Abstract
This study aimed to determine the effects of salinity on metal uptake and metallothionein (MT) mRNA levels in tilapia exposed to three metal ions. Male Oreochromis niloticus × O. aureus juveniles (hereafter, “tilapia”) were exposed to various concentrations (100, 500, and 1 ppm) of metal ions (Cd2+, Cu2+ and Zn2+) in freshwater and water with two levels of salinity (10 and 20 ppt) for 7 days. Tests were then performed to investigate the effects of salinity on metal concentrations and MT mRNA induction in the test subjects’ organs. Saline decreased cadmium (Cd) uptake and MT mRNA fold induction in various internal organs, but it did not enhance MT mRNA induction in the gills. Exposure to Cu2+ caused greater copper (Cu) levels in the brains, intestines and livers, but Cu uptake in the intestines and kidneys occurred only at 10 ppm. MT mRNA induction caused by Cu2+ was observed in various internal organs, but it occurred in the gills only at greater levels of salinity. Exposure at greater salinities also decreased zinc (Zn) uptake and MT mRNA induction in all organs except the gills. Although greater salinity decreased Cd and Zn uptake, the metal content in the water correlated with the MT mRNA levels in most of the organs, except for the intestines. In conclusion, metal accumulations in the livers and kidneys of tilapia correlated with MT mRNA levels. The levels of MT mRNA in the livers and kidneys of tilapia might therefore be used as biomarkers of exposure to Cd2+, Cu2+ and Zn2+ in water of various salinities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Fish metallothionein (MT) is induced by metal ions; therefore, its protein and mRNA levels are useful biomarkers of metal contamination in water (Chan et al. 1988, 2006; Chan 1994, 1995; George et al. 1996; Sarkar et al. 2006). MT levels are commonly used as biomarkers of metal exposure in a variety of marine and freshwater fish species (Linde et al. 1999, 2001; Li et al. 2004; Chan et al. 2006; Fernandes et al. 2008; Knapen et al. 2007; Linde-Arias et al. 2008). Tom et al. (2004) developed quantitative real-time polymerase chain reaction (PCR) analysis for biomonitoring with MT mRNA levels in fish (Oreochromis aureus) used as a biomarker of exposure. More recently, Kessabi et al. (2010) used real-time PCR detection of several biomarkers, including MT mRNA, to evaluate the environmental effects of the Mediterranean killifish Aphanius fasciatus on natural populations.
Some researchers, however, have raised concerns about inferring metal contamination from biomarker levels because biomarkers can be affected by reproductive or other biotic factors (Banks et al. 1999; Al-Yousuf et al. 2000). In addition, the bioavailability of metal ions can be affected by water parameters such as water hardness and salinity (Engel and Fowler 1979; Blust et al. 1992; Penttinen et al. 1998; Heijerick et al. 2003; Dutton and Fisher 2011), metal uptake would also vary in different organs and affected by MT (Pelgrom et al. 1995; Mazon and Fernandes 1999; Roesijadi 1996). These factors can ultimately affect metal uptake and tissue MT gene expression (Roesijadi 1996). The effects of temperature, salinity, calcium, and metal pre-exposure on metal accumulation and MT levels have been previously reported in various aquatic species (Barron and Albeke 2000; Hollis et al. 2001; Van Cleef-Toedt et al. 2001; Leung et al. 2002; Rainbow and Black 2005a, b; Zhang and Wang 2005, 2006, 2007; Grosell et al. 2007; Monserrat et al. 2007; Walker et al. 2007). Metal ions are water soluble and thus compete for uptake sites with other ions. For example, Baldisserotto et al. (2005) and Franklin et al. (2005) reported that Cd2+ and Ca2+ compete for the uptake channel in fish, and Sanders and Windom (1980) reported that arsenic (As)(V) uptake of phytoplankton is influenced by phosphate. Cases have been observed in which the uptake of metal ions serves to increase protection or tolerance against metal toxicities through metal pre-exposure, which stimulates MT induction (Cosson 1994; Unger and Roesijadi 1996; Wu and Hwang 2003).
Tilapia are commonly used as a bioindicator of water pollution due to their metal tolerance and availability in many polluted sites (Chan 1995; Lam et al. 1998; Zhou et al. 1998; Linde-Arias et al. 2008; Wang et al. 2009; Kwok et al. 2010). Tilapia are also useful in this regard because of their ability to accumulate metals (Shen et al. 1998; Zhou et al. 1998; Ay et al. 1999; Dang et al. 1999), their sensitive responses to pollutants through gene expression (Wong et al. 2001; Cheung et al. 2004) and their distribution between inland waters and estuarine regions in various parts of the world including Hong Kong. In addition, tilapia can tolerate poor water quality and resist viral, bacterial, and parasitic diseases. This fish is therefore an ideal pollutant-resistant species for the biomonitoring of water pollution. Tilapia are a Cu-resistant species [24-h LC50 value of 2.82 parts per million (ppm) and 96-h LC50 value of 1.52 ppm] relative to carp, which are sensitive to Cu2+ [24-h LC50 value of 200 parts per billion (ppb) and 96-h LC50 value of 50 ppb] (Lam et al. 1998). Significant MT mRNA induction has been found in the gills and livers of tilapia exposed to Cu2+ and Zn2+ for 7 days (Lam et al. 1998; Cheung et al. 2004). Real-time PCR is also a sensitive and reliable method for detecting MT mRNA fold induction after the administration of metal ions (Cheung et al. 2004; Tom et al. 2004; Chen and Chan 2009). Although tilapia inhabit a wide range of salinities, from 5 to 20 ppt, they grow optimally in brackish water and can even grow well in salinity of 40 ppt (Shelton and Popma 2006). In fact, tilapia is often used to study salinity adaptation because Na–K-ATPase in gills plays a major role in metal uptake and ion adjustments (Feng et al. 2002; Güuner et al. 2004). To investigate the possible effects of salinities on metal uptake and MT mRNA levels, we used tilapia MT mRNA levels as biomarkers of metal exposure. The aim of this study was to determine metal uptake and MT mRNA levels in the organs of tilapia exposed to metal ions at different levels of salinity.
