Abstract
Purpose
Both selenium (Se) and sulfate could largely affect methylmercury (MeHg) dynamics and phytoavailability in soil-rice systems, while their combined effects are less understood. Here, we aimed at exploring the potential effects of sulfate on MeHg accumulation in rice in the presence of Se.
Materials and methods
Rice was cultivated in inorganic Hg-spiked soils amended with Se only (selenite/selenate, “Se treatments”) or Se and sulfate (“Se + Sulfate treatments”). Soil parameters (e.g., pH and redox potential (Eh)), MeHg concentrations in soils, as well as MeHg or Se accumulation in rice plants were quantified during the rice growth period.
Results and discussion
Soil MeHg concentrations were generally comparable between Se + Sulfate and Se treatments. However, MeHg uptake by rice plants in Se + Sulfate treatments was 9–31 % lower than those in Se treatments, possibly due to the increased soil pH and formation of iron sulfides, which may reduce MeHg phytoavailability under sulfate amendment. Furthermore, sulfate input enhanced Se accumulation in root (especially in the presence of selenate), which could be responsible for the increased MeHg distribution in root and thus lower MeHg distribution in grain. Consequently, the reduced plant uptake of MeHg together with the decreased MeHg distribution in grain resulted in decline of grain MeHg concentrations in Se + Sulfate treatments (8–31 % lower compared to Se treatments).
Conclusions
Our results suggest that sulfate input with Se could further reduce MeHg accumulation in rice, which improved mechanistic understanding of MeHg behaviors in soil-rice systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Mercury (Hg), especially methylmercury (MeHg), is highly toxic and bioaccumulative (Clarkson 1997; Tchounwou et al. 2003). Microbial transformation of inorganic mercury (IHg) and massive production of MeHg in rice paddy soils could result in elevated MeHg levels in rice grain (up to 145 μg kg−1, Horvat et al. 2003). Consequently, consumption of MeHg-contaminated rice is considered as an important pathway of human exposure to MeHg in some Hg-contaminated areas (Feng et al. 2008; Zhang et al. 2010a). Considering that rice is a staple food in Asia and many other parts of the world, there is a great need to better understand Hg biogeochemistry in Hg-contaminated soil-rice systems.
Recently, there is growing evidence that selenium (Se) could efficiently reduce Hg accumulation in rice plants (i.e., Hg-Se antagonism, Zhang et al. 2012; Zhao et al. 2014; Wang et al. 2014a; Li et al. 2015). It is proposed that the formation of Hg-Se complexes in soil and/or high molecular weight Hg-Se complexes in rice root could be responsible for the observed Hg-Se antagonism. Furthermore, our recent study evidences that Se amendment could largely reduce net MeHg production in rice paddy soils, which could in turn reduce grain MeHg levels (Wang et al. 2016a). Nevertheless, the underlying mechanisms are far from clear. Particularly, the effects of key environmental factors such as sulfate on MeHg accumulation in rice are largely unknown, which hinders us from predicting effects of Se on risks of MeHg in contaminated rice paddy fields.
Sulfur cycling has been long believed to be a key biogeochemical process controlling Hg dynamics (e.g., Hg methylation and bioavailability) in wetland soils. On one hand, sulfate could affect the activity of sulfate-reducing bacteria (SRB, a principle microbial methylator for IHg, Gilmour et al. 1992) and thus microbial production of MeHg (Wang et al. 2014b). On the other hand, the products of sulfate reduction under flooded conditions (e.g., iron sulfides and Hg-S complex) have a great influence on IHg and MeHg availability (Skyllberg 2008; Skyllberg and Drott 2010). Similarly, in rice paddy soils, sulfate also plays a critical role in Hg biogeochemistry (Rothenberg and Feng 2012). Consequently, the unintended input of sulfate, either via fertilization or atmospheric deposition (Liu et al. 1990; Hu et al. 2002), could potentially affect the inhibitory effects of Se on Hg bioaccumulation. Our recent batch experiments provided initial evidence that Se could inhibit sulfate-mediated production of MeHg in soils (Wang et al. 2015, 2016b). However, the potential effects of co-application of sulfate with Se on MeHg accumulation in rice are still unknown and warrant investigation.
Here, we mainly aimed at exploring the effects of sulfate on MeHg accumulation in rice in the presence of Se. Because rice grain is an intensive bioaccumulator of MeHg (Zhang et al. 2010b), we focus on MeHg (instead of IHg) biogeochemistry in soil-rice systems. Rice was cultivated in IHg-spiked soils amended with Se (i.e., selenate or selenite) and different levels (0–960 mg kg–1) of sulfate. Soil parameters (e.g., pH and redox potential (Eh)), MeHg concentrations in soils, as well as MeHg or Se accumulation in rice plants were quantified during the rice growth period.
