Abstract
Chromium (Cr) is a biologically non-essential, carcinogenic and toxic heavy metal. The cultivation of Cr-tolerant genotypes seems the most favorable and environment friendly strategy for rehabilitation and remediation of Cr-contaminated soils. To prove this hypothesis and identify the Cr tolerance, the present study was performed to assess the physiological and biochemical response of sunflower genotypes to Cr stress. The seeds of six sunflower hybrids, namely FH-425, FH-600, FH-612, FH-614, FH-619, and FH-620, were grown in spiked soil for 12 weeks under increasing concentrations of Cr (0, 5, 10, and 20 mg kg−1). A seed germination test was also run under different concentrations of Cr (0, 5, 10, 200 mM) in petri dishes. Plants were harvested after 12 weeks of germination. Different plant attributes such as growth; biomass; photosynthesis; gas exchange; activity of antioxidant enzymes, i.e., superoxide dismutase (SOD), guaiacol peroxidase (POD), ascorbate (APX), and catalases (CAT); reactive oxygen species (ROS); lipid peroxidation; electrolyte leakage; and Cr concentration as well as accumulations in all plant parts were studied for the selection of the most Cr-tolerant genotype. Increasing concentration of Cr in soil triggered the reduction of all plant parameters in sunflower. Cr stress increased electrolyte leakage and production of reactive oxygen species which stimulated the activities of antioxidant enzymes and gas exchange attributes of sunflower. Chromium accumulation in the root and shoot increased gradually with increasing Cr treatments and caused reduction in overall plant growth. The accumulation of Cr was recorded in the order of FH-614 > FH-620 > FH-600 > FH-619 > FH-612 > FH-425. The differential uptake and accumulation of Cr by sunflower hybrids may be useful in selection and breeding for Cr-tolerant genotypes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anthropogenic activities, such as application of contaminated effluents, agrochemicals, phosphate fertilizers, industrial waste, sewage sludge, and pesticides, are the main cause of heavy metal contamination of soils (Adrees et al. 2015a; Rehman et al. 2015; Rizwan et al. 2016a). Along with anthropogenic activities, the natural sources like run off, forest fires, volcanic eruption, and production of sea salt aerosols are also a major cause of heavy metal release into the environment (Farid et al. 2015; Nagajyoti et al. 2010).
The wide industrial applications of chromium (Cr) make it a serious environmental pollutant. Chromium can be found in different states from Cr-I to Cr-VI while the most toxic form is Cr-VI, followed by Cr-III (Afshan et al. 2015). The extensive use of Cr6+ compounds in tanning, cooling water treatment, metal plating, and wood preservation industry is the main source of Cr contamination, and its bio-mobility and bioavailability in the environment (Ali et al. 2015).
In Pakistan, leather industry is the major source of Cr6+ release into the environment, which seriously affects the growth and development of living organisms (Ali et al. 2015). Different chemicals such as, chromium sulfate (CrSO4), formic acid (CH2O2), sodium chloride (NaCl), sodium bicarbonate (NaHCO3), magnesium sulfate (MgSO4), calcium hydroxide (Ca(OH)2), sulfuric acid (H2SO4), dyes, and fat liquors are being used during the tanning operation in leather industries (Ali et al. 2015).
Several studies have reported that Cr toxicity decreased the plant growth and biomass by generating reactive oxygen species at cellular and sub-cellular level (Atta et al. 2013; Bukhari et al. 2016a).
Under metal stress, a natural defense system has been evolved in plants with the involvement of both non-enzymatic and enzymatic antioxidants, synthesis of chelating agents, and production of osmolytes (Rizwan et al. 2015; Adrees et al. 2015a; Artiushenko et al. 2014). But the defense mechanism mainly depends upon the type of metal, plant species and genotype (Artiushenko et al. 2014).
Sunflower (Helianthus annuus L.) is a metal hyper-accumulator plant, capable of tolerating a certain concentration of Cr without showing toxic symptoms (Atta et al. 2013). But most of the times, Cr toxicity adversely affects growth and development of sunflower, that mainly depends upon metal concentration and growth medium (Fozia et al. 2008). The ability of sunflower to improve its biomass production helps the plants cope with Cr toxicity (Chae et al. 2014). Rizwan et al. (2016a, b) comprehensively reviewed the toxicity mechanisms in sunflower under other heavy metals like Pb, Cd, Cu, Ni, Zn, and Cr as well. Higher biomass production facilitates the accumulation of large amount of Cr from the growth media and its translocation towards the aerial plant parts (Atta et al. 2013; Cutright et al. 2010). The propensity of sunflower for the accumulation of metals, like Cr (Atta et al. 2013; Fozia et al. 2008), Cd (Júnior et al. 2015), Zn, Pb (Adesodun et al. 2010; Azad et al. 2011; Doncheva et al. 2013), Cu (Jiang et al. 2000; Kolbas et al. 2014), and As (Imran et al. 2013), has already been proved.
A significant enhancement was observed in metal extraction ability of a sunflower mutant which was 8.2, 9.2, and 7.5 times higher than that in control plants for Pb, Zn, and Cd, respectively, in aboveground plant parts (Nehnevajova et al. 2007). Furthermore, the biomass and oil of sunflower can be used for biodiesel, lubricants, and biogas production which should add an additional value and enhance the economic viability of phytoextraction (Harris et al. 2016; Del Gatto et al. 2015).
This suggests that existing varieties/cultivars of sunflower should be characterized for Cr uptake on the basis of their translocation ability. The present study was conducted to evaluate the Cr toxicity in terms of induced changes in morph-physiological and biochemical attributes of six sunflower hybrids and their potential to accumulation Cr from soil.
Materials and methods
Soil treatments, germination test, and growth conditions
A clay loam (25% sand, 22% silt, 53% clay) soil, collected at 0–15-cm depth from botanical garden of the Ayub Agriculture Research Institute (AARI), Faisalabad, Pakistan, was used in the present study. Prior to pot experiment, the soil was air-dried and passed through a 2-mm-diameter sieve to remove crop residues and stones and further characterized for chemical and physical properties (Table 1). Mature seeds of six sunflower genotypes (Faisalabad hybrid: FH-600, FH-612, FH-614, FH-619, FH-620, and FH-425) were collected from the Oilseed Research Institute, AARI, Faisalabad, Pakistan. To check the germination potential of these six hybrids, a test was carried out in petri dishes under different concentrations of Cr (0, 5, 10, 20 mM) in triplicates. Firstly, the seeds were washed with 10% H2O2 to remove germs and bacteria, followed by rinsing with distilled water. For germination test, ten seeds were placed in each petri dish and then incubated at 22 °C till germination in comparison to controls. Ten seeds were sown in each earthen pot containing 5 kg soil spiked with increasing Cr concentration (0, 5, 10, 20 mg kg−1 dry weight) under wire house conditions. After 15 days of germination, thinning was done to maintain five plants per pot and the pulled up plants were crushed carefully into the same pot. Each pot was fertilized with a 500-mL solution containing 2.19 g L−1 N (as (NH2)2CO), 0.5 g L−1 P (as (NH4)2HPO4), and 2.14 g L−1 K (as K2SO4). The fertilizer solution was applied after 15 and 30 days of germination. All glass wares were thoroughly rinsed with 10% HNO3 and then washed with distilled water to avoid contamination.