Materials and Methods
Tilapia, Fish Holding, and Exposure Tests
Male interspecific hybrid tilapia (female O. niloticus × male O. aureus) juveniles (4–6 cm in length) were purchased from a fish farm in mainland China that used clean (filtered) water supplied from an isolated reservoir filled with natural rainwater. The tilapia were kept in filtered freshwater in an aquarium room with a 16:8-h light-to-dark cycle at 25 ± 1 °C and were fed a high protein (48 %) diet (Mediaquafish; Japan Pet Drugs) daily for more than 1 month but less than two months prior to the exposure tests. The measured water temperature was 25.5 ± 1 °C, and the pH was 8.16 ± 0.10. These parameters were recorded daily. The tilapia used in this experiment weighed 29.3 ± 3.7 g and were 9.7 ± 0.4 cm in length. Artificial seawater was prepared using artificial sea salt (Marine Enterprises International). The fish were acclimatized stepwise to the desired levels of salinity from 5 to 10 to 20 ppt, with 7 days acclimation at each salinity level, and were then transferred to the testing tanks and maintained at those salinity levels for 3 days before the metal-exposure tests were performed.
The aqueous exposure tests (static with semirenewal) were performed for 7 days, and the fish were fed on the day 3 of the exposure experiment. The actual pH levels at 10 and 20 ppt salinity were 8.20 ± 0.12 and 8.25 ± 0.09, respectively. The exposure tests were performed at different concentrations (100, 500, and 1 ppm) of metal ions (Cd2+, Cu2+ and Zn2+; all with Cl− as the counter ion) with aeration in 30-L glass tanks. Ten tilapia were added to each tank with the desired metal concentrations and salinities. The same filtered water and the same salinities were used for the control condition but without the addition of the metal ions. Dissolved oxygen was maintained at 90–95 % saturation levels at 25 °C with an air pump. During the exposure period, a half tank (15 L) of water was replaced every 2 days, and water samples were collected for metal determination before the replacements. The concentrations of metal in the water were measured after acidification with hydrochloric acid. On day 7, the fish were killed by decreasing the water temperature gradually with ice water and then dissected to obtain brain, gill, intestine, kidney, liver, and muscle tissue. Minced organs were frozen in liquid nitrogen after washing with autoclaved deionized water and stored at −80 °C for further analysis of their metal concentrations and MT mRNA levels. The protocols for animal holding and the experiments were approved by the Department of Health with license from the Hong Kong SAR Government and the university’s Animal Ethics Committee, respectively.
Determination of Metal Concentrations
The organs obtained were weighed to the nearest 0.1 g and were extracted with 10 mL of 69 % analytical-grade nitric acid and 2 mL of 30 % hydrogen peroxide for 6–10 h at 105 °C. After acid digestion to obtain clear solutions, samples were filtered with ashless filter paper (Whatman no. 542) and diluted to 50 mL with ultra-pure deionized water. A similar procedure was performed with a bovine liver standard (National Institute of Standards and Technology, serial no. 1577b) for an acid digestion efficiency >90 %. Calibration curves for each of the metals were freshly prepared from 1,000 mg/L standard solutions of Cd, Cu, and Zn for atomic absorption spectrophotometry (Fluka) before each measurement. The Cd and Cu concentrations were determined using atomic absorption spectrophotometers (Varian SpectraAA 800 or Zeeman Z-8400) with graphite furnaces. Zn concentrations were measured using an acetylene gas flame on a Zeeman Z-2000 series atomic absorption spectrophotometer.
Extraction of RNA and Real-Time Quantitative Reverse Transcription PCR
Total RNA samples were prepared from 50 to 100 mg of organ tainted with 1 ml of TriPure Isolation Reagent (Roche, Indianapolis, Indiana, USA) on ice. After isopropanol precipitation, the RNA pellets were washed with 200 µl of 75 % ethanol, air dried by a SpeedVac for approximately 2 min, and dissolved in 40 µl of diethylpyrocarbonate-treated ddH2O at 70 °C for 10 min. The concentrations and purity of the total RNA were determined spectrophotometrically at 260 and 280 nm with a Hitachi U-2800 spectrophotometer with a 1-cm path quartz cuvette. The typical 260/280 nm ratios were between 1.6 and 2.0. The RNA samples were stored at −80 °C until use.
First-strand complementary DNA (cDNA) was synthesized from 2 μg of total RNA by reverse transcription according to the Applied Biosystems High-Capacity cDNA Reverse Transcription Kit protocol. The reactions were stored at −38 °C until further analysis. The integrity of the first-strand cDNA was confirmed by PCR amplification of the β-actin gene using conventional PCR with agarose gel electrophoresis. Oligonucleotide primers were designed according to a nucleotide sequence published in GenBank (tilapia β-actin; GenBank no. AB037865).
