Abstract
Patagonian salt marshes are not affected by pollution, but historical mining wastes are a continuous source of metals to salt marsh in San Antonio Bay. The present study evaluated the concentration of metals in sediments and used the halophyte Spartina spp. and the crab N. granulata as biomonitors. The levels of metals in sediment and organisms in SAB remained at levels corresponding to a slight enrichment or contamination. The highest levels corresponded to innermost sites of the Encerrado channel and close to the mining wastes. Spartina is a phytostabilizer so its aboveground tissues do not reflect the concentrations in the sediment; although, it retains the metals in its belowground tissues and in the rhizosediment. N. granulata showed to be a useful biomonitor for Pb, but not for the other metals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metals are especially interesting given their geochemical, nutritional, and/or toxicological significance. Although metals exist in nature, their extraction and remobilization, mediated by man, generates a clear increase in the environment, which can alter the ecological and biogeochemical balance of the ecosystem (Sadiq 1992).
The use of total concentration as criterion for establishing the potential effect of contamination on soils and/or sediments, or for discussing their mobility, implies that all metal forms have the same impact on the environment; this shows not to be true (Tessier et al. 1979). The predominant physical and chemical conditions in the environment will determine the distribution of metals in the different geochemical fractions, which in turn will affect their availability. The bioavailability of a metal in the sediment does not necessarily imply that it reaches organisms, depending on the intrinsic characteristics of the organism itself (species, age, susceptibility, etc.). Biomonitoring is a scientific technique for assessing environment, exposures to natural and synthetic chemicals, based on sampling and analysis of an individual organism’s tissues and fluids (Zhou et al. 2008).
Bioaccumulation is the net retention of a chemical in an organism across all exposure routes (diet, dermal, respiratory) and sources (water, sediment, food) as occurs in the natural environment (Spacie et al. 1995). On the other hand, bioconcentration is the process where a chemical is retained in an aquatic organism by absorption through the respiratory or dermal surface from its environment, not including the diet (Weisbrod et al. 2007). Bioaccumulation (BAF) and bioconcentration (BCF) factors are usually used to refer to the relationship between the concentration of metals in the organism and in the sediment or in the water, respectively (Barron 2002; Zhou et al. 2008).
The terms bioindicator and biomonitor apply to an organism (or part of an organism or a community of organisms) that represents the occurrence of contaminants based on specific symptoms, reactions, morphological changes or concentrations (Phillips and Rainbow 1993; MacFarlane et al. 2000). When the organism provides qualitative information of the environmental quality, it is called bioindicator and when it contains quantitative information, it is called biomonitor (Mertens et al. 2005; Markert 2007).
In aquatic environments, plants and animals have been widely used as biomonitors. Macrophytes are particularly useful for monitoring metals, as they may have a large capacity to accumulate and tolerate high metal concentrations. However, the selection of plant species as biomonitors depends on local conditions and their availability (Bonanno and Lo Giudice 2010). Besides, invertebrates of the aquatic trophic chain, generally associated with sediments, are also useful as biomonitors for metal contamination. Among them, decapods are known to have an effective accumulation of non-essential metals, such as Cd and Pb, and reflect environmental levels (Rainbow 1990). In particular, the crab Neohelice granulata, a key specie within estuarine ecosystems, plays an important role in the transfer of pollutants to their predators, such as birds and fishes. These characteristics allow considering its use as a potential biological indicator (Beltrame et al. 2011).
The present study evaluated the concentration of metals in sediments and organisms, halophytic plants and crabs as biomonitors of contamination of San Antonio Bay, a macrotidal system affected by mining wastes.
Methodology
Study site
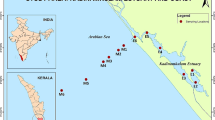
San Antonio Bay (SAB) is located in the northwest of San Matías gulf (40° 45′ S, 64° 55′ W), Río Negro province, Argentine Patagonia (Fig. 1). The scarce rainfall, the absence of freshwater inputs and high evaporation rates in the northern sector of the gulf, determine higher salinities, which contrast with the colder and less saline waters of the southern sector of the gulf (Fucks et al. 2011). The bay has an approximate total surface of 12,772 ha and the dominant feature of its coasts is the large tidal range (7-m high), denominated macrotidal system.
In the intertidal zone, presents well-differentiated physiographic units with particular biological community (Escofet et al. 1978): sandy or muddy mud flats, salt marshes (Spartina or Sarcocornia), mussels (Brachidontes rodriguezii), and “cangrejales” (large muddy tidal flats covered by N. granulata burrows). The dominant halophyte species in salt marshes are Spartina alterniflora and Sarcocornia perennis with coverage percentages of 2068 ha and 2124 ha, respectively. There are also patches of Spartina densiflora with less coverage, compared to a tidal plain extension of 10,111 ha (Isacch et al. 2006). The littoral is used as a resting, feeding, and nesting place by resident and migratory birds. In 1993, this area was declared Western Hemisphere Shorebird Reserve Network, site of international importance.
Sampling
Sediments, plants, and crabs were collected seasonally during 2013 and 2014. Sampled sites are shown in Fig. 1. On salt marshes (MA), three replicates of sediment without vegetation (S patch sediment) and sediment surrounding the rhizosphere of Spartina (R patch rhizosediment) in blocks (20 × 20 × 15 cm3) were taken. In addition, Spartina samples were collected at each site (aboveground and belowground tissues). Forty individuals of crab N. granulata were collected at four sampling sites in the marsh. Redox potential (Eh) and pH were also measured in situ in all sites, using an Altronix potenciometer.
Besides, seawater temperature (°C), salinity (psu), dissolved oxygen (DO%), pH, and Eh (mV) were measured with a multiparameter handheld instrument YSI model 556 MPS in tidal channel, within the Encerrado channel near the MA3 site and outside the channel near the MA8 site.
Sediments: characterization and metals analysis
The sediments were dried to constant weight at 60 °C. The particle size distribution was determined using plastic mesh sieves of 63-μm and 2-mm pore size, differentiating the fractions: gravel (> 2 mm), sand (between 2 mm and 63 μm), and silt-clay (< 63 μm). The organic matter content (OM) in the fraction < 63 μm was determined by loss on ignition at 450 °C for 4 h (Byers et al. 1978).
