Abstract
Bioindicators can be used to determine spatial and/or temporal variations of heavy metals in a certain coastal or marine environments. This study investigated the potential use of the burrowing crab Neohelice granulata from two different locations in the Bahía Blanca estuary, a moderately polluted ecosystem. Concentrations of zinc (Zn), nickel (Ni), and lead (Pb) in soft tissues of male and female crabs were measured. In addition, concentrations of the three metals in eggs were compared with concentrations in female crabs. No geographical differences were found for any of the three metals, whereas sexual and seasonal differences were obtained for Zn and Ni, with the winter season posing lower concentrations. Moreover, the three metals were detectable in eggs and were lower than concentrations in female crabs (except for Zn). Finally, the usefulness of this species as a potential bioindicator of heavy-metal pollution within this estuarine ecosystem is discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Marine ecosystems are subjected to an increasing pollution due to anthropogenic activities. Both the economic and demographic development of the coastal regions (i.e., increase of urban, industrial, and port settlements) generates an increased load of wastes that are discharged into the water, contaminating the aquatic environments with several organic and inorganic pollutants, such as pesticides, polychlorinated biphenyls, and heavy metals (Förstner and Wittmann 1983).
Three possible ways to perform the determination of heavy metal levels in this kind of ecosystems have been identified: (1) water and (2) sediment are the environmental matrixes where chemical analysis is the most direct approach to show the heavy-metal pollution status in the environment. Nevertheless, this makes it difficult to assess the influence and possible toxicity of such pollution on the organisms and ecosystems (Zhou et al. 2008); and (3) the use of biological matrixes. Thus, the determination of heavy-metal concentration in marine organisms is a better way to assess levels of contamination in the environment (Bryan et al. 1985; Phillips 1980; Rainbow 1995a; Zhou et al. 2008). In marine invertebrates, heavy metals tend to accumulate in tissues and organs of these organisms at higher levels than in the surrounding environment, although they can vary greatly among taxa (Rainbow 2002). According to Markert (2007), a bioindicator is an organism (or part of an organism or a community of organisms) that contains information on the quality of the environment (or a part of the environment). Thus, their use provides a better tool to establish geographical, spatial, and/or temporal variations in the bioavailable concentrations of toxic trace metals in marine environments.
When considering marine invertebrates as possible bioindicators, decapods crustaceans are generally excluded due to their ability to metabolically regulate essential metals until certain threshold environmental levels, [i.e., copper (Cu), zinc (Zn), and manganese (Mn)] (Bryan et al. 1985; Rainbow 1995b, 2002; MacFarlane et al. 2000; McPherson and Brown 2001). Nevertheless, when nonessential metals, such as lead (Pb) or cadmium (Cd), are considered, bioaccumulation occurs, thus reflecting levels in the environment (MacFarlane et al. 2000).
Neohelice granulata (Brachyura, Varunidae) is a semiterrestrial estuarine crab widely distributed along the Atlantic coast of South America from southern Brazil (23°S) to the northern Argentinean Patagonia (41°S) (Spivak et al. 1994). These benthic organisms inhabit the intertidal zone in tidal flats as well as salt marsh areas vegetated by Spartina densiflora, S. alterniflora, and Sarcocornia perennis (Spivak et al. 1994; Escapa et al. 2008). Crabs are herbivorous–detritivorous when associated with salt marshes and deposit feeders when living in mud flats (Iribarne et al. 1997). This species breeds from the middle of spring to the beginning of autumn (Ituarte et al. 2004). N. granulata is one of the most abundant macroinvertebrates inhabiting these estuarine environments (Boschi 1964; Spivak 1997) and represents an important link in the trophic web considering that all stages in the crab’s life cycle make them a relevant food component for other species, such as fishes, shellfishes, and birds. For this reason, and considering that N. granulata is usually a key species within these ecosystems, they could play a major role in the transference of pollutants to higher trophic levels.
A large population of N. granulata inhabits the Bahia Blanca Estuary in Argentina. This ecosystem is an excellent feeding and breeding site for several species, including shorebirds (Delhey et al. 2001; Petracci 2002). The estuary is under constant and increasing anthropogenic pressure because several urban settlements (350,000 habitants), including industrial developments (petrochemical complex, industrial park, oil refinery) and harbors, produce an impact on the environment through the discharge of sewages to the estuary without sufficient pretreatment and purification (Andrade et al. 2000; Ferrer et al. 2000; Tombesi et al. 2000). Agricultural activities also have an impact on the environment (Perillo et al. 2001; Botté et al. 2007).
Previous studies show low to medium heavy-metal concentrations in sediments, water, and particulate matter in the estuary (Andrade et al. 2000; Marcovecchio and Ferrer 2005; Botté et al. 2007, 2010; Fernández Severini et al. 2009). Several trace metals were also found in certain organisms from the estuary (e.g., fishes, crustaceans, diatoms, and halophytes) (Marcovecchio 1994; Marcovecchio et al. 1988a, b, 1996; Ferrer et al. 2000; Botté 2005; Fernández Severini et al. 2009). This indicates that the transference of heavy metals occurs between abiotic and biotic compartments.