To determine the relative fold induction of MT gene expression, quantitative real-time PCR with TaqMan probe assay was performed using the TaqMan Gene Expression Assay protocol as provided by Applied Biosystems. In this assay protocol, MT acted as the target gene, and actin was used as the housekeeping gene for normalization. The reaction was performed with the Applied Biosystems 7500 Fast Real-Time PCR System, and the nucleotide sequences of the primers used in this TaqMan assay are listed in Table 1. The cDNA was denatured at 95 °C for 20 s, and then 40 PCR cycles were performed with denaturation at 95 °C for 3 s with an extension at 60 °C for 30 s. Results were analyzed using the Applied Biosystems gene expression program based on ΔΔCt calculation (Overbergh et al. 2003; Tom et al. 2004). To ensure the validity of this ΔΔCt calculation, amplification efficiencies of the target gene and endogenous control were determined to be approximately equal; the calculation for this study was valid with a slope < 0.1 by plotting cDNA dilution versus ΔCt (slope = 0.064, figure not shown).
Statistical Analyses
All data were analyzed using the Prism 6 program (GraphPad Software) with one-way analysis of variance (ANOVA) set at a 95 % confidence level and by Tukey’s multiple comparison test (Figs. 1, 2, 3, 4, 5, 6). Table 3 lists the linear regressions, correlations, and interactions between the metal concentrations and the MT mRNA fold induction in different tilapia tissues after exposure to the metals. The data were calculated using Prism 6 program with linear regressions, two-tailed p values set at the 95 % confidence level, and two-way ANOVA (data not shown). Linear regressions, correlations, and interactions between exposure concentrations, MT mRNA fold induction, and metal concentrations in different tilapia organs under different salinities were used in our analysis. Data were analyzed using two-way ANOVA in Prism 6.
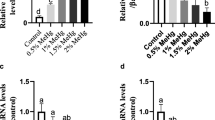
Concentrations of Cd in different organs of tilapia exposed to Cd2+ in freshwater and water at 10 and 20 ppt salinity. The results are represented as the mean ± SD of 10 replicates. The data were analyzed by one-way ANOVA (*p < 0.05, **p < 0.01, ***p < 0.001) comparing the same concentration-treated groups at different levels of salinity. Tukey’s multiple comparison test was used to compare the results of the concentration of Cd from different salinities. Columns without letters indicate no significant differences at the p < 0.05 level. Columns with different letters indicate significant differences between different salinities in the same concentration-treated group. Columns with the same letter indicate no significant differences between different salinities in the same concentration-treated group
Fold induction of MT mRNA levels in different organs of tilapia exposed to Cd2+ in freshwater and water at 10 and 20 ppt salinity. The results are represented as the mean ± SD of five replicates. The data were analyzed by one-way ANOVA (*p < 0.05, **p < 0.01, ***p < 0.001) comparing the same concentration-treated groups at different levels of salinity. Tukey’s multiple comparison test was used to compare the results of fold inductions from the different salinities. Significant differences indicated in columns with and without letters are as defined in Fig. 1
Concentrations of Cu in different organs of tilapia exposed to Cu2+ in freshwater and water at 10 and 20 ppt salinity. The results are represented as the mean ± SD of ten replicates. The data were analyzed by one-way ANOVA (*p < 0.05, **p < 0.01, ***p < 0.001) comparing the same concentration-treated groups at different levels of salinity. Tukey’s multiple comparison test was used to compare the results of the various concentrations of Cu from different levels of salinity. Significant differences indicated in columns with and without letters are as defined in Fig. 1
Induction of MT mRNA levels in different organs of tilapia exposed to Cu2+ in freshwater and water at 10 and 20 ppt salinity. The results are represented as the mean ± SD of five replicates. The data were analyzed by one-way ANOVA (*p < 0.05, **p < 0.01, ***p < 0.001) comparing the same concentration-treated groups at different levels of salinity. Tukey’s multiple comparison test was used to compare the results of fold inductions from the different salinities. Significant differences indicated in columns with and without letters are as defined in Fig. 1
Concentration of Zn in different organs of tilapia exposed to Zn2+ in freshwater and water at 10 and 20 ppt salinity. The results are represented as the mean ± SD of ten replicates. The data were analyzed by one-way ANOVA (*p < 0.05, **p < 0.01, ***p < 0.001) comparing the same concentration-treated groups at different levels of salinity. Tukey’s multiple comparison test was used to compare the results of Zn concentrations from the different salinities. Significant differences indicated in columns with and without letters are as defined in Fig. 1
Fold induction of MT mRNA levels in different organs of tilapia exposed to Zn2+ in freshwater and water at 10 and 20 ppt salinity. The results are represented as the mean ± SD of five replicates. The data were analyzed by one-way ANOVA (*p < 0.05, **p < 0.01, ***p < 0.001) comparing the same concentration-treated groups in different levels of salinity. Tukey’s multiple comparison test was used to compare the results of fold inductions from the different salinities. Significant differences indicated in columns with and without letters are as defined in Fig. 1
Results
Measurement of Metal Concentrations in the Aqueous Exposure Tests
The concentrations of the metals in the water collected from the exposure tests are listed in Table 2. The background level of Cd was <1 ppb (approximately 0.7–0.9 ppb), but it was greater for Cu (approximately 21–26 ppb) and Zn (approximately 61–63 ppb). The addition of artificial sea salt slightly increased the metal concentrations as reflected by the greater additional metal concentrations in the 20 ppt groups than in the 10 ppt groups or the controls in some samples. None of the treatments contained >10 % extra metal. Thus, the baseline metal ion levels were low and might not have affected the accumulations of other metal ions.