2 Materials and methods
2.1 Soil
Soil was sampled form a rice paddy field in Yixing, Jiangshu Province of China in 2014. After air-drying, the soil was sieved to an effective diameter of ≤2 mm and mixed homogenously. The soil is typical of Anthraquic Ustifluvents (widely distributed throughout Taihu area of the Yangtze Rive Delta, China, Du et al. 2007) and mainly composed of 86 ± 4 % slit and 7 ± 3 % clay, with pH of 5.5 ± 0.0 and organic carbon content of 2.1 ± 0.0 %. Reducible sulfate content in soil was 243 ± 2 mg kg–1. Total Se, Hg, and MeHg levels in soil were 0.9 ± 0.1 mg kg–1, 0.2 ± 0.0 mg kg–1, and 1.2 ± 0.2 μg kg–1 (n = 3), respectively.
2.2 Pot experiments
Soil was spiked with IHg (as 80 mg L–1 mercury nitrate monohydrate, Sigma-Aldrich) and equilibrated under flooded conditions for 20 days, after which differences in Hg geochemical fractionation or bioavailability became less evident between spiked and ambient Hg (Ma et al. 2015). The total Hg concentration in the spiked soil was 2.2 ± 0.1 mg kg–1 (n = 21), which was within the range of reported soil Hg concentrations (0.56–390 mg kg–1) in Hg-contaminated areas (e.g., due to discharge from chemical factories, Zhang et al. 2008; Huang et al. 2011).
After that, the IHg-spiked soils were amended with Se and different levels of sulfate. For Se application, Se (Na2SeO3, Se(IV) or Na2SeO4, Se(VI), Sigma-Aldrich) were added to achieve a target concentration of 3.0 mg Se kg–1 soil (herein referred to as Se(IV) or Se(VI) treatment). The Se level was selected mainly based on the toxic threshold of Se to rice plants (Mikkelsen et al. 1989). Concurrently, different levels of sulfate were added at concentrations of 240 or 960 mg sulfate kg–1 soil, which were one or four folds of the ambient sulfate level in the soil (243 mg kg–1) (herein referred to as Se + sulfate treatments, i.e., Se(IV) + 1S, Se(IV) + 4S, Se(VI) + 1S, and Se(VI) + 4S treatments). The total sulfate levels in soils fell into the range of sulfate levels reported in paddy soils (Wei 1981). A control treatment using IHg-spiked soil in the absence of Se and sulfate amendment was also included. Therefore, a total of seven treatments were set up with triplicate per treatment, i.e., control, Se(IV), Se(IV) + 1S, Se(IV) + 4S, Se(VI), Se(VI) + 1S, and Se(VI) + 4S treatments.
The pots experiments were set up in a glasshouse in Nanjing, Jiangsu Province, China. Rice seeds (Wufengyou2168, indica) were cultivated in a soil with low ambient Hg and Se levels (total Hg 93.4 ± 10.5 μg kg–1; MeHg 0.10 ± 0.05 μg kg–1; Se 0.6 ± 0.1 mg kg–1) for 30 days at ambient temperature before transplanting. Thereafter, the 1-month-old rice seedlings were transplanted into pots filled with 2.5 kg IHg-spiked soil per pot (two seedlings per pot). Rice was cultivated in pots for 120 days (June to October, 2014) under flooded conditions (by deionized water) at ambient temperature (15–38 °C). A total of 79 mg kg–1 P (Ca(HPO4)2·H2O), 150 mg kg–1 K (KCl), and 167 mg kg–1 N (CO(NH2)2) were supplied into each pot during the entire experimental period
2.3 Sampling
The redox potential (Eh, relative to the standard hydrogen electrode) and pH of the soils were monitored on days 45 (the beginning of the tillering stage), 80 (the beginning of the heading stage), and 140 (harvesting day) using a redox potential depolarization automatic analyzer equipped with a pH electrode (FJA-6, Nanjing Chuan-Di instrument & equipment, China). After removing surface water, the electrodes were inserted into the soils (5 cm below the soil surface, rooting zone) and allowed to equilibrate for 2 min for measuring Eh and pH. Meanwhile, soil samples (1–11 cm, rooting zone) were collected on days 20 (day of seedling transplantation), 80, and 140, respectively. Soil samples were packed in vacuum immediately to avoid oxidation and transferred to the laboratory in a portable ice box. The soil samples were homogenized and separated into subsamples in a glovebag (AtmosBag, Aldrich, filled with nitrogen gas) for analysis of total Hg, MeHg, and Se (details described below in Section 2.4) and water content. Water content in the soils was determined by drying soils at 105 °C for 48 h.
