester and Scholz 1997; Biester et al. 2002; Gustin et al. 2002; Sladek et al. 2002).
The main objective of our study is to assess contamination of tsunami sediments with mercury in the coastal zone of Thailand after the 26 December 2004 tsunami event. The study focuses on mobility and bioavailability of mercury species in tsunami sediments applying fractionation method. Possible relation of mercury to other chemical compounds or sediment characteristics is also discussed. We have not come across any earlier report on mercury fractionation in tsunami sediments.
Materials and methods
Sample collection and preparation for analysis
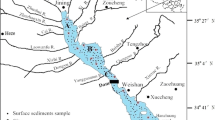
All the samples were collected within less than 50 days after the 26 December 2004 tsunami event from selected locations on Phuket Island (around Patong Bay) and along the coastline between Khao Lak and Kho Khao Island on the western coast of Thailand (Fig. 1, Table 1). Between the tsunami and sampling dates no rainfall was reported, therefore, at the time of collection the studied sediments were almost unaltered by redeposition processes. The entire layer of the tsunami deposit was sampled. If it was thicker than 5 cm, only the uppermost portion was collected. An additional sample (16) was taken for reference from an area out of reach of the tsunami wave. Samples were dried at room temperature to constant weight, packed in plastic bags, and transported to laboratories. They were divided into subsamples for sedimentological and chemical analysis. For analysis of mercury, the samples were slightly homogenized in an agate mortar, not to damage the grains. From the prepared samples, subsamples of uniform mass were collected and sieved through a copper sieve of mesh size 0.15 mm for further analysis.
Reagents and apparatus
The analytical agents used for mercury determination were of the highest available purity made by Merck (Darmstadt, Germany). All dilutions were made with deionized water from Milli-Q system (Millipore, France). Laboratory vessels used were made of boron-silica glass of the highest quality. Details of preparation of reagents and vessels prior analyses are presented in the work of Boszke et al. (2006).
Sequential extraction procedure
After review of the literature covering the determination of various species of mercury in solid samples, a sequential extraction procedure (Fig. 2) determining five fractions was established [based partially on the works of Wallschlager et al. (1996), Lechler et al. (1997), Bloom et al. (2003), Renneberg and Dudas (2001)].
Five grams of a sediment sample were treated with four subsequential extractions using chloroform, deionized water, 0.5 M HCl, and 0.2 M NaOH, followed by oxidative digestion with aqua regia (Fig. 2). The sample was shaken, centrifuged, and filtered after each stage of sequential extraction. The chloroform phase was reextracted with sodium thiosulphate and obtained solution was treated with concentrated nitric acid. The extracts obtained at separate stages were treated with 33% HCl and 0.033 M KBrO3/0.2 M KBr to oxidize all mercury species to Hg(II). Next, 12% NH2OH·HCl was added to the extract to remove free bromine. The sample obtained at the fourth stage was treated with an aqua regia and was heated in a glass apparatus consisting of water cooler and partial condenser. The same procedure such as at fifth stage was applied to determine the content of the total mercury in bulk sediment. Details of sequential extraction procedure are presented in the work of Boszke et al. (2006).
Mercury determination
Mercury was determined by cold-vapor atomic fluorescence spectroscopy (CV-AFS) on Millenium Merlin (PS Analytical, England). The calibration was performed with the mercury standard HgNO3 (Merck) with the nominal mercury content of 980 ± 020 ng l−1. The limit of detection and quantification of the method depends on the purity of the reagents used. The value of the limit of quantification (LOQ) of the method reached 0.8 ng Hg l−1 and was calculated according to the formulas presented by Konieczka et al. (2004).
Reference samples
Along with determination of the total mercury, routine analysis was made of the certified materials, including SRM 2711 (Montana Soil), SRM 2709 (San Joaquin Soil), and LGC 6137 (Estuarine Sediment). The total mercury concentrations obtained in our study, taking into regard the values of expanded uncertainty (Konieczka et al. 2004), 6,060 ± 351 ng g−1 (SRM 2711, n = 5), 1,440 ± 98 ng g−1 (SRM 2709, n = 6), 370 ± 21 ng g−1 (LGC 6137, n = 7) well correspond with those obtained for the certified materials of 6,250 ± 190, 1,400 ± 80 ng g−1, and 340 ± 50 μg g−1, respectively (Kowalski 2006). For comparison with the results of sequential extraction, one certified sample LGC 6137 was used. The sum of mercury concentrations obtained from particular fractions was 373 ng g−1 dry mass, while the corresponding sum obtained for the certified material was 340 ng g−1 dry mass. The method of sequential extraction is thus characterized by recovery of about 110% (range 108–112%) and good reproducibility (Table 2).