Concentrations of Cd and MT mRNA Induction in Organs of Tilapia Exposed to Cd2+
Greater concentrations of Cd were found in all of the tissues of tilapia exposed to different concentrations of Cd2+ in 0 ppt water. At greater salinities, Cd accumulation in the brains, intestines, kidneys, livers, and muscle tissues of the tilapia were significantly decreased, but not so in the gills, where only the 20 ppt salinity level had a significant effect on the decrease of Cd accumulation (Fig. 1). Similar patterns were found in MT mRNA inductions, and the gills of fish from tanks containing greater salinity (20 ppt) showed enhanced MT mRNA induction on exposure to Cd2+. Greater salinities also decreased the induction of MT mRNA in the intestine and kidney tissues of tilapia exposed to Cd2+ (Fig. 2).
Concentrations of Cu and MT mRNA Induction in Organs of Tilapia Exposed to Cu2+
Although the liver is the major organ to accumulate Cu, greater salinities decreased Cu accumulation in the tilapia. The Cu concentrations were two times greater in the liver of tilapia exposed to Cu2+ at 1 ppm in freshwater (Fig. 3). However, greater salinities decreased Cu concentrations to baseline levels of approximately 200–300 ppm. Intestines and kidneys contained greater concentrations of accumulated Cu at 10 ppt than at 0 or 20 ppt salinity. The exposure to Cu2+ caused greater Cu levels in the brains, intestines, and livers. Salinity at 10 ppt promoted Cu uptake in the intestines of tilapia exposed to Cu2+, but it decreased Cu uptake in the livers.
Significant induction of MT mRNA occurred in the gills, intestines, kidneys, and livers with exposure to greater Cu2+ concentrations in water at all levels of salinity. Thus, salinity appeared to have no significant effect on MT mRNA levels in the intestines, kidneys, or livers. Salinity had a significant effect only on the gills of tilapia exposed to Cu2+ (Fig. 4).
Concentrations of Zn and MT mRNA Induction in Organs of Tilapia Exposed to Zn2+
Accumulation of Zn occurred only in the intestines and livers, although greater salinities decreased the accumulation of Zn in these tissues (Fig. 5). Similar trends were found for MT mRNA levels (Fig. 6) in both livers and intestines. However, greater Zn2+ concentrations in the water induced greater MT mRNA induction, and greater salinities potentiated such MT mRNA induction in the gills of tilapia exposed to Zn2+. In other words, exposure to greater Zn concentrations was enough to promote greater Zn uptake and MT mRNA induction in the livers and intestines of tilapia, whereas exposure to greater salinities decreased Zn uptake and MT mRNA induction in the livers and intestines. However, greater salinities enhanced MT mRNA induction without causing accumulation of Zn in the gills of tilapia exposed to Zn2+.
Discussion
This study examined the potential effects of salinity levels on metal uptake in tilapia and investigated the possible causes of MT induction in tilapia tissues. Male tilapia (juveniles) were used for the 7-day exposure tests in water with different salinities and varying concentrations of Cd, Cu, and Zn ions. In our exposure tests, the MT mRNA levels in tilapia kidneys and livers correlated closely with the concentrations of Cd, Cu, and Zn accumulated in these tissues even under different salinities. However, the MT mRNA levels in gills did not correlate with the concentrations of the metals tested, nor did they correlate with Cu concentrations in the intestines (Table 3). Our results suggest that Cu might require other metal-binding proteins for its uptake or metabolism in the intestines. Greater salinities also decreased Cu accumulations in the livers (to background levels of 200–300 ppm) but not in other tissues. The baseline levels of Cu in tilapia livers are high compared with those of other fish species. Shen et al. (1998) and Lam et al. (1998) also reported high Cu levels in liver samples of tilapia collected from the Shing Mun River. Greater salinities significantly decreased Cd uptake in many different tissues, and similar effects also occurred with Zn2+ exposure, but this occurred only in the intestines, kidneys, and livers. Our results confirm that the level of salinity significantly (p < 0.01) affected metal uptake (mainly decrease) in the organs of tilapia exposed to different salinities except for that of Zn only in the intestines and kidneys.
The rationale of this study was to evaluate the use of MT mRNA levels (as measured using real-time PCR) as a biomonitor of metal exposure in fish (with tilapia as the model) that inhabit waters of varying salinities in river or estuarine regions. Before this study, we verified that the Environmental Protection Department of the Hong Kong Special Administrative Region Government had marine sediment data on metal contamination and freshwater metal levels. At some sample sites in the rivers or nullah (e.g., Fo Tan near Tolo Harbour), concentrations of Cu and Zn ranging from 10 to 1–2 ppm, and lower levels of Cd (0.1–10 ppb) have been measured over the past two decades (Environmental Protection Department 2014). However, concentrations of Cd in some contaminated sites in China may be ≤1–4 ppm (e.g., Wang et al. (2011) reported contamination in the Grand Canal). In our exposure tests, we used concentrations of 100–1 ppm to try to mimic the local situations.