On day 140, rice plants were harvested and separated into grain, straw, and root. All tissue samples were washed thoroughly by tap water and deionized water. Husk was manually removed from the whole grain to obtain brown rice. White rice was milled by a rice polisher using the whole grain. Iron plaque on root was removed according to a published method (Okkenhaug et al. 2012; Jia et al. 2015). Frozen dried (Labconco, USA) tissue samples were ground into fine powders by an IKA basic analytical mill (IKA A11, Germany) and analyzed for total Hg, MeHg, and Se.
2.4 Chemical analysis
For Se determination, soil samples (∼0.2 g dry weight) were digested with 8 mL concentrated HNO3 and HF (1:1 v/v) at 120 °C for 2.5 h in a microwave-digestion system (Ethos EZ, Milestone, Italy). Plant tissue samples (∼0.03 g dry weight) were pre-digested with 1 mL concentrated HNO3 for 18 h at room temperature, heated for 2 h at 120 °C, and then added with 0.2 mL concentrated H2O2 and heated at 90 °C for 30 min. The digested samples were filtered through a 0.45-μm membrane, and Se in the filtrates was analyzed by inductively coupled plasma mass spectrometry (NexION-300 ICP-MS, PerkinElmer, USA) in a collision cell mode with kinetic energy discrimination. Indium (In) was used as an internal standard.
Total Hg in soil was analyzed by Milestone DMA-80 direct mercury analyzer following US Environmental Protection Agency Method 7473. Methylmercury in soil samples were extracted with HNO3/CuSO4-CH2Cl2 (Liang et al. 2004). Plant tissues were digested with 25 % KOH/methanol (w/w) at 60 °C for 4 h. MeHg in digested solutions was determined by cold vapor atomic fluorescence spectrometry (CVAFS, Brooks Rand, USA) following US Environmental Protection Agency Method 1630. Blank and reference standard materials were digested and analyzed with each batch of samples (Electronic Supplementary Material, Table S1).
The total amount of reducible sulfate in soils was estimated by the exchangeable sulfate concentration: exchangeable sulfate in soils was extracted by a 0.5 M sodium bicarbonate solution at pH 8.5 (Weber et al. 2009) and determined by ion chromatography (Dionex ICS-1100, Thermo Fisher Scientific, USA).
2.5 Statistical analysis
Statistical significance was determined by Tukey’s multiple comparison test of one-way analysis of variance (ANOVA) (p = 0.05). All analysis was performed using SPSS, version 16.0.
3 Results
3.1 MeHg, Eh, and pH in soils
In this study, we are mainly concerned with the potential effects of sulfate on MeHg biogeochemistry and bioaccumulation in the presence of Se. Therefore, the effects of Se on MeHg bioaccumulation (i.e., treatments amended with Se only vs. the control) are not discussed in details but provided elsewhere (Wang et al. 2016a).
Soil MeHg levels during rice growth period were shown in the Electronic Supplementary Material, Fig. S1 (also reported in Wang et al. 2016b). Compared to the evident decrease in soil MeHg levels under Se amendment (Se vs. control), sulfate input had generally minor effects on soil MeHg concentrations (Se + Sulfate vs. Se). Meanwhile, a temporal variation in MeHg levels in all Se-amended soils was demonstrated: MeHg levels peaked on day 80 (21.1–30.7 μg kg–1) but were generally lower on day 20 (14.1–15.9 μg kg–1) or day 140 (15.5–24.4 μg kg–1).
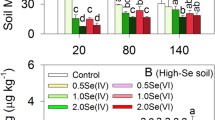
Sulfate input generally decreased soil Eh but increased pH on days 80 and 140 (Se + Sulfate vs. Se), especially under high dose of sulfate (Se(IV) + 4S or Se(VI) + 4S vs. Se) (Fig. 1). Coinciding with the temporal variation in soil MeHg levels, soil Eh was generally higher on day 80 (12.7–57.7 mV) than those on day 45 (8.1–26.8 mV) or 140 (–2.5 to –28.7 mV). Moreover, the dark grayish color in the soils was observed, especially under high dose of sulfate amendment, suggesting formation of iron sulfides. Meanwhile, pH values were generally higher in Se(IV)/Se(VI) + 4S treatments than those in Se(IV)/Se(VI) + 1S or Se treatments during the rice growth period.