In order to determine the pseudototal content of Cd, Pb, Cu, Zn, and Fe in the sediment, aqua regia extraction was performed (MacGrath and Cunliffe 1985; ISO 1995). This type of extraction is suitable for sediments or matrices with OM < 20% (ISO 1995) and is used in assessing the potential of soils and sediments to exert negative effects on biota (CCME 2001a). About 1 g of dry sediment (fraction < 63 μm) was digested with 10 ml of aqua regia (3:1 ratio of HCl and HNO3). It was evaporated in a hot plate at 85 °C for 2 h. The residue was recovered with 10 ml 5% HNO3 and centrifuged at 8000 rpm for 20 min at room temperature. The supernatant was brought to a final volume of 25 ml with 5% HNO3.
Plants: characterization and metals analysis
Plant biomass was separated in: aboveground tissue (AT: green leaves and stems) and belowground tissue (BT: roots and rhizomes). They were washed with tap water and rinsed with deionized water in ultrasonic bath for 2 min to promote the detachment of sediment adhered to the tissue.
Once plant tissues were dried to constant weight in an oven at 80 °C, they were grounded and homogenized with a butt-type grinder. About one gram of dry tissue was calcined in a furnace at 400 °C for 6 h, and digested with 3 ml of concentrated HNO3 at 80 °C to determine the different metal concentrations. This process was repeated three to four times until complete digestion. The residue was resuspended in 3% HNO3 and 6% HCl solution. It was centrifuged at 8000 rpm for 20 min and filled up to a final volume of 10 ml with a 3% HNO3 and 6% HCl solution (BOE 1991; Yoong 1998).
Crabs: characterization and metals analysis
Crabs were washed with tap water and rinsed with deionized water. The morphometric measurements were as follows: width (WC) and length (LC) of the carapace, total weight (TW), and wet soft tissue weight (SW).
For each site, sample groups were formed with ten individuals each. They were dried at 80 °C until constant weight and the moisture percentage was determined. The ash percentage was calculated by calcining in a furnace at 550 °C for 12 h (AOAC 1995). Determination of metal concentration in soft tissues was done following the same procedure as in plant tissue.
The metal concentration in sediment extracts was determined using an inductively coupled plasma optical emission spectrometer (ICP-OES) Agilent 720. The instrument detection limits (DL) were 0.002 mg/kg Cd, 0.01 mg/kg Pb, 0.01 mg/kg Cu, 0.01 mg/kg Zn, and 0.02 mg/kg Fe. The metal concentrations in biological extracts were performed with an atomic absorption spectrometer (AAS) IL 457. The DL were 0.01 mg/kg Cd, 0.20 mg/kg Pb, 0.05 mg/kg Cu, 0.01 mg/kg Zn, and 0.10 mg/kg Fe.
All samples for metal analysis (soils, sediments, and plant tissues) were processed by duplicate including blanks. Analytical grade reagents (Merck or Baker) were used. The accuracy of the methods was checked by the analysis of reference material. PACS-2 for sediments with recovery rates between 92 and 117%; BCR-060 for plants with recovery rates between 82 and 129%; and SRM-1566b for crabs with recovery rates between 98 and 106%. The variation coefficients tested for five replicates were always below 10%.
Statistical analyses
Statistical analyses were performed with the statistical package InfoStat (Di Rienzo et al. 2008). Non-parametric Mann Whitney (MW) and Kruskal Wallis (KW) tests were applied, as appropriate. In KW, pairwise comparisons were applied between the medians of the treatment ranges. The procedure used to judge the significance of multiple comparisons is the one described in Conover (1999). The Spearman coefficient was used for the correlation analyzes. A value of p < 0.05 was considered significant. In order to be able to include in the data matrix the largest amount of information, values < DL were replaced with the value corresponding to DL/2 (Gibbons and Coleman 2001; Tsakovski et al. 2009).
Results
Salt marsh sediments
According to the granulometry, the sediments were defined as sandy-gravel for MA3 and MA5-S and sandy for other sites. MA3 and MA5* presented the highest percentages of gravel (42% and 43%, respectively) and MA1 presented the highest content of the silt-clay (6%) (Table 1). The percentages of OM in the silt-clay fraction varied between 3.3 and 4.7%, with the maximum in MA3. The pH values were neutral to slightly alkaline (7.1–8.2) and the Eh ranged between − 145 and 186 mV, evidencing suboxic to oxic conditions.
The pseudototal concentrations of metals showed the following pattern: Cd (0.22–0.45 mg/kg) < Cu (4.1–15.3 mg/kg) < Pb (3.5–35.1 mg/kg) < Zn (26.4–103.5 mg/kg) < Fe (0.98–1.56%). Cd concentrations were higher in MA1 and MA8 (KW p < 0.05). Pb, Cu, and Zn concentrations were higher in MA1 and MA3 than in others sites (KW p < 0.05), while Fe concentrations were higher in MA1 (KW p < 0.05) than in others sites (Fig. 2).
When comparing metals between patches in each of sampling site, for MA1 Pb, Cu, and Zn concentrations were higher in R patch than in S patch (MW p < 0.05). The same pattern was observed for Zn in MA3 (MW p < 0.05), for Pb in MA5 (MW p < 0.05), for all metal in MA5* (MW p < 0.05) and for Pb and Cu in MA8 (MW p < 0.05) (Fig. 2).
In seawater, the temperature varied according to the seasonal period. It presented ranges between 7.9 and 23.7 °C in MA3 and ranges between 10.0 and 29.9 °C in MA8. The salinity was 36.1 ± 1.8 psu in MA3 and 37.6 ± 2.4 psu in MA8. The dissolved oxygen (DO) was always higher than 100% and the pH was similar in both sites (8.2–8.3).
Salt marsh plants
At sites MA1, MA3, and MA5, the data refer to Spartina densiflora, whereas in MA5* and MA8, they correspond to Spartina alterniflora, according to the species found at each place. For that reason, comparisons among sites were performed without considering the species.