We also plotted correlations between MT mRNA levels in the various organs of tilapia and the concentrations of metal ions in the water to which these fish were exposed. The results of a two-way ANOVA confirmed our comparisons of the MT mRNA levels in the gills and livers used for biomonitoring with significant correlations between exposure to different concentrations of metals in water and MT mRNA fold induction in different organs of tilapia. However, the intestinal levels of MT mRNA did not correlate significantly with Zn levels in the water (p = 0.056). The gills, kidneys, and liver are useful organs to study (Lam et al. 1998; Cheung et al. 2005). However, when tilapia were exposed to metal ions under different salinities, the MT mRNA levels in the gills did not correlate with the metal concentrations in the internal organs (Table 3). The Cu concentrations in the internal organs also did not correlate with intestinal MT mRNA levels (Table 3). Kidney and liver MT mRNA levels did, however, correlate with the concentrations of Cd, Cu, and Zn accumulated in the exposure tests under different salinities. When comparing the MT mRNA levels in the different organs of tilapia with the concentrations of the metals in the exposure water, the correlations were unaffected by the different levels of salinity used. Although greater salinity could decrease the uptake of Cd or Zn, the metal contents in the exposure water still correlated well (p < 0.05) with the MT mRNA levels in the different organs with the exception of Zn (p = 0.056) in the intestines.
Regarding MT mRNA fold induction, decreased gene expression was observed in the organs, except for the gills, of tilapia exposed to Zn2+ and Cd2+ at greater salinities (Fig. 6). Greater salinities increased the fold induction of MT mRNA in the gills of tilapia exposed to Cu2+, but no such effects were found in the other tissues (Fig. 4). Using black sea bream (Acanthopagrus schlegeli) as a model, Dang et al. also reported that MT in fish organs might contribute to Cu uptake, but such uptake might be primarily contributed by dietary, not waterborne, Cu uptake. Our results also confirm that the interaction between the exposure concentrations of Cu2+ or Cd2+ and salinity had little effect on MT mRNA fold induction in the intestine (p = 0.89) or in the liver (p = 0.36), respectively. These results suggest that the pathways of metal uptake and MT induction in fish tissues might be different for Cu2+ than for Cd2+ or Zn2+. In fact, metal ions might affect (1) the phosphorylation of a metal-responsive element-binding transcriptional factor called MTF-1 to induce MT gene transcription (LaRochelle et al. 2001) or (2) the oxidative stress response pathways (Mattie and Freedman 2004). In addition, because Cu and Zn are essential ions, the levels of these metals are tightly regulated with metal transporters and their own homeostasis system with Cu taken up mainly from the intestine and gills with ATP7A or 7B as found in zebrafish (Leung et al. 2014) or with ATP7A in tilapia intestinal tissues (Chen and Chan 2011). However, Zn transporters may be related to Cd uptake as has been found in humans with SLC39A8 and SLC39A14 (Lichten and Cousins 2009; Rentschler et al. 2014) or ZIP14 in mice gut (Jorge-Nebert et al. 2014).
Zhang and Wang (2007) reported that increases in salinity decreased Cd and Zn uptake in euryhaline black sea bream (A. schlegelii). However, the calcium-channel blocker verapamil could decrease metal uptake only in fish adapted to freshwater but not in fish adapted to marine water (Zhang and Wang 2007). A detailed molecular mechanism that involves metal transporters and governs metal uptakes has been well characterized in different fish species, but the information on this mechanism is fragmented (Bury et al. 2003; Galvez-Peralta et al. 2006; Zheng et al. 2010; Chen and Chan 2012; Zhu and Chan 2012). In addition, Zhang and Wang (2007) showed that the viscera contributes to 34–36 % of metal uptake or accumulation in high salinity with the gills contributing more to metal uptake in lower salinity. Dang et al. (2009) also found MT induction in dietary uptake of copper despite copper uptake could be saturated in an experiment with black seabream.
Our results also show that one of the major factors influencing the effects of metal ions and salinity on fish is the length of exposure. The effects of metal ions on fish increase over time, and prolonged exposure could have a significant effect on the metabolic rate. These effects are further complicated by variations in the types of metal and fish species. Thus, the effects of metal ions in decreasing the activities of Na–K-ATPase and carbonic anhydrase, along with major effects on osmoregulation and oxygen consumption (greater metabolic rates), have been well documented (Kolok et al. 2002; Monserrat et al. 2007). Therefore, prolonged exposure to salinity or metal stress would have profound effects on the metabolic rates of the organisms tested. For example, fathead minnows have greater metabolic rates after 96-h than after 24-h exposures regardless of the various concentrations of Cd2+ or Cu2+ ions and salinities used (Pistole et al. 2008). What is equally important is that the tilapia used in our experiments were juveniles with fast rates of growth. All of the exposure tests were performed for only 7 days, which eliminated the effects of the fast growth rate. Further investigation should be designed to examine the effects of metals and salinities on the oxygen consumption and metabolic rates of tilapia. Last, but not least, the involvement of metal transporters in metal uptakes in fish tissues also warrants further investigation.
Conclusion
In conclusion, MT mRNA levels in the kidneys and livers of tilapia can be used as biomarkers of exposure to Cd2+, Cu2+ and Zn2+ in waters of different salinities. Although salinity can decrease metal uptake in tilapia organs, the levels of MT mRNA in the kidneys and livers correlated with the concentrations of metal accumulated in the organs and the levels of metal ions found in the surrounding water.