Eh (a, b) or pH (c, d) in soils in different treatments with or without Se and sulfate amendment. Data are presented as means ± SD (n = 3). Different letters (i.e., a, b) indicate significant differences (one-way ANOVA with Tukey’s test, p < 0.05) among Se-amended treatments within the same day (i.e., day 20, 80, or 140)
3.2 Accumulation and distribution of MeHg and Se in rice plants
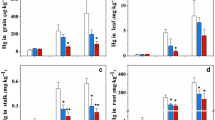
Uptake of MeHg by whole plants (μg pot–1) and tissue MeHg concentrations are shown in Figs. 2a and 3, respectively. In the presence of Se, sulfate input tended to reduce MeHg uptake (Se + Sulfate vs. Se). Uptake of MeHg was 9–26 % lower in Se(IV) + Sulfate treatments compared to Se(IV) treatments, and 22–31 % lower in Se(VI) + Sulfate compared to Se(VI) (Fig. 2a). Similarly, MeHg concentrations in brown rice or white rice were 8–31 % or 11–29 % lower in Se(IV) + Sulfate or Se(VI) + Sulfate treatments relative to their Se-amended counterparts, and significant declines were observed in Se(IV) + 4S and Se(VI) + 4S treatments (p < 0.05, Fig. 3). However, root or straw MeHg levels did not differ significantly among Se, Se(IV) + Sulfate, and Se(VI) + Sulfate treatments (Fig. 3). No significant difference in tissue biomass (root, straw, or brown rice) among treatments was noted either (Electronic Supplementary Material, Table S2).
Uptake of MeHg (a) or Se (b) by rice plants from soils in different treatments with or without Se and sulfate amendment. Data are presented as means ± SD (n = 3). Different letters (lowercase letters, i.e., a, b, for Se(IV); uppercase letters, i.e., A, B, for Se(VI)) indicate significant differences (one-way ANOVA with Tukey’s test, p < 0.05) among Se-amended treatments
Concentrations of MeHg in root, straw, brown rice, and white rice in different treatments: a Se(IV)-amended treatments; b Se(VI)-amended treatments. Data are presented as means ± SD (n = 3). Different letters (i.e., a, b) indicate significant differences (one-way ANOVA with Tukey’s test, p < 0.05) among Se-amended treatments
In response to sulfate input, MeHg distributions among tissues varied (Fig. 4). In the presence of Se(VI), the proportion of MeHg in root increased significantly from 4.1 % (Se(VI)) to 8.8 % (Se(VI) + 4S, p < 0.05), while that in brown rice decreased significantly from 92.3 % (Se(VI)) to 85.0 % (Se(VI) + 4S, p < 0.05, Fig. 4b). In comparison, MeHg distributions in all Se(IV)-amended treatments were less variable: no significant difference was found in MeHg distributions (in brown rice or root) between Se(IV) + Sulfate and Se(IV) treatments (Fig. 4a).
Mass distribution of MeHg in rice plant tissues in different treatments: a Se(IV)-amended treatments; b Se(VI)-amended treatments. Data are presented as means ± SD (n = 3). Different letters indicate (i.e., a, b) significant differences (one-way ANOVA with Tukey’s test, p < 0.05) among Se-amended treatments
Uptake of Se by whole plants (μg pot–1) and tissue Se concentrations are depicted in Fig. 2b and Fig. S2 (Electronic Supplementary Material), respectively. Sulfate input increased plant uptake of Se in Se(VI) treatments but had marginal effects on Se uptake in Se(IV) treatments (Fig. 2b), e.g., Se uptake increased from 66.7 ± 8.4 μg pot–1 in Se(VI) treatment to 78.5 ± 5.2 or 91.4 ± 10.5 μg pot–1 in Se(VI) + 1S or Se(VI) + 4S treatment. Particularly, Se levels in root showed the largest increase compared to other plant tissues (Electronic Supplementary Material, Fig. S2), e.g., 52 % increase in root (p < 0.05) compared to 25 % in rice grain and 8 % in straw, when comparing Se(VI) + 4S and Se(VI) (p > 0.05, Electronic Supplementary Material, Fig. S2B). Likewise, root Se concentrations tended to increase with sulfate doses in all Se(IV)-amended treatments, but no significant change was found (Electronic Supplementary Material, Fig. S2A). Moreover, different from MeHg, Se distributions were less affected by sulfate input: no significant differences were found between Se + Sulfate and Se treatments (Electronic Supplementary Material, Fig. S3).
4 Discussion
4.1 Sulfate input with Se further reduced MeHg uptake by rice plants
Inhibitory effects of Se on Hg accumulation in rice plants have been documented in a few recent studies (Li et al. 2015; Wang et al. 2016a), while the key environmental factors controlling Hg-Se antagonism were less studied. In this study, we demonstrated that sulfate input could further reduce MeHg uptake by rice plants in the presence of Se (i.e., Se + Sulfate vs. Se) under flooded conditions. The observed reduction should be attributed to the reduced MeHg phytoavailability following sulfate addition, rather than the variations in net MeHg production in soils due to sulfate input.
