Abstract
The detoxification process of trace metals in the estuarine burrowing crab Neohelice granulata, after previously being exposed to anthropogenic pressures in the field, is described for the first time. The objectives of this study were (a) to assess the metal content (Cd, Cu, Pb, Zn, Mn, Ni, Cr, Fe) in the sediments and the uptake of these elements in the hepatopancreas of N. granulata; (b) to quantify trace metal concentrations in the hepatopancreas before and after the detoxification experiment; and (c) to relate this information to metallothionein (MT) induction or reversibility. The detoxification assay was performed for 25 days with artificial seawater under controlled conditions in a culture chamber. The results showed higher uptake and bioaccumulation of Zn and Cu from the sediments, and the hepatopancreas exhibited increased levels of Zn and lower concentrations of the rest of the metals and MTs after the assay, mainly Fe and Mn that were significantly lower. We conclude that trace metals could be translocated to and accumulated in the hepatopancreas, the main metabolic organ, and then eliminated under controlled conditions with corresponding reversibility of MTs.

Graphical abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Within marine ecosystems, estuaries are among the most polluted coastal areas because their chemical and physical conditions provide an extraordinary capacity to accumulate different materials that are discharged on their shores (Marcovecchio et al. 2008). Industrial and domestic discharges are one of the most problematic issues since they carry several pollutants, such as heavy metals, microplastics, different persistent organic compounds (OCs, OPs, PAHs), and other emerging contaminants (Arias et al. 2010; Spetter et al. 2015; Fernández Severini et al. 2019; Villagrán et al. 2019a). Among these pollutants, trace metals are one of the main concerns for stakeholders in marine and coastal environment conservation: their deleterious effects on the biota have been well recorded in the international literature, especially those elements that are not essential and consequently can be accumulated, as they are not easily eliminated in the environment (Rainbow 1995; Nesto et al. 2007; Comoglio et al. 2011).
Concerning the indifference to information related to ocean pollution in industrialized countries from the North, the South Atlantic Ocean should be a significant concern because developing industries, rapid urbanization, and informal settlements have developed on the shores of the emerging countries. The increasing population levels, poor sanitary conditions, and a rise in the consumption level that has dramatically accelerated the waste generation rate, with toxic trace substances that are transported straight into the ocean, resulting in sensitive environments (Marcovecchio et al. 2008; Diodato et al. 2012). In this respect, most of the studies that analyze the influence of different effluent discharges in marine environments in the South Atlantic have focused on the accumulation patterns of metals in oysters like Crassostrea gigas (La Colla et al. 2019a) and other bivalves such as Brachidontes rodriguezii (Buzzi et al. 2017) and how they biomagnify through food webs (La Colla et al. 2019b; Vilches et al. 2019). But there are fewer studies on the response of the organisms to metal concentrations in SW Atlantic coastal systems, and they are mostly done in plants (Cressa truxillensis, Limonium brasiliense) (Pollicelli et al. 2018; Idaszkin et al. 2019), bivalves (Nacella magellanica, Mytilus edulis chilensis, Aulacomya atra atra) (Comoglio et al. 2011; Duarte et al. 2011; Giarratano et al. 2011, 2014; Di Salvatore et al. 2013), and few crustaceans (Neohelice granulata) (Giarratano et al. 2016) and polychaetes (Laeonereis acuta) (Dolagaratz et al. 2019).
How organisms eliminate toxic substances has scarcely been studied. However, analyses of the accumulation of trace metals in aquatic invertebrates and their association with reactive oxygen species (ROS) have been carried out (Chelomin et al. 2005; Valavanidis et al. 2006; Alé et al. 2019). The increase in ROS levels is mainly due to non-essential element exposure that induces intracellular damage. Thus, understanding the process of detoxification in organisms is fundamental as it has been a significant pathway for eliminating several toxic substances, especially in human-consumed species (El-Gamal 2011; Anacleto et al. 2015; Birnstiel et al. 2019). Studies have demonstrated that metallothioneins (hereafter MTs) and MT-like proteins (MTLPs) can react as metal-binding proteins that eliminate the excess of both essential and non-essential metals. In this way, they protect organisms from cellular damage, acting as specific biomarkers of these elements (Won et al. 2008).
First, MTs and MTLPs were identified in invertebrates in the digestive gland, hepatopancreas, and gills of marine mussels and crustaceans as cysteine-rich metal-binding proteins of low molecular weight, being essential for understanding the trace element pathways in metabolism and metal sequestration (Roesijadi 1981; Engel and Brouwer 1984; Viarengo et al. 1985). As a result, the invertebrates exposed to metals in the environment, or under laboratory conditions, exhibit enhanced levels of metals that are commonly associated with these metal-binding proteins. In invertebrates, the functions of MTs are respectively bioaccumulation of toxic metals and detoxification, homeostatic regulation of metals, and protection against oxidative stress, among others (Pedersen et al. 1994, Pedersen and Lundebye 1996, Pedersen et al. 1996, Pedersen et al. 1997; Legras et al. 2000; Brown et al. 2004; Pytharopoulou et al. 2008; Lemus et al. 2016; Capparelli et al. 2018), while in vertebrates, it has been less studied since most of them have a higher range of mobility and are, therefore, less representative in monitoring programs of the pollution in specific areas (Amiard et al. 2006). In marine vertebrates, MTs were studied in the gills of fish and kidneys of mammals, and recently in the brain of these groups (Wang et al. 2014). And it has been established that MTs are involved in the homeostatic processes of Cu and Zn and the protection against metal toxicity—such as Cd—and oxidative stress, and they also have a neuroprotective mechanism (Polizzi et al. 2014; Wang et al. 2014), assuming some similar functions as those reported for invertebrate species.
Nonetheless, other studies have discussed the use of these proteins as specific metal biomarkers as they might also be related to changes in other environmental factors or drivers, such as salinity, sex, stages, body size, seasonality, and tissues (Legras et al. 2000; Geffard et al. 2001; Morgan et al. 2004; Ladhar-Chaabouni et al. 2011; Cenov et al. 2018). Hence, it is also possible that the physiological response of detoxification in organisms due to MT induction might differ according to the physiology of each species and the different types of inputs and sources of metals within the environment (Establier et al. 1985; Buzzi and Marcovecchio 2016; Campos et al. 2016), and therefore, their role as specific metal biomarkers is still under discussion among scientists.
The uptake, biomarkers, mechanisms of sequestration, and detoxification of trace metals have scarcely been studied in the highly human-impacted coastal and estuarine environments in the Southwest Atlantic (Buzzi and Marcovecchio 2016; Negrin et al. 2019). Though the understanding of this process, and how organisms cope with cleaner conditions, could generate essential information for stakeholders for coastal monitoring and remediation programs.
The burrowing crab Neohelice granulata is a resident benthic varunid crab of the Southwestern Atlantic Ocean, and there is a vast literature on its biological and ecological characteristics. For that reason, it is known as an emergent model crab in developing countries (Spivak 2010 and references therein). Its biological characteristics make it a suitable bioindicator for these countries since it has a wide distribution throughout the year; it is easy to sample and has a high population recovery. As this widespread species is only found in coastal estuarine areas in the SW Atlantic, it is a specific indicator of these environments because they inhabit and eat from detritus associated with the surrounding sediments (Iribarne et al. 1997; Botto et al. 2011; Angeletti 2017). Besides, this species is known as an ecosystem engineer due to its high bioturbation abilities in the sediment-water interface (Angeletti et al. 2018), which also modifies the availability of resources and pollutants like trace elements (Andrade et al. 2019). As a result, the anthropogenic disturbance due to trace elements might be in their bioecology, accumulation abilities, and physiological trade-offs (Ferrer et al. 2003; Sabatini et al. 2009; Beltrame et al. 2010; Giarratano et al. 2016; Simonetti et al. 2018).
