Abstract
Sediment pollution by metals is of high interest considering that it can affect marine life. The estuaries' quality may be reflected by the environmental intertidal zone condition. Subsurface sediments collected at the nude tidal flats from three sampling stations in the Bahía Blanca Estuary were analyzed for total metals concentrations (Hg, Cd, Pb, and Cr), distribution, and geochemical partitioning. Most of the elements (Hg, Cd, and Cr) have shown highest concentration values in the industrial-influenced area. Maximum value of Pb was obtained where the main freshwater input discharges. Intertidal sediments have presented higher values of Cr than the subtidal ones. Cd and Pb contents near the industrial area were strongly higher in the subtidal zones. The distribution of Cd and Pb demonstrated the occurrence of a diffusion pattern from the land toward the sea, showing a dependence on both the metal itself and/or the source. Not all studied metals have shown the highest content in the fine fraction. The chemical partitioning in the fine fractions offered evidence that the tidal flats were an important source as well as sink of metals to the adjacent coastal area. The studies of intertidal sediments provide an integrative knowledge on the potential effects of different trace metals in the environment and they must be used in the contamination studies within coastal areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Estuarine areas are complex and dynamic aquatic environments where depositional processes are predominant. They principally act as sink and/or transitional way of several chemical pollutants as well as nutrients between freshwater–land and the open sea. Estuaries with extensive intertidal zones are of great interest from a biological point of view. Estuarine flats provide vital feeding grounds for migrant and native birds as well as nursery zones for fish; in addition, these areas are usually assigned to both industrialization and urbanization processes (Buggy and Tobin 2008). Contaminants use to be mostly found associated with fine sediment particles. Heavy metals concentrations in recently deposited surface sediments within the intertidal zone reflect updated water quality within the estuary (Spencer et al. 2003) and could also affect the bioassimilation and bioaccumulation of metals in aquatic organisms, resulting in potential long-term implications on human health and ecosystem (Ip et al. 2007). Physical, chemical, and biological interactions between tidal flats and saltwater estuarine system can have significant influences on the transport and distribution of trace metals. So, the transport of trace metals between intertidal areas and subtidal ones within estuaries depends on the partitioning of trace metals between dissolved and particulate phases (Botté et al. 2007), the association with different mineralogical fractions of the sediment (speciation), and physical–chemical phenomena like adsorption, desorption, precipitation, diffusion processes, etc. (Ramirez et al. 2005). Physical and chemical transport and biogeochemical interactions within these areas may be the key factors controlling land-derived natural and pollutant chemicals into the coastal areas. Besides, human activities have contributed to widespread coastal contamination; for example, they have altered the natural content of metals. The major contribution of heavy metals is of terrestrial origin: urban, mining, industrial and harbor developments, and other human practices near rivers and estuaries like agriculture activities. It is, therefore, important to understand the mechanisms of accumulation and geochemical distribution of trace metals in sediments as well as the spatial and temporal variance within the study area (Buggy and Tobin 2008; Ip et al. 2007).
Sediment grain size is one of the major controlling factors for the distribution of trace metals in coastal areas. As a matter of fact, coarse particles mainly consist of geological minerals, such as quartz and feldspars. A large proportion of trace metals in this fraction are in a crystalline solid state (usually in low concentration) and are environmentally immobile. On the other hand, fine particles such as clay and colloidal materials are generally surface active and contain organic matter and Fe/Mn oxide surface coatings, and consequently, they can play an important role in controlling trace metals depositional processes within the sediments (Ip et al. 2007). Measurement of total concentration as a criterion to assess metal contamination in the sediment environment is not enough to discriminate both the natural and anthropic sources (Adamo et al. 2005; Lacerda et al. 1988; Ramirez et al. 2005)
The geochemical partitioning of heavy metals by the application of selective extractants has been widely used to acquire information on the heavy metal origin and form of occurrence, as well as to identify the proportion of metals which are chemically mobile and bioavailable (Adamo et al. 2005; Lacerda et al. 1988; Marcovecchio and Ferrer 2005; Ramirez et al. 2005; Villaescusa-Celaya et al. 2000). So, metals availability from sediments may be a potential risk for aquatic biota, including marsh plants and associated organisms.
Bahía Blanca Estuary (BBE) has particular environmental characteristics, i.e., a small amount of sediment is supplied by the rivers within its catchment, while the circulation inhibits the import of sediment supplied by the sea; so, the principal sedimentary activity is the redistribution of sediment that originated from the erosion of both tidal flats and channel banks (Bokuniewicz 1995). Historically, the BBE has been receiving pollution from many sources: pesticides and fertilizers through continental runoff, raw sewage discharge from the cities, partially treated industrial effluent discharges, harbor-related operations, and aerial fallout, all of which might be potential hazards to this coastal marine system. In the BBE, the sediments have been characterized as containers of medium levels of metals—with a slight enrichment during several periods (Ferrer et al. 2000; Marcovecchio and Ferrer 2005; Marcovecchio et al. 1986; Marcovecchio et al. 2001; Pucci 1988; Pucci et al. 1979; Sericano and Pucci 1982; Villa 1988). Considering the absence of studies on heavy metal in the extensive tidal flat areas (intertidal sediments) in the BBE, it is necessary to know if those sediments could be acting as pollutant permanent sources to the ecosystem, like those that occur in other environments worldwide. Are intertidal sediments good regulators of trace metals within estuarine environment? Have rapid industrial development and urbanization within this system been responsible for the enrichment of trace metals in the muddy flats sediments of the BBE? Very high concentrations of several trace metals have been found in the benthic seaweed over the coast in the BBE (Botté, unpublished data) as well as lead in copepods (Marcovecchio et al. 2008). Moderate concentrations have been also recorded for other organism (fishes, shrimps, and crabs) within the estuary (Ferrer et al. 2000; Marcovecchio et al. 1988).
Within this framework, the main goals of this study were: (1) to determine the concentration and distribution of several heavy metals (Hg, Cd, Pb, and Cr) in tidal flats sediments from BBE, (2) to investigate the relationship of the same trace metal between sites under different anthropic activities, and (3) to evaluate the scale of chronological contaminant inputs to the BBE as well as the diagenetic remobilization through geochemical partitioning of trace metals in the silt–clay fraction. No study has yet reported on the value of using nude tidal flat sediments as bioindicators for Argentina coastal estuarine waters and salt marsh typical plants. Therefore, this work aims to determine whether relationships exist between intertidal and subtidal sediments in metal concentrations within BBE, Argentina.