Results
The sampling sites were located in a range of locations presenting variable morphological conditions, degree of tsunami damages, and pre-tsunami human impact (Fig. 1, Table 1). Samples 1 and 2 were collected on a narrow isthmus of a peninsula which encompasses the southern part of Patong Bay and was completely flooded by the tsunami waves. Both the samples were taken from local small depressions/ponds. Samples 3–8 were taken from a low lying terrain, adjacent to a small river in the southern part of Patong city. Samples 9–13 were collected in Nam Khem village, which was largely damaged. Samples 14 and 15 were taken in inundated zone in vicinity of Bang Mor village. Sample 16 was taken as a reference sample in a neighborhood of Thung Tuk village. It represents a soil top layer, which developed on former coastal sands. All the samples belong to grain size classes ranging from coarse silt to medium sand (Table 1).
The concentrations of total mercury and the contributions of particular mercury fractions in the total concentration are shown in Table 3. The mean concentration of the total mercury (calculated as a sum of mercury concentrations in individual fractions) is 119 ± 50 ng g−1 dry mass (range 66–230). Most of mercury is bound to sulphides (F5) 75 ± 6% (range 62–86%), organomercury compounds (F1) 14 ± 7% (range 4–26), and humic matter (F4) 9 ± 7% (range 1–27). The remaining water (F2) and acid soluble (F3) fractions contribute 0.8 ± 1.0% (range 0.1–3.6) and 0.9 ± 0.5% (range 0.2–2.1) of the total mercury, respectively. The reference sample has revealed similar concentrations to that observed in the tsunami sediments. However, in case of mercury fractions related to organomercury (F1) and acid soluble (F3) compounds, the reference sample represents lower limit values.
There is not much difference in the total content of mercury between the sampling locations. Patong City and Nam Khem village reveal the widest ranges, including samples with the highest and the lowest concentration of mercury. These two locations are also characterized by the highest contribution of F1 fraction in the total mercury content. The F2 fraction has the highest amount (several times higher than in remaining areas) in the vicinity of Bang Mor village. Differences in the F3 fraction contribution are very small. Its highest contribution is found in samples from Patong Bay region. The latter region is also characterized by the lowest values of the F4 fraction and, along with vicinity of Bang Mor village—the highest content of the F5 fraction.
The variance analysis (ANOVA) did not show statistically significant differences (p > 0.05) in the mean concentration of total mercury and its fractions taken from Patong Bay, Patong, Nam Khem, Bang Mor, and Thung Tuk. Statistically significant differences have been only for fraction F2, both in mercury concentration (F(4,11) = 24.82480; p = 0.000019) and in contribution to the total mercury content (F(4,11) = 32.11399; p = 0.000005). The mean concentration of mercury in F2 fraction and its contribution to the total concentration were higher for samples collected from Bang Mor, 2.8 ± 0.9 ng g−1 and 3.1 ± 0.7%, respectively, than for the other areas. For the sum of water- and acid-soluble mercury (exchangeable mercury), differences in mercury concentration (F(4,11) = 7.547631; p = 0.003531) and in the fraction contribution (F(4,11) = 7.383665; p = 0.003839) are observed. They are again found for sediment samples from Bang Mor where mercury concentration in these fractions and their contribution to the total concentration were 3.6 ± 1.3 ng g−1 and 3.9 ± 1.1%, respectively.