Previous studies demonstrated that sulfate input may facilitate microbial production of MeHg under sulfate-limiting conditions by enhancing SRB activities (Gilmour et al. 1992; Han et al. 2010), or reduce MeHg production by binding IHg with reduced sulfur species (Han et al. 2010; Benoit et al. 1999). However in this study, sulfate input had minor effects on net MeHg production and thus MeHg levels in soils in the presence of Se, which is consistent with the results of our previous studies (Wang et al. 2015, 2016b). Therefore, the reduced MeHg uptake by plants following sulfate addition (especially in all Se(VI)-amended treatments) was unlikely attributed to the variations in soil MeHg levels. The generally weak effects of sulfate on soil MeHg levels was possibly due to the formation of Hg-Se complexes in soils (Zhang et al. 2012; Li et al. 2015; Wang et al. 2016a, b), which was less available to methylating microbes (Yang et al. 2008; Truong et al. 2014). Further discussions about the effects of sulfate and Se amendment on net MeHg production in soils could be found in a companion paper (Wang et al. 2016b).
Meanwhile, our results demonstrated lower Eh but higher pH in Se + Sulfate treatments, especially in Se(IV) + 4S or Se(VI) + 4S treatment, than those in Se(IV) or Se(VI) treatment on days 80 and 140 (Fig. 1). Those observations are in line with previous study that sulfate input in flooded soils could facilitate microbial reduction of sulfate, which decreased Eh and increased pH (Lamers et al. 1998; Jia et al. 2015). The increased pH would immobilize MeHg in soils, as reported previously (Ullrich et al. 2001), resulting in reduced MeHg availability to rice plants. Meanwhile, S2– produced following sulfate reduction could react with Fe2+ or Fe3+ to form iron sulfides (Lamers et al. 1998; Rickard and Luther 2007), as reflected by the black color gradually shown in the soils in this study. The accumulation of iron sulfides in sulfate-added soils would also reduce MeHg phytoavailability via surface complexation (Skyllberg 2008; Skyllberg and Drott 2010). Consequently, sulfate input into the Se-amended soils would further reduce MeHg phytoavailability and thus MeHg uptake by rice plants (Se + Sulfate vs. Se) under flooded conditions during rice cultivation, as observed in this study (Fig. 2a).
The significantly decreased net MeHg production in Se(IV) + 4S compared to Se(IV) on day 140 (Electronic Supplementary Material, Fig. S1A) could be due to the accumulation of iron sulfides in soils, which further immobilize IHg in soils (Skyllberg and Drott 2010; Jonsson et al. 2012). The relatively high net MeHg methylation on day 80 could be attributed to the higher Eh values during heading stage (e.g., due to radial oxygen loss, Schmidt et al. 2011), which may result in oxidation of reduced sulfur species (e.g., iron sulfides) and thus release of IHg from soils. Positive effects of sulfate on MeHg levels in Se-amended soils were reported under oxic/sub-oxic conditions, probably due to re-oxidation of iron sulfides and thus release of IHg for methylation (Wang et al. 2016b). However in this study, Eh in soils were relatively low throughout the experimental period, probably due to the flooded conditions and minor effects of rice root radical oxygen loss on the overall redox potential changes in the soils, and thus may lead to the limitation of changes in net MeHg production and facilitate reducing MeHg phytoavailability in soils.
4.2 Sulfate input reduced MeHg distribution in grain and grain MeHg levels
Besides its inhibitory effects on MeHg uptake by plants, sulfate input also influenced MeHg distribution among plant tissues in the presence of Se. As mentioned above, there was a trend that Se accumulation in root increased with increasing sulfate doses, especially in all Se(VI)-amended treatments (Electronic Supplementary Material, Fig. S2). The enhanced root Se levels following sulfate addition could subsequently increase production of selenocysteine and other organic Se species (Sors et al. 2005), which would bind strongly with MeHg within root. Similarly, it has been reported that IHg could bind strongly with organic Se species in the root of Indian mustard and green onion (Mounicou et al. 2006; McNear et al. 2012). Consequently, MeHg would be retained in the root to a larger extent in Se(VI) + Sulfate treatments compared to Se(VI) treatment. Thus MeHg distributions in brown rice were generally lower in Se(VI) + Sulfate treatments than those in Se(VI) treatment (Fig. 4), which partly explain the reduced grain MeHg levels following sulfate input. Therefore, both (1) reduced MeHg phytoavailability and thus MeHg uptake by plants, and (2) reduced MeHg distribution in grain, could be responsible for the decreased grain MeHg levels following sulfate input in all Se(VI)-amended treatments. However in all Se(IV)-amended treatments, less evident changes in root Se levels following sulfate addition and thus insignificant changes in MeHg distribution in root or grain could be less important in explaining the reduced grain MeHg levels (Se(IV) + Sulfate vs. Se(IV)).