2 Materials and Methods
2.1 Study Area
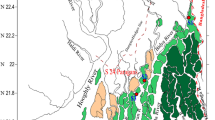
The BBE, with an approximate total area of 2,300 km2, is situated between 38°45′–39°40′ S and 61°45′–62°30′ W in Buenos Aires Province (Fig. 1). It has an elongated shape with NW–SE orientation, nearly 80 km in length, with depths ranging from 3 to 22 m and widths varying from 200 m to 3–4 km, characterized by a semidiurnal tidal regime (mean tidal range of 2–3.3 m; Perillo et al. 2001; Piccolo and Perillo 1990). One of the most important deep harbor systems of Argentina is located in the inner zone, and due to frequent shipping of cargo and transport vessels, the Principal Channel requires regular dredging. The uniform vertical distribution of the main oceanographic parameters is a consequence of the prevailing strong N and NW winds, which in turn supply a water mixing, create energetic tidal currents (Perillo et al. 2001), and coupled with the very low supply of fluvial sediment acting upon Bahía Blanca in an erosional mode (Bokuniewicz 1995). Based on the salinity distribution, the inner region of the estuary may be defined as partially mixed with a strong tendency to be vertically homogeneous, though it presents a number of marginal areas seasonally functioning such as hypersaline ones; in fact, the salinity for the study site (inner zone) is strongly influenced by local climatic conditions (Botté et al. 2007; Freije and Marcovecchio 2004). Other important characteristic of the system is its high turbidity (Andrade et al. 2000). The main tributaries are the Sauce Chico River (drainage area of 1,600 km2) in the head of the estuary and the Napostá Grande Creak (drainage area of 920 km2) upward Bahía Blanca city on the northern margin (Perillo et al. 2001). The average flow rate for both tributaries is low (1.72 and 1.05 m3 s−1, respectively, for the 2006–2007 period; Melo and Limbozzi 2008). The fluvial input from other smaller tributaries (Saladillo García Stream, Maldonado Stream, and Galván Stream) is intermittent and negligible within the estuary.
2.2 Sediment Sampling
Metal contents (Hg, Cd, Pb, and Cr) in tidal flats surface sediments from three sampling sites within the inner area of BBE (see Fig. 1) were recorded. They were Cuatreros Port (PC; utmost internal area of the estuary), Maldonado (M; close to the former municipal waste dumping), and Galván Port (PG; near the large industrial nucleus). The sediment samples were collected from July 2000 to January 2002 on a monthly frequency basis (N = 20). Samples were kept in polyethylene bags, immediately carried to the laboratory, and stored at 4 C for less than 48 h. Afterwards, subsamples directed to determine grain size composition, total metal contents, and metals geochemical partitioning in the <63-µm fraction were removed. The samples, including for mercury analysis (Marcovecchio et al. 1986; Hylander et al. 2000; Ram et al. 2003), were oven dried at 50 ± 5 C for 4 days. Large debris and fragments of biota were removed before grounding. All of the sediment samples were then ground in a porcelain mortar. In two opportunities, subsamples were sifted through stainless steel meshes until fine particles (<63 μm) were obtained (for Cd, Pb, and Cr analyses in the silt–clay fraction and geochemical partitioning). All homogenized samples were stored in plastic ware within desiccators until their analytical treatment.
2.3 Analysis of Trace Elements
Metal concentrations (Cd, Pb, and Cr) in the total fraction (TF) as well as in the <63-µm one (finest fraction [FF]) were made according to the method described by Dalziel and Baker (1983) and modified by Marcovecchio et al. (1988). About 0.500 g of sediment samples were weighted and placed into acid-washed Pyrex tubes. To each tube, 1.0 ml of perchloric acid and about 10 ml of nitric acid in different steps were added. Then tubes were placed in a glycerin heating bath at controlled temperature until complete mineralization. After cooling, 0.7% nitric acid was added to the residue up 10 ml into centrifuge tubes prior to undergoing metal concentration analyses. The trace element concentrations of the solutions were measured by atomic absorption spectroscopy with air/acetylene flame (AAS, Perkin Elmer 2380). Total mercury (HgT) analyses were developed following the methodology described in the article of De Marco et al. (2006) using the cold vapor flameless atomic absorption spectroscopy technique.
The geochemical partitioning of Cd, Pb, and Cr in the fine fraction of the sediments was also determined. The methodology involves a four-step sequential extraction with different mixtures of solvents beginning by weight at exactly 1 g of fine sediments and put into polycarbonate flask (50 ml; Lacerda et al. 1988; Maddock and Lopes 1988; Megalatti et al. 1983). The last fraction was obtained by difference between total concentration in the FF and the sum of the four extraction fractions. The five geochemical fractions were: FI, exchangeable adsorbed metals; FII, oxidizable complexed metals; FIII, metals in carbonates; FIV, reducible compounds; and, FV, residual metals (Marcovecchio and Ferrer 2005).
The detection limit of the method (MDL) was calculated as the standard deviation (SD) of 12 blank replicates (Federal Register 1984). The MDL for HgT sediment analysis was about 0.02 µg g−1, while the MDL for Cd, Pb, and Cr were 0.27, 2.15, and 0.29 µg g−1, respectively. All of the relative SDs of the replicate samples were <20%. For the analytical quality control, reagent blanks, certified reference materials (CRM; Pond Sediments, R.M. No. 2, NIES, Japan), and analytical grade reagents (Merck or Baker) were used. The recovery percentages for all trace metals in CRM were higher than 90%.
2.4 Particle Size Analysis
Granulometric fractions in tidal flat sediments were determined at IADO's Marine Geology Area, applying the wet sieving method according to Krumbein and Pettijohn (1938). The obtained fractions represent gravel, sand (coarse, middling, fine, and finer), and mud (silt plus clay).
2.5 Statistical Analysis
In the present study, analysis of variance (ANOVA), Tukey's test, Pearson's correlation (PC), and principal component analysis (PCA) were conducted using STATISTICA 7.0 (StatSoft, Inc. 2004). In order to study the general characteristics of the sediments at the BBE, the concentrations of trace metals of the tidal flats sediments were used as the input data in the ANOVA as well as in the PC and PCA.