Plant sampling and analysis
Plants were harvested after 10 weeks of treatment. The roots were carefully detached from the soil. Deionized water was used to wash the plant samples. All plants were carefully separated into the roots, stems, and leaves. Then, growth parameters like plant height, root and stem length, leaves and flowers per plant, leaf area, and fresh and dry biomass of all plant parts including achene were measured. A simple electric balance was used for biomass measurements and leaf area was measured by leaf area meter (L1-2000, L1-COR, USA). The samples were then oven dried at 70 °C, until constant weight, for further analysis.
Pigment content assay
After 10 weeks of treatment, the second uppermost fully extended fresh leaves were used for chlorophyll (a, b, total chlorophylls) and carotenoids contents. The pigments were extracted in the dark with 85% (v/v, Sigma) aqueous acetone solution at 4 °C by insistent shaking until complete discoloration of the leaves. The extract was centrifuged at 4000 × rpm at 4 °C for 10 min, and the supernatant was used for light absorbance at 663, 644, and 452.5 nm with spectrophotometer (Halo DB-20/DB-20S, Dynamica Company, London, UK) (Metzner et al. 1965). The concentrations of chlorophylls and carotenoids were calculated according to the adjusted extinction coefficients and following equations given by Lichtenthaler (1987).
Chromium content analysis
Plant samples were dried at 70 °C and the known weight of plant root, stem, and leaves was placed in muffle furnace at 650 °C for 6 h. Plant samples turned into ash after 6 h. The ash was dissolved in a combination of 3 mL nitric acid (HNO3) and 2-mL hydrochloric acid (HCl) solution. The extract were diluted to 50 mL with distilled water and analyzed for Cr content by using flame atomic absorption spectrometry (novA A400 Analytik Jena, Germany), following Ehsan et al. (2013).
Cr concentration was calculated by the following formula:
where
Cr accumulation was calculated as follows:
The translocation factor (TF) was calculated as the ratio of shoot Cr concentration to root Cr concentration and shoot Cr concentration to leaf Cr concentration (Baker and Whiting 2002).
Determination of electrolyte leakage, MDA, and H2O2
The method described by Dionisio-Sese and Tobita (1998) was followed to measure electrolyte leakage (EL). After 8 weeks of treatment, the uppermost fully expanded leaves were cut into pieces of about 5-mm length and placed in test tubes filled with 8 mL of distilled water. The electrical conductivity of initial medium (EC1) was recorded after 2 h of incubation in a water bath at 32 °C. To measure electrical conductivity of second medium (EC2), the same samples were autoclaved for 20 min at 121 °C to release all the electrolytes into the solution. The samples were cooled to 25 °C and pH/conductivity meter (model 720, INCO-LAB Company, Kuwait) was used to record EC2. Finally, the EL was computed using the following equation:
The malondialdehyde (MDA), a product of lipid peroxidation in leaf and root tissues of plants, was measured by the thiobarbituric acid (TBA) reaction as described by Heath and Packer (1968) with some minor amendments mentioned by Zhang and Kirham (1994) and Dhindsa et al. (1981). Leaf and root samples (0.25 mg) were homogenized in 5 mL of 0.1% trichloro acetic acid (TCA). The resultant was centrifuged for 5 min at 10,000×g. Four-milliliter 20% TCA containing 0.5% TBA was mixed in 1-mL aliquot of the supernatant. The mixture was suddenly cooled in ice bath after heating at 95 °C for 30 min. After centrifugation for 10 min at 10,000×g, the reading for nonspecific absorption at 600 nm was subtracted from the absorbance of supernatant recorded at 532 nm. An extinction coefficient of 155 mM−1 cm−1 was applied to calculate MDA content.
The hydrogen peroxide (H2O2) content was analyzed colorimetrically as presented by Jana and Choudhuri (1981). Leaf/root tissues (50 mg) were homogenized with 3 mL of phosphate buffer (50 mM, pH 6.5) to get H2O2 extract. The homogenate was centrifuged for 25 min at 6000×g. To measure H2O2 contents, 1 mL of 0.1% titanium sulfate in 20% (v/v) H2SO4 was mixed with 3 mL of extracted solution, and then mixture was centrifuged for 15 min at 6000×g. The supernatant was analyzed at 410 nm. The extinction coefficient of 0.28 μmol−1 cm−1 was applied to calculate H2O2 contents.
Gas exchange attributes and SPAD value
Eight weeks after Cr exposure, gas exchange parameters such as stomatal conductance (Gs), photosynthetic rate (A), water use efficiency (A/E), and transpiration rate (E) were measured from the second upper most fully expanded leaf, using portable infrared gas analyzer (LCA-4 ADC, Analytical Development Company, Hoddesdon, England) (Shakoor et al. 2014; Farid et al. 2015). Readings were recorded between 11:00 to 13:00 by clamping the middle part of the leaf in the chamber of the instrument to keep temperature and photon flux density unchanged. Chlorophyll measurement, in terms of soil plant analysis development (SPAD) value, was performed on second upper most fully expanded leaf with the help of SPAD meter (SPAD-502).
Evaluation of antioxidant enzymes and protein content
Antioxidant enzymes including guaiacol peroxidase (POD), superoxide dismutase (SOD), ascorbate (APX), and catalase (CAT) in the leaves and roots were assessed spectrophotometrically. After 8 weeks of treatment, second fully expanded leaf and root samples were taken for enzymatic analysis. The samples (1.0 g) were quickly frozen in liquid nitrogen (N2) and ground with pre-cooled pestle and mortar. This ground samples were homogenized with 0.05 M phosphate buffer (pH 7.8) and filtered through four layers of muslin cloth and then centrifuged for 10 min at 12,000×g, at 4 °C. The final supernatant was used for the estimation of POD and SOD activities according to Zhang (1992). Soluble protein content was measured from same supernatant using Coomassie brilliant blue G-250 as a dye and albumin as a standard as reported by Bradford (Bradford 1976).
The activity of catalase (CAT, EC 1.11.1.6) was measured according to the method of Aebi (1984). The sample mixture consisted of 2.8 mL 50-mM phosphate buffer with 2 mM CA (pH 7.0), 100 μL enzyme extract, and 100 μL H2O2 (300 mM). The CAT activity was recorded by measuring the decrease in absorbance at 240 nm as a consequence of H2O2 disappearance (ε = 39.4 mM−1 cm−1).