The hepatopancreas is a dynamic organ that is mostly related to digestive and detoxification functions, but also to gonadal development processes as it is responsible for the production of vitellogenins, the precursors of eggs (Vogt 2002; Negro and Collins 2017). It is also known to be a target organ for detoxification because different systems, such as cytochrome P450, lysosomes, and MTs, were detected (Pedersen et al. 1997; Vogt 2002; Ahearn et al. 2004). Therefore, to study of the toxicity in this organ is of great interest because previous studies have shown that metals can cause injury to the hepatopancreas at the histopathological and biochemical levels, with possible modifications of its principal functions (Wang et al. 2008).
The aims of the present study were (a) to assess the metal content (Cd, Cu, Pb, Zn, Mn, Ni, Cr, Fe) in the sediments and the uptake of these elements in the hepatopancreas of N. granulata; (b) to quantify the trace metal concentrations in the hepatopancreas before and after the detoxification experiment; and (c) to relate this information to MT induction or reversibility. This information is fundamental for relating MT expression to bioaccumulation and the concentration of trace metals in sediments to generate approaches to entirely aboard this process in this emergent bioindicator species.
Materials and methods
Study area and field sampling
This study was carried out in the Bahía Blanca estuary (BEE) (38° 44′–39° 27′ S and 61° 45′–62° 30′ W), a mesotidal Patagonian estuarine system located in Buenos Aires province (Argentina) to the southwest of the Atlantic Ocean (Fig. 1). It is the second largest estuary in Argentina after La Plata, with an extension of almost 3000 km2, and is characterized by tidal flats and channels with islands and a few salt marsh areas. Moreover, it is a homogeneous estuary, and the sedimentology is based on silty clays on the flats and sand in most of the deeper parts of the Main Channel (Perillo et al. 2001; Cuadrado et al. 2004). This wetland has been the subject of different human impacts due to the presence of a large petrochemical complex, a thermoelectric power plant, and one of the largest deep ports in South America, being regularly navigated by large cargo ships. Also, it is an essential place for families of small-scale artisanal fishing that carry out their small economies in the estuary (Truchet et al. 2019a). Nevertheless, industrial and domestic discharges are thrown directly into the estuary without any pretreatment and with high concentrations of heavy metals and other pollutants that are harmful to the biota (Berasategui et al. 2018; Villagrán et al. 2019b).
The samples were taken in February 2018 (summer in the southern hemisphere) from the middle part of the estuary at Rosales Port (hereafter RP) (Fig. 1), a small-scale artisanal fishery port and also a naval port. This port is located in the coastal town of Punta Alta (General Rosales district with ~ 60,000 inhabitants) and is characterized by maritime transport and eutrophication caused by the untreated sewage plant in Punta Alta with high impacts on the biota (Berasategui et al. 2018). RP has extensive tidal flats with siliciclastic depositions and fine sediments from fine sand to mud, the total organic matter is low, and it ranges between 5 and 12% in the tidal flats, whereas the pH and potential redox (Eh, mV) ranges are between 5.5 and 8.5 and − 125 and 328, respectively (Fernández et al. 2016). The upper intertidal and supratidal zones are colonized by benthic microbial communities that form biofilms and microbial mats; the presence of the toxic bacteria Escherichia coli was detected in the sediments and water from the area (Pan et al. 2013; Spetter et al. 2015). The intertidal area is mainly vegetated by Spartina alterniflora and also by Sarcocornia spp. in the supratidal zone. These tidal flats and salt marshes are dominated by the varunid burrowing crab N. granulata, followed by the rocky crab Cyrtograpsus angulatus and C. altimanus and the southernmost population of the fiddler crab Leptuca uruguayensis (Spivak 1997; Truchet et al. 2019b).
The environmental parameters in the water column were measured in situ with a HANNA HI 9828 multisensor probe recording the following values: pH 8.05; salinity 32 psu, and a temperature of 22.08 °C. One hundred twenty adult males of N. granulata were collected by hand from a tidal flat at RP with a width of carapace > 15 mm, according to Angeletti and Cervellini (2015) for the BBE, and they were stored in plastic containers with estuarine water and transported to the laboratory. Three replicates of sediment were also taken from the intertidal zone and were stored in plastic bags and refrigerated.
Detoxification experiment
Once in the laboratory, 60 individuals were anesthetized by freezing, and the biometric data (i.e., carapace, mm, and total weight, g) were recorded. Then, the organisms were dissected in ten pools of six individuals (n = 10, 6 organisms each) to obtain the hepatopancreas (non-detoxified or untreated pools, ND). Five pools were used for MT determination and the rest for trace metal determination. The remaining crabs were placed in 8 aerated glass tanks (8 per tank) with artificial seawater in a chamber set for day/night temperatures, photoperiod, and humidity according to the summer climatic conditions of the BBE.
Each tank was divided by perforated septums to avoid fights between the males (a typical behavior in N. granulata) and to allow circulation and oxygenation of the water. During the detoxification experiment, the crabs were fed (food represented 1% of their total body weight) every 2 days. The tanks were cleaned the day after they were fed to avoid any possible contamination in the water due to the excretion and feces of the organisms. This procedure was followed for 25 days until the experiment was completed.
Finally, the organisms were randomly selected in ten pools of six individuals (n = 10, 6 organisms each) (detoxified or treated pools, D), biometric data was taken, and dissection was also performed to obtain the hepatopancreas for both trace metal and MT determination, following the same procedure as described above for the ND crabs. The material for trace metal determination and MT assay was stored at − 20 °C and − 80 °C, respectively, until the analysis. Prevention was taken to avoid cross-contamination during the whole process, and all the materials were washed with non-ionic soap, HNO3 (5%), and distilled water following the recommendations of APHA-AWWA-WEF (1998).
Trace metal determination
For trace metal determination, the pools of the hepatopancreas of ND and D crabs were lyophilized until constant weight, and the sediment samples were dried in an oven at 60 °C until constant weight. In the case of the sediments, large debris and fragments of biota were removed before grinding and sieving to obtain the finest fraction (< 63 μm). Tissue samples were also grown and homogenized. Finally, subsamples of approximately 0.5 g of dry material were digested with a mixture of 5 ml of HNO3 and 1 ml of HClO4 (5:1) (Merck) in a glycerine bath at 110 ± 10 °C until the digestion was completed (Botte et al. 2010). The final extract was poured into a centrifuge tube, and it was filled up to 10 ml with HNO3 (0.7%) for analytical metal determinations by inductively coupled plasma optical emission spectroscopy (ICP-OES Optima 2100 DV Perkin Elmer).
Reagent blanks, certified reference materials (CRM), and analytical grade reagents were used for analytical quality control. Recovery percentages of all trace metals in CRM (n°6, NIE Tsukuba, Japan, mussel tissue flour) were higher than 90%. The method detection limit (MDL) was calculated for each metal by multiplying the standard deviation of 20 blank replicates by Student’s t value at the 99% confidence level at n − 1 degree of freedom (Federal Register 1984; EPA 2016) resulting in the following values (in μg g−1): Cd: 0.157, Cu: 1.366, Pb: 1.861, Cr: 1.654, Zn: 2.821, Mn: 17.341, Ni: 1.944, Fe: 31.102. In the cases where metal concentrations did not exceed the MDL, they were replaced with half of its value (MDL/2).