3 Results
3.1 Trace Metal Concentrations in the Inner Area of Bahía Blanca Estuary
The mean, SD, median, and range of trace metals concentrations within the tidal flats subsuperficial sediment samples at the BBE are summarized in Table 1. The elemental concentrations of the sediments (TF) at the intertidal area were calculated from the 20 sediment samples taken in each of the three coastal areas. Table 1 reports that the HgT concentration fluctuated between nondetectable and 0.50 µg g−1 dry weight, with the highest value in PG. Hg was not detected in approximately 45% of the campaigns at PC site, while Hg was not detected in 35% and 30% of analyzed samples in PG and M sites, respectively, and most of reported values were slightly higher than the method detection limit (MDL = 0.02 µg g−1). So, the concentrations that were reported as “lesser than the detection limit (DL)” were substituted by one half the DL when the statistical analysis was applied (Jones and Clarke 2005). Total Cd and Cr concentrations varied between 0.63–1.17 and 6.17–13.26 µg g−1 dry weight, respectively, also with the highest values within sediments from the above-mentioned site. Unlike this, Pb contents ranged between 10.59 and 17.87 µg g−1 dry weight, with a maximum mean value obtained at PC. The reported differences between metal concentrations in sediments of those areas confirmed the effect of the anthropic activities, taking into account that PG (industrial area) usually presented the highest values. In the same places but in the subtidal part of the Principal Channel, the evaluated metals indicate that Cr concentration was always high at intertidal sediments; meanwhile, Cd contents are slightly high in the tidal flats from PC and M. But in PG, the Cd concentration is strongly high in the subtidal area, probably due to its intensive use as a route of cargo vessel. On the other hand, mean Pb concentrations were superior in those sediments from the bottom of the Principal Channel, particularly once again within the industrial zone. In addition, the one-way ANOVA (met the assumptions: Levene's test for homogeneity of variances and normal p–p graph) of the corresponding mean values as calculated for each sampling site has showed only highly significant differences (p ≪ 0.01) between them in the case of Cd and Pb [F(2,57)Cd = 17.059 and F(2,57)Pb = 7.756]. Tukey's honestly significant difference (HSD) test indicated that Cd-PG was significantly different from the other two places and Pb-M differs from Pb-PC as well as from Pb-PG (Fig. 2).
In order to assess trace metal contamination in this region, their concentrations were compared against the reference values within the estuary (Table 2) as the background levels for this region. The mean concentration of Pb in the tidal flats sediments (intertidal areas) were lower than the reference site values, while the concentrations of Cd and Cr were slightly higher than the reference one and the concentrations of Hg were significantly lower.
Temporal variation of trace metal concentrations showed that none of them have a clear seasonal distribution trend (see Fig. 3). Nevertheless, Cr and Pb contents slightly fall at all three sites during winter and spring (July to October), and both metals show a similar trend along the year. Hg concentrations were extremely low, with only two significant increases between September and March.
Other statistical analyses of the surface sediments in the intertidal BBE area were performed to further evaluate the relationship between evaluated sites and trace metal concentrations. Table 3 presents the Pearson's correlation (PC) coefficient matrixes between trace elements and study areas for the intertidal sediments. The Cr element was positively and significantly correlated with Cd and Pb (p < 0.01), but at only one site (M), weakly indicating a possible common natural origin as it has been suggested by other authors (Ramirez et al. 2005). None of the metals have strongly correlated with each other at both PC and PG sites, indicating the occurrence of different sources into the estuary. Considering simultaneously all sites together (e.g., inner region of the estuary) and with a p < 0.05, other correlations (Cr with Hg and Pb with Cd) have been observed. In order to evaluate the patterns of trace metal contamination in the study areas under different anthropic impact, a multivariate statistical analysis (PCA) was also performed. PCA is one of the analytical tools used to assess metal behavior in the aquatic system (Ip et al. 2007). The biplot graph of the first two principal components (PCs) of the surface intertidal samples is depicted in Fig. 4 where PC1–PC2 accounted for nearly 76% of the total variance. Cd, Pb, and Cr formed a group away from Hg, but they did not show a strong association among them. The M site was slightly isolated from other sites, and it was dominated by the first component (PC1). The results of PC and PCA showed good agreement with each other.
Metal concentrations within the FF of sediments (<63 µm) have shown the following: Cd concentrations were higher in the fine fraction than in the coarse one at the three sites (mean values=1.25, 1.51, and 1.30 µg g−1 at PC, M, and PG, respectively). The same distribution pattern was observed for both Pb (mean values=19.83 and 21.35 µg g−1) and Cr (mean values=12.26 and 12.22 µg g−1) at PC and M sites, respectively. These facts fully agreed with the concept that fine fraction favors metal adsorption processes. Meanwhile, both Pb (mean value=13.91 µg g−1) and Cr (mean value=10.43 µg g−1) have shown a different distribution model at PG where their concentrations were slightly lesser in the fine fraction than in the coarse one. This could be suggesting a recent incorporation into the system.
3.2 Sediments Physical Properties
Within the BBE, the silt–clay content (<63 µm) of the benthic sediments decreases southward in the direction of the estuary mouth, showing a typical sediment transport mechanism in the direction of the ocean (Gelós et al. 2004). The fine-grained intertidal sediments (FF) were principally concentrated where the hotspots of trace metals are located (96.3% at PC and 82.5% at PG). On the other hand, the other site (M) presented the lower content of FF with 67% of silt–clay. These results and previous data (Marcovecchio and Ferrer 2005) indicated that fine particles might be a major carrier for transporting trace metals from the head source area to the coastal zone. The distribution of fine grain sediments reflects the physical transportation of sediments and associated trace metals (Ip et al. 2007). The distribution of grain size is controlled by the physical transportation of sediment, including sediment aggregation and deposition, gravitational circulation, tidal pumping, and tidal trapping (Ip et al. 2007). When fine particle-reactive metals enter estuarine waters, many are quickly adsorbed onto suspended particulate matter in the water column and removed to the surface sediment layer at the bottom (Buggy and Tobin 2008) or even tend to be accumulated in places where waves and currents are absent or weak (Bokuniewicz 1995). Resuspension of surface sediment by increased turbulence and during ebb tides lead to sediment-bound contaminants re-entering the water column and moving to the estuarine system into the marine zone (Buggy and Tobin 2008), resulting in particulates transported down the estuary or exchanged between different depositional regions. This system has been characterized by high turbidity, particularly in its inner region. One explanation for the relatively low concentrations of trace metals in the sediments at the area far from the Principal Channel (site M) of the estuary is the low effect of the estuarine tide. Finally, the sediment colors at three sites are predominantly brown at the surface layer, becoming from gray–brown to black with increasing depth. The inner site of the three evaluated sites was characterized by sediments with slightly more sulfide smell, showing its anoxic conditions.