Discussion
Total mercury concentration
The total mercury content is similar in the tsunami sediments and in the reference sample—it may point to similar source area for former coastal sediments forming modern soils and the modern tsunami sediments. The values of mercury concentration calculated as a sum of mercury concentrations in individual fractions obtained in this study (66–230 ng g−1 d.w.) are comparable to total mercury established in the sediments of the Thailand seas. According to Pollution Control Department of Thailand, average total mercury concentration in the sediment samples collected from 106 stations across 22 provinces covering the area of the Upper Gulf, Western Coast of the Gulf, ESB (Eastern Seaboard), and Andaman Sea was from 138 ng g−1 d.w. (range 5–2,135) in 1998, 67 ng g−1 (range 3–872) in 1999, to 230 ng g−1 (range < 100–350 ng g−1) in 2001 (vide Chongprasith et al. 2006). Global background mercury concentration ranges from 10 to 200 ng g−1 (Fergusson 1990; Kabata-Pendias and Pendias 1999). However, marine sediments are usually characterized by low background concentrations. For example, in sediments from Yatsushiro Sea (Japan) background concentration is 59 ± 13 ng g−1 (Tomiyasu et al. 2000) and in sediments from Adriatic Sea is 20 ng g−1 (Fabbri et al. 2001).
Fractionation
Mercury in organomercury compounds
Organomercury compounds are those in which methyl (CH3), ethyl (C2H5), and phenyl groups (C6H5) link to a mercury atom via a carbon atom. Organomercury species such as methylmercury are at least an order of magnitude more mobile than inorganic mercury species, and thus are more toxic and more readily bioaccumulated. Methylation is influenced by environmental variables which affect both the availability of mercuric ions for methylation and the growth of the methylating microbial populations. Methylation rates are higher under anoxic conditions, with low pH and in freshwater compared to saltwater (Stein et al. 1996; Ullrich et al. 2001; Boszke et al. 2003).
In the studied tsunami sediments, contribution of organomercury species in the total mercury concentration is higher (even five times) than in reference sample from Thung Tuk. In the reference sample, contribution of organomercury compounds in the total mercury content is 5% and mercury concentration in this fraction is 7 ng g−1. In the tsunami sediments, average mercury concentration in this fraction is 14 ng g−1, with maximum value of 32 ng g−1. It shows that the tsunami sediments may present bigger environmental hazard.
Comparison of the obtained results with contribution of certain mercury fractions in other sediments may be semiquantitative only due to differences in applied fractionation methods. Exactly the same extraction method was used by Boszke et al. (2006) for analysis of freshwater sediment samples from Warta river (Poland) and of estuarine sediment. The total mercury concentration in these sediments, calculated as a sum of mercury concentrations in individual fractions were 130 ± 71 ng g−1 (range 51–307) and 373 ng g−1 for river and estuarine sediments, respectively. In Warta river, sediments contribution of mercury bound with organomercury species was 17 ± 18% (range 0.03–65), but in estuarine sediment only 2.2% (Boszke et al. 2006).
Usually, concentration of methylmercury in sediments varies between 1 and 1.5% of total mercury, while it makes less than 0.5% in marine and estuarine sediments (Ullrich et al. 2001). In the bottom sediments of the Gdańsk Bay (Poland), South China Sea (Malaysia), and Bering Sea (Russia), the contributions of methylmercury were 0.02–2.27, 0.02–0.27, and 0.02–0.7%, respectively (Kannan and Falandysz 1998). Higher contributions were found for bottom sediments of the harbor in Hamburg, where its value varied from 2.5 to 8.1 (Wilken and Hintelmann 1991) and in bottom sediments from Minamata Bay (Japan) where contribution organomercury compounds varied from 1 to 4%, while in the Kagoshima Bay (Japan) it was from 7 to 37% (Sakamoto et al. 1995; Eguchi and Tomiyasu 2002). Kannan and Falandysz (1998) suggest that value of percentage contribution of the methylmercury in the total mercury concentration could be a useful index describing the degree of pollution in a given water reservoir. According to them, in unpolluted areas this index takes values lower than one. In the studied samples of tsunami sediments and in the reference sample, the index is higher than one (range 4–26), so we may treat them as polluted. Relatively high contributions of the mercury from this fraction in the total mercury concentrations in samples treated with the method applied in this study may be partly explained by no complete selectivity of organic solvents, not only the organomercury compounds but also part of the mercury bound to organic matter may be extracted (Eguchi and Tomiyasu 2002).
Water-soluble mercury
The fraction called water-soluble mercury includes mercury species present in pore water. Usually mercury is not present in the form of water-soluble ionic species (e.g. Hg(OH)2, HOHgCl, HgCl2) in the water phase but as species bound to organic matter (without a Hg–carbon bond) or suspended mineral particles (Renneberg and Dudas 2001; Wallschlager et al. 1996, 1998b; Biester et al. 2002). Mercury species extracted by water may be easily transported by natural processes and serve as the substrate for mercury methylation process (Stein et al. 1996; Ullrich et al. 2001; Boszke et al. 2003).