The activity of ascorbate peroxidase (APX, EC 1.11.1.11) was measured according to the method of Nakano and Asada (1981). The sample mixture consisted of 2.7 mL 25-mM potassium phosphate buffer with 2 mM CA (pH 7.0), 100 μL H2O2 (300 mM, 100 μL ascorbate (7.5 mM), and 100 μL enzyme extract. The oxidation activity of ascorbate was recorded by measuring the change in wavelength at 290 nm (ε = 2.8 mM−1 cm−1).
Statistical analysis
All values presented in this study are mean of three replicates. Analysis of variance (ANOVA) was done by using a statistical package, SPSS version 16.0 (SPSS, IBM, 2009) followed by Tukey’s post hoc test between the means of treatments to determine the significant difference and standard deviation.
Results
Seed germination and growth traits
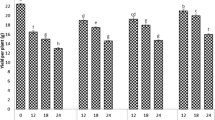
The variations in sunflower growth parameters including plant height, root length, number of leaves, number of flowers, and leaf area are shown in Fig. 1. Chromium application reduced seed germination (Table 2) and growth of all cultivars of sunflower as compare to control treatment. However, the reduction in germination and growth was Cr concentration and genotype-dependent. The germination and growth gradually decreased with increasing concentration of Cr. Maximum decline was observed in the plants grown under the highest Cr concentration (20 mg kg−1) while FH-425 proved to be the most vulnerable genotype at the highest Cr concentration. The genotypic tolerance to increasing Cr concentration, regarding all growth parameters and germination, was in the order of FH-614 > FH-620 > FH-600 > FH-619 > FH-612 > FH-425.
Effect of Cr on plant height (a), root length (b), number of leaves (c), number of flower (d), and leaf area (e) in six different varieties of sunflower grown in soil with increasing Cr concentrations (0, 5, 10, and 20 mg kg−1). Values are demonstrated as means of three replicates along with standard deviation. Different letters indicate that values are significantly different at P < 0.05
Biomass
The difference in biomass accumulation among the six varieties of sunflower, exposed to different levels of Cr, is presented in Fig. 2. Increasing Cr concentration in growth media significantly decreased the biomass like leaf, stem, and root fresh and dry weight of plants. However, different varieties showed different trend for each parameter. For leaf fresh and dry weight as well as stem dry weight, the response of different varieties was as follow:
Effect of Cr on leaf fresh weight (a), leaf dry weight (b), stem fresh weight (c), stem dry weight (d), root fresh weight (e), and root dry weight (f) in six different varieties of sunflower grown in soil with increasing Cr concentrations (0, 5, 10, and 20 mg kg−1). Values are demonstrated as means of three replicates along with standard deviation. Different small letters indicate that values are significantly different at P < 0.05
For stem fresh weight, root fresh weight, and root dry weight, the results are presented in Fig. 2c, e, f. FH-614 proved to be the most tolerant genotype at all Cr levels. However, the overall genotypic response to Cr, regarding these traits, is presented below:
Chlorophyll content and carotenoids
Chlorophyll (a, b and total chlorophyll) and carotenoids concentrations significantly decreased in all the varieties of sunflower with increasing Cr concentrations in soil (0, 5, 10, 20 mg kg−1) as given in Fig. 3. Regarding genotypes, FH-614 was found to be the most tolerant and FH-425 the most susceptible variety at all the Cr levels. The general trend of chlorophyll (a, b, total chlorophyll) and carotenoids among the six varieties, as affected by Cr, is given below:
Effect of Cr on chlorophyll a (a), chlorophyll b (b), total chlorophyll (c), and carotenoids (d) in six different varieties of sunflower grown in soil with increasing Cr concentrations (0, 5, 10, and 20 mg kg−1). Values are demonstrated as means of three replicates along with standard deviation. Different small letters indicate that values are significantly different at P < 0.05
Gas exchange attributes
The Cr-induced effects on gas exchange attributes of sunflower varieties are given in Fig. 4. Generally, the increasing Cr concentration significantly decreased water use efficiency (Fig. 4a), transpiration rate (Fig. 4b), net photosynthetic rate (Fig. 4c), and stomatal conductance (Fig. 4d) of sunflower as compared to those in the control. However, the genotypic response of sunflower, to Cr stress, was variable. The lowest and highest gas exchange attributes were shown by FH-425 and FH-614, respectively, at each Cr treatment, as compare to the other varieties. The overall response of genotypes was in the order as follows:FH-614 > FH-620 > FH-600 > FH-619 > FH-612 > FH-425.
Effect of Cr on water use efficiency (a), transpiration rate (b), net photosynthetic rate (c), and stomatal conductance (d) in six different varieties of sunflower grown in soil with increasing Cr concentrations (0, 5, 10, and 20 mg kg−1). Values are demonstrated as means of three replicates along with standard deviation. Different letters indicate that values are significantly different at P < 0.05
Soluble protein and SPAD value
Soluble protein (leaf and root) and SPAD value of all the varieties of sunflower showed significant reduction with increasing Cr concentrations, as compare to those in the control (Fig. 5a, b, c).