The objective of this work was focused on determining concentrations of Zn, nickel (Ni), and lead (Pb) in soft tissues of the burrowing crab N. granulata, which inhabits the intertidal areas of the Bahía Blanca estuary. Differences between sexes, sites, and seasonality were analyzed for these heavy metals. The presence of these metals was measured in crab eggs and compared with levels in females crab soft tissue. Finally, all of the information obtained for N. granulata within the Bahía Blanca estuary was integrally considered to assess its ability as a potential pollution bioindicator for this environment.
Materials and Methods
Study Area and Sampling
The Bahía Blanca estuary is located on the Southwest coast of the Buenos Aires Province, Argentina (38°45′ to 39° 40′S and 61°45′ to 62°30′W). It is a mesotidal coastal plain estuary extending over 2,300 km2 and formed by several tidal channels, extensive tidal flats with patches of low salt marshes, and islands (Piccolo et al. 2008).
From the head to the mouth of the estuary, several physicochemical characteristics (e.g., water salinity, water temperature, sediment composition, organic matter content, and pH) vary (Freije and Marcovecchio 2004; Freije et al. 2008; Piccolo et al. 2008). This is important because the heavy-metal content in sediment and water in estuaries is related to these characteristics (Förstner and Wittmann 1983; Salomon and Förstner 1984). Moreover, these extrinsic factors may influence metal concentrations in crustaceans (Swaileh and Adelung 1995; Turoczy et al. 2001).
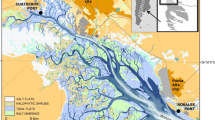
Two sites were selected according to their position within the Bahía Blanca estuary (Fig. 1). Cuatreros Port (CP) is located in the inner part of the estuary and is close to the town of General Cerri. This area is influenced by the Sauce Chico River and the Saladillo de Garcia stream discharges, which drain large extensions of agricultural fields (Perillo et al. 2001; Limbozzi and Leitao 2008). This site is characterized by a water salinity range of 20.8–31.9 psu (practical salinity unit), a water temperature range of 8.5–23.3 °C, and a sediment composition of mainly silt–clay (96.3 %) (Botté 2005).
The other site is Villa del Mar (VM), a small village located near Punta Alta City. It is located in the middle-outer part of the estuary and is further away from the industrial area. This site has a water salinity range of 30.88–36.57 psu, a water temperature water range of 6.2–22.6 °C (Negrin 2010), and a sediment composition of a mixture of mud and sand (87 and 13 %, respectively) (Pratolongo et al. 2010).
Crabs were collected bimonthly from both sites from October 2008 to August 2009. Specimens of both sexes were hand-picked during low tide at the crab beds on mud flats with no vegetation. At the laboratory, they were washed with distillated water and preserved at −20 °C in the freezer until analysis. Mature crabs with maximum carapace width (20–30 mm for female and 25–35 mm for male crabs) were selected. When available, both ovigerous and nonovigerous female crabs were treated separately. Ovigerous female crabs were only present during warmer seasons, such as spring and summer.
Laboratory Procedures
Crabs were dissected to separate soft tissue from carapace; soft tissues were pooled from five or six individuals according to sex, site, and season (n = two to five pooled samples for each case) and were placed in cleaned Petri dishes in an oven at 50 °C until constant weight was achieved. Dried samples were homogenized by crushing in a porcelain mortar. All equipment used for dissection and drying of samples was previously cleaned with diluted nitric acid (0.7 % v/v) to prevent contamination.
Eggs were removed from the female crab’s pleon and cleaned with distilled water. Egg masses were dried until constant weight and pooled according to sampling site. Pooled samples of each site (from three to four crabs) were homogenized. After drying, egg masses were ground in a porcelain mortar and prepared for extraction.
Acid digestion of crab tissue and eggs samples was performed according to the methodology described by Marcovecchio and Ferrer (2005). Subsamples between 250 and 500 mg were mineralized with 3 ml concentrated nitric acid and 1 ml concentrated perchloric acid and placed in a heated glycerin bath at a temperature of 120 ± 10 °C. After acid digestion, the residue was transferred to centrifuge tubes and completed with diluted nitric acid (0.7 %) up to 10 ml. Samples digestion was replicated to ensure the reproducibility of the results.
Heavy-metal concentrations (Zn, Ni, and Pb) were determined by atomic absorption spectroscopy with air-acetylene flame using a Perkin-Elmer AA-2380. All concentrations are expressed in parts per million (μg g−1) on a dry weight (dw) basis. The method detection limit (MDL) was experimentally calculated as the SD of 12 blank replicates. The MDLs, expressed in μg g−1, were as follows: Zn 0.88, Ni 1.54, and Pb 0.29. The %RSD (percentage of relative SD) of the replicate samples were between 10 and 20 %. For both the analytical quality control and reagent blanks, as well as calibration-curve build-up, certified reference materials [CRMs (mussel tissue flour, reference material no. 6, provided by the National Institute for Environmental Studies, Tsukuba, Japan)] and analytical-grade reagents (Merck or Baker) were used. The recovery percentages for the three metals in CRM were >90 %. For samples that were lower than the applied analytical MDL, a value of one half the detection limit was assigned, and the sample was included in the data set for statistical treatment (Jones and Clarke 2005).