3.3 Partitioning of Metals in the Tidal Flats Sediments
Information on the chemical partitioning of trace metals can provide further evidence of the potential interactions of trace metals between intertidal and subtidal areas within an estuary. The geochemical fractions considered are the exchangeable (FI), oxidizable (FII = organic complexes and sulfides), carbonatic (FIII), reducible (FIV = absorbed by iron and manganese oxides), and residual or lithogenous (FV = metals associated with silicate and stable minerals) ones.
The results of metal sequential extraction (Cd, Pb, and Cr) within the FF (<63 µm) of the sediment samples in the intertidal area of BBE (in the present study) and the surrounding subtidal one (Marcovecchio and Ferrer 2005) are reported in Fig. 5. The means and ranges of the trace metals in different geochemical fractions of the tidal flats sediments are summarized in Table 4.
Geochemical partitioning (in percent) of metals in the FF of sediments at the three sites (a) and comparison with respect to values from the subtidal area (b; after Marcovecchio and Ferrer 2005)
The residual geochemical fraction has shown a decrease in the concentrations of Cd and Pb in the intertidal sediments (Fig. 5a), in comparison with the subtidal ones (Fig. 5b) in the inner area of the BBE. The Cd was principally adsorbed to the carbonate fraction (FIII, since 55% to 59%), meanwhile Pb to the organic complexes and sulfides (FII, since 36% to 45%). In the case of Pb, the other important fraction was carbonate (FIII), varying between 17% and 24%, followed in importance by the reducible fraction (FIV) but not in all three evaluated sites. On the other hand, this metal was slightly represented in the exchangeable fraction (FI) although with lesser than 11%. Salinity is also a factor affecting the settlement of sediment. An increase in salinity from a freshwater zone to a marine zone favors the adsorption of trace metals on particulates, induces flocculation, and further encourages the aggregation and deposition of sediment (Ip et al. 2007). This factor can contribute to different concentrations of trace metals in various geochemical fractions. As salinity in the inner zone of BBE showed values up to almost 37 psu during the same period of study (Botté et al. 2007), this could explain the observed variation.
In Fig. 5a, b, the geochemical partitioning of Cr showed a little differentiation between the intertidal zone and the subtidal one. Cr have presented more than 75% in the lithogenous phase (FV), indicating that it was included into the structure of clay or other stable mineral, and consequently, present low mobility and scarce availability within the studied system. In both areas (intertidal and subtidal) for all study sites, chromium was also represented in the FII (between 11% and 15%). Besides, in the samples from tidal flat sediments, this trace metal appears associated to Fe/Mn oxides (FIV = 5–8%), particularly in the zone faraway of industrial and harbor activities.
Finally, the low content of Cd, Pb, and Cr in the exchangeable fraction was very important because it means that their potential toxicity decreases.
Also shown in Fig. 5 are the concentrations presented in Table 4 that denote the following: (1) Cd in almost all fractions was higher in the subtidal area; (2) Pb reported the same tendency but with a great increase of 7.9 µg g−1; (3) Cr showed not only the main variation along the time, but also an increase in all labile fractions in samples from tidal flats sediments.
According to Marcovecchio and Ferrer (2005), the potentially bioavailable fraction (PBF) includes both FI + FII (exchangeable adsorbed metals + oxidizable complexed metals). When PBF was analyzed (Fig. 6), the highest values for the three sites correspond to Pb. Besides, the three trace metals analyzed have presented different distributions with respect to the study sites. The PBF values for those metals suggested that a significant amount of them could be easily assimilated by the organisms within the inner wetlands. This information must be taken into account because the aforementioned elements are considered as potentially toxic substances to the biota.
4 Discussion
The four analyzed heavy metals (Hg, Cd, Pb, and Cr) were found in almost all tidal flat sediment samples within the BBE. Their distribution during the 20-month sampling period was not associated with the season. If anthropogenic inputs were relatively constant, temporal variation would be dictated by in situ environmental factors (Buggy and Tobin 2008). The industrial area included within the BBE is one of the heavy metals sources to this environment, considering the reports on sediments, suspended particulate matter, and water (i.e., Marcovecchio and Ferrer 2005; Botté et al. 2007) as well as those on biota from the area (i.e., Fernández Severini et al. 2009). Through these studies, the role of industrial activities as one of the reliable input of metals into the estuary has been highlighted, even though other significant sources have been opportunely identified, including Bahía Blanca city sewage system discharge, harbors operation (including the dredging of the navigation channels), as well as the occurrence of extensive neighboring land areas directed for agricultural and livestock production (Freije and Marcovecchio 2004). Therefore, the mean concentrations of metals (except Hg) in the TF represent the industrial area (PG) and the influence of freshwater input (PC) as the main sites that could be acting as sources to the system.
Mean Cd and Pb concentrations within the TF of intertidal sediments were lower than those reported earlier for close sites within the Principal Channel (Marcovecchio and Ferrer 2005) in the area influenced by industrial and harbor activities. In the case of Cr, both areas closer to the Principal Channel (PG and PC) showed a lower mean concentration in the subtidal sediments. This could be indicating a landward transport or input due to the terrestrial area with settlement into supratidal and intertidal regions and also atmospheric transport followed by wet or dry deposition. Furthermore, total Hg contents were much lower than those corresponding to previous reports during the 1980s and 1990s (De Marco et al. 2006; Marcovecchio et al. 2001) and they were of the same magnitude along the whole inner zone of the BBE.
On the other hand, Cd, Pb, and Cr have presented higher concentrations in the finest fraction (<63 µm) than in the TF of tidal flats sediments in the inner sites principally affected by freshwater. As regards the industrial zone, only Cd has shown a similar behavior because both Pb and Cr were slightly more concentrated in the TF. Although there is not enough information on metal concentrations in the sediment fine fraction, a comparison with previous reports must be done. Grecco et al. (2006) have studied three semienclosed docks at the PG area, reported similar trends to those included here for Cd and Cr, but found higher Pb values, probably because one of the sites receives a direct input of industrial effluents with high load of fine material. According to the results provided by other authors for different estuaries or coastal areas (Buggy and Tobin 2008; Giordano et al. 1992; Villaescusa-Celaya et al. 2000), Cd, Cr, and Pb contents herein found were below those levels recognized as characteristic of polluted environments.