Effect of on soluble protein in the root (a), soluble protein in the leaves (b), and SPAD value (c) in six different varieties of sunflower grown in soil with increasing Cr concentrations (0, 5, 10, and 20 mg kg−1). Values are demonstrated as means of three replicates along with standard deviation. Different letters indicate that values are significantly different at P < 0.05
The FH-425 showed the lowest SPAD value in each treatment as compared to the other five varieties and the highest values were observed in FH-614, at all the Cr concentrations. Similar pattern was found in soluble protein content of the roots and leaves. The sequence of SPAD and soluble protein content in all the varieties is given below:
Electrolyte leakage, MDA. and H2O2
Oxidative stress in the different varieties of sunflower was evaluated by measuring the concentration of electrolyte leakage (EL), hydrogen peroxide (H2O2), and malondialdehyde (MDA) in the leaves and root of sunflower (Supplementary Fig. 1). Increasing concentration of Cr significantly increased the EL, H2O2, and MDA concentration in all the varieties of sunflower. FH-614 showed tolerance to Cr, as compared to the rest of the varieties at all the levels of Cr concentration (5, 10, 20 mg kg−1) while the maximum concentrations of EL, H2O2, and MDA were measured in FH-425. The increased concentrations of EL, H2O2, and MDA within the six FH varieties were as follows:
Antioxidant enzyme activity
The activity of different antioxidant enzymes SOD, POD, CAT, and APX in the leaves and roots was measured to investigate the effects of Cr on antioxidant capacity of sunflower (Supplementary Fig. 2). Increasing concentration of Cr in soil affected the normal functioning of antioxidant defense system of sunflower plant in all the varieties. It was interesting that at initial concentrations of Cr (5 and 10 mg kg−1), the activity of antioxidant enzymes (SOD, POD, APX, CAT) was increased, as compared to that in the control. At higher Cr concentration (20 mg kg−1), the activity of these enzymes exhibited a declining trend. Enzymatic activity trend at different treatments of Cr was as follows:
According to the results, the comparison among the varieties for enzymatic activity in the roots and leaves, at different Cr concentrations, is presented below:
Chromium concentration, accumulation, and translocation factor
Cr uptake in the different cultivars of sunflower was significantly increased when plants were grown in spiked soil with gradually increasing Cr concentration, i.e., Cr 5, 10, and 20 mg kg−1 as compared to that in the control plants (Table 3). The translocation factor of Cr from the root to the shoot and then from the shoot to the leaf is given in (Table 4). The maximum and minimum concentration and accumulation of Cr at all the stress levels were found in FH-614 and FH-425, respectively. At each Cr treatment, the uptake and accumulation trend was as follows:
Discussion
Increasing heavy metal (HM) concentration in water and soil is becoming a major health issue around the globe due to its direct effects on biota via food chain (Shakoor et al. 2014; Shaheen and Rinklebe 2015). Previous studies widely reported a sever reduction in seed germination, plant growth, biomass, and yield under HM stress (Farid et al. 2015; Adrees et al. 2015a; Ramzani et al. 2016). Similarly, in present study, the increasing concentration of Cr in the soil reduced the germination potential, growth, and biomass of the six hybrids of sunflower (Figs. 1 and 2, Table 2). It has been observed that the smaller concentrations of HMs are beneficial for sunflower seeds as described by Imran et al. (2013). Under lower concentration (2–4 mg L−1) of arsenic (As), an increase was observed in radicle and plumule length of seedlings while the same was not detected under Cr stress. Similar to our results, Cd decreased the seed germination rate and imposed seed dormancy (Junior et al. 2016), while sunflower seeds have higher seed germination rate as compared to soybean and brassica under lead (Pb) and cadmium (Cd) stress (Lee et al. 2013). The similar results were also observed in different varieties of maize (Akhtar et al. 2017), sunflower (Saleem et al. 2015; Atta et al. 2013), Brassica napus (Gill et al. 2016a), and mung bean (Jabeen et al. 2016) under Cd and Cr stress. Different varieties showed variable behavior under increasing Cr concentration (Figs. 1 and 2). Higher concentrations of Cr (10 and 20 mg kg−1) reduced leaf area, plant height, root length, and biomass but the number of leaves and flowers remained unaffected. The similar results were found in sunflower under Cd (Cornuet et al. 2016), Pb (Azad et al. 2011; Kastori et al. 1998), Cu (Jiang et al. 2000; Kolbas et al. 2014, 2015), Co, Ni, and Cr (Gopal and Khurana 2011) stress. The decline in plant’s physiological parameters was due to the rapid uptake of Cr, and the results were clear in the form of toxic symptoms on plant leaves (chlorosis and necrosis), lower plant height and reduced number of leaves (Júnior et al. 2014). Presence of heavy metals in growth media hampers the uptake of other essential minerals, which are required for normal plant growth and development. So, the development of toxic symptoms might be due to the deficiency of essential minerals owing to Cr stress (Kötschau et al. 2013; Rivelli et al. 2014).
Photosynthetic pigments are very sensitive to HM stress and therefore can be trustworthy indicators to monitor HM toxicity (Anjum et al. 2015). Significant reduction in chlorophyll (a, b and total chlorophyll) and carotenoids, with increasing Cr concentration (5, 10, 20 mg kg−1), was attributed to inhibited uptake and translocation of mineral nutrients (Atta et al. 2013; Kötschau et al. 2013). FH-614 proved to be the most tolerant sunflower hybrid to Cr stress and showed very little reduction in photosynthetic pigments, as compared to the other varieties. Sunflower showed similar results under Cr (Saleem et al. 2015), Cd (De Maria et al. 2013), and Pb (Azad et al. 2011; Kastori et al. 1998) application. Similar trend was observed in Brassica napus, mung bean, and tobacco under Cr stress by Gill et al. (2016b), Jabeen et al. (2016), and Bukhari et al. (2016b), respectively. This phenomenon also supported by the reduction in chlorophylls and carotenoids in Brassica napus under Cd (Ehsan et al. 2014; Farid et al. 2015), Pb (Shakoor et al. 2014; Kanwal et al. 2014), and Cu (Habiba et al. 2015), and in cotton under Ni (Zaheer et al. 2015) and Pb (Anwaar et al. 2015) stress.
In present study, a significant variation was observed in the gas exchange attributes of all the sunflower varieties which might be the consequence of decreased chlorophylls, carotenoid content, and activities of CO2-fixating enzymes (Rizwan et al. 2017). Furthermore, the HMs interrupt metabolic activities in plant organelles and tissues, particularly in the chloroplast and leaves, which subsequently change the structure and functions of photosystem II by disturbing electron transport chain (Qadir et al. 2004; Di Cagno et al. 2001). Many studies have reported the alteration in water status of plants under HM stress (Barceló and Poschenrieder 1990; Perfus-Barbeoch et al. 2002). Atta et al. (2013) stated that higher concentration of Cr reduced the water use efficiency, transpiration, photosynthesis, and stomatal conductance in the leaves of sunflower hybrids, and reduction rate was directly proportional to the Cr concentration. Our results are in accordance with recent studies under different heavy metals such as Cr on mung bean and Brassica (Jabeen et al. 2016; Afshan et al. 2015), As + Cd and Cr + Al on maize (Anjum et al. 2015, 2016a), and Pb + Cd + Cu + Ni on Brassica napus (Shakoor et al. 2014; Ehsan et al. 2014; Habiba et al. 2015; Kanwal et al. 2014; Farid et al. 2015).
Soil plant analysis development (SPAD) and soluble proteins content significantly decreased in all the six hybrid varieties of sunflower under increasing Cr concentration (Fig. 5). Higher concentration of heavy metals in the rhizosphere and plant tissues inhibits the nutrient and water uptake and their translocation from the roots to the aboveground parts of plant as observed by Farooq et al. (2016) and Rizwan et al. (2017). Meanwhile, the production of ROS (O2 ·−, ·OH) and EL also decreased the soluble proteins in plant tissues (Ehsan et al. 2014). Similar results were found under Cr (Gill et al. 2015), Cd (Wahid et al. 2008), Cu (Adrees et al. 2015a), Ni (Kanwal et al. 2014), and Pb (Shakoor et al. 2014). Gupta et al. (2009) reported that the decrease in soluble protein content in Zea mays, grown hydroponically, was due to the oxidative damage caused by the Pb. Park et al. (2012) and Ghani (2011) reported the similar results in Brassica. The results of the present investigation are in line with previous findings that the excess Cr concentration decreased the leaf greenness (pigment content), an indicator of higher SPAD value (Gill et al. 2015; Singh et al. 2013). This decrease in SPAD value might be due to the anatomical deformation of chloroplast under metal stress (Najeeb et al. 2011).