Data Analysis
A statistical package was selected: Infostat 5.1 for Windows (Grupo Infostat Professional, FCA, Universidad Nacional de Córdoba, Argentina). For Zn and Ni, three-way analysis of variance (ANOVA) was performed to analyze possible interactions among sex, location, and season. Fisher’s least significant difference test was used for multiple comparisons. For Pb, no statistical analysis was performed because most of the values were lower than the detection limit.
Results
Mean seasonal concentrations of Zn and Ni in soft tissues of burrowing male and female crabs N. granulata from the two study sites are presented in Fig. 2. For both metals, concentrations in VM and CP showed no significant differences throughout the year (three-way ANOVA, p = 0.14 for Zn and p = 0.06 for Ni).
Mean concentrations of Zn and Ni showed differences not only between sexes but also between seasons (Fig. 2). In the case of Zn, mean concentrations in male crabs were greater than in female crabs only during summer (37.09 ± 0.49 vs. 34.74 ± 0.66 μg g−1 dw; VM and CP averaged). These results were consistent with the statistical analysis. First, a significant interaction between sex and season was found (three-way ANOVA, p = 0.006). Moreover, significant differences were found for sex (female crabs had significantly greater levels than male crabs; three-way ANOVA, p = 0.013) and season (crabs during winter and spring had significantly lower levels than crabs during summer and autumn; three-way ANOVA, p = 0.0002). In contrast, mean concentrations of Ni were greater in female than in male crabs during all seasons except for winter, in which concentrations were similar for both sexes [9.532 ± 0.006 μg g−1 dw for female vs. 9.55 ± 0.02 μg g−1 dw for male crabs (VM and CP averaged)]. As it was for Zn statistical analysis for Ni showed a significant interaction between sex and season (three-way ANOVA, p = 0.004); moreover, significant differences between sex (male significantly greater than female crabs; three-way ANOVA, p < 0.0001) and seasons (crabs in winter had significantly lower levels than crabs during the remaining seasons; three-way ANOVA, p < 0.0001) were found. Pb was lower than the detection limit for most samples except for female crabs from VM and CP during the spring (3.18 ± 2.98 and 4.66 ± 0.85 μg g−1 dw, respectively). For this reason, statistical analysis of this metal was not performed.
Ovigerous female crabs were only found during spring (October 2008 in VM and CP) and summer (December 2008 in VM and CP). As a consequence, only one pooled egg sample was obtained for VM and CP crabs during each season. For this reason, statistical analysis could not be performed to test differences between sites and season.
Concentrations of Ni and Pb in eggs were lower than minimum concentrations found in female crabs in all cases except for Pb in eggs from VM (Table 1). For Zn, the results were quite different. Considering both sites and both seasons, concentrations in eggs were greater than maximum concentrations found in female crabs (Table 1). The mean difference between the maximum concentration of Zn in female crabs and the concentration in eggs was 12.32 ± 6.74 μg g−1 dw in VM and 18.01 ± 0.64 μg g−1 dw in CP.
Discussion
The use of marine organisms for assessment and monitoring of marine pollution is growing worldwide, and the selection of biomonitors and bioindicators frequently includes benthic invertebrates and seaweeds (e.g., Phillips 1977 and Rainbow 1988; Rainbow and Phillips 1993; Rainbow 1995a; Villares et al. 2002; Zauke et al. 2003; Zhou et al. 2008). This is related to the capability of these organisms to take up and accumulate several contaminants, thus reflecting the status of the pollution of a certain environment (Rainbow 1995a, 2007; Chen et al. 2005). According to Rainbow (1995a) a biomonitor should be sedentary, easy to identify, abundant, long-lived, available for sampling year round, and be widely distributed. They should also be relatively tolerant to environmental stressors, be net accumulators of the pollutants, and provide enough tissue for analysis. In this study, the burrowing crab N. granulata was selected as a potential bioindicator of the Bahía Blanca estuary. It is noteworthy that this species possesses almost all of the above-mentioned characteristics, which suggests the potential use of this crab as a bioindicator in this estuarine system.
Difference between sexes was the first factor considered in this study. Sex is a biological intrinsic factor that may influence the concentration of heavy metals in crabs (Devescovi and Lucu 1995; Swaileh and Adelung 1995; MacFarlane et al. 2000; Turoczy et al. 2001; Barrento et al. 2009). The obtained results showed significant differences between sexes for Zn and Ni. Concentrations of Ni were greater in male than in female crabs, and concentrations of Zn in female crabs were greater than in male crabs. Beltrame (2008), working with both sexes of N. granulata from Mar Chiquita Lagoon, Argentina, obtained no significant differences between sexes for Ni. Nevertheless, levels on Zn in female crabs were significantly greater than levels in male crabs, which coincides with the results of the present study. Beltrame et al. (2011) suggested that the difference found in levels of Zn between sexes could be associated with differences in the uptake and elimination rates of male versus female crabs or with a difference in the demand of this essential metal between both sexes. Sex differences in trace-metal accumulation are not commonly found in decapods crustaceans (White and Rainbow 1987; Kannan et al. 1995; Swaileh and Adelung 1995; Turoczy et al. 2001). Nevertheless, there are some cases in which a difference between sexes was found (Jeckel et al. 1996; Sastre et al. 1999). In a previous study, Simonetti et al. (2012) found no significant differences between sexes for Cu and Cd in soft tissues of N. granulata from the Bahía Blanca estuary. Regarding the results of the current study, the observed sexual variation of metal concentrations indicates that sampling of female and male crabs would be a likely alternative to develop Cd- and Cu-biomonitoring programs. Meanwhile, samples of both sexes should be included in Zn and Ni studies.