Metallothionein determination
For the MT determination, the method of Viarengo et al. (1997) and Linde et al. (2001) was adopted with modifications by Buzzi and Marcovecchio (2016). Pools of ND and D hepatopancreas tissue were ice-cold homogenized (1:4) (in saccharose 0.5 M, Tris-HCl 0.02 M, DTT 1 mM, and PMSF 0.5 mM, pH: 8.6) and then centrifuged at 30,000g for 45 min at 4 °C. Then, MTs were purified by two successive extractions with pure cold ethanol and chloroform. The pellets were dried under a soft N2 flux (analytical quality) dissolved in NaCl 250 mM and EDTA buffer 4 mM and finally reacted with DTNB 0.43 mM in phosphate buffer 0.2 M. MT quantification was spectrophotometrically determined at 412 nm, using a solution of reduced glutathione (GSH, 307.32 g mol−1) as a reference standard. MT concentrations were quantified based on the cysteine content of 18-cysteine residues per mol (Serra-Batiste et al. 2010), assuming a similar thiol SH group content in N. granulata. And MT concentrations were reported as nmol MT per gram (g) of wet tissue (wt).
Morphological analysis
The hepatosomatic index (HSI), condition index (CI), and percentage of humidity (H) in the hepatopancreas were estimated for the crabs in both treatments as follows:
Data analysis
For the trace metal analysis, ANOVA (p > 0.05) was used to determine possible differences between the ND and D experiences. Statistical differences between the MT concentrations in both treatments (ND and D) were analyzed using the nonparametric Mann-Whitney test (p > 0.05) because of the rejection of the homoscedasticity of variance assumption. Finally, the biota-sediment factor (BSAF) was calculated as the ratio between the metal concentrations in the hepatopancreas and the fine fraction of the intertidal sediments (< 63 μm):
Results
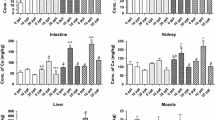
The morphological indices evaluated showed similar results for both conditions, and although the CI was 10% higher in D crabs, it was not statistically significant (Table 1). All trace metals except Cd were detected in the fine sediments in the following order: Fe > Mn > Zn > Cu > Pb ≥ Cr > Ni (Table 2). In the hepatopancreas of both the ND and D groups, the order was different: Fe > Cu > Zn > Mn> Ni > Cd (Pb, Cr < MDL), and only the Mn concentrations were below the MDL after the assay (Fig. 2). A significantly (p < 0.05) lower Fe concentration was observed after detoxification, whereas Mn became undetectable. Besides, Zn significantly increased in value, whereas metals, such as Cd, Cu, and Ni, did not show any significant differences between the ND and D but tended to decrease in D (Fig. 2).
The Mann-Whitney test between both treatments demonstrated that MTs decreased significantly after the assay in D organisms by almost 75% (Fig. 3), showing strong reversibility of these proteins (p < 0.05). Finally, the BSAF values showed bioaccumulation and uptake of metals in the crabs from the sediments as follows: Cu > Zn > Ni > Mn > Fe (Table 2).
Discussion
The burrowing crab Neohelice granulata is a widespread species in the South Atlantic Ocean. In this extensive marine system, several developing countries are located, and therefore, in recent decades, it has been considered as an emergent animal model for bioecological and ecotoxicological studies in these countries (Spivak 2010). The burrowing ability of this species produces the bioturbation of sediments, and consequently, it can modify the metal fate and distribution by mixing or resuspending subsurface and surface sediments or altering biogeochemical processes. Hence, the bioturbation of sediments might enhance the metal biodisponibility for organisms and probably the uptake of toxic metals, causing enzymatic stress and other physiological damage, as it was proved in other benthic invertebrates, such as annelids, amphipods, and mollusks (Andrade et al. 2019).
Regarding the sediments, and in comparison with the worldwide PEL (probable effect level) and TEL (threshold effect level) values (Table 2), the metal concentrations in the fraction of fine sediments were of low to medium risk to the biota but higher than in the inner part of the estuary (Buzzi and Marcovecchio 2016; Buzzi et al. 2017; Buzzi and Marcovecchio 2018), probably due to the input of untreated sewage water (Spetter et al. 2015; Villagrán et al. 2019a). The BSAF indicates that the organisms mostly uptake Cu and Zn from sediments, and the same pattern was found by Simonetti et al. (2018) in the total soft tissue (muscle, gills, hepatopancreas, reproductive, digestive, and nervous system) of the same species of crab from the same region. However, trace metal concentrations in the hepatopancreas were higher than in soft tissue and this could be explained by a dilution factor caused by using the total tissues and organs for the trace metal analysis. Nevertheless, other studies by Beltrame et al. (2009, 2010), Giarratano et al. (2016), and Marinho et al. (2018) detected higher values of toxic trace metals like Pb, Cd, Mn, and Cr in the hepatopancreas of the same species from other Southwestern Atlantic coastal systems. In other worldwide estuarine and coastal regions in emerging countries, other species of crabs have also tended to show higher levels of toxic metals, such as Cd and Pb (i.e., Jewett and Naidu 2000; Legras et al. 2000; Al-Mohanna and Subrahmanyam 2001), but the essential metals, like Cu, Zn, and Fe, found in N. granulata in this study were still higher than those reported in these studies, probably due to untreated sewage discharges that carry high levels of trace metals which are deposited in the sediments and are bioaccumulated in organisms (Spetter et al. 2015).
The information mentioned above demonstrates the invaluable and accurate use of an organ like the hepatopancreas for biomonitoring programs in this widespread and ecological key species of the South Atlantic. Considering that the hepatopancreas is involved in detoxification and excretory activities, its functions and structure are likely to be affected when the crustaceans are exposed to certain pollutants (Wu et al. 2008). In crustaceans, it is also responsible for the production of vitellogenin, because the lipids that are transported to the oocytes are stored in the hepatopancreas (Negro 2015). Therefore, it is possible that the damage at the different levels in this organ (enzymatic, metabolic, histological) due to different xenobiotic pollutants, like trace metals, might have a persistent effect on reproduction and the next generation of crabs (Simonetti et al. 2013; Negro and Collins 2017). In this sense, it would be relevant to analyze the reactive oxygen species (ROS), as a complementary biomarker of pollution by trace metals that induce oxidative stress at a cellular level, since it has been proven that MTs could reduce ROS activity (Bocchetti and Regoli 2006; Freitas et al. 2012; Figueira et al. 2012; Alé et al. 2019).
Our results showed that morphological indices and body condition are not suitable for assessing crab health status in depuration experiments since the values were similar in both treatments, and even for other authors, neither long (a year) nor a short detoxification period reflect any significant differences (Arini et al. 2014; Anacleto et al. 2015). In this respect, Buzzi and Marcovecchio (2018) and Villagrán et al. (2019b) explained that in estuarine invertebrates, these indices would be more associated with differences in the inputs and sources of pollutants that are discharged to the estuary. Therefore, they would be better for measuring the uptake of trace elements at different sites with different xenobiotic discharges.
One of our main goals was to assess the recovery abilities of N. granulata after being relocated from the field where they were exposed to untreated domestic discharges, to the laboratory. Variables, such as sex, temperature, salinity, and size, were discounted in this study, which was to assess whether MT reversibility or induction was only due to trace metal excretion and if these proteins acted as metal-binding proteins in N. granulata. After 25 days of detoxification, the MT levels significantly decreased, following the same pattern found by Buzzi and Marcovecchio (2016) in a different area of the same estuary. The total levels of MTs in both conditions were lower than those reported by Buzzi and Marcovecchio (2016), although we found higher metal concentrations in the sediments and tissues than those authors. These results could be explained by the effects of higher levels of metals in the sewage waters on the enzymatic and protein activities that sometimes are inhibited infield exposures (Santana et al. 2018). However, trace metal concentrations in the sewage discharge from Punta Alta city were not determined in this study.