The use of metals geochemical partitioning within contaminated estuarine sediments has been recognized as a strong tool to identify the influence of human activities upon heavy metals distribution at naturals systems (Baptista Neto et al. 2000; Giordano et al. 1992; Lacerda et al. 1988; Salomons and Fösrtner 1984). Hence, a significant decrease on the residual fraction (the major sink for trace metals) or, oppositely, an increase on the other geochemical fractions would be indicating a strong anthropic pressure associated with environmental impact processes. In this sense, several metals (Cd, Pb) showed lesser preference for the residual phase, resulting in an increase in metals biological availability and thus enhancing metal toxicity.
4.1 Mercury
Two important increases were simultaneously observed at the three sampling sites and that would be an indication of an Hg-specific input into this environment. The values of this element have changed along the whole considered region, from levels lower than the detection limit of the applied analytical method to maximum ones near the area most influenced by industries and traffic of cargo vessel. Another source may be the internal cycling/recycling of older contaminated sediments or introduced from the atmosphere. However, all areas presented a homogeneous distribution with very low values in comparison with former data in subtidal sediment samples (Table 5). Furthermore, the reports on the decrease of Hg concentrations in the BBE since 1983 and up to the present reflected important changes within anthropic activities as developed on this area, such as better technology into the industrial processes, most adequate management of effluents and garbage, etc. (De Marco et al. 2006; Marcovecchio et al. 2001).
Finally, the information obtained through several studies would allow to say that Hg stretch out to accumulate in the subtidal sediments as well as in other coastal zones in the world. Within this area, this element is extensively used; however, it is not introduced directly in the system. This element is retained within special containers.
4.2 Cadmium
Elevated Cd concentrations found in estuarine sediments are related to the fact that this element is more ubiquitous in the environment due to a variety and congregation of anthropogenic activities, which provide different sources of metals to intertidal sediments, supported by atmospheric deposition, urban runoff, or different discharges (Emmerson et al. 1997). Intertidal nude sediments in the BBE presented a slightly higher average in the area situated in the middle of an industrial nucleus. Nevertheless, none of the industrial plants of that area use cadmium in their daily processes. Besides, it is more concentrated in the sediments from the subtidal area. Notwithstanding, in the other sites, the content is higher in the intertidal sediments. All these could mean that this metal has different sources within the system. Keeping in mind that this metal is highly toxic even at low concentrations, its accumulation within tidal flat sediments may be largely important as they can act as a metal reservoir, and so a potential Cd source for the estuary. But subtidal sediments more influenced by vessels and ships can also act as an important sink at first and then source to the surrounding environment. Previous records on subtidal sediments have shown values slightly higher but similar in samples from nude tidal flats in the medium region of the estuary (Table 5). There is scarce information linked to this topic in our country. Our results were lower than those reported as typical for moderate to largely contaminated sites (see Table 5).
On the other hand, Cd geochemical partitioning has shown the maximum percentages for the inner region in the same fraction (carbonatic). These results were different from those reported by Marcovecchio and Ferrer (2005) who determined that Cd PBF was not as high (range between 3.8% and 13.4% vs 13% and 40%) over the Principal Channel (subtidal area). Thus, it seemed that processes occurring mainly within the carbonatic fraction take on a significant role in the biogeochemical distribution of cadmium in the nude tidal flats, hindering the transference to more mobile fractions which could be incorporated by organisms.
The accumulation of cadmium in sediments could represent a potential risk to sediment-dwelling organisms living within these tidal flats. Concerning this, specific studies directed to evaluate this topic have not at present been developed, even though the Canadian guidelines for marine/estuarine sediment quality point out concentrations greater than 0.7 mg kg−1 as a limit where this kind of processes could start (CCME, Canadian Council of Ministers of the Environment 1999).
4.3 Lead
Recorded values of Pb on tidal flat sediments were higher within the recreational port/freshwater input areas as well as near the industrial one. There is a refinery within the studied area which used up to 2005 this element as catalyst in the cracking process of heavy hydrocarbons and the Galván creek directly received the effluents from several industries. The Sauce Chico River is influenced by the agricultural and rural development areas located in the surrounding zones and indirectly receives discharges from some factories. The main Pb contribution to the system should be the subtidal sediments with high concentrations (Marcovecchio and Ferrer 2005), which suggest that tidal flats would not be acting as a sink of this element.
As far as the partitioning of lead is concerned, a significant increase was observed in the fraction FII (oxidizable) at all the studied area. So, organic complexes and sulfides appeared to be a more important sink for lead than the other mobile phases. Besides, it was the unique element that reached the highest amounts associated to FIV (reducible compounds). This fraction represents metals absorbed on iron or manganese oxides which are excellent scavengers for trace metals. These results are consistent with the observations of other researchers, particularly those who have worked on uncontaminated areas (Giordano et al. 1992).
4.4 Chromium
The inner region of the BBE showed a similar spatial and temporal variation. The observed levels were similar to or lesser than those reported for other coastal areas in the country as well as in the world (Table 5). The fact that its concentration was higher within the intertidal sediment suggests the occurrence of a transport process from land to the coastal marine environment. Our results were quite different to Cr values reported in sediments from La Plata river estuary, Argentina (Bilos et al. 1998) where the determined higher contents reflected the influence of the most industrialized and populated region. In addition, our results were lower than those from environments recognized as polluted ones (i.e., Mexico, Spain, and China). Thus, BBE sediments do not show chromium enrichment with respect to other coastal areas. Iron–chromium catalysts are recently used in the area of study and seemed to be an explanation of the low concentration. Moreover, there were no differences between sites at industrial area and others; it also suggests a narrow adsorption to organic matter within sediments (Turner and Millward 2002), which is also related with the high occurrence of suspended particulate matter.