According to Depledge and Fossi (1994), an effective biological indicator must reflect levels of environmental contamination, and the relationship should remain constant both spatially and temporally. For that reason, these two extrinsic factors were also considered in the current study. No significant differences between CP and VM were found for Zn and Ni. This reflects the potential usefulness of using this species as a bioindicator of this environment. Nevertheless, the varying seasonal metal concentration needs further attention. For Ni, concentrations in crabs during winter were significantly lower than in those during the remaining seasons. In contrast, Zn concentrations were significantly greater in crabs during summer and autumn than in crabs during spring and winter. Although concentrations of Pb were lower than the detection limit in most crab samples, spring was the only season for crabs with detectable values. To obtain decreased metal levels in crabs during winter is usually expected. This may be related to varying seasonal growth rates, reproductive cycles, phytoplankton productivity, and salinity and temperature of the water (Swaileh and Adelung 1995; Beltrame et al. 2008). A decrease in winter Cu levels in Carcinus mediterraneus was described by Devescovi and Lucu (1995), and they attributed this decrease to metabolic reactions induced by nutritional status. Swaileh and Adelung (1995), working with the cumacean crustacean Diastylis rathkei, found the lowest Cu, Zn, Cd, and Pb concentrations in crabs during the winter months (August and December), suggesting that increased metal concentrations in crabs during summer months corresponds to the main growth period of this species. Beltrame et al. (2009) described a seasonal variation for Cu, Cd, Cr, and Mn in N. granulata from Mar Chiquita Lagoon, Argentina, with crabs in spring and summer having the highest concentrations. These are the seasons with the most biological activities compared with the winter months when the crabs spend most of their time deeply burrowed in the mud (Ituarte et al. 2004). Finally, Simonetti et al. (2012) also found a significant decrease in levels of Cu and Cd in N. granulata from the Bahía Blanca estuary during winter. It is worth noting that Botté et al. (2010) found that concentrations of several metals in tidal flat sediments from the Bahía Blanca estuary were not associated with season. This supports the idea that seasonal variations in metal concentrations in N. granulata may be more related to internal than external conditions.
However, seasonal variation among the concentrations of the three analyzed metals in crab samples could be a constraint when a species is selected as a bioindicator. Nevertheless, a possible solution may be to take all of the samples at the same time of year in further biomonitoring programs as suggested by Fialkowski et al. (2003).
One more consideration is necessary: the distinction between essential and nonessential metals. Zn is an essential metal for decapod crustaceans, being a key component of many enzymes, including carbonic anhydrase (Rainbow 1997, 2007). Several studies have shown that these metals do not accumulate but are regulated until a threshold exposure level beyond which they are accumulated proportionally to its external levels (White and Rainbow 1982, 1985; Rainbow and White 1989; MacFarlane et al. 2000; Rainbow 2002). For N. granulate, the threshold level for Zn is still unknown. Although metals in superficial sediments were not measured in this research, a previous study reported sediments containing a mean Zn concentration of 33 ± 7 μg g−1 dw (Hempel et al. 2008). A subsequent study reported a range of Zn concentrations from 24.22 to 82.01 μg g−1 dw (Botté et al. 2009). Considering the range obtained in crab samples in this research (30.34–38.47 μg g−1 dw), these results suggest that N. granulata in the Bahía Blanca estuary may regulate (and not bioaccumulate) Zn concentrations within a certain range according to metabolic requirements. MacFarlane et al. (2000) also described Zn regulation in the semaphore crab Heloecius cordiformis, in which levels in hepatopancreas tissue were maintained at approximately one third to one half that of sediment concentrations. Therefore, N. granulata may not be an appropriate candidate for a Zn-pollution bioindicator.
In contrast, Ni and Pb are nonessential metals; therefore, they are not regulated by decapod crustaceans, and accumulation can occur according to external exposure (Rainbow 2002; Phillips and Rainbow 1993; MacFarlane et al. 2000). Botté et al. (2009) reported a range of Ni concentrations from 5.30 to 17.39 μg g−1 dw and a range of Pb concentrations from 6.28 to 30.28 μg g−1 dw in sediments from the Bahía Blanca estuary. Ni concentrations obtained in crab samples in this study ranged from 9.53 to 17.68 μg g−1 dw; therefore, N. granulata may bioaccumulate Ni according to levels available in the environment. These results suggest that this species seems to be a good bioindicator for Ni pollution.