Concerning the metal concentrations and possible metal kinetics in N. granulata, Zn increased whereas Fe and Mn decreased and most of the trace metals showed no statistical differences but tended to diminish after the detoxification assay slowly. Arini et al. (2014) explained that the regulation of some trace elements, like Zn (an essential metal) in the visceral mass of Corbicula fluminea, is rapid with high induction of MT synthesis: this element increased after 28 days of depuration. They coincided with a significant MT increase, whereas after a year of decontamination, the MTs and Zn were significantly lower, with a quick regulation of this metal. In our study, the values of Zn increased after 25 days of detoxification. Consequently, a process similar to that of the bivalves may occur in decapods, where Zn is translocated from different parts of the body to the primary excretory organ, increasing in concentration after 28 days of depuration, and then it can be quickly expelled (Arini et al. 2014). This element, along with Cu, is an essential enzymatic cofactor for MT synthesis in invertebrates, playing a vital role in the homeostasis of essential elements (Viarengo 1989; Anacleto et al. 2015; Arini et al. 2015). Zn shows different behaviors in benthic organisms: being reduced, increased, or maintained after detoxification, even after a long-term period of detoxification (do Amaral et al. 2005; Bergey and Weis 2007; Arini et al. 2014; Anacleto et al. 2015). The ability to exhibit different kinetics might be related to the metal bioavailability, indicating a quicker depuration of metals in the dissolved form, whereas metals present in amorphous granules are kept for a more extended time in the tissues (Legras et al. 2000; Mouneyrac et al. 2001; Wallace et al. 2003; Reichmuth et al. 2010; Lemus et al. 2016). Further studies should be carried out to assess the affinity with Zn movements with MT induction at different times of depuration and in different phases (metallic granules or dissolved) of the metal in N. granulata.
Although it was not significant, N. granulata tended to remove almost 40% of the Cd content from the hepatopancreas. Overall, the removal of Cd, as well as other non-essential or toxic elements, by benthic organisms, such as C. fluminea, Carcinus maenas, Mytilus sp., and Gammarus pulex, is difficult (Viarengo 1989; Pedersen and Lundebye 1996; Khan et al. 2012; Arini et al. 2014). However, Chiodi Boudet et al. (2015, 2019) recorded a significant decrease in Cd accumulation after short-term detoxification in the crustacean Palaemonetes sp. Thus, it is probable that some crustaceans might have quicker mechanisms of detoxification of this element than mollusks, enabled by MT or MTLP induction and the form of storing Cd in the cells, which was not recorded in our study.
Fe and Mn levels significantly decreased in D crabs, whereas Cu and Ni tended to decrease but without any statistical differences. It is essential to point out that the mechanisms of uptake and elimination of metals other than Cd and Zn have been studied less frequently. Mn is an essential metal for cellular metabolism, but in excess, it can act as a neurotoxin in marine animals (Baden et al. 1995; Baden and Eriksson 2006), or it can cause other effects such as shell disease in the blue crab Callinectes sapidus (Weinstein et al. 1992) and intestinal morphology damage in the prawn Macrobrachium nipponense (Ding et al. 2020). Mohamad (2008) reported a significant decrease in Mn levels from the baseline values in the hepatopancreas of the crab Liocarcinus depurator after a short depuration period. Though the hepatopancreas is the main detoxifying organ in crustaceans, the high absorption rate exceeds the excretion rate of Mn under conditions of high bioavailability, leading to a net accumulation of the metal in the organ.
On the contrary, the significant decrease in the Mn concentration in D N. granulata to values below the MDL may be because, under depuration conditions, the excretion rate of Mn far exceeds the absorption rate. Kobayashi et al. (2006) explained that Mn is a metal that induces the synthesis of MTs, entirely depending on the production of glycoproteins in mammals. Nevertheless, there is no information about the molecular activity of MT synthesis with Mn in invertebrates, but it is possible that this metal also binds to these proteins so that it is quickly detoxified.
Conclusions
In the present study, we analyzed the uptake and detoxification process of trace metals in N. granulata from RP. Cu and Zn were the principal metals that were absorbed and bioaccumulated in the hepatopancreas of N. granulata. Moreover, the levels of both metals in the sediments and the crabs from RP were higher than the values reported at other sites within the EBB, which is probably due to the discharge of untreated sewage discharge at RP.
A short detoxification period of 25 days did not modify the morphological indices in N. granulata, such as the humidity or the hepatosomatic and condition indices. Under controlled laboratory conditions, where sexual and environmental variables were not considered, it was possible to determine the recovery capacities of MTs after trace metal detoxification. Therefore, the property of these proteins as key biomarkers of these pollutants was enhanced in N. granulata. Along with the significant decrease in MTs in the hepatopancreas, significantly lower levels of Fe and Mn were observed, whereas the other metals tended to diminish but not in a significant way. However, the Zn value increased, suggesting that in this species, the behavior of this element is probably related to metal bioavailability and the storage form in the cells.
Finally, we consider that it is necessary to continue studying the detoxification of this species in other places in the estuary to find out how this organism copes with the different sources of metal inputs in this human-impacted estuary (i.e., agricultural and cattle activities, industrial wastes, others) from an ecotoxicological and physiological perspective.
References
Ahearn GA, Mandal PK, Mandal A (2004) Mechanisms of heavy-metal sequestration and detoxification in crustaceans: a review. J Comp Physiol B 174(6). https://doi.org/10.1007/s00360-004-0438-0
Alé A, Liberatori G, Vannuccini ML, Bergami E, Ancora S, Mariotti G, Bianchi N, Galdoporpora JM, Desimone MF, Cazenave J, Corsi I (2019) Exposure to a nanosilver-enabled consumer product results in similar accumulation and toxicity of silver nanoparticles in the marine mussel Mytilus galloprovincialis. Aquat Toxicol 211:46–56. https://doi.org/10.1016/j.aquatox.2019.03.018
Al-Mohanna S, Subrahmanyam MN (2001) Flux of heavy metal accumulation in various organs of the intertidal marine blue crab, Portunus pelagicus (L.) from the Kuwait coast after the Gulf War. Environ Int 27(4):321–326. https://doi.org/10.1016/s0160-4120(01)00063-0