Oxidative stress and electrolyte leakage in plants under heavy metal stress have been widely reported in recent studies (Gallego et al. 2005. In the present study, similar results have been found under Cr stress in sunflower hybrids regarding oxidative damage and electrolyte production (Supplementary Fig. 1). Increase in ROS and EL under escalating Cr concentration in all the hybrids showed different effects. The FH-614 showed tolerance to ROS and EL production by activating the ROS scavenging antioxidant defense system as described by Shakoor et al. (2014) and Farid et al. (2015). The increasing concentration of Cr in the plant cells and tissues causes K+ efflux, which is abundant in plant cells mediated by two ion channel groups (Demidchik et al. 2014). Similarly, the ROS are generated by the reaction associated with K+ leakage in plants under metal stress. In most cases, the biosynthesis of ROS, redox regulation, and oxidative stress is started in plants by the production of O2 − via single-electron reduction from triplet oxygen (O2) (Demidchik 2012). Further, the sequential formation of H2O2 and OH− is done by the addition of two electrons, respectively (Halliwell and Gutteridge 1999). Many studies reported that plants produced H2O2, O2 −, and OH, and caused EL in response to heavy metals (Wang et al. 2008; Ehsan et al. 2014), salinity (Arshad et al. 2016; Di Cagno et al. 1998), pathogens (Giovanini et al. 2006), drought (Noman et al. 2015), hyperthermia (Dong et al. 2009), herbicides (Song et al. 2007), hypothermia (Edreva et al. 1998), and other stresses. Our results are in accordance with many studies performed under different heavy metals such as Brassica napus and Alternanthera bettzickiana under Cr, Cd, Pb, Cu, and Ni (Afshan et al. 2015; Ehsan et al. 2014; Shakoor et al. 2014; Habiba et al. 2015; Kanwal et al. 2014; Tauqeer et al. 2016); mung bean and wheat under Cr (Jabeen et al. 2016; Adrees et al. 2015b; Ali et al. 2015); and cotton under Pb, Cd, (Farooq et al. 2013), Zn (Anwaar et al. 2015), and Ni (Khaliq et al. 2016).
Naturally, plants have developed an antioxidant defense system consisting of some specific metabolites and antioxidant enzymes which help plants survive and adapt under HM stress (Shakoor et al. 2014; Adrees et al. 2015b). The oxidative stress, generated by ROS, alters the activities of antioxidant enzymes (Supplementary Fig. 2). This vital approach helps the plants reduce HM stress (Shamsi et al. 2014), while increasing concentration of ROS, coupled with growth inhibition, further deteriorates the activities of antioxidant enzymes (Schützendübel et al. 2001; Shakoor et al. 2014). At lower concentration of Cr (5 and 10 mg kg−1), the activities of all the enzymes SOD, POD, CAT, and APX were increased, as compared to those in the controls, followed by a decline at higher Cr concentration (20 mg kg−1). The sunflower hybrids responded differently to applied Cr stress. FH-614 developed a good oxidative defense system and increased its enzymatic activities to reduce the oxidative damage. On the other hand, FH-425 could not enhance enzymatic activities to counter act the oxidative stress and hence showed more toxic effects, as compared to FH-614. Chen and Murata (2011) concluded that under higher metal stress, the antioxidant enzyme activities were reduced due to overproduction of ROS. These observations indicate the ability of plants to withstand Cr stress, by stimulating antioxidant enzyme activities, up to a certain extent. But at higher metal concentration, plant loses its capability to cope with the deleterious effects of Cr (Belhaj et al. 2016).
The present study revealed a variable response of different plant parts to Cr stress in terms of antioxidant enzyme activities. Higher activity of CAT and SOD was observed in the roots as compared with the leaves. However, POD and APX behaved opposite to CAT and SOD (Supplementary Fig. 2. Previously, it has been reported that the activities of POD were higher in Brassica napus leaves as compared to those in the roots under Cu stress (Habiba et al. 2015), while CAT and SOD showed an opposite response to Cd stress in hydroponics (Ehsan et al. 2014). The fluctuations in the activities of antioxidant enzymes might be due to the difference in plant species, type and concentration of metal stress as well as growth conditions (Gallego et al. 2005).
During active growth stages, plants rapidly uptake mineral elements and also absorb organic and inorganic contaminants attached with them, from the growth medium. These contaminants are then translocated to aboveground parts of the plants (Rizwan et al. 2017; Ali et al. 2015). The contaminants cause toxicity in plants by destroying plant tissues and cells (Adrees et al. 2016). The plants can tolerate a certain level of heavy metal stress which varies with plant species, genotype, and concentration of metal in the growth media (Arshad et al. 2016; Bukhari et al. 2015). The present study revealed a variable response of different sunflower hybrids to the same concentration of Cr (Table 2). FH-614 contained higher amount of Cr in all the plant parts with overall better growth and biomass production, as compared to the FH-425. The difference in Cr concentration and plant growth attributes shows the effectiveness of antioxidant defense system to counteract the Cr toxicity (Belhaj et al. 2016; Meers et al. 2005a). Oil seed crops are considered to be the hyper-accumulator of heavy metals due to their higher biomass, rapid uptake, and greater translocation factor from the roots to the shoots (Rizwan et al. 2017). Increased Cr concentration was inversely proportional to the plants’ physiological and biochemical formations while it was directly proportional to the ROS and EL, which reduced the overall plant growth (Adrees et al. 2015a; Ali et al. 2013). The similar trend of Cr toxicity and accumulation was found in sunflower, Brassica napus, mung bean, castor bean, wheat, and barley (Fozia et al. 2008; Afshan et al. 2015; Ali et al. 2013).
Conclusions
The present study concluded that the increasing concentration of Cr caused a significant decline in growth, biomass, photosynthesis, gas exchange attributes, and antioxidant enzyme activities of sunflower hybrids, by elevating ROS production and electrolyte leakage, in genotype-dependent manner. FH-614 accumulated the highest amount of Cr and proved to be the most tolerant sunflower hybrid with least toxic effects, as compared with the other genotypes, by virtue of strong antioxidant defense system. However, FH-425 was found to be the most vulnerable to Cr toxicity. The FH-614 could be used for further studies to uncover the molecular basis of underlying Cr tolerance mechanism. The present study also indicates the possibility of employing sunflower for the phyto-management of heavy metal.