Although Pb concentrations were detectable in sediments from the Bahía Blanca estuary, in most of the crab samples the concentrations were lower than the detection limit (except for spring). Similar results were found in N. granulata from CP, where Pb concentrations in soft tissues were, in all cases, lower than the detection limit (Ferrer 2001). When Marcovecchio and Ferrer (2005) performed Pb geochemical fractioning within the finest fraction (<63 mm) of Bahía Blanca estuarine sediments, they found a range between 4.5 and 15 % of the potentially bioavailable fraction (PBF), indicating the potential level of metal assimilation by organisms in the Bahía Blanca estuary. Considering the low percentages of PBF for Pb that were previously mentioned, as well as the facility of Pb to complex with organic matter in estuarine environments (Town and Filella 2002; Shank et al. 2004; Louis et al. 2009)—added to the high charge of organic matter in the Bahía Blanca estuary (Marcovecchio and Freije 2004; Marcovecchio et al. 2009)—together these could explain why concentrations of this nonessential metal in N. granulata are in most cases lower than the detection limit. Further investigation on this subject must be conducted to clearly understand the behavior of this species and their relationship with Pb. Nevertheless, the presence of Pb in crabs, at least during spring, may indicate bioaccumulation from exposure to the environment, which suggests that this species could also be used as a bioindicator of this metal. Simonetti et al. (2012) found a positive bioaccumulation of Cd (a nonessential metal) in N. granulata, suggesting the bioavailability of this metal in the Bahía Blanca estuary. This result reinforces the potential use of this species as a bioindicator of metal pollution of this environment.
Finally, Ni, Zn and Pb were also measured in eggs from both locations and compared with levels in corresponding female crabs. Although concentrations of Ni and Pb in eggs were in all cases lower than the concentrations in female crabs from both sites, the concentrations of Zn were quite different: Eggs always had greater concentrations of Zn relative to female crab soft tissue. Beltrame et al. (2009) found similar results for N. granulata from Mar Chiquita Lagoon, Argentina. Although levels of Ni and Pb were greater in the female crab soft tissue compared with eggs, concentrations of Zn were greater in eggs than in female crabs. The results of the present work are of great importance considering that it appears to be indicate bioaccumulation in this species before hatch.
Therefore, concentrations of these metals in eggs may be associated with the direct transference of these metals by the mother as well as by external exposure because incubation of the eggs is external; thus, eggs come into direct contact with sediment and water when they are laid (Ituarte et al. 2004).
Considering the results of this study, N. granulata (adults and eggs) seem to be an adequate species to be used as a bioindicator of heavy-metal pollution in the Bahía Blanca estuary. This could be a useful tool for future environmental monitoring programs and to evaluate the evolution of heavy-metal pollution within this environment.
Conclusion
A good bioindicator accumulates contaminants from the environment and accurately reflects environmental levels. Considering that previous studies have shown that the Bahía Blanca estuary is a moderately polluted ecosystem, an efficient tool is required to monitor the changing pollutant concentrations over time within this system.
In this study, three heavy metals (Zn, Ni, and Pb) were measured in the crab N. granulata. According to the results, there were found significant differences between sexes regarding Zn and Ni accumulation. For this reason, it would be necessary to treat them separately in further analysis. In contrast, no significant differences between locations were obtained regarding levels of heavy metals in crabs. However, a seasonal variation in Zn and Ni levels was found; moreover, Pb was detected in crabs only during spring. For this reason, seasonality should be considered in further biomonitoring programs. The comparison of Zn concentrations in N. granulata with levels in the environment suggests that this species may regulate Zn according to metabolic requirements. Nevertheless, this species may bioaccumulate Ni and Pb according to levels in the environment. Finally, the presence of heavy metals found in eggs is of great importance considering that crab eggs are eaten by several species, such as shorebirds, seabirds, and fishes.
Based on the results of this study, it can be concluded that N. granulata is indeed an appropriate candidate for bioindication of Ni and Pb pollution in the Bahía Blanca estuary. Moreover, the knowledge of metal accumulation has an additional importance considering that this is a key species within the Bahía Blanca estuary by playing a major role in the transference of pollutants to greater trophic-level species, such as seabirds and shorebirds, including Olrog’s gull, Larus atlanticus, which is considered to be a vulnerable species (IUCN 2011).