Amiard J, Amiardtriquet C, Barka S, Pellerin J, Rainbow P (2006) Metallothioneins in aquatic invertebrates: their role in metal detoxification and their use as biomarkers. Aquat Toxicol 76(2):160–202. https://doi.org/10.1016/j.aquatox.2005.08.015
Anacleto P, Maulvault AL, Nunes ML, Carvalho ML, Rosa R, Marques A (2015) Effects of depuration on metal levels and health status of bivalve molluscs. Food Control 47:493–501. https://doi.org/10.1016/j.foodcont.2014.07.055
Andrade VS, Wiegand C, Pannard A, Gagneten AM, Pédrot M, Bouhnik-Le Coz M, Piscart C (2019) How can interspecific interactions in freshwater benthic macroinvertebrates modify trace element availability from sediment? Chemosphere 125594:125594. https://doi.org/10.1016/j.chemosphere.2019.125594
Angeletti S (2017) Efecto bioturbador del cangrejo Neohelice granulata sobre la distribución y transporte de sedimento en ambientes intermareales próximos al límite sur de su distribución geográfica: Un estudio poblacional comparado. Doctoral Thesis, Universidad Nacional del Sur. Available at: http://repositoriodigital.uns.edu.ar/bitstream/123456789/3388/1/Tesis_Angeletti.pdf
Angeletti S, Cervellini PM (2015) Population structure of the burrowing crab Neohelice granulata (Brachyura, Varunidae) in a southwestern Atlantic salt marsh. Lat Am J Aquat Res 43(3):539–547. https://doi.org/10.3856/vol43-issue3-fulltext-15
Angeletti S, Cervellini PM, Lescano L (2018) Burrowing activity of the Neohelice granulata crab (Brachyura, Varunidae) in Southwest Atlantic intertidal area. Ciencias Marinas 44(3):155–167. https://doi.org/10.7773/cm.v44i3.2851
APHA-AWWA-WEF (1998) In: Clesceri LS, Greenberg AE, Eaton AD (Eds.) Standard methods for the examination of water and wastewater, 20th ed. American Public Health Association, Washington (USA)
Arias AH, Fernández Severini MD, Delucchi F, Freije RH, Marcovecchio JE (2010) Persistent pollutants monitoring in a South Atlantic coastal environment. Case study: the Bahía Blanca estuary. In: Ortiz AC, Griffin NB (Eds.). Pollution monitoring. Nova Science Publishers, New York, USA, pp 1–30
Arini A, Daffe G, Gonzalez P, Feurtet-Mazel A, Baudrimont M (2014) Detoxification and recovery capacities of Corbicula fluminea after an industrial metal contamination (Cd and Zn): a one-year depuration experiment. Environ Pollut 192:74–82. https://doi.org/10.1016/j.envpol.2014.04.012
Arini A, Gourves PY, Gonzalez P, Baudrimont M (2015) Metal detoxification and gene expression regulation after a Cd and Zn contamination: an experimental study on Danio rerio. Chemosphere 128:125–133. https://doi.org/10.1016/j.chemosphere.2015.01.022
Baden SP, Eriksson SP (2006) Role, routes and effects of manganese in crustaceans. Oceanogr Mar Biol Annu Rev 44:61–83
Baden SP, Eriksson SP, Weeks JM (1995) Uptake, accumulation and regulation of manganese during experimental hypoxia and normoxia by the decapod Nephrops norvegicus (L.). Mar Pollut Bull 31:93–102
Beltrame MO, De Marco SG, Marcovecchio JE (2009) Influences of sex, habitat, and seasonality on heavy-metal concentrations in the burrowing crab (Neohelice granulata) from a coastal lagoon in Argentina. Arch Environ Contam Toxicol 58(3):746–756. https://doi.org/10.1007/s00244-009-9405-9
Beltrame MO, De Marco SG, Marcovecchio JE (2010) The burrowing crab Neohelice granulata as potential bioindicator of heavy metals in estuarine systems of the Atlantic coast of Argentina. Environ Monit Assess 172(1–4):379–389. https://doi.org/10.1007/s10661-010-1341-7
Berasategui AA, Biancalana F, Fricke A, Fernández Severini MD, Uibrig R, Dutto MS, Marcovecchio JE, Calliari DL, Hoffmeyer MS (2018) The impact of sewage effluents on the fecundity and survival of Eurytemora americanain a eutrophic estuary of Argentina. Estuar Coast Shelf S 211:208–216. https://doi.org/10.1016/j.ecss.2017.08.034
Bergey LL, Weis JS (2007) Molting as a mechanism of depuration of metals in the fiddler crab, Uca pugnax. Mar Environ Res 64(5):556–562. https://doi.org/10.1016/j.marenvres.2007.04.009
Birnstiel S, Soares-Gomes A, da Gama BAP (2019) Depuration reduces microplastic content in wild and farmed mussels. Mar Pollut Bull 140:241–247. https://doi.org/10.1016/j.marpolbul.2019.01.044
Bocchetti R, Regoli F (2006) Seasonal variability of oxidative biomarkers, lysosomal parameters, metallothioneins and peroxisomal enzymes in the Mediterranean mussel Mytilus galloprovincialis from Adriatic Sea. Chemosphere 65(6):913–921. https://doi.org/10.1016/j.chemosphere.2006.03.049
Botte SE, Freije RH, Marcovecchio JE (2010) Distribution of several heavy metals in tidal flats sediments within Bahía Blanca estuary (Argentina). Water Air Soil Pollut 210:371–388. https://doi.org/10.1007/s11270-009-0260-0
Botto F, Gaitán E, Mianzan H, Acha M, Giberto D, Schiariti A, Iribarne O (2011) Origin of resources and trophic pathways in a large SW Atlantic estuary: an evaluation using stable isotopes. Estuar Coast Shelf S 92(1):70–77. https://doi.org/10.1016/j.ecss.2010.12.014
Brown RJ, Galloway TS, Lowe D, Browne MA, Dissanayake A, Jones MB, Depledge MH (2004) Differential sensitivity of three marine invertebrates to copper assessed using multiple biomarkers. Aquat Toxicol 66(3):267–278. https://doi.org/10.1016/j.aquatox.2003.10.001
Buchman MF (1999) NOAA Screening Quick Reference Tables, NOAA. HAZMAT. Report 99-1, Seattle WA. Coastal Protection and Restoration Division, National Oceanic and Atmospheric Administration, p. 12
Buzzi NS, Marcovecchio JE (2016) A baseline study of the metallothioneins induction and its reversibility in Neohelice granulata from the Bahía Blanca estuary (Argentina). Mar Pollut Bull 112(1–2):452–458. https://doi.org/10.1016/j.marpolbul.2016.07.007
Buzzi NS, Marcovecchio JE (2018) Heavy metal concentrations in sediments and in mussels from Argentinean coastal environments, South America. Environ Earth Sci 77(8). https://doi.org/10.1007/s12665-018-7496-1
Buzzi NS, Oliva AL, Arias AH, Marcovecchio JE (2017) Assessment of trace metal accumulation in native mussels (Brachidontesrodriguezii) from a South American temperate estuary. Environ Sci Pollut R 24(18):15781–15793. https://doi.org/10.1007/s11356-017-9237-5
Campos BG, Cruz ACF, Buruaem LM, Rodrigues APC, Machado WTV, Abessa DMS (2016) Using a tiered approach based on ecotoxicological techniques to assess the ecological risks of contamination in a subtropical estuarine protected area. Sci Tot Environ 544:564–573. https://doi.org/10.1016/j.scitotenv.2015.11.124
Capparelli MV, Gusso-Choueri PK, de Souza Abessa DM, McNamara JC (2018) Seasonal environmental parameters influence biochemical responses of the fiddler crab Minuca rapax to contamination in situ. Comp Biochem Physiol Part C 216:93–100. https://doi.org/10.1016/j.cbpc.2018.11.012
Cenov A, Perić L, Glad M, Žurga P, Lušić DV, Traven L, Bihari N (2018) A baseline study of the metallothioneins content in digestive gland of the Norway lobster Nephrops norvegicus from Northern Adriatic Sea: body size, season, gender and metal specific variability. Mar Pollut Bull 131:95–105. https://doi.org/10.1016/j.marpolbul.2018.03.002