References
Al-Yousuf MH, El-Shahawi MS, Al-Ghasis SM (2000) Trace metals in liver, skin and muscle of Lethrinus lentjan fish species in relation to body length and sex. Sci Total Environ 256:87–94
Ay O, Kalay M, Tamer L, Canli M (1999) Copper and lead accumulation in tissues of a freshwater fish Tilapia zillii and its effect on the branchial Na, K-ATPase activity. Bull Environ Contam Toxicol 62:160–168
Baldisserotto B, Chowdhury MJ, Wood CM (2005) Effects of dietary calcium and cadmium on cadmium accumulation, calcium and cadmium uptake from the water, and their interactions in juvenile rainbow trout. Aquat Toxicol 72:99–117
Banks SD, Thomas P, Baer KN (1999) Seasonal variations in hepatic and ovarian zinc concentrations during the annual reproductive cycle in female channel catfish (Ictalurus punctatus). Comp Biochem Physiol C 124:65–72
Barron MG, Albeke S (2000) Calcium control of zinc uptake in rainbow trout. Aquat Toxicol 50:257–264
Blust R, Kockelbergh E, Baillieul M (1992) Effects of salinity on the uptake of cadmium by the brine shrimp Artemia franciscana. Mar Ecol Prog Ser 84:245–254
Bury NR, Walker PA, Glover CN (2003) Nutritive metal uptake in teleost fish. J Exp Biol 206:11–23
Chan KM (1994) PCR-cloning of goldfish and tilapia metallothionein complementary DNAs. Biochem Biophys Res Commun 205(1):368–374
Chan KM (1995) Metallothionein: potential biomarker for monitoring heavy metal pollution in fish around Hong Kong. Mar Pollut Bull 31:411–415
Chan KM, Davidson WS, Fletcher GL (1988) Metallothionein messenger RNA: potential molecular indicator of metal exposure. In: Nriagu JO (ed) Aquatic toxicology and water quality management. Advances in environmental science and technology, vol. 22. John Wiley & Sons, New York/Toronto, pp 89–109
Chan KM, Ku LL, Chan PC, Cheuk WK (2006) Metallothionein gene expression in zebrafish embryo-larvae and ZFL cell-line exposed to heavy metal ions. Mar Environ Res 62:S83–S87
Chen DS, Chan KM (2009) Changes in the protein expression profiles of the hepa-T1 cell line when exposed to Cu2+. Aquat Toxicol 94:163–176
Chen DS, Chan KM (2011) PCR-cloning of tilapia7A cDNA and its mRNA levels in tissues of tilapia following copper administrations. Aquat Toxicol 105:717–727
Chen DS, Chan KM (2012) Identification of hepatic copper-binding proteins from tilapia by column chromatography with proteomic approaches. Metallomics 4:820–834
Cheung AP, Lam TH, Chan KM (2004) Regulation of tilapia metallothionein gene expression by heavy metal ions. Mar Environ Res 58:389–394
Cheung APL, Lam VKL, Chan KM (2005) Tilapia metallothionein genes: PCR-cloning and gene expression studies. Biochim Biophys Acta 1731:191–201
Cosson RP (1994) Heavy metal intracellular balance and relationship with metallothionein induction in the gills of carp. After contamination by Ag, Cd, and Hg following pretreatment with Zn or not. Biol Trace Elem Res 46(3):229–245
Dang Z, Lock RA, Flik G, Wendelaar Bonga SE (1999) Metallothionein response in gills of Oreochromis mossambicus exposed to copper in fresh water. Am J Physiol 277:320–331
Dang F, Zhong H, Wang W-X (2009) Copper uptake kinetics and regulation in a marine fish after waterborne copper acclimation. Aquat Toxicol 94:2328–2344
Dutton J, Fisher NS (2011) Salinity effects on the bioavailability of aqueous metals for the estuarine killifish Fundulus heteroclitus. Environ Toxicol Chem 30(9):2107–2114
Engel DW, Fowler BA (1979) Factors influencing cadmium accumulation and its toxicity to marine organisms. Environ Health Perspect 28:81–88
Environmental Protection Department (2014) Data and statistics in Environmental Protection Department, Hong Kong Special Administrative Region Government. Available at: http://epic.epd.gov.hk/EPICRIVER/river/result/report
Feng SH, Leu JH, Yang CH, Fang MJ, Huang CJ, Hwang PP (2002) Gene expression of Na+-K+-ATPase alpha 1 and 3 subunits in gills of the teleost, Oreochromis mossambicus, adapted to different environmental salinity. Mar Biotech 4:379–391
Fernandes D, Bebianno MJ, Porte C (2008) Hepatic levels of metal and metallothioneins in two commercial fish species of the Northern Iberian shelf. Sci Total Environ 391:159–167
Franklin NM, Glover CN, Nicol JA, Wood CM (2005) Calcium/cadmium interactions at uptake surfaces in rainbow trout: waterborne versus dietary routes of exposure. Environ Toxicol Chem 24:2954–2964
Galvez-Peralta M, He L, Jorge-Nebert LF, Wang B, Miller ML, Eppert B et al (2006) ZIP8 zinc transporter: indispensible role for both multiple-organ organogenesis and hematopoiesis in utero. PLoS ONE 7(5):e36055
George SG, Hodgson PA, Tytler P, Todd K (1996) Inducibility of metallothionein mRNA expression and cadmium tolerance in larvae of a marine teleost, the turbot (Scophthalmus maximus). Fundam Appl Toxicol 33:91–99