With regard to seasonal variation, Cd concentration in AT was detected in summer and autumn in MA5* and in autumn and winter in MA8 and it was < DL in all the other cases. In BT, Cd concentration ranged from < DL to 0.38 mg/kg. Comparing among sites, Cd concentration was higher in MA5* and MA8 than in others sites (KW p < 0.05). Pb concentration in AT varied between < DL and 2.40 mg/kg, being higher in MA1 and MA3 than in others sites (KW p < 0.05). In BT, Pb concentration varied between < DL and 7.50 mg/kg, being MA1 higher than MA3, and both of them higher than the rest (KW p < 0.05). Considering the seasonal variation, in summer, it presented highest Pb concentration in MA3, MA5, MA5*, and MA8 (KW p < 0.05). Cu concentration in AT varied between 1.38 and 4.32 mg/kg, being higher in MA5* than the rest (KW p < 0.05). Seasonal variation was found in MA3, with the minimum concentration in winter. Cu levels in BT varied between 2.4 and 8.2 mg/kg, being maximum in MA1 and minimum in MA5 (KW p < 0.05). Significant seasonal variation was found in MA1 being the highest concentrations measured in summer and spring and, in MA5 in summer and autumn. Zn concentrations in AT varied between 10.2 and 34.0 mg/kg, being maximum in MA3 and minimum in MA8 (KW p < 0.05). Zn concentrations in BT ranged from 18.1 to 145.2 mg/kg, with the highest values in MA1 and MA3 and the lowest one in MA8 (KW p < 0.05). Finally, Fe concentrations in AT ranged between 384.4 and 875.1 mg/kg and between 713.0 and 2257.0 mg/kg in BT. In AT, Fe was higher in MA3 and MA8 than the other sites (KW p < 0.05), whereas in BT, it was highest in MA8 followed by MA1 (KW p < 0.05) (Fig. 3).
Metals concentrations in aboveground and belowground tissues of Spartina (mean ± SD, N = 6). Su, summer; Wi, winter; Au, autumn; Sp, spring. Small letters indicate significant differences among seasons for each site (KW p < 0.05). Capitals letters indicate differences significant differences among sites (KW p < 0.05)
At sites where it was possible to compare between aboveground and belowground tissues, Cd, Pb, Cu, Zn, and Fe concentrations were higher in BT than in AT (MW p < 0.05).
In this regard, metal uptake by the plants was assessed through the bioaccumulation factor (BAF), which was calculated as the ratio between metal concentration in BT and in rhizosediment (Yoon et al. 2006). It was higher than 1 only for Zn, meanwhile for the rest of the elements, BAF was lower than 1 and in the following order: Cu > Cd > Pb > Fe (Table 2). In addition, translocation factor (TF) was calculated to relate metal concentration in AT and in BT (Yoon et al. 2006). This factor was always lower than 1 for the essential elements Cu, Zn, and Fe, close to 1 for Pb in all sites except for MA1, and greater than 1 for Cd in MA5* (Table 2).
Crab N. granulata
The adult male crabs presented sizes between 27.6 and 30.0 mm of WC and between 23.0 and 24.9 mm of LC. The TW ranged from 11.7 to 15.8 g and SW between 1.4 and 1.7 g. The moisture percentage varied between 79 and 82%, and the ash percentage between 19 and 24% (Table 3).
The metal concentrations in soft tissue of N. granulata presented the following order Cd < Pb < Zn < Cu < Fe (Fig. 4). Cd levels ranged from 0.52 to 1.7 mg/kg and showed seasonal variation only in MA1, being highest concentrations in winter (KW p < 0.05). Among sites, Cd concentrations were higher in MA5 and MA8 than in MA1 and MA3 (KW p < 0.05). Pb concentrations ranged from < DL to 6.4 mg/kg. With respect to seasonal variation for each site, Pb concentration in MA1 was lower in spring than in the rest of the seasons (KW p < 0.05), in MA3 was higher in summer and autumn (KW p < 0.05), in MA5 greater in autumn and winter (KW p < 0.05), and in MA8 did not present significant differences. Among sites, Pb concentrations were highest in MA1, intermediate in MA3, and lowest in MA5 and MA8 (KW p < 0.05). Cu concentrations ranged from 192 to 415 mg/kg. In general, the levels were maximum in winter and minimum in spring (KW p < 0.05), except in MA8 that did not show significant differences. Among sites, MA1 and MA3 presented the highest Cu concentrations and MA5 and MA8 the lowest ones (KW p < 0.05). Zn concentrations varied between 90 and 147 mg/kg. In MA1 and MA3, the highest concentrations were found in autumn-winter (KW p < 0.05), in MA5, the concentration was lowest in spring (KW p < 0.05), and in MA8, the concentration was highest in autumn (KW p < 0.05). Among sites, Zn concentrations were higher in MA1 than in other sites (KW p < 0.05). Finally, Fe concentrations ranged from 493 to 1613 mg/kg. In MA1, higher values were obtained in autumn-winter than in spring (KW p < 0.05); in MA3, no significant differences were found; in MA5, concentrations were highest in autumn and winter (KW p < 0.05); and in MA8, they were higher in autumn than in summer (KW p < 0.05). Among sites, Fe concentrations were highest in MA1, followed by MA8 and MA5, and lowest in MA3 (KW p < 0.05).
Metals concentrations in soft tissues of crab N. granulata (mean ± SD, mg/kg, N = 6–8). Su, summer; Wi, winter; Au, autumn; Sp, spring. Small letters indicate significant differences among seasons for each site (KW p < 0.05). Capitals letters indicate significant differences among sites (KW p < 0.05)
The biota accumulation factor (BAF) for crabs is the ratio of the concentration of an element in the organism to the concentration of the same element in the sediment. BAF for Pb and Fe were smaller than 1, while those of Cd, Cu, and Zn were higher (Table 4). Cu and Zn were significantly higher at sites MA5* and MA8 than at the other sites (KW p < 0.05).