Chelomin VP, Zakhartsev MV, Kurilenko AV, Belcheva NN (2005) An in vitro study of the effect of reactive oxygen species on subcellular distribution of deposited cadmium in digestive gland of mussel Crenomytilus grayanus. Aquat Toxicol 73:181–189. https://doi.org/10.1016/j.aquatox.2005.03.009
Chiodi Boudet LN, Polizzi P, Romero MB, Robles A, Marcovecchio JE, Gerpe MS (2015) Histopathological and biochemical evidence of hepatopancreatic toxicity caused by cadmium in white shrimp, Palaemonetes argentinus. Ecotox Environ Safe 113:231–240. https://doi.org/10.1016/j.ecoenv.2014.11.019
Chiodi Boudet L, Mendieta J, Romero MB, Dolagaratz A, Polizzi P, Marcovecchio JE, Gerpe M (2019) Strategies for cadmium detoxification in the white shrimp Palaemonetes argentinus from clean and polluted field locations. Chemosphere. 236:124224. https://doi.org/10.1016/j.chemosphere.2019.06.194
Comoglio L, Amin O, Botté SE, Marcovecchio JE (2011) Use of biomarkers in resident organisms as a tool for environmental monitoring in a cold coastal system, Tierra del Fuego Island. Ecotox Environ Safe 74:382–393. https://doi.org/10.1016/j.ecoenv.2010.10.005
Cuadrado DG, Ginsberg SS, Gómez EA (2004) Geomorfología. In: Piccolo MC, Hoffmeyer MS (eds) Ecosistema del Estuario de Bahía Blanca. Instituto Argentino de Oceanografía (IADO-CONICET), Bahía Blanca, pp 37–39
Di Salvatore P, Calcagno JA, Ortíz N, Ríos de Molina M del C, Sabatini SE (2013) Effect of seasonality on oxidative stress responses and metal accumulation in soft tissues of Aulacomya atra, a mussel from the South Atlantic Patagonian coast. Mar Environ Res 92:244–252. https://doi.org/10.1016/j.marenvres.2013.10.004
Ding Z, Chen X, Kong Y, Shao X, Zhang Y, Ye J (2020) Dietary manganese requirement and its effects on antioxidant enzyme activities, intestinal morphology and microbiota in oriental river prawn Macrobrachium nipponense (De Haan). Aquaculture 516:734622. https://doi.org/10.1016/j.aquaculture.2019.734622
Diodato S, Comoglio L, Camilión C, Amin O (2012) Responses of the resident rocky crab (Halicarcinus planatus, Decapoda) to natural stressors and effluent discharges in Ushuaia Bay, Tierra del Fuego, Argentina. J Exp Mar Biol Ecol 436-437:11–18. https://doi.org/10.1016/j.jembe.2012.08.011
Do Amaral CRM, de Freitas RM, Paulo Machado Torres J, Christian Pfeiffer W (2005) Bioaccumulation and depuration of Zn and Cd in mangrove oysters (Crassostrea rhizophorae, Guilding, 1828) transplanted to and from a contaminated tropical coastal lagoon. Mar Environ Res 59(4):277–285. https://doi.org/10.1016/j.marenvres.2004.05.004
Dolagaratz A, Chiodi Boudet L, Romero MB, Polizzi P, Marcovecchio JE, Gerpe M (2019) Toxicological responses of Laeonereis acuta (Polychaeta, Nereididae) after acute, subchronic and chronic exposure to cadmium. Ecotox Environ Safe 149:217–224. https://doi.org/10.1016/j.ecoenv.2017.11.048
Duarte CA, Giarratano E, Amin OA, Comoglio LI (2011) Heavy metal concentrations and biomarkers of oxidative stress in native mussels (Mytilus edulis chilensis) from Beagle Channel coast (Tierra del Fuego, Argentina). Mar Pollut Bul 62(8):1895–1904. https://doi.org/10.1016/j.marpolbul.2011.05.031
El-Gamal MM (2011) The effect of depuration on heavy metals, petroleum hydrocarbons, and microbial contamination levels in Paphia undulata (Bivalvia: Veneridae). Czech J Anim Sci 56(8):345–354. https://doi.org/10.17221/2395-CJAS
Engel DW, Brouwer M (1984) Trace-metal binding proteins in marine mollusks and crustaceans. Mar Env Res 13:177–194
EPA Environmental Protection Agency’s (2016) Definition and procedure for the determination of the method detection limit, 40 CFR 136 Appendix B; Revision 2, EPA Office of Water, EPA 821-R-16-006
Establier R, Gomez-Parra A, Basco J (1985) Superficial accumulation of heavy metals in near shore marine sediments: an objective index of environmental pollution. Bull Environ Contam Toxicol 35:348–353
Federal Register (1984) Definition and procedure for determination of the method detection limit. EPA, 40 CFR Part 136, Appendix B, Revision 1.11 1 (11), pp. 198–199
Fernández Severini MD, Villagrán DM, Buzzi NS, Chatelain Sartor G (2019) Microplastics in oysters (Crassostrea gigas) and water at the Bahía Blanca Estuary (Southwestern Atlantic): an emerging issue of global concern. Reg Stud Mar Sci 32:100829. https://doi.org/10.1016/j.rsma.2019.100829
Fernández EM, Spetter CV, Martinez AM, Cuadrado DG, Avena MJ, Marcovecchio JE (2016) Carbohydrate production by microbial mats communities in tidal flat from Bahía Blanca estuary (Argentina). Environ Earth Sci 75(8). https://doi.org/10.1007/s12665-016-5405-z
Ferrer LD, Santiago Andrade J, Contardi ET, Asteasuain RO, Marcovecchio JE (2003) Copper and zinc concentrations in Bahía Blanca Estuary (Argentina), and their acute lethal effects on larvae of the crab Chasmagnathus granulata. Chem Spec Bioavailab 15(1):7–14. https://doi.org/10.3184/095422903782775271
Figueira E, Branco D, Antunes SC, Gonçalves F, Freitas R (2012) Are metallothioneins equally good biomarkers of metal and oxidative stress? Ecotox Environ Safe 84:185–190. https://doi.org/10.1016/j.ecoenv.2012.07.012
Freitas R, Costa E, Velez C, Santos J, Lima A, Oliveira C, Rodrigues AM, Quintino V, Figueira E (2012) Looking for suitable biomarkers in benthic macroinvertebrates inhabiting coastal areas with low metal contamination: comparison between the bivalve Cerastoderma edule and the Polychaete Diopatranea politana. Ecotox Environ Safe 75:109–118. https://doi.org/10.1016/j.ecoenv.2011.08.019
Geffard A, Amiard-Triquet C, Amiard J-C, Mouneyrac C (2001) Temporal variations of metallothionein and metal concentrations in the digestive gland of oysters (Crassostrea gigas) from a clean and a metal-rich site. Biomarkers 6(2):91–107. https://doi.org/10.1080/13547500010000860
Giarratano E, Gil MN, Malanga G (2011) Seasonal and pollution-induced variations in biomarkers of transplanted mussels within the Beagle Channel. Mar Pollut Bull 62(6):1337–1344. https://doi.org/10.1016/j.marpolbul.2011.03.037
Giarratano E, Gil MN, Malanga G (2014) Biomarkers of environmental stress in gills of ribbed mussel Aulacomya atra atra (Nuevo Gulf, Northern Patagonia). Ecotox Environ Safe 107:111–119. https://doi.org/10.1016/j.ecoenv.2014.05.003
Giarratano E, Gil MN, Marinho CH, Malanga G (2016) Metals from mine waste as potential cause of oxidative stress in burrowing crab Neohelice granulata from San Antonio bay. Ecotox Environ Safe 132:68–76. https://doi.org/10.1016/j.ecoenv.2016.05.029
Idaszkin YL, Márquez F, Mateos-Naranjo E, Pollicelli M, Saraví Cisneros H (2019) Multidimensional approach to evaluate Limonium brasiliense as source of early biomarkers for lead pollution monitoring under different saline conditions. Ecol Indic 104:567–575. https://doi.org/10.1016/j.ecolind.2019.05.041
Iribarne O, Bortolus A, Botto F (1997) Between-habitat differences in burrow characteristics and trophic modes in the south western Atlantic burrowing crab Chasmagnathus granulata. Mar Ecol Prog Ser 155:137–145. https://doi.org/10.3354/meps155137