Inhibitory effects of sulfate on Se uptake by plants have been reported previously. For instance, sulfate addition has been demonstrated to inhibit selenate uptake in plants, such as wheat and Indian mustard (Terry et al. 2000; Sors et al. 2005), mainly because selenate uptake could be linked with sulfate transporter in the root. Differently in this study, increased Se accumulation in root following sulfate input was observed in Se (especially in all Se(VI)) amended treatments, which was largely unexplained. The sulfate input-induced increase in root Se could probably be explained by the inhibitory effects of sulfate on the formation of iron plaque on root surface: sulfate input facilitated formation of iron sulfides in the anoxic soils, which decreased the mobility of Fe2+ in the rhizosphere and thus transfer of Fe2+ to root surface. This may subsequently reduce iron plaque formation on root surface (Fan et al. 2010) and increase Se uptake by root, in view of the inhibitory effects of iron plaque on root uptake of Se (Zhou et al. 2007; Huang et al. 2015). Future studies are necessary to better explain the positive effects of sulfate input on Se accumulation in rice root. Besides, more evident increase in Se bioaccumulation following sulfate addition was observed in all Se(VI)-amended treatments than those in all Se(IV)-amended treatments (Fig. 2b, Electronic Supplementary Material, Fig. S2), which could subsequently lead to the significantly reduced MeHg distribution in grain for Se(VI) + 4S (compared to Se(VI)). This could possibly be explained by the higher phytoavailability of selenate (Terry et al. 2000; Sors et al. 2005).
5 Conclusions
Our previous studies provided initial evidences for the importance of MeHg-Se antagonism in soils (i.e., Se inhibited net MeHg production in soils, Wang et al. 2016a) and indicated the potential effects of sulfate and Se on net MeHg production in soils (Wang et al. 2015, 2016b). Based on those observations, we further explored the potential effects of sulfate on MeHg accumulation in rice in the presence of Se in this study. Our results indicated that sulfate could further reduce MeHg accumulation in rice plants (especially in grain) in the presence of Se under flooded conditions during rice cultivation, probably through reducing MeHg phytoavailability and decreasing MeHg distribution in rice grain (especially in all Se(VI)-amended treatments). The negative effects of sulfate input on MeHg uptake by plants could be attributed to the increased pH and/or accumulation of reduced sulfur species (e.g., iron sulfides) in soils. The decreased MeHg distribution in grain was possibly explained by the enhanced Se accumulation and thus retention of MeHg in root. Although the mechanisms of Hg, Se, and sulfate interactions are far from clear at this stage, our study is suggestive of many research avenues. For instance, mechanistic studies are necessary to better understand the potential effects of sulfate input on MeHg phytoavailability in soils in the presence of Se. Besides, it would also be interesting to further explore the effects of sulfate input on Se accumulation in rice root under anoxic conditions, which may modify MeHg-Se interaction in root and thus MeHg translocation within rice plants.
Meanwhile, our results provided initial evidence that co-application of sulfate and Se could further reduce grain MeHg levels, compared to treatments amended with Se only. Further field studies are necessary to confirm the possibility of using Se + sulfate fertilization to reduce MeHg accumulation in rice and to mitigate the risk of MeHg in Hg-contaminated farmland.