Discussion
Pseudototal concentrations of metals were generally higher in sediments of the Encerrado channel than in the other sites, as indicated by Pb, Cu, and Zn values. Concentrations of these elements in the innermost site of the channel presented the following values close to the limits for the protection of the aquatic life according to the Canadian legislation (CCME 2001b): 30.2 mg/kg, 18.7 mg/kg, and 124 mg/kg, respectively. The R patches of the site presented remarkable higher concentrations of Pb, Cu, and Zn than the S patches, suggesting probable influence of vegetation.
Metal uptake by the plants was assessed through BAF. This factor proved to be above one only for Zn, probably due to its importance as a component of enzymes for protein synthesis and energy production and maintains the structural integrity of biomembranes (Hänsch and Mendel 2009). The Fe biogeochemistry plays an important role in the control of its own absorption and other elements (Feng et al. 2017). In this sense, low BAF for Fe are consistent with the high concentrations found in rhizosediments, probably in the form of oxyhydroxides. Plants capable of releasing oxygen from the roots promote the formation of Fe-rich rhizoconcretions. One of the major consequences of radial oxygen release from plant roots is the oxidation of Fe (II) (mainly as iron sulfides) and the precipitation of oxihydroxides as rhizoconcretions. These may also constitute a retention substrate for the other elements such as Mn, Zn, Cd, and Pb, limiting their entry into the tissues (Sundby et al. 1998; Vale et al. 2003; Redondo Gómez 2013). In general, low metal BAF are expected in plants capable of releasing oxygen from the roots, as in the case of Spartina (Vale et al. 1990; Williams et al. 1994).
Translocation factor was greater than 1 for Cd in MA5*. It must be taken into account that the compounds released by the plants to facilitate the absorption of Zn can promote the entry of Cd, since both elements possess similar chemical properties (Almeida et al. 2011). In general, it was observed that in places where the concentrations in rhizosediments were higher, BAF and TF were lower (MA1 and MA3).
The lower metal concentration in aerial parts compared to that in roots could be related to the development of mechanisms such as compartmentation, which would control ion transport into leaves, thereby improving plant tolerance to heavy metals (Mateos Naranjo et al. 2008). This would indicate that plants avoid entering excess of metals and the translocation to aerial tissues, as it has been reported for different Spartina species (Windham et al. 2003; Duarte et al. 2009; Cambrollé et al. 2011; Almeida et al. 2011; Redondo Gómez 2013). Considering the high capacity to immobilize the metals by accumulation in the roots or precipitation in the rhizosediment, Spartina has been suggested, by the latter authors, as a phytostabilizer genus. Although metals remaining in the roots are generally considered “out of trouble” as far as release to the environment is concerned, further studies are needed regarding the turnover of nutritive roots and the potential release of metals from decomposing roots (Weis and Weis 2004).
Metal accumulation in Spartina depends on the species, metal content in sediment and their bioavailability. In the case of SAB, the values recorded in Spartina did not exceed those that can be phytotoxic (5–30 mg/kg Cd, 30–300 mg/kg Pb, 20–100 mg/kg Cu, and 100–400 mg/kg Zn) (Prasad et al. 2006; Kabata Pendias 2011). Despite Zn concentration in belowground tissues that was within the phytotoxic range, (145.2 mg/kg), it must be taken into account that the content was determined without removing rizhoconcretions. Zn would be associated to rizhoconcretions and not inside of the root. This agree with a low translocation of Zn towards aboveground tissues.
By contrast, they were comparable to those reported in sites with low or moderate pollution, such as the Rawson marsh (Idaszkin et al. 2014), Bahía Blanca estuary (Hempel et al. 2008) and even SAB (Idaszkin et al. 2015, 2017) (Table 5). In highly contaminated areas such as the estuary of rivers Odiel and Tinto in Spain, levels of metals such as Pb, Cu, and Zn in tissues of S. densiflora and S. maritima are 10 to 100 times greater than those found in this study (Cambrollé et al. 2008). In the case of S. alterniflora from Yangtze River estuary in China, metal levels were much higher than those from SAB (Quan et al. 2007).
Salinity influences the uptake and excretion of metals in plants; however, there is controversy about how this mechanism works. The increase of the salinity of the overlying water could increase the excretion of salts by S. alterniflora, associated to an increase in the excretion of metals (Weis and Weis 2004). This mechanism has been proposed for the excretion of metals such as Cd, Cr, Cu, Fe, Hg, Mn, Ni, Pb, and Zn in Spartina (Burke et al. 2000; Windham et al. 2001). On the other hand, in response to saline stress, the halophytes excrete a greater amount of Na and decrease the uptake and the excretion of Ca. As a consequence of the above, a lower excretion of divalent metals, such as Pb and Zn, could occur (Brown et al. 2006; Mahon and Carman 2008). Also, studies in S. densiflora report that salinity favors tolerance to the toxic action of Zn (Redondo Gómez et al. 2011).
Salinity in SAB generally is 36 psu, which may be restricting the metal uptake and its translocation. Spartina is a phytostabilizer plant that maintains metals in the rhizosediment with very low translocation towards aerial tissues. This results in low transfer in the food chain. However, it should be mentioned that in this study, the content of metals in dead or dry tissue was not measured, which could contain concentrations higher than those measured in living tissue (shoots and leaves green). Senescent tissues usually accumulate contaminants in greater proportion than photosynthetic or young leaves (Cambrollé et al. 2008; Salla et al. 2011).
Regarding decapod crustaceans, sex is a biological factor that can affect the metal concentrations in their tissues (MacFarlane et al. 2000). For N. granulata, some studies have reported no sexual differences for Cu and Cd (Beltrame et al. 2011), while others indicated differences between sexes for Cu, Zn, Ni, and Pb (Ferrer 2001; Simonetti et al. 2011, 2013). In this study, metal levels were evaluated in male crabs, in order to decrease the variability during different stages of the reproductive cycle. Cd and Cu levels were higher in winter in most sites, while for Zn and Fe, these were higher in autumn. Pb levels, meanwhile, were maximum in summer in the innermost sites of the Encerrado channel. During autumn and winter, crabs are less active and have a lower metabolic rate (Ituarte et al. 2004, 2006). The higher concentrations of metals in this period could be the result of less excretion and a change in their mode of feeding (higher debris content), due to their lower activity outside the burrows.