References
Adesodun JK, Atayese MO, Agbaje TA, Osadiaye BA, Mafe OF, Soretire AA (2010) Phytoremediation potentials of sunflowers (Tithonia diversifolia and Helianthus annuus) for metals in soils contaminated with zinc and lead nitrates. Water Air Soil Pollut 207:195–201
Adrees M, Ali S, Rizwan M, Ibrahim M, Abbas F, Farid M, Zia-ur-Rehman M, Irshad MK, Bharwana SA (2015a) The effect of excess copper on growth and physiology of important food crops: a review. Environ Sci Pollut Res 22:8148–8162. doi:10.1007/s11356-015-4496-5
Adrees M, Ali S, Rizwan M, Zia-ur-Rehman M, Ibrahim M, Abbas F, Farid M, Qayyum MF, Irshad MK (2015b) Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: a review. Ecotoxicol Environ Safe 119:186–197. doi:10.1016/j.ecoenv.2015.05.011
Adrees M, Saleem F, Jabeen F, Rizwan M, Ali S, Khalid S, Ibrahim M, Iqbal N, Abbas F (2016) Effects of ambient gaseous pollutants on photosynthesis, growth, yield and grain quality of selected crops grown at different sites varying in pollution levels. Arch Agron Soil Sci 62:1195–1207
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Afshan S, Ali S, Bharwana SA, Rizwan M, Farid M, Abbas F, Ibrahim M, Mehmood MA, Abbasi GH (2015) Citric acid enhances the phytoextraction of chromium, plant growth, and photosynthesis by alleviating the oxidative damages in Brassica napus L. Environ Sci Pollut Res 22:11679–11689
Akhtar T, Zia-ur-Rehman M, Naeem A, Nawaz R, Ali S, Murtaza G, Maqsood MA, Azhar M, Khalid H, Rizwan M (2017) Photosynthesis and growth response of maize (Zea mays L.) hybrids exposed to cadmium stress. Environ Sci Pollut Res 24:5521–5529
Ali S, Farooq MA, Yasmeen T, Hussain S, Arif MS, Abbas F, Bharwana SA, Zhang G (2013) The influence of silicon on barley growth, photosynthesis and ultra-structure under chromium stress. Ecotoxicol Environ Saf 89:66–72
Ali S, Bharwana SA, Rizwan M, Farid M, Kanwal S, Ali Q, Ibrahim M, Gill RA, Khan MD (2015) Fulvic acid mediates chromium (Cr) tolerance in wheat (Triticum aestivum L.) through lowering of Cr uptake and improved antioxidant defense system. Environ Sci Pollut Res 22:10601–10609
Anjum SA, Tanveer M, Hussain S, Bao M, Wang L, Khan I, Ullah E, Tung SA, Samad RA, Shahzad B (2015) Cadmium toxicity in maize (Zea mays L.): consequences on antioxidative systems, reactive oxygen species and cadmium accumulation. Environ Sci Pollut Res 22:17022–17030. doi:10.1007/s11356-015-4882-z
Anjum SA, Ashraf U, Khan I, Tanveer M, Saleem MF, Wang L (2016a) Aluminum and chromium toxicity in maize: implications for agronomic attributes, net photosynthesis, physio-biochemical oscillations, and metal accumulation in different plant parts. Water Air Soil Pollut 227:326. doi:10.1007/s11270-016-3013-x
Anwaar SA, Ali S, Ali S, Ishaque W, Farid M, Farooq MA, Najeeb U, Abbas F, Sharif M (2015) Silicon (Si) alleviates cotton (Gossypium hirsutum L.) from zinc (Zn) toxicity stress by limiting Zn uptake and oxidative damage. Environ Sci Pollut Res 22:3441–3450
Arshad M, Ali S, Noman A, Ali Q, Rizwan M, Farid M, Irshad MK (2016) Phosphorus amendment decreased cadmium (Cd) uptake and ameliorates chlorophyll contents, gas exchange attributes, antioxidants, and mineral nutrients in wheat (Triticum aestivum L.) under Cd stress. Arch Agron Soil Sci 62:533–546
Artiushenko T, Syshchykov D, Gryshko V, Čiamporová M, Fiala R, Repka V, Martinka M, Pavlovkin J (2014) Metal uptake, antioxidant status and membrane potential in maize roots exposed to cadmium and nickel. Biologia 69:1142–1147
Atta MI, Bokhari TZ, Malik SA, Wahid A, Saeed S, Gulshan AB (2013) Assessing some emerging effects of hexavalent chromium on leaf physiological performance in sunflower (Helianthus annuus L.) Int J Sci Eng Res 4:945–949
Azad HN, Shiva AH, Malekpour R (2011) Toxic effects of lead on growth and some biochemical and ionic parameters of sunflower (Helianthus annuus L.) seedlings. Curr Res J Biol Sci 3:398–403
Baker AJ, Whiting SN (2002) In search of the Holy Grail—a further step in understanding metal hyperaccumulation. New Phytol 155:1–4
Barceló JU, Poschenrieder C (1990) Plant water relations as affected by heavy metals stress: a review. J Plant Nutr 13:1–37
Belhaj D, Jerbi B, Medhioub M, Zhou J, Kallel M, Ayadi H (2016) Impact of treated urban wastewater for reuse in agriculture on crop response and soil ecotoxicity. Environ Sci Pollut Res 23:15877-15887
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bukhari SA, Shang S, Zhang M, Zheng W, Zhang G, Wang TZ, Shamsi IH, Wu F (2015) Genome-wide identification of chromium stress-responsive microRNAs and their target genes in tobacco (Nicotiana tabacum) roots. Environ Toxicol Chem 34:2573–2582
Bukhari SA, Wang R, Wang W, Ahmed IM, Zheng W, Cao F (2016a) Genotype-dependent effect of exogenous 24-epibrassinolide on chromium-induced changes in ultrastructure and physicochemical traits in tobacco seedlings. Environ Sci Pollut Res 23:18229–18238
Bukhari SA, Zheng W, Xie L, Zhang G, Shang S, Wu F (2016b) Cr-induced changes in leaf protein profile, ultrastructure and photosynthetic traits in the two contrasting tobacco genotypes. Plant Growth Regul 79:147–156
Chae MJ, Jung GB, Kang SS, Kong MS, Kim YH, Lee DB (2014) Evaluation of the feasibility of phytoremediation of soils contaminated with Cd, Pb and Zn using sunflower, corn and castor plants. Korean J Soil Sci Fert 47:491–495
Chen TH, Murata N (2011) Glycinebetaine protects plants against abiotic stress: mechanisms and biotechnological applications. Plant Cell Environ 34:1–20