Contributions of water-soluble mercury in tsunami sediments are very low and, except sediments collected from Bang Mor, are lower than in the reference sample. In comparison to the tsunami sediments, contribution of water-soluble mercury in sediments of Warta river is higher (2.1 ± 0.9%, range 1.1–3.8), but contribution of water-soluble mercury in estuarine sediment is lower (0.3%) (Boszke et al. 2006). In general, the contribution of water-soluble mercury species is very small and often the concentrations determined are below the limit of detection of applied methods (Kot et al. 2002; Kot and Matyushkina 2002). The same order of values was determined in some studies which applied similar fractionation method. For example, in river Ji Yun sediments in China this fraction constitutes 0.05–1.22% of total mercury (Ching and Hongxiao 1985), and in marine sediments from the Gdańsk Bay in Poland less than 4% (Bełdowski and Pempkowiak 2003).
Acid-soluble mercury
The fraction called acid soluble, include strongly bound Hg species extracted by solution of acids, such as HCl (Ching and Hongxiao 1985; Lechler et al. 1997). Solutions of acids extract—operationally defined ‘‘reactive mercury species” or “bioavailable inorganic mercury” bonded to iron monosulphides (i.e. AVS), iron and manganese hydroxides and carbonates. They can also include the species bound to organic matter and adsorbed on surface of minerals (Lechler et al. 1997; Bloom et al. 2003; Shi et al. 2005). Some authors determined “bioavailable” mercury in the fraction extracted by acids as an approximation of conditions met in the alimentary tract of mammals (Barnett and Turner 2001; Bloom et al. 2003).
In the most of tsunami sediments, contribution of acid-soluble mercury is higher (even seven times) than in the reference sample from Thung Tuk. In the latter one, the contribution of acid-soluble mercury was 0.3% and mercury concentration in this fraction was 0.4 ng g−1. In tsunami sediments, average mercury concentration was 0.9 ng g−1, with maximum value of 1.7 ng g−1. It shows that the tsunami sediments may present bigger environmental hazard. Contribution of acid-soluble mercury in the tsunami sediments is higher than in the sediments of Warta river (0.4 ± 0.1%, range 0.2–0.7) and estuarine sediment (0.2%) (Boszke et al. 2006). Comparable or higher values were obtained by similar extraction methods from some freshwater and marine sediments. In the sediments of the Ji Yun river (China), this contribution was from 0.34 to 1.55% (Ching and Hongxiao 1985). According to Peng and Wang (1985), the mean contribution of mercury in this fraction in the rivers Ji Yun and Zijan sediments (China) was 3.4%, and the maximum contribution of this fraction (13.1%) was found in the vicinity of waste outflow, the concentration of this mercury fraction in that sample was 262,000 ng g−1. The concentration of divalent mercury Hg(II) in the marine sediments ranged from 4.81 ± 5.73 to 8.97 ± 5.46 ng g−1 (5.1 ± 2.8 to 12.9 ± 14.9%) and were lower in freshwater sediments 0.538 ± 0.176 ng g−1 (9.2 ± 6.1%) (Kannan and Falandysz 1998). The contribution of methylmercury and Hg(II) in the concentration of total mercury is on average lower than 10% in the sediments (Kannan and Falandysz 1998).
Mercury bound to humic matter
Organic matter is important component of sediments and soils. It is also in a significant degree, responsible for binding metals. Organic mercury includes mercury Hg(II) complexes with organic ligands, e.g. humic and fulvic acids, amino acids (but without a Hg–carbon bond). Binding of mercury in organic matter is mainly due to the reduced sulphur species in such functional groups as thiol group (R-SH), disulphide group (R-SS-R), or disulphane group (R-SSH) (Xia et al. 1999). Besides sulphur, some contribution in binding mercury in organic matter is brought by oxygen and nitrogen atoms but their significance is lower (Hesterberg et al. 2001). It has been indicated that organic matter can bind up to 95% of divalent mercury species (Meili 1997). A significant part of organic matter is the humic matter whose contribution in the total organic matter is ∼ 25% in bottom sediments, 20% in marine water, 60% in the river water, and 70% in marsh areas in river catchment areas (Weber 1993).