References
Andrade JS, Pucci AE, Marcovecchio JE (2000) Cadmium concentrations in the Bahía Blanca estuary, (Argentina). Potential effects of dissolved cadmium on the diatom Thalassiosira curviseriata. Oceanologia 42(4):505–520
Barrento S, Marques A, Teixeira B, Carvalho ML, Vaz- Pires P, Nunes ML (2009) Accumulation of elements (S, As, Br, Sr, Cd, Hg, Pb) in two populations of Cancer pagurus: ecological implications to human consumption. Food Chem Toxicol 47:150–156
Beltrame MO (2008) Dinámica biogeoquímica de nutrientes y metales pesados en ambientes intermareales de la laguna costera de Mar Chiquita: Potenciales efectos ecotoxicológicos sobre especies claves del ecosistema. Doctoral thesis, Universidad Nacional del Sur, Argentina
Beltrame MO, Marco SG, Marcovecchio JE (2008) Cadmium and zinc in Mar Chiquita Coastal Lagoon (Argentina): salinity effects on lethal toxicity in juveniles of the burrowing crab Chasmagnathus granulatus. Arch Environ Contam Toxicol 55(1):78–85
Beltrame MO, De Marco SG, Marcovecchio JE (2009) Influences of sex, habitat, and seasonality on heavy-metal concentrations in the burrowing crab (Neohelice Granulata) from a coastal lagoon in Argentina. Arch Environ Contam Toxicol 58(3):746–756
Beltrame MO, De Marco SG, Marcovecchio JE (2011) The burrowing crab Neohelice granulata as potential bioindicator of heavy metals in estuarine systems of the Atlantic Coast of Argentina. Environ Monit Assess 172(1–4):379–389
Boschi EE (1964) Los crustáceos decápodos Brachyura del litoral bonaerense (R. A.). Bol Inst Biol Mar 6:1–75
Botté SE (2005) El rol de la vegetación en el ciclo biogeoquımico de los metales pesados en humedales del estuario de Bahía Blanca. Doctoral thesis, Universidad Nacional del Sur, Argentina
Botté SE, Freije RH, Marcovecchio JE (2007) Dissolved heavy metal (Cd, Pb, Cr, Ni) concentrations in surface water and porewater from Bahía Blanca Estuary Tidal Flats. Bull Environ Contam Toxicol 79:415–421
Botté SE, Arlenghi J, Del Blanco L, Asteasuain AA, Marcovecchio JE (2009) Variación entre pleamar y bajamar en la concentración de metales en el sedimento de planicie del estuario de Bahía Blanca. Primera Reunión Argentina de Geoquímica de la Superficie (IRAGSU). Córdoba, Argentina
Botté SE, Freije RH, Marcovecchio JE (2010) Distribution of several heavy metals in tidal flats sediments within Bahía Blanca Estuary (Argentina). Water Air Soil Pollut 210:371–388
Bryan GW, Langston WJ, Hummerstone LG, Burr GR (1985) A guide to the assessment of heavy metal contamination in estuaries using biological indicators. Mar Biol Assoc UK, Occasional Publication No. 4, pp 1–92
Chen M-H, Chen C-Y, Chou H-Y, Wen T-C (2005) Gender and size effects of metal bioaccumulation on the rock crab, Thalamita crenata, in Dapeng Bay, southwestern Taiwan. Mar Pollut Bull 50:463–484
Delhey JKV, Carrete M, Martinez MM (2001) Diet and feeding behavior of Olrog’s gull Larus atlanticus in Bahía Blanca. Argentina Ardea 89(2):319–329
Depledge MH, Fossi MC (1994) The role of biomarkers in environmental assessment. 2. Invertebrates. Ecotoxicology 3:161–172
Devescovi M, Lucu C (1995) Seasonal changes of the copper level in shore crabs Carcinus mediterraneus. Mar Ecol Prog Ser 120:169–174
Escapa M, Perillo GME, Iribarne O (2008) Sediment dynamics modulated by burrowing crab activities in contrasting SW Atlantic intertidal habitats. Estuar Coast Shelf Sci 80:365–373
Fernández Severini MD, Botté SE, Hoffmeyer M, Marcovecchio JE (2009) Spatial and temporal distribution of cadmium and copper in water and zooplankton in the Bahía Blanca Estuary, Argentina. Estuar Coast Shelf Sci 85:57–66
Ferrer LD (2001) Estudio de los diversos metales pesados en sedimentos del estuario de Bahía Blanca y sus efectos tóxicos sobre el cangrejo Chasmagnathus granulata. Doctoral thesis, Universidad Nacional del Sur, Argentina
Ferrer LD, Contardi ET, Andrade JS, Asteasuain RO, Pucci AE, Marcovecchio JE (2000) Environmental cadmium and lead concentrations in the Bahía Blanca estuary (Argentina). Potential toxic effects of Cd and Pb on crab larvae. Oceanologia 42(4):493–504
Fialkowski W, Rainbow PS, Smith BD, Zmudzinski L (2003) Seasonal variation in trace metal concentrations in three talitrid amphipods from the Gulf of Gdansk, Poland. J Exp Mar Biol Ecol 288:81–93
Förstner U, Wittmann GTW (1983) Metal pollution in the aquatic environment. Springer, Berlin
Freije RH, Marcovecchio JE (2004) Oceanografía química. In: Piccolo MC, Hoffmeyer M (eds) Ecosistema del Estuario de Bahía Blanca. Instituto Argentino de Oceanografía (IADO-CONICET), Bahía Blanca, pp 69–78
Freije RH, Spetter CV, Marcovecchio JE, Popovich CA, Botté SE, Negrín V et al (2008) Water chemistry and nutrients of the Bahía Blanca Estuary. In: Neves R, Baretta JW, Mateus M (eds) Perspectives on integrated coastal zone management in South America. IST Press, Lisbon, pp 241–254