Cornuet JY, Bakoto R, Bonnard O, Bussière S, Coriou C, Sirguey C, Sterckeman T, Thunot S, Visse MI, Nguyen C (2016) Cadmium uptake and partitioning during the vegetative growth of sunflower exposed to low Cd2C concentrations in hydroponics. Plant Soil. doi:10.1007/s11104-016-2839-8
Cutright T, Gunda N, Kurt F (2010) Simultaneous hyperaccumulation of multiple heavy metals by Helianthus annuus grown in a contaminated sandy-loam soil. Int J Phytoremediation 12:562–573
De Maria S, Puschenreiter M, Rivelli AR (2013) Cadmium accumulation and physiological response of sunflower plants to Cd during the vegetative growing cycle. J Plant Soil Environ 59:254–261
Del Gatto A, Mengarelli C, Pedretti EF, Duca D, Pieri S, Mangoni L, Signor M, Raccuia SA, Melilli MG (2015) Adaptability of sunflower (Helianthus annuus L.) high oleic hybrids to different Italian areas for biodiesel production. Ind Crop Prod 75:108–117
Demidchik V (2012) Reactive oxygen species and oxidative stress in plants. In: Shabala S (ed) Plant stress physiol. CAB International, Wallingford, pp 24–58
Demidchik V, Straltsova D, Medvedev SS, Pozhvanov GA, Sokolik A, Yurin V (2014) Stress-induced electrolyte leakage: the role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J Exp Bot 65:1259–1270
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased level of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Di Cagno R, Guidi L, De Gara L, Soldatini GF (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9
Di Cagno R, Guidi L, De Gara L, Soldatini GF (2001) Combined cadmium and ozone treatments affect photosynthesis and ascorbate-dependent defences in sunflower. New Phytol 151:627–636. doi:10.1046/j.1469-8137.2001.00217.x
Dionisio-Sese ML, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9
Doncheva S, Moustakas M, Ananieva K, Chavdarova M, Gesheva E, Vassilevska R, Mateev P (2013) Plant response to lead in the presence or absence EDTA in two sunflower genotypes (cultivated H. annuus cv. 1114 and interspecific line H. annuus H. argophyllus). Environ Sci Pollut Res 20:823-833
Dong CH, Zolman BK, Bartel B, Lee BH, Stevenson B, Agarwal M, Zhu JK (2009) Disruption of Arabidopsis CHY1 reveals an important role of metabolic status in plant cold stress signaling. MolePlant 2:59–72
Edreva A, Yordanov I, Kardjieva R, Gesheva E (1998) Heat shock responses of bean plants: involvement of free radicals, antioxidants and free radical/active oxygen scavenging systems. Biol Plantarum 41:185–191
Ehsan S, Ali S, Noureen S, Farid M, Shakoor MB, Aslam A, Bharwana SA, Tauqeer HM (2013) Comparative assessment of different heavy metals in urban soil and vegetables irrigated with sewage/industrial waste water. Ecoterra 35:37–53
Ehsan S, Ali S, Noureen S, Mahmood K, Farid M, Ishaque W, Shakoor MB, Rizwan M (2014) Citric acid assisted phytoremediation of cadmium by Brassica napus L. Ecotoxicol Environ Saf 106:164–172. doi:10.1016/j.ecoenv.2014.03.007
Farid M, Ali S, Ishaque W, Shakoor MB, Niazi NK, Bibi I, Dawood M, Gill RA, Abbas F (2015) Exogenous application of ethylenediamminetetraacetic acid enhanced phytoremediation of cadmium by Brassica napus L. IJEST 12:3981–3992
Farooq MA, Ali S, Hameed A, Ishaque W, Mahmood K, Iqbal Z (2013) Alleviation of cadmium toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes; suppressed cadmium uptake and oxidative stress in cotton. Ecotoxicol Environ Saf 96:242–249
Farooq MA, Ali S, Hameed A, Bharwana SA, Rizwan M, Ishaque W, Farid M, Mahmood K, Iqbal Z (2016) Cadmium stress in cotton seedlings: physiological, photosynthesis and oxidative damages alleviated by glycinebetaine. S Afr J Bot 104:61–68
Fozia A, Muhammad AZ, Muhammad A, Zafar MK (2008) Effect of chromium on growth attributes in sunflower (Helianthus annuus L.) J Environ Sci 20:1475–1480
Gallego SM, Kogan MJ, Azpilicueta CE, Peña C, Tomaro ML (2005) Glutathionemediated antioxidative mechanisms in sunflower (Helianthus annuus L.) cells in response to cadmium stress. Plant Growth Regul 46:267–276. doi:10.1007/s10725-005-0163-z
Ghani A (2011) Varietal differences in canola (Brassic anapus L.) for the growth, yield and yield components exposed to cadmium stress. J Anim Plant Sci 21:57–59
Gill RA, Zang L, Ali B, Farooq MA, Cui P, Yang S, Ali S, Zhou W (2015) Chromium-induced physio-chemical and ultrastructural changes in four cultivars of Brassica napus L. Chemosphere 120:154–164
Gill RA, Ali B, Cui P, Shen E, Farooq MA, Islam F, Ali S, Mao B, Zhou W (2016a) Comparative transcriptome profiling of two Brassica napus cultivars under chromium toxicity and its alleviation by reduced glutathione. BMC Genomics 17:885
Gill RA, Zhang N, Ali B, Farooq MA, Xu J, Gill MB, Mao B, Zhou W (2016b) Role of exogenous salicylic acid in regulating physio-morphic and molecular changes under chromium toxicity in black-and yellow-seeded Brassica napus L. Environ Sci Pollut Res 23:20483–20496
Giovanini MP, Puthoff DP, Nemacheck JA, Mittapalli O, Saltzmann KD, Ohm HW, Shukle RH, Williams CE (2006) Gene-for-gene defense of wheat against the Hessian fly lacks a classical oxidative burst. Mol Plant Microbe In 19:1023–1033
Gopal R, Khurana N (2011) Effect of heavy metal pollutants on sunflower. African J Plant Sci 5:531–536
Gupta DK, Nicoloso FT, Schetinger MR, Rossato LV, Pereira LB, Castro GY, Srivastava S, Tripathi RD (2009) Antioxidant defense mechanism in hydroponically grown Zea mays seedlings under moderate lead stress. J Hazard Mater 172:479–484
Habiba U, Ali S, Farid M, Shakoor MB, Rizwan M, Ibrahim M, Abbasi GH, Hayat T, Ali B (2015) EDTA enhanced plant growth, antioxidant defense system, and phytoextraction of copper by Brassica napus L. Environ. Sci Pollut Res 22:1534–1544. doi:10.1007/s11356-014-3431-5
Halliwell B, Gutteridge JM (1999) Free radicals in biology and medicine. Oxford University Press, Oxford