In contrast to the tsunami sediments, where organomercury compounds (F1) constituted the second fraction with the greatest contribution of mercury, the mercury bound with humic matter is the second in Warta river sediments 23 ± 9% (range 4–36) and in estuarine sediment 4.6% (Boszke et al. 2006). Contribution of mercury species bound to humic matter obtained in with other works applying similar fractionation scheme shows a wide range of values. In the bottom sediments of the rivers Ji Yun and Zijan (China), the contribution was from 2.7 to 77.4% (Peng and Wang 1985). The lowest contribution of the mercury species bound to organic matter was found in a sample from the region of waste release from a plant producing chlorine alkaline compounds, although it was 54,000 ng g−1, while the concentration of mercury in a sample with the greatest contribution of the organic matter bound mercury was 4,400 ng g−1 (Peng and Wang 1985). Other authors (Ching and Hongxiao 1985) reported the contribution of humic acid bound mercury in the bottom sediments from the same river Ji Yun as varying from 6.52 to 23.7% (800–46,650 ng g−1). In marine sediments, the participation of fulvic acid bound mercury is small and does not exceed a few percent (9% at maximum), while the contribution of humic acid bound mercury is much greater—about 20% (reaching 54%) (Bełdowski and Pempkowiak 2003). In general, greater contributions of mercury bound to organic matter are found in sediments from not polluted rivers and are smaller in highly polluted. For example, the contribution of mercury bound to humic matter in bottom sediments samples from Carson river (USA) was < 40% in the region not flowing through mill tailing and gradually decreased to a few percent in the region highly polluted with mercury (Lechler et al. 1997).
Mercury bound to sulphides
In the presence of sulphides, the mercuric ion becomes tightly bound to them as insoluble HgS and is not available for methylation. Sulphide activity may be the main factor influencing the availability of Hg(II) and the concentration of methylmercury in sediment. If conditions become aerobic due to a decrease in the organic load or seasonal turnover, sulphide can be oxidized to sulphate, releasing the mercury in the ionic form Hg(II), which is available for methylation (Stein et al. 1996; Ullrich et al. 2001; Boszke et al. 2003).
Like in the tsunami sediments, the greatest contribution to the total mercury concentration was for mercury bound to sulphides, in the sediments of Warta river—58 ± 17% (range 20–81%) and in estuarine sediment—93% (Boszke et al. 2006). In general, the contribution of mercury bound to sulphides was found to be greater in sediments and soil samples characterized by reduction conditions (Lechler et al. 1997; Bełdowski and Pempkowiak 2003). For instance, the contribution of mercury bound to sulphides in the bottom sediments of Gdańsk Bay (Poland) was close to 40%, with a maximum value of 96% in the bottom sediments characterized by reduction conditions (Bełdowski and Pempkowiak 2003). In a soil profile the contribution of residual mercury was found to increase with depth of the profile, from 8.1% in the surface layer (0–30 cm) to 69% in the layer at 50–80 cm (Lechler et al. 1997). High contribution of mercury bound to sulphides—71.4% (mean) (range 38.4–96.1) was found in the bottom sediments from the Kogushima Bay (Japan), where mercury sulphide is formed also from H2S and mercury chloride liberated from fumarole gasses coming from under the sea bottom (Sakamoto et al. 1995).
Correlations
Statistically significant positive correlations (p < 0.05) have been found between the total mercury concentration in sediment and mercury concentration in fractions F4, F5, sum of F1 + F4, and sum of F1 + F2 + F3 + F4 (called as bioavailable fraction). Statistically significant positive correlation has been found also between mercury concentration in bioavailable fraction and F4, F5, and F1 + F4, between exchangeable fraction (F2 + F3) and mercury concentration in water-soluble fraction. Sum of mercury concentration in fraction F1 and F4 correlates with mercury concentration in fraction F4 and F5. Except fraction F5, there are positive correlations between mercury concentrations and its contributions. From ecotoxicological point of view interesting is negative correlation between contribution of organomercury compounds and concentration of mercury in fractions F4 and F5. Contribution of organomercury compounds is negatively correlated with contribution of mercury associated with humic matter and contribution of mercury sulphide fraction is negatively correlated with contribution sum of F1 and F4. For example, in sediments from Gulf of Thailand was found strong positive correlation between contributions of methylmercury and organic matter fraction and negative correlation between contribution of methylmercury and contribution of mercury sulphide (Bloom et al. 2003).