Since decapod crustaceans are not able to regulate non-essential metals such as Cd and Pb, they accumulate them in amounts directly proportional to external concentrations (MacFarlane et al. 2000; Rainbow 2002). Pb concentrations were highest in individuals from the innermost site of the Encerrado channel, according to the sediment concentrations. While Cd concentrations were highest outside the channel. On the other hand, N. granulata regulates essential metals such as Cu and Zn. Cu is part of the respiratory pigment hemocyanin and Zn is part of numerous enzymes (Rainbow 1997).
Non-essential metals as well as the excess of essential metals must be detoxified. They may be regulated in the hepatopancreas and detoxified by several pathways: by binding to proteins (metallothioneins); by accumulation in vacuoles in the form of sulfide granules; by elimination in feces and urine or accumulated in hemolymph or other detoxification organs (Rainbow 2007; Sa et al. 2008; Henry et al. 2012). Therefore, metals are regulated to a certain threshold, above which they are accumulated proportionally to the external levels. In this study, except for individuals from the innermost site of the Encerrado channel, there were similar among sites, which could be related to the fact that sediment concentrations did not exceed thresholds established for the protection of the aquatic life (CCME 2001b).
Cu and Zn are essential metals and decapod crustaceans have several routes of detoxification of excess, tolerating relative high concentrations. Thus, in the sites with higher concentrations of metals in sediments, the BAF remained low. In the case of Cd, it is a non-essential metal and its detoxification is slow, so that at small concentrations in the environment, a net accumulation in the organisms is observed. Studies have shown that accumulated concentrations of Cd in crabs increase with dissolved Cd exposure without significant increase in excretion (Rainbow 2002, 2007).
Metals concentrations in N. granulata were similar to those reported by Giarratano et al. (2016) and relatively higher than those reported by Gil et al. (2006) in organisms of the tidal plain of this bay, except in the case of Pb that was slightly higher (Table 6). Concentrations of the same order of magnitude for this species have been reported in the estuary of Bahía Blanca (Buenos Aires), a site with moderate pollution (Simonetti et al. 2013), being smaller in most metals except Cd.
Essential and non-essential metals possess the ability to produce reactive oxygen species (ROS) that cause DNA damage, lipid peroxidation, and enzymatic inhibition. Studies in N. granulata of the BSA did not present a differentiated oxidative stress between organisms of the Encerrado channel and outside of it (Giarratano et al. 2016). The toxicity of metals in the organism is not directly related to the total accumulated concentration since the metals can be stored in unavailable forms, such as granules or metallothionein complexes (Rainbow 2007). It is also possible that crabs from contaminated areas have developed a genetic tolerance to metals, indicating strong ecotoxicological selective pressure (Luoma and Rainbow 2008).
It has been reported for N. granulata that the increase in salinity decreases the toxicity and accumulation of Cd (Vitale et al. 1999) and Cu (Lauer et al. 2012). Also, in other crabs such as Carcinus maenas, Zn accumulation decreased with increasing salinity (Niyogi et al. 2015). These authors indicate that higher salinity increases the tolerance to these metals. SAB receive low fresh water input from rainfalls and evaporation rate is high. In this study, the surrounding seawater had salinity between 36 and 37 psu, and salinities of 39 psu have been also reported in the bay (Montemayor et al. 2014). The high salinity of the environment could favor the metal tolerance of crabs from SAB.
Conclusions
The levels of metals in sediment and organisms in SAB remained at levels corresponding to a slight enrichment or contamination. The highest levels correspond to innermost sites of the Encerrado channel and close to the mining wastes.
Spartina is a phytostabilizer plant so its aboveground tissues do not reflect the concentrations in the sediment; instead, it retains the metals in its belowground tissues and in the rhizosediment. The metal levels in the rhizosediment were higher than in the adjacent sediment evidencing the influence of the vegetation, but only in the innermost site of the channel.
The pools of metals bioaccumulated by Spartina were much lower in the aboveground tissues, as seen by the lower translocation rates and by the metal concentrations. The necromass from root system becomes important to metal budget of the sediment, not only due to its input of metals, but also due to the increase of organic matter (Duarte et al. 2010). On the other hand, since crabs’ herbivory is mainly on young leaves would not represent a significant source of metals for these organisms (Salla et al. 2011).
N. granulata showed to be a useful biomonitor for Pb, but not for the other metals. This may be because the concentrations in the environment do not reach threshold levels being the crabs able to regulate the uptake and detoxification of excess metals. In this work, metals have been measured in total soft tissues. It is possible that different results could be obtained when specific tissues are analyzed. Crustaceans use physiological and biochemical detoxification and storage mechanisms in particular organs to protect other tissues and organs from the harmful effects of the toxic metals. There are several internal and external factors affecting the patterns of metal accumulation in crustaceans, such as those described in Marsden and Rainbow (2004).
Present information is of interest in order to have an update of the metal levels in different matrices of the ecosystem (sediments and organisms) of the SAB. Currently, a remediation plan is being carried out at the study site, which consists in the removal of mining wastes and affected soil. If movement of soils is not carried out with care, it is possible that dispersion of metal-enriched particles towards the Bay occurs. It is necessary to have an environmental control that monitors this situation, during and after this process has been completed.
References
Almeida, C. M. R., Mucha, A. P., & Vasconcelos, M. T. (2011). Role of different salt marsh plants on metal retention in an urban estuary (Lima estuary, NW Portugal). Estuarine, Coastal and Shelf Science, 91, 243–249.
AOAC. (1995). Official methods of analyses of Association of Analytical Chemist (16th ed.). DC: Washington.
Barron, M.G. (2002) Chapter 32: Bioaccumulation and bioconcentration in aquatic organisms. In: Hoffman DJ et al. (Eds.), Handbook of ecotoxicology 2nd ed. Boca Raton London New York Washington, D.C.
Beltrame, M. O., De Marco, S. G., & Marcovecchio, E. J. (2011). The burrowing crab Neohelice granulata as potential bioindicator of heavy metals in estuarine systems of the Atlantic coast of Argentina. Environmental Monitoring and Assessment, 172, 379–389.