The geochemical partitioning of Cr in the inner region of the BBE reflected an increase in three fractions (exchangeable, oxidizable, and reducible) compared with previous results on the subtidal sediments (Marcovecchio and Ferrer 2005). This fact is suggesting a recent input of this metal, possibly as a result of a new source within the area. Keeping in mind that most of Cr was geochemically linked to the lithogenous fraction, our results allow to sustain that this metal preferentially appeared associated with the oxidizable fraction within the studied coastal sediments, which agreed with previous reports by other authors (i.e., Lacerda et al. 1988) who have highlighted similar trends at different eutrophicated estuarine systems. In other sites recognized as highly contaminated, the carbonatic fraction has demonstrated to be the more important one (Ponce-Velez and Botello 1991). The importance of the oxidizable fraction in both the organic-rich estuaries and those with natural eutrophic conditions such as the BBE (Freije and Marcovecchio 2004) results in a synergistic interaction between organic and metal contamination, since the partial reducing conditions of these areas allow the remobilization of metals from the reducible form (Lacerda et al. 1988).
The evaluation of the PBF for the three evaluated metals has shown significant percentages, mainly in the cases of lead and chromium, a fact that indicated they could be assimilated by the organisms within this environment.
5 Conclusions
Immobilized forms of metals within estuarine sediments constitute a potential hazard to quality water, considering that they can be released into the aquatic environment due to chemical changes (e.g., increase of salinity, decrease of pH, change in Eh conditions, microbial activity). The effect of coastal human activities on heavy metals distribution has been evaluated using surface tidal flats sediments from the nude intertidal areas of the BBE. Between heavy metals, Hg, Cd, Pb, and Cr are considered as not essential to biota, as well as extremely toxic even at very low concentrations.
Although Cd and Pb showed significant differences between sites, the distribution in the inner region of the estuary has shown to be homogenous enough. The relationship of the concentration with the sediments grain size confirmed the preferential association of metals with fine fraction. The absence of correlation between Hg, Cd, Pb, and Cr suggest different sources or different effects of the waters covering the extensive tidal flats. Samples have shown many differences in metals speciation. The area most influenced by industrial and harbor activities has shown the highest Pb and Cr potentially bioavailable geochemical fraction, even Pb also presented high bioavailability within all inner region. It is very important to highlight that it represents a high risk for plants and animals, considering that these metals could be easily mobilized and consequently bioconcentrated or bioaccumulated by organism (Ponce-Velez and Botello 1991), including the human population. The enrichment of metals in the intertidal sediments could be attributed to land source or to the deposition of the dissolved and particulate trace metals in the water column at the estuarine area.
Although it appears that all studied metal inputs from industrial sources overreached natural ones within the BBE, the measured concentrations were lower than those reported for other heavily industrialized estuaries. In addition, the agricultural and rural developments which surround and influence streams and rivers outflowing within the estuary must also be considered. Several metals (Cd, Pb, and Cr) are included in the composition of fertilizers, which are intensively used within this area, and so they may constitute a significant source of metals for the estuarine system (Caccia et al. 2003). Cr was strongly associated to silicates and other minerals, limiting their potential toxicity as pollutants. But also, it showed a moderate bioavailability. On the other hand, Cd and particularly Pb presented low association with the residual phase and Pb showed a very high availability within the inner region.
Do the sediments act effectively retaining heavy metals? The present results allow and sustain that the intertidal sediments can act as a source of several metals and also as a sink to others (i.e., Cr). The intertidal sediments provide much more complete information than estuarine sediments do. In order to develop pollution control strategies and approaches to water quality management in a coastal area, the study of temporal as well spatial distribution of contaminants like metals is necessary. The concentrations obtained in the TF can be assumed as the background values for the system and they are a relevant reference for the whole marine coastal area of Argentina. Therefore, it is important to implement and execute appropriate studies directed to monitor and control pollutant actions in order to avoid both alterations in the biodiversity as well as in the diagenesis of sediments from the BBE coastal area. In this sense, nude tidal flat sediments may provide an effective information source about ecosystem status influenced by different human pressures, which represents not only regional but also international importance through comparatives studies within similar estuarine systems.
References
Adamo, P., Arienzo, M., Imperato, M., Naimo, D., Nardi, G., & Stanzione, D. (2005). Distribution and partition of heavy metals in surface and sub-surface sediments of Naples city port. Chemosphere, 61, 800–809.
Amín, O., Ferrer, L., & Marcovecchio, J. (1996). Heavy metal concentrations in littoral sediments from the Beagle Channel, Tierra del Fuego, Argentina. Environmental Monitoring and Assessment, 41, 219–231.
Andrade, S., Pucci, A., & Marcovecchio, J. E. (2000). Cadmium concentrations in the Bahía Blanca Estuary (Argentina). Potential effects of dissolved cadmium on the diatom Thalassiosira curviseriata. Oceanologia, 42, 505–520.
Andrade, S., Poblet, A., Scagliola, M., Vodopivez, C., Curtosi, A., Pucci, A., et al. (2001). Distribution of heavy metals in surface sediments from an Antarctic marine ecosystem. Environmental Monitoring and Assessment, 66, 147–158.
Baptista Neto, J. A., Smith, B. J., & McAllister, J. J. (2000). Heavy metal concentrations in surface sediments in a nearshore environment, Jurujuba Sound, Southest Brazil. Environmental Pollution, 109, 1–9.
Bilos, C., Colombo, J. C., & Rodríguez Presa, M. J. (1998). Trace metals in suspended particles, sediments and Asiatic clams (Corbicula fluminea) of the Río de la Plata Estuary, Argentina. Environmental Pollution, 99, 1–11.
Bokuniewicz, H. (1995). Sedimentary systems of coastal plain estuaries. In G. M. E. Perillo (Ed.), Geomorphology and sedimentology of estuaries. Developments in sedimentology (Vol. 53, pp. 49–67). Amsterdam: Elsevier.
Botté, S. E., Freije, R. H., & Marcovecchio, J. E. (2007). Dissolved heavy metal (Cd, Pb, Cr, Ni) concentrations in surface water and porewater from Bahía Blanca estuary tidal flats. Bulletin of Environmental Contamination and Toxicology, 79, 415–421.
Buggy, C. J. & Tobin, J. M. (2008). Seasonal and spatial distribution of metals in surface sediment of an urban estuary. Environmental Pollution, 155, 308–319.
Caccia, V. G., Millero, F. J., & Palanques, A. (2003). The distribution of trace metals in Florida Bay sediments. Marine Pollution Bulletin, 46, 1420–1433.