Jewett SC, Naidu AS (2000) Assessment of heavy metals in red king crabs following offshore placer gold mining. Mar Pollut Bull 40(6):478–490. https://doi.org/10.1016/S0025-326X(00)00037-0
Khan FR, Bury NR, Hogstrand C (2012) Copper and zinc detoxification in Gammarus pulex (L.). J Exp Biol 215(5):822–832. https://doi.org/10.1242/jeb.062505
Kobayashi K, Kuroda J, Shibata N, Hasegawa T, Seko Y, Satoh M, Tohyama C, Takano H, Imura N, Sakabe K, Fujishiro H, Himeno S (2006) Induction of metallothionein by manganese is completely dependent on interleukin-6 production. J Pharmacol Exper Ther 320(2):721–727. https://doi.org/10.1124/jpet.106.112912
La Colla NS, Botté SE, Fiori SM, Dos Santos EP, Labudía AC (2019a) First records of metal concentrations in the Pacific oyster (Crassostrea gigas) from a Southwest Atlantic estuary. Environ Geochem Health 41:1321–1338. https://doi.org/10.1007/s10653-018-0217-6
La Colla NS, Botté SE, Marcovecchio JE (2019b) Mercury cycling and bioaccumulation in a changing coastal system: from water to aquatic organisms. Mar Poll Bull 140:40–50. https://doi.org/10.1016/j.marpolbul.2018.12.051
Ladhar-Chaabouni R, Machreki-Ajmi M, Hamza-Chaffai A (2011) Use of metallothioneins as biomarkers for environmental quality assessment in the Gulf of Gabès (Tunisia). Environ Monitor Assess 184(4):2177–2192. https://doi.org/10.1007/s10661-011-2108-5
Legras S, Mouneyrac C, Amiard JC, Amiard-Triquet C, Rainbow PS (2000) Changes in metallothionein concentrations in response to variation in natural factors salinity, sex, weight and metal contamination in crabs from a metal-rich estuary. J Exp Mar BiolEcol 246:259279–259279. https://doi.org/10.1016/S0022-0981(99)00187-2
Lemus M, Salazar R, Lapo B, Chung K (2016) Metalotioneínas en bivalvos marinos. Lat Am J Aquat Res 44(2):202–215. https://doi.org/10.3856/vol44-issue2-fulltext-2
Linde AR, Sánchez-Galán S, Vallés-Mota P, García-Vázquez E (2001) Metallothionein as bioindicator of freshwater metal pollution: European eel and brown trout. Ecotox Environ Safe 49(1):60–63. https://doi.org/10.1006/eesa.2001.2042
Marcovecchio JE, Botté SE, Delucchi F, Arias AH, Fernández Severini MD, De Marco S, Tombesi N, Andrade S, Ferrer LD, Freije RH (2008) Pollution processes in Bahía Blanca Estuarine Environment. In: Neves R, Baretta JW, Mateus M (eds) Perspectives on integrated coastal zone management in South America. IST Press, Lisboa, pp 301–314
Marinho CH, Giarratano E, Gil MN (2018) Metal biomonitoring in a Patagonian salt marsh. Environ Monitor Assess 190(10):598. https://doi.org/10.1007/s10661-018-6975-x
Mohamad F (2008) Uptake of manganese into the exoskeleton of the swimming crab Liocarcinus depurator (L.) in relation to biomonitoring and biosorption. Doctoral Thesis, p 250
Morgan AJ, Stürzenbaum SR, Winters C, Grime GW, Aziz NAA, Kille P (2004) Differential metallothionein expression in earthworm (Lumbricus rubellus) tissues. Ecotox Environ Safe 57(1):11–19. https://doi.org/10.1016/j.ecoenv.2003.08.022
Mouneyrac C, Amiard-Triquet C, Amiard J, Rainbow P (2001) Comparison of metallothionein concentrations and tissue distribution of trace metals in crabs (Pachygrapsus marmoratus) from a metal-rich estuary, in and out of the reproductive season. Comp Biochem Phys C 129(3):193–209. https://doi.org/10.1016/s1532-0456(01)00193-4
Negrin VL, Botté SE, La Colla NS, Marcovecchio JE (2019) Uptake and accumulation of metals in Spartina alterniflora salt marshes from a South American estuary. Sci Tot Environ 649:808–820. https://doi.org/10.1016/j.scitotenv.2018.08.357
Negro CL (2015) Histopathological effects of endosulfan to hepatopancreas, gills and ovary of the freshwater crab Zilchiopsis collastinensis (Decapoda: Trichodactylidae). Ecotox Environ Safe 113:87–94. https://doi.org/10.1016/j.ecoenv.2014.11.025
Negro CL, Collins P (2017) Histopathological effects of chlorpyrifos on the gills, hepatopancreas and gonads of the freshwater crab Zilchiopsis collastinensis. Persistent effects after exposure. Ecotox Environ Safe 140:116–122. https://doi.org/10.1016/j.ecoenv.2017.02.030
Nesto N, Romano S, Moschino V, Mauri M, Da Ros L (2007) Bioaccumulation and biomarker responses of trace metals and micro-organic pollutants in mussels and fish from the Lagoon of Venice, Italy. Mar Pollut Bull 55(10–12):469–484. https://doi.org/10.1016/j.marpolbul.2007.09.009
Pan J, Bournod CN, Pizani N, Cuadrado DG, Carmona NB (2013) Characterization of microbial mats from a siliciclastic tidal flat (Bahía Blanca estuary, Argentina). Geomicrobiol J 30:1–10. https://doi.org/10.1080/01490451.2012.757998
Pedersen SN, Lundebye A-K (1996) Metallothionein and stress protein levels in shore crabs (Carcinus maenas) along a trace metal gradient in the Fal estuary (UK). Mar Environ Res 42(1–4):241–246. https://doi.org/10.1016/0141-1136(96)00077-3
Pedersen KL, Pedersen SN, Højrup P, Andersen JS, Roepstorff P, Knudsen J, Depledge MH (1994) Purification and characterization of a cadmium-induced metallothionein from the shore crab Carcinus maenas (L.). Biochem J 297(3):609–614. https://doi.org/10.1042/bj2970609
Pedersen SN, Pedersen KL, Højrup P, Depledge MH, Knudsen J (1996) Primary structures of decapod crustacean metallothioneins with special emphasis on freshwater and semi-terrestrial species. Biochem J 319(3):999–1003. https://doi.org/10.1042/bj3190999
Pedersen SN, Lundebye A-K, Depledge MH (1997) Field application of metallothionein and stress protein biomarkers in the shore crab (Carcinus maenas) exposed to trace metals. Aquat Toxicol 37(2–3):183–200. https://doi.org/10.1016/s0166-445x(96)00816-8
Perillo GME, Piccolo MC, Parodi E, Freije RH (2001) The Bahia Blanca estuary, Argentina. In: Seeliger U, Kjerfve B (eds) Coastal marine ecosystems of Latin America. Springer, Heidelberg, pp 205–217
Polizzi PS, Romero MB, Chiodi Boudet LN, Das K, Denuncio PE, Rodríguez DH, Gerpe MS (2014) Metallothioneins pattern during ontogeny of coastal dolphin, Pontoporia blainvillei, from Argentina. Mar Pollut Bul 80(1–2):275–281. https://doi.org/10.1016/j.marpolbul.2013.10.037
Pollicelli MP, Idaszkin YL, Gonzalez-José R, Márquez F (2018) Leaf shape variation as a potential biomarker of soil pollution. Ecotox Environ Safe 164:69–74. https://doi.org/10.1016/j.ecoenv.2018.08.003
Pytharopoulou S, Sazakli E, Grintzalis K, Georgiou CD, Leotsinidis M, Kalpaxis DL (2008) Translational responses of Mytilus galloprovincialis to environmental pollution: integrating the responses to oxidative stress and other biomarker responses into a general stress index. Aquat Toxicol 89(1):18–27. https://doi.org/10.1016/j.aquatox.2008.05.013
Rainbow PS (1995) Physiology, physiochemistry and metal uptake—a crustacean perspective. Mar Pollut Bull 31(1–3):55–59
Reichmuth JM, Weis P, Weis JS (2010) Bioaccumulation and depuration of metals in blue crabs (Callinectes sapidus Rathbun) from a contaminated and clean estuary. Environ Pollut 158(2):361–368. https://doi.org/10.1016/j.envpol.2009.09.009