BOE. (1991). Normas microbiológicas, límites de contenido en metales pesados y métodos analíticos para su determinación en los productos de la pesca y acuicultura con destino al consumo humano. Boletín Oficial del Estado, España, 195, 5937–5941.
Bonanno, G., & Lo Giudice, R. (2010). Heavy metal bioaccumulation by the organs of Phragmites australis (common reed) and their potential use as contamination indicators. Ecological Indicators, 10, 639–645.
Brown, C. E., Pezeshki, S. R., & De Laune, R. D. (2006). The effects of salinity and soil drying on nutrient uptake and growth of Spartina alterniflora in a simulated tidal system. Environmental and Experimental Botany, 58, 140–148.
Burke, D. J., Weis, J. S., & Weis, P. (2000). Release of metals by the leaves of the salt marsh grasses Spartina alterniflora and Phragmites australis. Estuarine, coastal and Shelf Science, 51, 153–159.
Byers, S. C., Mills, E. L., & Stewart, P. L. (1978). A comparison of methods of determining organic carbon in marine sediments, with suggestions for a standard method. Hydrobiologia, 58(1), 43–47.
Cambrollé, J., Redondo-Gómez, S., Mateos-Naranjo, E., & Figueroa, M. E. (2008). Comparison of the role of two Spartina species in terms of phytostabilization and bioaccumulation of metals in the estuarine sediment. Marine Pollution Bulletin, 56, 2037–2042.
Cambrollé, J., Mateos-Naranjo, E., Redondo-Gómez, S., Luque, T., & Figueroa, M. E. (2011). The role of two Spartina species in phytostabilization and bioaccumulation of Co, Cr, and Ni in the Tinto-Odiel estuary (SW Spain). Hydrobiologia, 671, 95–103.
CCME (2001a). Canadian Council of Ministers of the Environment. Canadian sediment quality guidelines for the protection of aquatic life: Introduction. Updated. In: Canadian environmental quality guidelines, 1999, Canadian Council of Ministers of the Environment, Winnipeg.
CCME (2001b). Canadian Council of ministers of the Environment [online] http://www.ccme.ca/en/resources/canadian_environmental_quality_guidelines. Access Feb 2017.
Conover, W. J. (1999). Practical nonparametric statistics. New York: John Wiley y Sons.
Di Rienzo, J. A., Casanoves, F., Balzarini, M. G., Gonzalez, L., Tablada, M., & Robledo, C. W. (2008). InfoStat, versión 2008. Grupo InfoStat: FCA, Universidad Nacional de Córdoba, Argentina.
Duarte, B., Almeida, P. R., & Caçador, I. (2009). Spartina maritima (cordgrass) rhizosediment extracellular enzymatic activity and its role in organic matter decomposition processes and metal speciation. Marine Ecology, 30(1), 65–73.
Duarte, B., Caetano, M., Almeida, P. R., Vale, C., & Caçador, I. (2010). Accumulation and biological cycling of heavy metal in four salt marsh species, from Tagus estuary (Portugal). Environmental Pollution, 158, 1661–1668.
Escofet, A. M., Orensanz, J. M., Olivier, S., & Scarabino, V. (1978). Biocenología bentónica del golfo San Matías (Río Negro, Argentina): metodología, experiencias y resultados del estudio ecológico de un gran espacio geográfico en América Latina. An Inst Cienc Mar Limnol Univ Autón Méx, 5, 59–82.
Feng, H., Qian, Y., Kirk Cochran, J., Zhu, Q., Hu, W., Yan, H., et al. (2017). Nanoscale measurement of trace element distributions in Spartina alterniflora root tissue during dormancy. Scientific Reports, 7, 40420.
Ferrer, L. (2001). Tesis Doctoral: Estudio de diversos metales pesados en sedimentos del estuario de Bahía Blanca y sus efectos tóxicos sobre el cangrejo Chasmagnathus granulata. Universidad Nacional del Sur 212 pp.
Ferrer, L., Andrade, S., Asteasuain, R., & Marcovecchio, J. (2006). Acute toxicities of four metals on the early life stages of the crab Chasmagnathus granulata from Bahía Blanca estuary, Argentina. Ecotoxicology and Environmental Safety, 65, 209–217.
Fucks, E. E., Scalise, A. H., & Schnack, E. J. (2011). Evaluación de alternativas para la conservación y manejo del frente costero en Las Grutas, Río Negro. Informe Final. Provincia de Rio Negro y Consejo Federal de Inversiones. Pp 15–26.
Giarratano, E., Gil, M. N., Marinho, C. H., & Malanga, G. (2016). Metal from mine waste as potential cause of oxidative stress in burrowing crab Neohelice granulata from San Antonio bay. Ecotoxicology and Environmental Safety, 132, 68–76.
Gibbons, R. D., & Coleman, D. E. (2001). Statistical methods for detection and quantification of environmental contamination (p. 139). New York: John Willey & Sons.
Gil, M. N., Torres, A., Harvey, M., & Esteves, J. L. (2006). Metales pesados en organismos marinos de la zona costera de la Patagonia Argentina Continental. Revista de Biología Marina y Oceanografía, 41(2), 167–176.
Hänsch, R., & Mendel, R. R. (2009). Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Current Opinion in Plant Biology, 12, 259–266.
Hempel, M., Botté, S. E., Negrin, V. L., Chiarello, M. N., & Marcovecchio, J. E. (2008). The role of the smooth cordgrass Spartina alterniflora and associated sediments in the heavy metal biogeochemical cycle within Bahía Blanca estuary salt marshes. Journal of Soils and Sediments, 8, 289–297.
Henry, R. P., Lucu, C., Onken, H., & Weihrauch, D. (2012). Multiple functions of the crustacean gill: osmotic/ionic regulation, acid-base balance, ammonia excretion, and bioaccumulation of toxic metals. Frontiers in Physiology, 3, 431.
Idaszkin, Y. L., Bouza, P. J., Marinho, C. H., & Gil, M. N. (2014). Trace metal concentrations in Spartina densiflora and associated soil from a Patagonian salt marsh. Marine Pollution Bulletin, 89, 444–450.