Grosell M, Blanchard J, Brix KV, Gerdes R (2007) Physiology is pivotal for interactions between salinity and acute copper toxicity to fish and invertebrates. Aquat Toxicol 84:162–172
Güuner Y, ÖOzden O, ÇCaĞuirgan H, Altunok M, Kizak V (2004) Effects of salinity on the osmoregulatory functions of the gills in Nile Tilapia (Oreochromis niloticus). Turk J Vet Anim Sci 29:1259–1266
Heijerick DG, Janssen CR, De Coen WM (2003) The combined effects of hardness, pH, and dissolved organic carbon on the chronic toxicity of Zn to D. magna: development of a surface response model. Arch Environ Contam Toxicol 44:210–217
Hollis L, Hogstrand C, Wood CM (2001) Tissue-specific cadmium accumulation, metallothionein induction, and tissue zinc and copper levels during chronic sublethal cadmium exposure in juvenile rainbow trout. Arch Environ Contam Toxicol 41:468–474
Jorge-Nebert LF, Gálvez-Peralta M, Figueroa JL, Somarathna M, Hojyo S, Fukada T et al (2014) Comparing gene expression during cadmium uptake and distribution: untreated versus oral Cd-treated wild-type and ZIP14 knockout mice. Toxicol Sci 143(1):26–35
Kessabi K, Navarro A, Casado M, Saïd K, Messaoudi I, Piña B (2010) Evaluation of environmental impact on natural populations of the Mediterranean killifish Aphanius fasciatus by quantitative RNA biomarkers. Mar Environ Res 70:327–333
Knapen D, Reynders H, Bervosts L, Verheyen E, Blust R (2007) Metallothionein gene and protein expression as a biomarker for metal pollution in natural gudgeon populations. Aquat Toxicol 82:163–172
Kolok AS, Hartman MW, Sershan J (2002) The physiology of copper tolerance in fathead minnows: insight from an intraspecific, correlative analysis. Environ Toxicol Chem 21:1730–1735
Kwok CK, Yang SM, Mak NK, Wong CK, Liang Y, Leung SY et al (2010) Ecotoxicological study on sediments of Mai Po marshes, Hong Kong using organisms and biomarkers. Ecotoxicol Environ Safe 73:541–549
Lam KL, Ko PW, Wong JK-Y, Chan KM (1998) Metal toxicity and metallothionein gene expression studies in common carp and tilapia. Mar Environ Res 46(1–5):563–566
LaRochelle O, Gagné V, Charron J, Soh JW, Séguin C (2001) Phosphorylation is involved in the activation of metal-regulatory transcription factor 1 in response to metal ions. J Biol Chem 276:41879–41888
Leung KM-Y, Svavarsson J, Crane M, Morritt D (2002) Influence of static and fluctuating salinity on cadmium uptake and metallothionein expression by the dogwhelk Nucella lapillus. J Exp Mar Biol Ecol 274:175–189
Leung KP, Chen D-S, Chan KM (2014) Understanding copper sensitivity in zebrafish (Danio rerio) through the intracellular localization of copper transporters in a hepatocyte cell-line ZFL and the tissue expression profiles of copper transporters. Metallomics 6:1057–1067
Li WH, Chan PC, Chan KM (2004) Metal uptake in zebrafish embryo-larvae exposed to metal-contaminated sediments. Mar Environ Res 58:829–832
Lichten LA, Cousins RJ (2009) Mammalian zinc transporters: nutritional and physiologic regulation. Annu Rev Nutr 29:53–176
Linde AR, Sánchez-Galán S, Klein D, García-Vázquez E, Summer KH (1999) Metallothionein and heavy metals in brown trout (Salmo trutta) and European eel (Anguilla anguilla): a comparative study. Ecotox Environ Safe 44:168–173
Linde AR, Sánchez-Galán S, Vallés-Mota P, García-Vázquez E (2001) Metallothionein as bioindicator of freshwater metal pollution: European eel and brown trout. Ecotox Environ Safe 49:60–63
Linde-Arias AR, Inácio AF, de Alburquerque C, Freire MM, Moreira JC (2008) Biomarkers in an invasive fish species, Oreochromis niloticus, to assess the effects of pollution in a highly degraded Brazilian River. Sci Total Environ 399:186–192
Mattie MD, Freedman JH (2004) Copper-inducible transcription: regulation by metal- and oxidative stress-responsive pathways. Am J Physiol Cell Physiol 286:293–301
Mazon AF, Fernandes MN (1999) Toxicity and differential tissue accumulation of copper in the tropical freshwater fish, Prochilodus scrofa (Prochilodontidae). Bull Environ Contam Toxicol 63:791–804
Monserrat JM, Martinez PE, Geracitano LA, Amado LL, Martins CM, Pinho GL et al (2007) Pollution biomarkers in estuarine animals: critical review and new perspectives. Comp Biochem Physiol C 146:221–234
Overbergh L, Giulietti A, Valckx D, Decallonne B, Bouillon R, Mathieu C (2003) The use of real-time reverse transcriptase PCR for the quantification of cytokine gene expression. J Biomol Tech 14:33–43
Pelgrom SM, Lamers LP, Lock RA, Balm PH, Bonga SE (1995) Interactions between copper and cadmium modify metal organ distribution in mature tilapia, Oreochromis mossambicus. Environ Pollut 90(3):415–423