There are also no clear correlations to grain size statistics, which was observed typically in other studies related to other heavy metals (Förstner and Wittman 1979; Singh et al. 1999). Mercury is often characterized by untypical grain size distribution in sediments. For example, in sediments of the Odra river (Poland) gradual decrease in the mercury concentration with increasing grain size from fine to coarse fraction has been observed for sediments taken from the river bed but not for sediments taken from river banks (Boszke et al. 2004). In tsunami sediments, positive correlations were found between contribution mercury sulphide fraction and medium silt, very fine silt and clay content. In these sediments, negative correlations were found between medium silt, very fine silt and clay content and contribution of F1 + F4 (sum of fraction) and “bioavailable” fraction (sum of F1, F2, F3, and F4).
The significance of mercury as a pollutant follows specific character of this metal different from other heavy metals. The chemical and phase speciation of mercury in aquatic systems is especially complex and relationships between mercury and different heavy metals and other parameters are not clear and have different patterns, as exemplified by the studied tsunami sediments (Szczuciński et al. 2005).
Conclusions
In the tsunami sediments from coastal zone of Thailand, mercury occurs mainly in the form of mercury sulphide, hardly soluble and hardly bioavailable. Although the concentration of organomercury species in the studied samples is low, these species may pose a real threat to man and other living organisms because of their extreme toxicity and capability of bioaccumulation and biomagnification in trophic chains. Though, total mercury content is similar in the tsunami sediments and in the reference sample, the highly mobile organomercury fraction contribution is higher in tsunami sediments. Water-soluble mercury and acid-soluble mercury (exchangeable mercury) can easily enter the aquatic system and accumulate in organism. There is, however, also possible that heavy rainfall during summer monsoon will cause dilution and removal of significant portion of the exchangeable mercury.
References
Altaff K, Sugumaran J, Naveed MS (2005) Impact of tsunami on meiofauna of Marina beach, Chennai, India. Curr Sci 89:34–38
Barnett MO, Turner RR (2001) Bioaccessibility of mercury in soils. Soil Sediment Contam 10:301–316
Bełdowski J, Pempkowiak J (2003) Horizontal and vertical variabilities of mercury concentration and speciation in sediments of Gdańsk Basin, Southern Baltic Sea. Chemosphere 52:645–654
Biester H, Scholz C (1997) Determination of mercury phase in contaminated soils. Mercury pyrolysis versus sequential extractions. Environ Sci Technol 31:233–239
Biester H, Muller G, Schöler HF (2002) Binding and mobility of mercury in soils contaminated by emissions from chlor-alkali plants. Sci Total Environ 284:191–203
Bloom NS, Preus E, Katon J, Hilter M (2003) Selective extractions to assess the biogeochemically relevant fractionation of inorganic mercury in sediment and soils. Anal Chim Acta 479:233–248
Boszke L, Głosińska G, Siepak J (2002) Some aspects of speciation of mercury in a water environment. Pol J Environ Stud 11:285–298
Boszke L, Kowalski A, Głosińska G, Szarek R, Siepak J (2003) Selected factors affecting the speciation of mercury in the bottom sediments: an overview. Pol J Environ Stud 12:5–13
Boszke L, Kowalski A, Siepak J (2004) Grain size partitioning of mercury in sediments of the middle Odra river (Germany/Poland). Water Air Soil Pollut 159:125–138
Boszke L, Kowalski A, Siepak J (2006) Fractionation of mercury in sediments of the Warta river (Poland). In: Pawłowski L, Dudzińska M, Pawłowski A (eds) Environmental engineering science in Poland. CRC Press, Boca Raton (in press)
Calmano W (1983) Chemical extraction of heavy metals in polluted river sediments in central Europe. Sci Total Environ 28:77–90