CCME (Canadian Council of Ministers of the environment) (1999). Canadian sediment quality guidelines for the protection of aquatic life: Cadmium. In Canadian environmental quality guidelines, 1999, Canadian Council of Ministers of the Environment, Winnipeg.
Conaway, C. H., Squire, S., Mason, R. P. & Flegal, A. R. (2003). Mercury speciation in the San Francisco Bay estuary. Marine Chemistry, 80, 199–225.
Dalziel, J. & Baker, C. (1983). Métodos analíticos para medir la presencia de metales mediante espectrofotometría de absorción atómica. FAO, Documento Técnico de Pesca, 212, 15–22.
De Marco, S., Botté, S. E., & Marcovecchio, J. (2006). Mercury distribution in abiotic and biological compartments within several estuarine systems from Argentina: 1980–2005 period. Chemosphere, 65, 213–223.
Emmerson, R. H. C., O'Reilly-Weise, S. B., Macleod, C. L., & Lester, J. N. (1997). A multivariate assessment of metal distribution in inert-tidal sediments of the Blackwater Estuary, UK. Marine Pollution Bulletin, 34, 960–968.
Federal Register (1984). Definition and procedure for determination of the method detection limit, EPA, 40 CFR Part 136, Appendix B, Revision 1.11, pp. 198–199.
Fernández Severini, M. D., Botté, S. E., Hoffmeyer, M., & Marcovecchio, J. E. (2009). Spatial and temporal distribution of cadmium and copper in water and zooplankton in the Bahía Blanca Estuary, Argentina. Estuarine, Coastal and Shelf Science, 85, 57–66. doi:10.1016/j.ecss.2009.03.019.
Ferrer, L. D. (2001). Estudio de diversos metales pesados en sedimentos del estuario de Bahía blanca y sus efectos tóxicos sobre el cangrejo Chasmagnathus granulata. Doctoral Thesis, Univerisidad Nacional del Sur, Argentina, 213 pp.
Ferrer, L., Contardi, E., Andrade, S. J., Asteasuain, R., Pucci, A. E., & Marcovecchio, J. E. (2000). Environmental cadmium and lead concentrations in the Bahía Blanca Estuary (Argentina). Potential toxic effects of Cd and Pb on crab larvae. Oceanologia, 42, 493–504.
Freije, R. H. & Marcovecchio, J. E. (2004). Oceanografía química. In M. C. Piccolo & M. Hoffmeyer (Eds.), Ecosistema del estuario de bahía blanca (pp. 69–78). Argentina: Bahía Blanca.
Gelós, E. M., Marcos, A. O., Spagnuolo, J. O., & Schillizzi, R. A. (2004). Textura y mineralogía de sedimentos. In M. C. Piccolo & M. Hoffmeyer (Eds.), Ecosistema del estuario de bahía blanca (pp. 43–50). Argentina: Bahía Blanca.
Giordano, R., Musmeci, L., Ciaralli, I., Vernillo, M., Chirico, A., Piccioni, A., et al. (1992). Total contents and sequential extractions of mercury, cadmium, and lead in coastal sediments. Marine Pollution Bulletin, 24, 350–357.
Grecco, L. E., Marcos, A. O., Gómez, E. A., Botté, S. E., & Marcovecchio, J. E. (2006). Natural and anthropogenic input of heavy metals in sediments from Bahía Blanca Estuary (Argentina). Journal of Coastal Research, SI 39 (Proceedings of the 8th International Coastal Symposium), 1021–1025.
Hempel, M., Botté, S. E., Negrin, V. L., Chiarello, M. N., & Marcovecchio, J. E. (2008) The role of the smooth cordgrass Spartina alterniflora and associated sediments in the heavy metal biogeochemical cycle within Bahía Blanca estuary salt marshes. Journal of Soils and Sediments, 8, 289–297.
Hylander, L. D., Maili, M., Oliveira, L. J., de Castro e Silva, E., Guimarães, J. R. D., Araujo, D. M., et al. (2000). Relationship of mercury with aluminum, iron and manganese oxy-hydroxides in sediments from the Alto Pantanal, Brazil. Science of the Total Environment, 260, 97–107.
Ip, C. C. M., Li, X.-D., Zhang, G., Wai, O. W. H., & Li, Y.-S. (2007). Trace metal distribution in sediments of the Pearl River Estuary and the surrounding coastal area, South China. Environmental Pollution, 147, 311–323.
Jones, R. P., & Clarke, J. U. (2005). Analytical chemistry detection limits and the evaluation of dredged sediment. ERDC/TN EEDP-04-36, U.S. Army Engineer Research and Development Center, Vicksburg, MS.
Krumbein, W. C. & Pettijohn, F. J. (1938). Manual of sedimentary petrography. New York: Appleton–Century–Crofts.
Lacerda, L. D., Souza, C. M. M., & Pestana, M. H. D. (1988). Geochemical distribution of Cd, Cu, Cr, and Pb in sediment of estuarine areas along the Southeastern Brazilian coast. In U. Seeliger, L. D. Lacerda & S. R. Patchineelam (Eds.), Metals in coastal environments of Latin America (pp. 86–99). Berlin: Springer.
Maddock, J. E. L. & Lopes, C. E. A. (1988). Behavior of pollutant metals in aquatic sediments. In U. Seeliger, L. D. Lacerda & S. R. Patchineelam (Eds.), Metals in coastal environments of Latin America (pp. 100–105). Berlin: Springer.
Marcovecchio, J. E. & Ferrer, L. D. (2005). Distribution and geochemical partitioning of heavy metals in sediments of the Bahía Blanca Estuary, Argentina. Journal of Coastal Research, 21, 826–834.
Marcovecchio, J. E., Lara, R. J., & Gómez, E. (1986). Total mercury in marine sediments near a sewage outfall. Relation with organic matter. Environmtal Technology Letters, 7, 501–507.
Marcovecchio, J. E., Moreno, V. J., & Perez, A. (1988). Determination of heavy metal concentrations in biota of Bahía Blanca, Argentina. Science of the Total Environment, 75, 181–190.
Marcovecchio, J. E., Asteasuain, R., Rusansky, C., Ferrer, L., Andrade, S., & Asteasuain, A. (1997). Estudio de la calidad de agua en la ria de Bahía Blanca. Capítulo 1: Quimica Marina. Technical Report. Instituto Argentino de Oceanografía (IADO), Bahía Blanca, Argentina, 48 pp.