References
Benoit JM, Gilmour CC, Mason RP, Heyes A (1999) Sulfide controls on mercury speciation and bioavailability to methylating bacteria in sediment pore waters. Environ Sci Technol 33(6):951–957
Clarkson TW (1997) The toxicology of mercury. Crit Rev Clin Lab Sci 34(4):369–403
Du GH, Zhang GL, Gong ZT (2007) Placement of paddy soils of the Yangtze Delta in the Chinese Soil Taxonomy. Soils 39(5):684–691 (in Chinese with English abstract)
Fan JL, Hu ZY, Ziadi N, Xia X, Wu CYH (2010) Excessive sulfur supply reduces cadmium accumulation in brown rice (Oryza sativa L.). Environ Pollut 158:409–415
Feng XB, Li P, Qiu GL, Wang SF, Li GH, Shang LH, Meng B et al (2008) Human exposure to methylmercury through rice intake in mercury mining areas, Guizhou Province, China. Environ Sci Technol 42(1):326–332
Gilmour CC, Henry EA, Mitchell R (1992) Sulfate stimulation of mercury methylation in fresh-water sediments. Environ Sci Technol 26(11):2281–2287
Han S, Narasingarao P, Obraztsova A, Gieskes J, Hartmann AC, Tebo BM, Allen EE (2010) Mercury speciation in marine sediments under sulfate-limited conditions. Environ Sci Technol 44(10):3752–3757
Horvat M, Nolde N, Fajon V, Jereb V, Logar M, Lojen S, Jacimovic R et al (2003) Total mercury, methylmercury and selenium in mercury polluted areas in the province Guizhou, China. Sci Total Environ 304:231–256
Hu ZY, Xu CK, Zhao YY, Wang TJ, Zhang HC, Cao ZH (2002) Dynamics of atmospheric sulphur deposition on rapeseed/rice rotation in selected area of south China. China Environ Sci 22(1):11–15 (in Chinese with English abstract)
Huang B, Wang M, Yan LX, Sun WX, Zhao YC, Shi XZ, Weindorf DC (2011) Accumulation, transfer, and environmental risk of soil mercury in a rapidly industrializing region of the Yangtze River Delta, China. J Soils Sediments 11:607–618
Huang QQ, Wang Q, Lou Z, Yu Y, Jiang RF, Li HF (2015) Effects of root iron plaque on selenite and selenate dynamics in rhizosphere and uptake by rice (Oryza sativa). Plant Soil 388(1):255–266
Jia Y, Bao P, Zhu YG (2015) Arsenic bioavailability to rice plant in paddy soil: influence of microbial sulfate reduction. J Soils Sediments 15:1960–1967
Jonsson S, Skyllberg U, Nilsson MB, Westlund PO, Shchukarev A, Lundberg E, Björn E (2012) Mercury methylation rates for geochemically relevant HgII species in sediments. Environ Sci Technol 46(21):11653–11659
Lamers LPM, Tomassen HBM, Roelofs JGM (1998) Sulfate-induced eutrophication and phytotoxicity in freshwater wetlands. Environ Sci Technol 32(2):199–205
Li YF, Zhao JT, Li YY, Li HJ, Zhang JF, Li B, Gao YX et al (2015) The concentration of selenium matters: a field study on mercury accumulation in rice by selenite treatment in Qingzhen, Guizhou, China. Plant Soil 391(1):195–205
Liang L, Horvat M, Feng XB, Shang LH, Li H, Pang P (2004) Re-evaluation of distillation and comparison with HNO3 leaching/solvent extraction for isolation of methylmercury compounds from sediment/soil samples. Appl Organomet Chem 18(6):264–270
Liu CQ, Cao SQ, Chen GA, Wu XJ (1990) Sulphur in the agriculture of China. Acta Pedol Sinica 27(4):398–404 (in Chinese with English abstract)
Ma L, Zhong H, Wu YG (2015) Effects of metal-soil contact time on the extraction of mercury from soils. Bull Environ Contam Toxicol 94:399–406
McNear DH Jr, Afton SE, Caruso JA (2012) Exploring the structural basis for selenium/mercury antagonism in Allium fistulosum. Metallomics 4(3):267–276
Mikkelsen RL, Mikkelsen DS, Abshahi A (1989) Effects of soil flooding on selenium transformations and accumulation by rice. Soil Sci Soc Am J 53:122–127
Mounicou S, Shah M, Meija J, Caruso JA, Vonderheide AP, Shann J (2006) Localization and speciation of selenium and mercury in Brassica juncea—implications for Se-Hg antagonism. J Anal At Spectrom 21(4):404–412
Okkenhaug G, Zhu YG, He JW, Li X, Lou L, Mulder J (2012) Antimony (Sb) and arsenic (As) in Sb mining impacted paddy soil from Xikuangshan, China: differences in mechanisms controlling soil sequestration and uptake in rice. Environ Sci Technol 46(6):3155–3162
Rickard D, Luther GW III (2007) Chemistry of iron sulfides. Chem Rev 107:514–562
Rothenberg SE, Feng XB (2012) Mercury cycling in a flooded rice paddy. J Geophys Res 117:G03003,1–16
Schmidt H, Eickhorst T, Tippkötter R (2011) Monitoring of root growth and redox conditions in paddy soil rhizotrons by redox electrodes and image analysis. Plant Soil 341(1):221–232
Skyllberg U (2008) Competition among thiols and inorganic sulfides and polysulfides for Hg and MeHg in wetland soils and sediments under suboxic conditions: illumination of controversies and implications for MeHg net production. J Geophys Res 113(G00C03):1–14