Ching IL, Hongxiao T (1985) Chemical studies of aquatic pollution by heavy metals in China. In: Irgolic KJ, Martel AE (eds) Environmental inorganic chemistry. VCH Publishers, pp 359–371
Chongprasith P, Utoomprurkporn W, Wilairatanadilok W (2006) Mercury situation in Thailand. Available from: http://www.marinepcd.org/hgtaskforce/index.htm
Danielsen F, Sørensen MK, Olwig MF, Selvam V, Parish F, Burgess ND, Hirashi T, Karunagaran VM, Rasmussen MS, Hansen LB, Quarto A, Suryadiptura N (2005) The Asian tsunami: a protective role for coastal vegetation. Science 310:643
Eguchi T, Tomiyasu T (2002) The speciation of mercury in sediments from Kagoshima Bay and Minamata Bay, Southern Kyusyu, Japan, by fractional extraction/cold-vapor AAS (in Japan). Bunseki Kagaku 51:859–864
Fabbri D, Gabbianelli G, Locatelli C, Lubrano D, Trombini C, Vassura I (2001) Distribution of mercury and other heavy metals in core sediments of the northern Adriatic Sea. Water Air Soil Pollut 129:143–153
Fergusson JE (1990) The heavy elements—chemistry, environmental impact and health effects. Pergamon Press, Oxford
Förstner U, Wittman GT (1979) Metal pollution in the aquatic environment. Springer, Berlin Heidelberg New York
Gustin MS, Biester H, Kim CS (2002) Investigation of the light-enhanced emission of mercury from naturally enriched substrates. Atmos Environ 36:3241–3254
Hall GEM, Vaive JE, Beer R, Hoashi M (1996) Selective leaches revisited, with emphasis on the amorphous Fe oxyhydroxide phase extraction. J Geochem Explor 56:59–78
Hesterberg D, Chou JW, Hutchison KJ, Sayers DE (2001) Bonding of Hg(II) to reduced organic sulfur in humic acid as affected by S/Hg ratio. Environ Sci Technol 35:2741–2745
Jackson TA (1998a) Mercury in aquatic ecosystems. In: Langston WJ, Bebianno MJ (eds) Metal metabolism in aquatic environments. Chapman & Hall, London, pp 77–158
Jackson TA (1998b) The biogeochemical and ecological significance of interactions between colloidal minerals and trace elements. In: Parker A, Rae JE (eds) Environmental interactions of clays. Springer, Berlin Heidelberg New York, pp 93–205
Kabata-Pendias A, Pendias H (1999) Biogeochemistry of trace elements. Państwowe Wydawnictwo Naukowe. Warszawa (in Polish)
Kannan K, Falandysz J (1998) Speciation and concentrations of mercury in certain coastal marine sediments. Water Air Soil Pollut 103:129–136
Kathiresan K, Rajendran N (2005) Coastal mangrove forests mitigated tsunami. Estuar Coast Shelf Sci 65:601–606
Konieczka P, Namieśnik J, Zygmunt B, Bulska E, Świtaj-Zawadka A, Naganowska A, Kremer E, Rompa M (2004) Quality assessment and quality control of analytical results—QA/QC. Centre of Excellence in Environment Analysis and Monitoring, Gdańsk (in Polish)
Kot FS, Matyushkina LA (2002) Distribution of mercury in chemical fraction of contaminated urban soils of Middle Amur, Russia. J Environ Monit 4:803–908
Kot FS, Matyushkina LA, Nikorych VN, Polivichenko VG (2002) Mercury in chemical and thermal fraction of soils of Eastern Ukrainian Polissya. Soil Sci 3:94–101
Kotwicki L, Szczuciński W (2006) Meiofauna assemblages, sediment characteristic of the Thailand sandy beaches after the 2004 tsunami event. Phuket Marine Biological Center Bulletin (in press)
Kowalski A (2006) Determination of the total mercury and its species in the environmental samples. Doctoral thesis. Adam Mickiewicz University, Poznan (in Polish)
Lechler PJ, Miller JR, Hsu LC, Desilets MO (1997) Mercury mobility at the Carson River superfund site, west-central Nevada, USA—interpretation of mercury speciation data in mill tailing, soils, and sediments. J Geochem Explor 58:259–267
Liu PLF, Lynett P, Fernando H, Jaffe BE, Fritz H, Higman B, Morton R, Goff J, Synolakis C (2005) Observations by the International Tsunami Survey Team in Sri Lanka. Science 308:1595
Meili M (1997) Mercury in lakes and rivers. In: Sigel A, Sigel H (eds) Mercury and its effects on environment and biology. Metal ions in biological systems. Marcel Dekker, New York, pp 21–51