Idaszkin, Y. L., Lancelotti, J. L., Bouza, P. J., & Marcovecchio, J. E. (2015). Accumulation and distribution of trace metals within soils and the austral cordgrass Spartina densiflora in a Patagonian salt marsh. Marine Pollution Bulletin, 101(1), 457–465.
Idaszkin, Y. L., Lancelotti, J. L., Pollicelli, M. P., Marcovecchio, J., & Bouza, P. (2017). Comparison of phytoremediation potential capacity of Spartina densiflora and Sarcocornia perennis for metal polluted soils. Marine Pollution Bulletin, 118, 297–306.
Isacch, J. P., Costa, C. S. B., Rodríguez-Gallego, L., Conde, D., Escapa, M., Gagliardini, D. A., & Iribarne, O. O. (2006). Distribution of saltmarsh plant communities associated with environmental factors along a latitudinal gradient on the south-west Atlantic coast. Journal of Biogeography, 33, 888–900.
ISO International Organization for Standardization. (1995). Soil quality, extraction of trace elements soluble in aqua Regia. ISO, 11466, 1–6.
Ituarte, R., Spivak, E., & Luppi, T. (2004). Female reproductive cycle of the southwestern Atlantic estuarine crab Chasmagnatus granulatus (Brachyura: Grapsoidea: Varunidae). Scientia Marina, 68, 127–137.
Ituarte, R. B., Bas, C., Luppi, T., & Spivak, E. D. (2006). Interpopulational differences in the female reproductive cycle of the southwestern Atlantic estuarine crab Chasmagnathus granulatus Dana, 1851 (Brachyura: Grapsoidea: Varunidae). Scientia Marina, 70(4), 709–718.
Kabata Pendias, A. (2011). Trace elements in soils and plants. 4th Edition. Taylor & Francis Group, Boca Raton pp 548.
Lauer, M. M., de Olivera, C. B., Yano, N. L. I., & Bianchini, A. (2012). Copper effects on key metabolic enzymes and mitochondrial membrane potential in gills of the estuarine crab Neohelice granulata at different salinities. Comparative Biochemistry and Physiology - Part C, 156, 140–147.
Luoma, S. N., & Rainbow, P. S. (2008). Metal contamination in aquatic environments: science and lateral management (p. 573). Cambridge: Cambridge University Press.
MacFarlane, G. R., Booth, D. J., & Brown, K. R. (2000). The semaphore crab, Heloecious cordiformis: bio-indication potential for heavy metals in estuarine systems. Aquatic Toxicology, 50, 153–166.
MacGrath, S. P., & Cunliffe, C. H. (1985). A simplified method for the extraction of the metals Fe, Zn, Cu, Ni, Cd, Pb, Cr, Co and Mn from soils and sludges. Journal of the Science of Food and Agriculture, 36, 794–798.
Mahon, S., & Carman, K. R. (2008). The influence of salinity on the uptake, distribution, and excretion of metals by the smooth cordgrass, Spartina alterniflora (Loisel), grown in sediment contaminated by multiple metals. Estuaries and Coasts, 31, 1089–1097.
Markert, B. (2007). Definitions and principles for bioindication and biomonitoring of trace metals in the environment. Journal of Trace Elements in Medicine and Biology, 21(S1), 77–82.
Marsden, I. D., & Rainbow, P. S. (2004). Does the accumulation of trace metals in crustaceans affect their ecology – the amphipod example? Journal of Experimental Marine Biology and Ecology, 300, 373–408.
Mateos Naranjo, E., Redondo Gómez, S., Cambrollé, J., Luque, T., & Figueroa, E. (2008). Growth and photosynthetic responses to zinc stress of an invasive cordgrass, Spartina densiflora. Plant Biology, 10, 754–762.
Mertens, J., Luyssaert, S., & Verheyen, K. (2005). Use and abuse of trace metal concentrations in plant tissue for biomonitoring and phytoextraction. Environmental Pollution, 138, 1–4.
Montemayor, D. I., Canepuccia, A. D., Pascual, J., & Iribarne, O. O. (2014). Aboveground biomass pattern of dominant Spartina species and their relationship with selected abiotic variables in Argentinian SW Atlantic marshes. Estuaries and Coasts, 37, 411–420.
Niyogi, S., Blewett, T. A., Gallegher, T., Fehsenfeld, S., & Wood, C. (2015). Effects of salinity on short-term waterborne zinc uptake, accumulation and sub-lethal toxicity in the green shore crab (Carcinus maenas). Aquatic Toxicology, 178, 132–140.
Phillips, D. J. H., & Rainbow, P. S. (1993). Biomonitoring of trace aquatic contaminants (p. 371). London: Elsevier Science.
Prasad,, M., Sajwan, K., & Naidu, R. (Eds) (2006). Trace elements in the environment: biogeochemistry, biotechnology, and bioremediation. 1st Edition. CRC Press. Boca Raton pp. 744.
Quan, W. M., Han, J. D., Shen, A. L., Ping, X. Y., Qian, P. L., Li, C. J., Shi, L. Y., & Chen, Y. Q. (2007). Uptake and distribution of N, P and heavy metals in three dominant salt marsh macrophytes from Yangtze River Estuary, China. Marine Environmental Research, 64, 21–37.
Rainbow, P. S. (1990). Heavy metal levels in marine invertebrates. In R. W. Furness & P. S. Rainbow (Eds.), Heavy metals in the marine environment (pp. 67–79). Boca Raton, Florida: CRC Press.
Rainbow, P. S. (1997). Ecophysiology of trace metal uptake in crustaceans. Estuarine, Coastal and Shelf Science, 44, 169–175.
Rainbow, P. S. (2002). Trace metal concentrations in aquatic invertebrates: why and so what? Environmental Pollution, 120, 497–507.
Rainbow, P. S. (2007). Trace metal bioaccumulation: models, metabolic availability and toxicity. Environment International, 33, 576–582.
Redondo Gómez, S. (2013). Bioaccumulation of heavy metals in Spartina. Functional Plant Biology, 40, 913–921.