Roesijadi GI (1981) The significance of low molecular weight, metallothionein-like proteins in marine invertebrates: current status. Mar Environ Res 4:167–179
Sabatini SE, Chaufan G, Juárez ÁB, Coalova I, Bianchi L, Eppis MR, Ríos de Molina M del C (2009) Dietary copper effects in the estuarine crab, Neohelice (Chasmagnathus) granulata, maintained at two different salinities. Comp Biochem Phys C 150(4):521–527. https://doi.org/10.1016/j.cbpc.2009.07.006
Santana MS, Yamamoto FY, Sandrini-Neto L, FilipakNeto F, Ortolani-Machado CF, Oliveira Ribeiro CA, Prodocimo MM (2018) Diffuse sources of contamination in freshwater fish: detecting effects through active biomonitoring and multi-biomarker approaches. Ecotox Environ Safe 149:173–181. https://doi.org/10.1016/j.ecoenv.2017.11.0360
Serra-Batiste M, Cols N, Alcaraz LA, Donaire A, González-Duarte P, Vašák M (2010) The metal-binding properties of the blue crab copper specific CuMT-2: a crustacean metallothionein with two cysteine triplets. JBIC J Biol Inorg Chem 15(5):759–776. https://doi.org/10.1007/s00775-010-0644-z
Simonetti P, Botté SE, Fiori SM, Marcovecchio JE (2013) Burrowing crab (Neohelice granulata) as a potential bioindicator of heavy metals in the Bahía Blanca estuary, Argentina. Arch Environ Contam Toxicol 64(1):110–118. https://doi.org/10.1007/s00244-012-9804-1
Simonetti P, Botte SE, Marcovecchio JE (2018) Heavy metal bioconcentration factors in the burrowing crab Neohelice granulata of a temperate ecosystem in South America: Bahía Blanca estuary, Argentina. Environ Sci Pol Res 25(34):34652–34660. https://doi.org/10.1007/s11356-018-3404-1
Spetter CV, Buzzi NS, Fernández EM, Cuadrado DG, Marcovecchio JE (2015) Assessment of the physicochemical conditions sediments in a polluted tidal flat colonized by microbial mats in Bahía Blanca estuary (Argentina). Mar Pollut Bull 9:491–505. https://doi.org/10.1016/j.marpolbul.2014.10.008
Spivak ED (1997) Los crustáceos decápodos del Atlántico sudoccidental (25°-55°S): distribución y ciclos de vida. Investig Mar 25:69–91. https://doi.org/10.4067/s0717-71781997002500006
Spivak ED (2010) The crab Neohelice (=Chasmagnathus) granulata: an emergent animal model from emergent countries. Helgoland Mar Res 64:149–154. https://doi.org/10.1007/s10152-010-0198-z
Truchet DM, Noceti MB, Villagrán DM, Orazi MM, Medrano MC, Buzzi NS (2019a) Fishers’ ecological knowledge about marine pollution: what can FEK contribute to ecological and conservation studies of a Southwestern Atlantic estuary? J Ethnobiol 39(4):584–606. https://doi.org/10.2993/0278-0771-39.4.584
Truchet DM, Buzzi NS, Carcedo MC, Marcovecchio JE (2019b) First record of the fiddler crab Leptuca (=Uca) uruguayensis in the Bahía Blanca estuary (Buenos Aires, Argentina) with comments on its biology in South America. Reg Stud Mar Sci 27:100539. https://doi.org/10.1016/j.rsma.2019.100539
Valavanidis A, Vlahogianni T, Dassenaki M, Scoullos M (2006) Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotox Environ Safe 64(2):178–189. https://doi.org/10.1016/j.ecoenv.2005.03.013
Viarengo A, Palmero S, Zanicchi G, Capelli R, Vaissiere R, Orunesu M (1985) Role ofmetallothioneins in Cu and Cd accumulation and elimination in the gill and digestive gland cells of Mytilus galloprovincialis . Lam Mar Environ Res 16:23–26
Viarengo A, Ponzano E, Dondero F, Fabbri R (1997) A simple spectrophotometric method for metallothionein evaluation in marine organisms: an application to Mediterranean and Antarctic molluscs. Mar Environ Res 44(1):69–84. https://doi.org/10.1016/s0141-1136(96)00103-1
Vilches FO, Bobinac MA, Labudía AC, Paso Viola MN, Marcovecchio JE, Cappozzo HL, Panebianco MV (2019) Metals concentration and bioaccumulation in the marine-coastal trophic web from Buenos Aires province southern coast. Argentina Chem Ecol 35:1–23. https://doi.org/10.1080/02757540.2019.1604693
Villagrán DM, Fernández Severini MD, Biancalana F, Spetter CV, Fernández EM, Marcovecchio JE (2019a) Bioaccumulation of heavy metals in mesozooplankton from a human-impacted south western Atlantic estuary (Argentina). J Mar Res 77:217–241. https://doi.org/10.1357/002224019826887362
Villagrán DM, Truchet DM, Buzzi NS, Forero AD, Fernández Severini MD (2019b) A baseline study of microplastics in the burrowing crab (Neohelice granulata) from a template Southwestern Atlantic estuary. Mar Pollut Bull 150:110686. https://doi.org/10.1016/j.marpolbul.2019.110686
Vogt G (2002) Functional anatomy. In: Holdich DM (ed) Biology of freshwater crayfish. Blackwell, Oxford, pp 53–151
Wallace WG, Lee B-G, Luoma SN (2003) Subcellular compartmentalization of Cd and Zn in two bivalves. I. Significance of metal-sensitive fractions (MSF) and biologically detoxified metal (BDM). Mar Ecol Prog Ser 249:184–197
Wang L, Yan B, Liu N, Li Y, Wang Q (2008) Effects of cadmium on glutathione synthesis in hepatopancreas of freshwater crab, Sinopotamon yangtsekiense. Chemosphere 74(1):51–56. https://doi.org/10.1016/j.chemosphere.2008.09.025
Wang W-C, Mao H, Ma D-D, Yang W-X (2014) Characteristics, functions, and applications of metallothionein in aquatic vertebrates. Front Mar Sci 1. https://doi.org/10.3389/fmars.2014.00034
Weinstein JE, West T, Bray J (1992) Shell disease and metal content of blue crabs, Callinectes sapidus, from the Albemarle-Pamlico Estuarine System, North Carolina. Arch Environ Contam Toxicol 23(3):355–362
Won E-J, Raisuddin S, Shin K-H (2008) Evaluation of induction of metallothionein-like proteins (MTLPs) in the polychaetes for biomonitoring of heavy metal pollution in marine sediments. Mar Pollut Bull 57(6–12):544–551. https://doi.org/10.1016/j.marpolbul.2008.02.025
Wu J-P, Chen H-C, Huang D-J (2008) Histopathological and biochemical evidence of hepatopancreatic toxicity caused by cadmium and zinc in the white shrimp, Litopenaeus vannamei. Chemosphere 73(7):1019–1026. https://doi.org/10.1016/j.chemosphere.2008.08.019
Acknowledgments
The authors would like to thank Lic. F. García for his help during field sampling and the ICP-OES analysis, and all the staff of Área de Oceanografía Química (IADO, CONICET-UNS); Dr. W. Melo (GIS specialist) for the map, and to M.Sc. R Scoffield and Prof. R. Hilson Foot (English native speakers) for revisiting the language; and also the reviewer of this manuscript for her/his invaluable and critical suggestions that enhanced the value of this study. This research is part of the doctoral thesis of Lic. D. M. Truchet granted by Consejo Nacional de Investigaciones Científico Tecnológicas (CONICET) and a part of a funding granted to Dr. P. Simonetti (ANPCyT, PICT 2015-2076) by the Agencia Nacional de Promoción Científica y Tecnológica.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Truchet, D.M., Buzzi, N.S., Simonetti, P. et al. Uptake and detoxification of trace metals in estuarine crabs: insights into the role of metallothioneins. Environ Sci Pollut Res 27, 31905–31917 (2020). https://doi.org/10.1007/s11356-020-09335-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09335-6