Peng A, Wang Z (1985) Mercury in river sediment. In: Irgolic KJ, Martel AE (eds) Environmental inorganic chemistry. VCH Publishers, pp 393–400
Renneberg AJ, Dudas MJ (2001) Transformations of elemental mercury to inorganic and organic forms in mercury and hydrocarbon co-contaminated soils. Chemosphere 45:1103–1109
Sakamoto H, Tomiyasu T, Yonehara N (1995) The content and chemical forms of mercury in sediments from Kagoshima Bay, in comparison with Minamata Bay and Yatsushiro Sea, Southwestern Japan. Geochem J 29:97–105
Shi JB, Liang LN, Jiang GB, Jin XL (2005) The speciation and bioavailability of mercury in sediments of Haihe River, China. Environ Int 31:357–365
Singh AK, Hasnain SI, Banerjee DK (1999) Grain size and geochemical partitioning of heavy metals in sediments of the Damoder River—a tributary of the lower Ganga, India. Environ Geol 39:90–98
Sladek C, Gustin MS, Kim CS, Biester H (2002) Application of three methods for determining mercury speciation in mine waste. Geochem Explor Environ Anal 2:369–376
Stein ED, Cohen Y, Winer AM (1996) Environmental distribution and transformation of mercury compounds. Crit Rev Environ Sci Technol 26:1–43
Szczuciński W, Niedzielski P, Rachlewicz G, Sobczyński T, Zioła A, Kowalski A, Lorenc S, Siepak J (2005) Contamination of tsunami sediments in a coastal zone inundated by the 26 December 2004 tsunami in Thailand. Environ Geol 49:321–331
Tessier A, Campbell P, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51:844–851
Tomiyasu T, Nagano A, Yonehara N, Sakamoto H, Rifardi, Oki K, Akagi H (2000) Mercury contamination in the Yatsushiro Sea, south-western Japan: spatial variations of mercury in sediment. Sci Total Environ 257:121–132
Ullrich SM, Tanton TW, Abdrashitowa SA (2001) Mercury in the aquatic environment: a review of factors affecting methylation. Crit Rev Environ Sci Technol 31:241–293
United Nations Environment Protection—UNEP (2005) After the tsunami. Rapid environmental assessment. United Nations Environment Programme
Young S, Balluz L, Malilay J (2004) Natural and technologic hazardous material releases during and after natural disasters: a review. Sci Total Environ 322:3–20
Wallschlager D, Desai MVM, Wilken R-D (1996) The role of humic substances in the aqueous mobilization of mercury from contaminated floodplain soils. Water Air Soil Pollut 90:507–520
Wallschlager D, Desai MVM, Spengler M, Windmöler CC, Wilken R-D (1998a) Mercury speciation in floodplain soils and sediments along a contaminated river transect. J Environ Qual 27:1034–1044
Wallschlager D, Desai MVM, Spengler M, Windmöler CC, Wilken R-D (1998b) How humic substances dominate mercury geochemistry in contaminated floodplain soils and sediments. J Environ Qual 27:1044–1054
Weber JH (1993) Review of possible paths for abiotic methylation of mercury(II) in aquatic environment. Chemosphere 51:2063–2077
Wilken RD, Hintelmann H (1991) Mercury and methylmercury in sediments and suspended particles from the river Elbe, North Germany. Water Air Soil Pollut 56:427–437
Xia K, Skyllberg UL, Bleam WF, Bloom RP, Nater EA, Helmke PA (1999) X-ray absorption spectroscopic evidence for the complexation of Hg(II) by reduced sulfur in soil humic substances. Environ Sci Technol 33:257–261
Acknowledgments
The field and laboratory work was supported by Adam Mickiewicz University in Poznan, Poland. We appreciate logistical support provided by Department of Mineral Resources of Kingdom of Thailand and Embassy of Poland in Thailand. We would like to thank Darunee Saisuttichai, Tinnakorn Tatong, and Thawatchai Tepsuwan for field assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boszke, L., Kowalski, A., Szczuciński, W. et al. Assessment of mercury mobility and bioavailability by fractionation method in sediments from coastal zone inundated by the 26 December 2004 tsunami in Thailand. Environ Geol 51, 527–536 (2006). https://doi.org/10.1007/s00254-006-0349-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-006-0349-3