Redondo Gómez, S., Andrades Moreno, L., Mateos Naranjo, E., Parra, R., Valera Burgos, J., & Arcoa, R. (2011). Synergic effect of salinity and zinc stress on growth and photosynthetic responses of the cordgrass, Spartina densiflora. Journal of Experimental Botany, 62(15), 5521–5530.
Sa, M. G., Valenti, W. C., & Zanotto, F. P. (2008). Dietary copper absorption and excretion in three semi-terrestrial grapsoid crabs with different levels of terrestrial adaptation. Comparative Biochemistry and Physiology - Part C, 148, 112–116.
Sadiq, M. (1992). Toxic metal chemistry in marine environments. New York: Marcel Dekker 390 pp.
Salla, V., Hardaway, C. J., & Sneddon, J. (2011). Preliminary investigation of Spartina alterniflora for phytoextraction of selected heavy metals in soils from Southwest Louisiana. Microchemical Journal, 97, 207–212.
Simonetti, P., Botté, S. E., Fiori, S. M., & Marcovecchio, J. E. (2011). Heavy-metal concentrations in soft tissues of the burrowing crab Neohelice granulata in Bahía Blanca Estuary, Argentina. Archives of Environmental Contamination and Toxicology, 62, 243–253. https://doi.org/10.1007/s00244-011-9692-9.
Simonetti, P., Botté, S. E., Fiori, S. M., & Marcovecchio, J. E. (2013). Burrowing crab (Neohelice granulata) as a potential bioindicator of heavy metals in the Bahía Blanca Estuary, Argentina. Archives of Environmental Contamination and Toxicology, 64, 110–118.
Spacie, A., McCarty, L. S., & Rand, G. M. (1995). Bioaccumulation and bioavailability in multiphase systems. In G. M. Rand (Ed.), Fundamentals of Aquatic Toxicology (pp. 493–521). Washington, DC: Taylor & Francis.
Sundby, B., Vale, C., Caçador, I., Catarino, F., Madureira, M., & Caetano, M. (1998). Metal-rich concretions on the roots of the salt marsh plants: mechanism and rate of formation. Limnology Oceanography, 43, 245–252.
Tessier, A., Campbell, G. C., & Bisson, M. (1979). Sequential extraction procedure for the speciation of particulate trace metals. Analytical Chemistry, 51(7), 844–851.
Tsakovski, S., Kudlak, B., Simeonov, V., Wolska, L., Garcia, G., Dassenakis, M., & Namiesnik, J. (2009). N-way modelling of sediment monitoring data from Mar Menor lagoon, Spain. Talanta, 80, 935–941.
Vale, C., Catarino, F. M., Cortesao, C., & Cacador, M. I. (1990). Presence of metal-rich rhizoconcretions on the roots of Spartina maritima from the salt marshes of the Tagus Estuary, Portugal. Science of the Total Environment, 97(98), 617–626.
Vale, C., Caetano, M., & Raimundo, J. (2003). Incorporation of trace elements on iron-rich concretions around plant roots of Tagus Estuary salt marsh (Portugal). J Soils & Sediments, 3(3), 208–212.
Vitale, A. M., Monserrat, J. M., Castillo, P., & Rodriguez, E. M. (1999). Inhibitory effects of cadmium on carbonic anhydrase activity and ionic regulation of the estuarine crab Chasmagnathus granulata (Decapoda, Grapsidae). Comparative Biochemistry and Physiology - Part C, 122, 121–129.
Weis, J., & Weis, P. (2004). Metal uptake, transport and release by wetland plants: implications for phytoremediation and restoration. Environment International, 30, 685–700.
Weisbrod, A. V., Burkhard, L. P., Arnot, J., Mekenyan, O., Howard, P. H., Russom, C., Boethling, R., Sakuratani, Y., Traas, T., Bridges, T., Lutz, C., Bonnell, M., Woodburn, K., & Parkerton, T. (2007). Workgroup report: review of fish bioaccumulation databases used to identify persistent, bioaccumulative, toxic subtances. Environmental Health Perspectives, 115(2), 255–261.
Williams, T. P., Bubb, J. M., & Lester, J. N. (1994). Metal accumulation within salt marsh environments: a review. Marine Pollution Bulletin, 28(5), 277–290.
Windham, L., Weis, J. S., & Weis, P. (2001). Patterns and processes of mercury (Hg) release from leaves of two dominant salt marsh macrophytes, Phragmites australis and Spartina alterniflora. Estuaries, 24, 787–795.
Windham, L., Weis, J. S., & Weis, P. (2003). Uptake and distribution of metals in two dominant salt marsh macrophytes, Spartina alterniflora (cordgrass) and Phragmites australis (common reed). Estuarine, Coastal and Shelf Science, 56, 63–72.
Yoon, J., Cao, X., Zhou, Q., & Ma, L. Q. (2006). Accumulation of Pb, Cu, and Zn in native plant growing on a contaminated Florida site. Science of the Total Environment, 368, 456–464.
Yoong, K. S. (1998). Determination of cadmium, chromium, cobalt, lead, and nickel in plant tissue. In Y. P. Kalra (Ed.), Handbook of reference methods for plant analysis (pp. 193–199). Boca Raton: CRC Press.
Zhou, Q., Zhang, J., Fu, J., Shi, J., & Jiang, G. (2008). Biomonitoring: an appealing tool for assessment of metal pollution in the aquatic ecosystem. Analytica Chimica Acta, 606, 153–150.
Acknowledgments
The authors are grateful to Mr. Bernabé Urtubey for checking the grammar and spelling of the manuscript. We appreciate the comments and corrections of the anonymous reviewers, which effectively contributed to the improvement of this manuscript.
Funding
This study was partially funded by CONICET, through a doctoral fellowship to the first author, and Secretaria de Ciencia y Técnica of Universidad Nacional de la Patagonia San Juan Bosco (PI 1281 to MG and CM).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marinho, C.H., Giarratano, E. & Gil, M.N. Metal biomonitoring in a Patagonian salt marsh. Environ Monit Assess 190, 598 (2018). https://doi.org/10.1007/s10661-018-6975-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-018-6975-x