Penttinen S, Kostamo A, Kukkonen JVK (1998) Combined effects of dissolved organic material and water hardness on toxicity of cadmium to Daphnia magna. Environ Toxicol Chem 17(12):2498–2503
Pistole DH, Peles JD, Taylor K (2008) Influence of metal concentrations, percent salinity, and length of exposure on the metabolic rate of fathead minnows (Pimephales promelas). Comp Biochem Physiol C 148:48–52
Rainbow PS, Black WH (2005a) Physiochemistry or physiology: cadmium uptake and effects of salinity and osmolality in three crabs of different ecologies. Mar Ecol Prog Ser 286:217–229
Rainbow PS, Black WH (2005b) Cadmium, zinc and the uptake of calcium by two crabs, Carcinus maenas and Eriocheir sinensis. Aquat Toxicol 72:45–65
Rentschler G, Kippler M, Axmon A, Raqib R, Skerfving S, Vahter M et al (2014) Cadmium concentrations in human blood and urine are associated with polymorphisms in zinc transporter genes. Metallomics 6:885–891
Roesijadi G (1996) Metallothionein and its role in toxic metal regulation. Comp Biochem Physiol C 113:117–123
Sanders JG, Windom HL (1980) The uptake and decrease of arsenic species by marine algae. Estuar Coast Mar Sci 10:555–567
Sarkar A, Ray D, Shrivastava AN, Sarker S (2006) Molecular biomarkers: their significance and application in marine pollution monitoring. Ecotoxicology 15:330–340
Shelton WL, Popma TJ (2006) Biology. In: Lim C, Webster CD (eds) Tilapia, biology, culture, and nutrition. Haworth Press, pp 1–49
Shen L-H, Lam KL, Ko PW, Chan KM (1998) Metal concentrations and analysis of metal binding protein fractions from the liver of tilapia collected from Shing Mun River. Mar Environ Res 46:597–600
Tom M, Chen N, Segev M, Herut B, Rinkevich B (2004) Quantifying fish metallothionein transcript by real time PCR for 1st utilization as an environmental biomarker. Mar Pollut Bull 48:705–710
Unger ME, Roesijadi G (1996) Increase in metallothionein mRNA accumulation during Cd challenge in oysters preexposed to Cd. Aquat Toxicol 34(3):185–193
Van Cleef-Toedt KA, Kaplan LA, Crivello JF (2001) Killifish metallothionein messenger RNA expression following temperature perturbation and cadmium exposure. Cell Stress Chaperones 6:351–359
Walker PA, Bury NR, Hogstrand C (2007) Influence of culture conditions on metal induced responses in a cultured rainbow trout gill epithelium. Environ Sci Technol 41:6505–6513
Wang F, Leung AO, Wu SC, Yang MS, Wong MH (2009) Chemical and ecotoxicological analyses of sediments and elutriates of contaminated rivers due to e-waste recycling activities using a diverse battery of bioassays. Environ Pollut 157:2082–2090
Wang X, Han J, Xu L, Gao J, Zhang Q (2011) Effects of anthropogenic activities on chemical contamination within the Grand Canal, China. Environ Monit Assess 177:127–139
Wong CKC, Yeung HY, Woo PS, Wong MH (2001) Specific expression of cytochrome P4501A1 gene in gills, intestine, liver of tilapia exposed to coastal sediments. Aquat Toxicol 54:69–80
Wu S-M, Hwang PP (2003) Copper or cadmium pretreatment increases the protection against cadmium toxicity in Tilapia larvae (Oreochromis mossambicus). Zool Stud 42:179–185
Zhang L, Wang W-X (2005) Effects of Zn pre-exposure on Cd and Zn bioaccumulation and metallothionein levels in two species of marine fish. Aquat Toxicol 73:353–369
Zhang L, Wang W-X (2006) Alteration of dissolved cadmium and zinc uptake kinetics by metal pre-exposure in the Black Sea bream (Acanthopagrus schlegeli). Environ Toxicol Chem 25:1312–1321
Zhang L, Wang W-X (2007) Waterborne cadmium and zinc uptake in a euryhaline teleost Acanthopagrus schlegelii acclimated to different salinities. Aquat Toxicol 84:173–181
Zheng D, Kille P, Feeney GP, Cunningham P, Handy RD, Hogstrand C (2010) Dynamic transcriptomic profiles of zebrafish gills in response to zinc supplementation. BMC Genom 11:553–559
Zhou HY, Cheung RYH, Chan KM, Wong MH (1998) Metal concentrations in sediments and tilapia collected from inland waters of Hong Kong. Water Res 32:3331–3340
Zhu JY, Chan KM (2012) Mechanism of cadmium-induced cytotoxicity on the ZFL zebrafish liver cell line. Metallomics 4:1064–1076
Acknowledgments
The authors thank the Department of Biochemistry (Faculty of Science) for the provision of a research assistantship to A. Shek and for its support of this research with a high degree of academic freedom.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shek, A.C.S., Chan, K.M. Effects of Salinity on Metal Uptake and Metallothionein mRNA Levels in the Organs of Tilapia Exposed to Cadmium, Copper, and Zinc Ions. Arch Environ Contam Toxicol 68, 622–635 (2015). https://doi.org/10.1007/s00244-014-0123-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-014-0123-6