Hempel M, Botté SE, Negrin VL, Chiarello MN, Marcovecchio JE (2008) The role of the smooth cordgrass Spartina alterniflora and associated sediments in the heavy metal biogeochemical cycle within Bahía Blanca estuary salt marshes. J Soils Sed 8:289–297
Iribarne O, Bortolus A, Botto F (1997) Between-habitat differences in burrow characteristics and trophic modes in the southwestern Atlantic burrowing crab Chasmagnathus granulata. Mar Ecol Prog Ser 155:132–145
Ituarte R, Spivak E, Luppi T (2004) Female reproductive cycle of the Southwestern Atlantic estuarine crab Chasmagnathus granulatus (Brachyura: Grapsoidea: Varunidae). Sci Mar 68:127–137
Jeckel WH, Roth RR, Ricci L (1996) Patterns of trace-metal distribution in tissues of Pleoticus muelleri (Crustacea: Decapoda: Solenoceridae). Mar Biol 125:297–306
Jones RP, Clarke JU (2005) Analytical chemistry detection limits and the evaluation of dredged sediment. ERDC/TN EEDP-04–36. United States Army Engineer Research and Development Center, Vicksburg
Kannan K, Yasunaga Y, Iwata H, Ichihashi H, Tanabe S, Tatsukawa R (1995) Concentrations of heavy metals, organochlorines, and organotins in Horseshoe Crab, Tachypleus tridentatus, from Japanese coastal waters. Arch Environ Contam Toxicol 28:40–47
Limbozzi F, Leitao TE (2008) Characterization of Bahia Blanca main existing pressures and their effects on the state indicators for surface and groundwater quality. In: Neves R, Baretta JW, Mateus M (eds) Perspectives on integrated coastal zone management in South America. IST Press, Lisbon, pp 315–331
Louis Y, Garnier C, Lenoble V, Omanović D, Mounier S, Pizěta I (2009) Characterisation and modelling of marine dissolved organic matter interactions with major and trace cations. Mar Environ Res 67:100–107
MacFarlane GR, Booth DJ, Brown KR (2000) The semaphore crab, Heloecius cordiformis: Bio-indication potential for heavy metals in estuarine systems. Aquat Toxicol 50:153–166
Marcovecchio JE (1994) Trace metals residues in several tissues of two crustacean species from the Bahía Blanca estuary in Argentina. Environ Monit Assess 29:65–73
Marcovecchio JE, Ferrer L (2005) Distribution and geochemical partitioning of heavy metals in sediments of the Bahía Blanca Estuary, Argentina. J Coastal Res 21:826–834
Marcovecchio JE, Freije RH (2004) Efectos de la intervención antrópica sobre sistemas marinos costeros: El estuario de Bahía Blanca. Anales de la Academia Nacional de Ciencias Exactas, Físicas y Naturales (ANCEFN). Argentina 56:115–132
Marcovecchio JE, Moreno VJ, Perez A (1988a) Determination of heavy metal concentrations in biota of Bahia Blanca, Argentina. Sci Total Environ 75:181–190
Marcovecchio JE, Moreno VJ, Perez A (1988b) The sole, Paralichthys sp., as an indicator species of heavy metal pollution in the Bahía Blanca estuary trophic web. In: Seeliger U, Lacerda LD, Patchineelam SR (eds) Metals in coastal environments of Latin America. Springer, Heidelberg, pp 122–129
Marcovecchio JE, Moreno VJ, Perez A (1996) Bio-magnification of total mercury in Bahía Blanca estuary shark. Mar Pollut Bull 17:276–278
Marcovecchio JE, Spetter CV, Botté SE, Delucchi F, Arias AH, Fernández Severini MD et al (2009) Inorganic nutrients and organic matter tidal time-scale variation in a mesotidal estuary: Bahía Blanca, Argentina. J Chem Ecol 25(6):453–465
Markert B (2007) Definitions and principles for bioindication and biomonitoring of trace metals in the environment. J Trace Elem Med Biol 21(Suppl 1):77–82
McPherson R, Brown K (2001) The bioaccumulation of cadmium by the Blue Swimmer Crab Portunus pelagicus L. Sci Total Environ 279:223–230
Negrin VL (2010) El rol de las marismas del estuario de Bahía Blanca en el ciclo bio-geoquímico de nutrientes inorgánicos y de materia orgánica. Doctoral thesis, Universidad Nacional del Sur, Bahía Blanca, Argentina
Perillo GME, Piccolo MC, Parodi E, Freije RH (2001) The Bahía Blanca estuary, Argentina. In: Seeliger U, Kjerfve B (eds) Coastal marine ecosystems of Latin America. Springer, Heidelberg, pp 205–217
Petracci PF (2002) Diet of sanderling in Buenos Aires Province, Argentina. Waterbirds 25(3):366–370
Phillips DJH (1977) The use of biological indicator organisms to monitor trace metal pollution in marine and estuarine environments—a review. Environ Pollut 13:281–317
Phillips DJH (1980) Quantitative aquatic biological indicators: their use to monitor trace metal and organochlorine pollution. Applied Science Publishers, London
Phillips DJH, Rainbow PS (1988) Barnacles and mussels as biomonitors of trace elements: a comparative study. Mar Ecol Prog Ser 49:83–93
Phillips DJH, Rainbow PS (1993) Biomonitoring of trace aquatic contaminants. Chapman and Hall, London