Skyllberg U, Drott A (2010) Competition between disordered iron sulfide and natural organic matter associated thiols for mercury(II)—an EXAFS study. Environ Sci Technol 44(4):1254–1259
Sors TG, Ellis DR, Salt DE (2005) Selenium uptake, translocation, assimilation and metabolic fate in plants. Photosynth Res 86(3):373–389
Tchounwou PB, Ayensu WK, Ninashvili N, Sutton D (2003) Review: environmental exposure to mercury and its toxicopathologic implications for public health. Environ Toxicol 18(3):149–175
Terry N, Zayed AM, De Souza MP, Tarun AS (2000) Selenium in higher plants. Annu Rev Plant Physiol Plant Mol Biol 5:401–432
Truong HYT, Chen YW, Saleh M, Nehzati S, George GN, Pickering IJ, Belzile N (2014) Proteomics of Desulfovibrio desulfuricans and X-ray absorption spectroscopy to investigate mercury methylation in the presence of selenium. Metallomics 6:465–475
Ullrich SM, Tanton TW, Abdrashitova SA (2001) Mercury in the aquatic environment: a review of factors affecting methylation. Crit Rev Environ Sci Technol 31(3):241–293
Wang X, Tam NFY, Fu S, Ametkhan A, Ouyang Y, Ye ZH (2014a) Selenium addition alters mercury uptake, bioavailability in the rhizosphere and root anatomy of rice (Oryza sativa). Ann Bot 114(2):271–278
Wang X, Ye ZH, Li B, Huang LN, Meng M, Shi JB, Jiang GB (2014b) Growing rice aerobically markedly decreases mercury accumulation by reducing both Hg bioavailability and the production of MeHg. Environ Sci Technol 48(3):1878–1185
Wang YJ, Dang F, Zhon H, Wei ZB, Li P (2015) Effects of sulfate and selenite on mercury methylation in a mercury-contaminated rice paddy soil under anoxic conditions. Environ Sci Pollut Res 23(5):4602–4608
Wang YJ, Dang F, Evans RD, Zhong H, Zhao JT, Zhou DM (2016a) Mechanistic understanding of MeHg-Se antagonism in soil-rice systems: the key role of antagonism in soil. Sci Rep 6:19477,1–11
Wang YJ, Dang F, Zhao JT, Zhong H (2016b) Selenium inhibits sulfate-mediated methylmercury production in rice paddy soil. Environ Pollut 213:232–239
Weber FA, Voegelin A, Kretzschmar R (2009) Multi-metal contaminant dynamics in temporarily flooded soil under sulfate limitation. Geochim Cosmochim Acta 73(19):5513–5527
Wei QF (1981) In: Institute of Soil Science, Academia Sinica (ed) Proceedings of Symposium on Paddy Soils. Science Press, Beijing, pp 439–443
Yang DY, Chen YW, Gunn JM, Belzile N (2008) Selenium, mercury in organisms: interactions and mechanisms. Environ Rev 16:71–92
Zhang JF, Qu LY, Feng XB, Zhang W, Guo YN, Lin K, Li M (2008) Farmland mercury contamination in the vicinity of an organic chemical factory in Guizhou, China. Chin J Geochem 27:424–430
Zhang H, Feng XB, Larssen T, Qiu GL, Vogt RD (2010a) In inland China, rice, rather than fish is the major pathway for methylmercury exposure. Environ Health Perspect 118(9):1183–1188
Zhang H, Feng XB, Larssen T, Shang LH, Li P (2010b) Bioaccumulation of methylmercury versus inorganic mercury in rice (Oryza sativa L.) grain. Environ Sci Technol 44(12):4499–4504
Zhang H, Feng XB, Zhu JM, Sapkota A, Meng B, Yao H, Qin HB et al (2012) Selenium in soil inhibits mercury uptake and translocation in rice (Oryza sativa L.). Environ Sci Technol 46(18):10040–10046
Zhao JT, Li YF, Li YY, Gao YX, Li B, Hu Y, Zhao YL et al (2014) Selenium modulates mercury uptake and distribution in rice (Oryza sativa L.) in correlation with mercury species and exposure level. Metallomics 6(10):1951–1957
Zhou XB, Shi WM, Zhang LH (2007) Iron plaque outside roots affects selenite uptake by rice seedlings (Oryza sativa L.) grown in solution culture. Plant Soil 290(1):17–28
Acknowledgments
Financial support was provided to Huan Zhong by the National Natural Science Foundation of China (41273087). We are grateful for the valuable comments from anonymous reviewers on this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Jean-Paul Schwitzguébel
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
Supplementary data. Concentrations of MeHg in soils during rice cultivation, recovery rates of the certified reference materials, biomass of rice tissues, concentrations and mass distributions of Se in rice tissues. (DOC 1.46 mb)
Rights and permissions
About this article
Cite this article
Wang, Y., Wei, Z., Zeng, Q. et al. Amendment of sulfate with Se into soils further reduces methylmercury accumulation in rice. J Soils Sediments 16, 2720–2727 (2016). https://doi.org/10.1007/s11368-016-1453-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-016-1453-y