Marcovecchio, J. E., Andrade, S., Ferrer, L. D., Asteasuain, R. O., De Marco, S. G., Gavio, M. A., et al. (2001). Mercury distribution in estuarine environments from Argentina: The detoxification and recovery of salt marshes after 15 years. Wetlands Ecology and Management, 9, 317–322.
Marcovecchio, J. E., Botté, S. E., Delucchi, F., Arias, A. H., Fernández Severini, M. D., De Marco, S. G., et al. (2008). Pollution processes in Bahía Blanca estuarine environment. In R. Neves, J. Baretta & M. Mateus (Eds.), Perspectives on integrated coastal zone management in South America (pp. 301–314). Lisbon: IST.
Megalatti, N., Robbe, O., Marchandise, P., & Astruk, M. (1983). A new chemical extraction procedure in the fractionation of heavy metals in sediments. In U. Förstner (Ed.), Proceeding of international conference heavy metals in the environment (Vol. 4, pp. 1090–1093). Edinburgh: CEP Consultants.
Melo, W. D. & Limbozzi, F. (2008). Geomorphology, hydrological systems and land use of Bahía Blanca estuarine region. In R. Neves, J. Baretta & M. Mateus (Eds.), Perspectives on integrated coastal zone management in South America (pp. 317–331). Lisbon: IST.
Perillo, G. M. E., Piccolo, C., Parodi, E., & Freije, R. H. (2001). Bahía Blanca Estuary, Argentina. In U. Seeliger & B. Kjerfve (Eds.), Coastal marine ecosystems of Latin America, ecological studies (Vol. 144, pp. 205–217). Berlin: Springer.
Piccolo, M. C. & Perillo, M. E. G. (1990). Physical characteristics of the Bahía Blanca Estuary (Argentina). Estuarine, Coastal and Shelf Science, 31, 303–317.
Ponce-Velez, G. & Botello, A. V. (1991). Aspectos geoquímicos y de contaminación por metales pesados en la Laguna de Términos, Campeche. Hidrobiológica, 1, 1–10.
Pucci, A. E. (1988). Heavy metals in water and sediments of the Blanca Bay, Argentina. In U. Seeliger, L. D. de Lacerd & S. R. Patchineelam (Eds.), Metals in coastal environments of Latin America (pp. 9–15). Berlin: Springer.
Pucci, A. E., Freije, R. H., Asteasuain, R. O., Zavatti, J. R., & Sericano, J. L. (1979). Evaluación de la contaminación de las aguas y sedimentos de la Bahía Blanca. Contribución Científica I.A.D.O. (52), Bahía Blanca, Argentina.
Pucci, A. E., Freije, R. H., Asteasuain, R. O., Zavatti, J. R., & Sericano, J. L. (1980). Evaluación de la contaminación de las aguas y sedimentos de la Bahía Blanca. Contribución Científica I.A.D.O. (56), Bahía Blanca, Argentina.
Ram, A., Rokade, M. A., Borole, D. V., & Zingde, M. D. (2003). Mercury in sediments of Ulhas estuary. Marine Pollution Bulletin, 46, 846–857.
Ramirez, M., Massolo, S., Frache, R., & Correa, J. A. (2005). Metal speciation and environmental impact on sandy beaches due to El Salvador copper mine, Chile. Marine Pollution Bulletin, 50, 62–72.
Rosales-Hoz, L., Cundy, A. B., & Bahena-Manjarrez, J. L. (2003). Heavy metals in sediments cores from a tropical estuary affected by anthropogenic discharges: Coatzacoalcos estuary, Mexico. Estuarine, Coastal and Shelf Science, 58, 117–126.
Ruiz, F. (2001). Trace metals in estuarine sediments from the southwestern Spanish coast. Marine Pollution Bulletin, 42, 482–490.
Salomons, W. & Fösrtner, U. (1984). Metals in the hydrocycle. 3. Sediments and the transport of metals. Berlin: Springer.
Sericano, J. L. & Pucci, A. E. (1982). Cu, Cd and Zn in Blanca Bay surface sediments, Argentina. Marine Pollution Bulletin, 13, 429–431.
Spencer, K. L., Cundy, A. B., & Croudace, I. W. (2003). Heavy metal distribution and early-diagenesis in salt marsh sediments from the Medway, Kent, UK. Estuarine, Coastal and Shelf Science, 57, 43–54.
StatSoft, Inc. (2004). STATISTICA (data analysis software system), version 7. Retrieved from http://www.statsoft.com.
Turner, A. & Millward, G. E. (2002). Suspended particles: Their role in estuarine biogeochemical cycles. Estuarine, Coastal and Shelf Science, 55, 857–883.
Villa, N. (1988). Spatial distribution of heavy metals in seawater and sediments from coastal areas of the southeastern Buenos Aires Province, Argentina. In U. Seeliger, L. D. de Lacerda & S. R. Patchineelam (Eds.), Metals in coastal environments of Latin America (pp. 33–44). Berlin: Springer.
Villaescusa-Celaya, J. A., Gutiérrez-Galindo, E. A., & Flores-Muñoz, G. (2000). Heavy metals in the fine fraction of coastal sediments from Baja California (Mexico) and California (USA). Environmental Pollution, 108, 453–462.
Acknowledgements
The authors are greatly indebted to Lic. Federico Delucchi (IADO) for his help in samples processing and chemical analysis; Dr. Laura E. Grecco and Dr. Angel O. Marcos (UNS) for their assistance with the geochemical partitioning analysis. Thanks to Raul Asteasuain and M. Nedda Chiarello (IADO) for their assistance during sampling and laboratory work. Mrs. Mariela Gherbi has kindly reviewed the English version of this manuscript. Thanks also to the anonymous referee who greatly contributed with a critical reading and valuable corrections to the improvement of the original manuscript. This research was funded through grants by ANPCyT (PICT No. 07-11636/02 and PICT 0945/06) from Argentina.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Botté, S.E., Freije, R.H. & Marcovecchio, J.E. Distribution of Several Heavy Metals in Tidal Flats Sediments within Bahía Blanca Estuary (Argentina). Water Air Soil Pollut 210, 371–388 (2010). https://doi.org/10.1007/s11270-009-0260-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-009-0260-0