Piccolo MC, Perillo GME, Melo W (2008) The Bahía Blanca estuary: an integrated overview of its geomorphology and dynamics. In: Neves R, Baretta JW, Mateus M (eds) Perspectives on integrated coastal zone management in South America. IST Press, Lisbon, pp 219–229
Pratolongo PD, Perillo GME, Piccolo MC (2010) Combined effects of waves and plants on a mud deposition event at a mudflat–saltmarsh edge in the Bahía Blanca estuary. Estuar Coast Shelf Sci 87:207–212
Rainbow PS (1995a) Biomonitoring of heavy metal availability in the marine environment. Mar Pollut Bull 31:183–192
Rainbow PS (1995b) Physiology, physiochemistry and metal uptake—a crustacean perspective. Mar Pollut Bull 31(1–3):55–59
Rainbow PS (1997) Trace metal accumulation in marine invertebrates: marine biology or marine chemistry? J Mar Biol Assoc UK 77:195–210
Rainbow PS (2002) Trace metal concentrations in aquatic invertebrates: why and so what? Environ Pollut 120:497–507
Rainbow PS (2007) Trace metal bioaccumulation: models, metabolic availability and toxicity. Environ Int 33:576–582
Rainbow PS, Phillips DJH (1993) Cosmopolitan biomonitors of trace metals. Mar Pollut Bull 26(11):593–601
Rainbow PS, White SL (1989) Comparative strategies of heavy metal accumulation by crustaceans: zinc, copper and cadmium in a decapod, an amphipod and a barnacle. Hydrobiologia 174:245–262
Salomons W, Förstner U (1984) Metals in the Hydrocycle. Springer-Verlag, Berlin, Heidelberg, New York, Tokyo, p 349
Sastre MP, Reyes P, Ramos H, Romero R, Rivera J (1999) Heavy metal bioaccumulation in Puerto Rican Blue Crabs (Callinectes spp.). Bull Mar Sci 64(2):209–217
Shank GC, Skrabal SA, Whitehead RF, Kieber RJ (2004) Strong copper complexation in an organic-rich estuary: the importance of allochthonous dissolved organic matter. Mar Chem 88:21–39
Simonetti P, Botté SE, Fiori SM, Marcovecchio JE (2012) Heavy metals concentration in soft tissues of the burrowing crab, Neohelice granulata, in the Bahía Blanca Estuary, Argentina. Arch Environ Contam Toxicol 62(2):243–253
Spivak ED (1997) Cangrejos estuariales del Atlántico sudoccidental (25–41°S) (Crustacea: Decapoda: Brachyura). Invest Mar Valparaíso 25:105–120
Spivak E, Anger K, Luppi TA, Bas C, Ismael D (1994) Distribution and habitat preferences of two grapsid crab species in Mar Chiquita Lagoon (Province of Buenos Aires, Argentina). Helgol Mar Res 48:59–78
Swaileh KM, Adelung D (1995) Effect of body size and season on the concentrations of Cu, Cd, Pb and Zn in Diastylis rathkei from Keil Bay, Western Baltic. Mar Pollut Bull 31(1–3):103–107
Tombesi NB, Pistonesi MF, Freije RH (2000) Physico-chemical characterization improvement evaluation of primary treated municipal waste water in the City of Bahía Blanca (Argentina). Ecol Environ Conserv 6(2):147–151
Town RM, Filella M (2002) Implications of natural organic matter binding heterogeneity on understanding lead (II) complexation in aquatic systems. Sci Total Environ 300:143–154
Turoczy NJ, Mitchell BD, Levings AH, Rajendram VS (2001) Cadmium, copper, mercury, and zinc concentrations in tissues of the King Crab (Pseudocarcinus gigas) from southeast Australian waters. Environ Int 27:327–334
Villares R, Puente X, Carballeira A (2002) Seasonal variation and background levels of heavy metals in two green seaweeds. Environ Pollut 119:79–90
White S, Rainbow PS (1982) Regulation and accumulation of copper, zinc and cadmium by the shrimp Palaemon elegans. Mar Ecol Prog Ser 8:95–101
White SL, Rainbow PS (1985) On the metabolic requirements for copper and zinc in molluscs and crustaceans. Mar Environ Res 16:215–229
White SL, Rainbow PS (1987) Heavy metal concentrations and size effects in the mesopelagic decapod crustacean Systellaspis debilis. Mar Ecol Prog Ser 37:147–151
Zauke GP, Clason B, Savinov VM, Savinova T (2003) Heavy metals of inshore benthic invertebrates from the Barents Sea. Sci Total Environ 306(1–3):99–110
Zhou Q, Zhang J, Fu J, Shi J, Jiang G (2008) Biomonitoring: an appealing tool for assessment of metal pollution in the aquatic ecosystem. Anal Chim Acta 606:135–150
Acknowledgments
The authors thank M. Nedda Chiarello and Javier Arlenghi (IADO) for valuable help in the laboratory analyses. This research was supported by a grant funded by the National Council of Scientific and Technological Researches (CONICET–Argentina) and with the 2008 Latin America E. Alexander Bergstrom Memorial Research Award from the Association of Field Ornithologists.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Simonetti, P., Botté, S.E., Fiori, S.M. et al. Burrowing Crab (Neohelice granulata) as a Potential Bioindicator of Heavy Metals in the Bahía Blanca Estuary, Argentina. Arch Environ Contam Toxicol 64, 110–118 (2013). https://doi.org/10.1007/s00244-012-9804-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-012-9804-1