Harris TM, Hottle TA, Soratana K, Klane J, Landis AE (2016) Life cycle assessment of sunflower cultivation on abandoned mine land for biodiesel production. J Clean Prod 112:182–195
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Imran MA, Khan RM, Ali Z, Mahmood T (2013) Toxicity of arsenic (As) on seed germination of sunflower (Helianthus annuus L.) Int J Phys Sci 8:840–847
Jabeen N, Abbas Z, Iqbal M, Rizwan M, Jabbar A, Farid M, Ali S, Ibrahim M, Abbas F (2016) Glycinebetaine mediates chromium tolerance in mung bean through lowering of Cr uptake and improved antioxidant system. Arch Agron Soil Sci 62:648–662. doi:10.1080/03650340.2015.1082032
Jana S, Choudhuri MA (1981) Glycolate metabolism of three submerged aquatic angiosperms during aging. Aquat Bot 12:345–354
Jiang W, Liu D, Li H (2000) Effects of Cu2C on root growth, cell division, and nucleolus of Helianthus annuus L. Sci Total Environ 256:59–65. doi:10.1016/S0048-9697(00)00470-8
Júnior CA, Mazzafera P, Arruda MA (2014) A comparative ionomic approach focusing on cadmium effects in sunflowers (Helianthus annuus L.) Environ Exp Bot 107:180–186. doi:10.1016/j.envexpbot.2014.06.002
Júnior CA, de Sousa BH, Galazzi RM, Koolen HH, Gozzo FC, Arruda MA (2015) Evaluation of proteome alterations induced by cadmium stress in sunflower (Helianthus annuus L.) cultures. Ecotoxicol Environ Saf 119:170–177
Junior CAL, Oliveira SR, Mazzafera P, Arruda MAZ (2016) Expanding the information about the influence of cadmium on the metabolism of sunflowers: evaluation of total, bioavailable, and bioaccessible content and metallobiomolecules in sunflower seeds. Environ Exp Bot 125:87–97. doi:10.1016/j.envexpbot.2016.02.003
Kanwal U, Ali S, Shakoor MB, Farid M, Hussain S, Yasmeen T, Adrees M, Bharwana SA, Abbas F (2014) EDTA ameliorates phytoextraction of lead and plant growth by reducing morphological and biochemical injuries in Brassica napus L. under lead stress. Environ Sci Pollut Res 21:9899–9910
Kastori R, Plesničar M, Sakač Z, Panković D, Arsenijević-Maksimović I (1998) Effect of excess lead on sunflower growth and photosynthesis. J Plant Nutr 21:75–85. doi:10.1080/01904169809365384
Khaliq A, Ali S, Hameed A, Farooq MA, Farid M, Shakoor MB, Mahmood K, Ishaque W, Rizwan M (2016) Silicon alleviates nickel toxicity in cotton seedlings through enhancing growth, photosynthesis, and suppressing Ni uptake and oxidative stress. Arch Agron Soil Sci 62:633–647
Kolbas A, Marchand L, Herzig R, Nehnevajova E, Mench M (2014) Phenotypic seedling responses of a metal-tolerant mutant line of sunflower growing on a Cu-contaminated soil series: potential uses for biomonitoring of Cu exposure and phytoremediation. Plant Soil 376:377–397. doi:10.1007/s11104-013-1974-8
Kolbas A, Kidd P, Guinberteau J, Jaunatre R, Herzig R, Mench M (2015) Endophytic bacteria take the challenge to improve Cu phytoextraction by sunflower. Environ Sci Pollut Res 22:5370–5382. doi:10.1007/s11356-014-4006-1
Kötschau A, Büchel G, Einax JW, Fischer C, Von Tümpling W, Merten D (2013) Mapping of macro and micro elements in the leaves of sunflower (Helianthus annuus) by laser ablation-ICP-MS. Microchem J 110:783–789. doi:10.1016/j.microc.2012.12.011
Lee KK, Cho HS, Moon YC, Ban SJ, Kim JY (2013) Cadmium and lead uptake capacity of energy crops and distribution of metals within the plant structures. KSCE J Civ Eng 17:44–50. doi:10.1007/s12205-013-1633-x
Lichtenthaler HK (1987) Chlorophylls and carotenoids pigments of photosynthetic biomembranes In: Colowick SP, Kaplan NO (ed) Methods Enzymol vol 148, pp 350–382
Meers E, Ruttens A, Geebelen W, Vangronsveld J, Samson R, Vanbroekhoven K, Vandegehuchte M, Diels L, Tack FM (2005a) Potential use of the plant antioxidant network for environmental exposure assessment of heavy metals in soils. Environ Monit Assess 120:243–267. doi:10.1007/s10661-005-9059-7
Metzner H, Rau H, Senger H (1965) Untersuchungen zur Synchronisierbakeit einzelner Pigmentmangel-Mutation von Chlorella. Planta 65:186–194 (in German)
Nagajyoti PC, Lee KD, Sreekanth TVM (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8:199–216
Najeeb U, Jilani G, Ali S, Sarwar M, Xu L, Zhou WJ (2011) Insight into cadmium induced physiological and ultra-structural disorders in Juncus effusus L. and its remediation through exogenous citric acid. J Hazard Mater 186:565–574
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Nehnevajova E, Herzig R, Federer G, Erismann KH, Schwitzguébel JP (2007) Chemical mutagenesis—a promising technique to increase metal concentration and extraction in sunflowers. Int J Phytoremediation 9:149–165
Noman A, Ali S, Naheed F, Ali Q, Farid M, Rizwan M, Irshad MK (2015) Foliar application of ascorbate enhances the physiological and biochemical attributes of maize (Zea mays L.) cultivars under drought stress. Arch Agron Soil Sci 61:1659–1672
Park J, Kim JY, Kim KW (2012) Phytoremediation of soil contaminated with heavy metals using Brassica napus. Geosyst Eng 15:10–18
Perfus-Barbeoch L, Leonhardt N, Vavasseur A, Forestier C (2002) Heavy metal toxicity: cadmium permeates through calcium channels and disturbs the plant water status. Plant J 32:539–548
Qadir S, Qureshi MI, Javed S, Abdin MZ (2004) Genotypic variation in phytoremediation potential of Brassica juncea cultivars exposed to Cd stress. Plant Sci 167:1171–1181
Ramzani PMA, Khan WD, Iqbal M, Kausar S, Ali S, Rizwan M, Virk ZA (2016) Effect of different amendments on rice (Oryza sativa L.) growth, yield, nutrient uptake and grain quality in Ni-contaminated soil. Environ Sci Pollut Res doi:10.1007/s11356-016-7038-x
Rehman MZ, Rizwan M, Ghafoor A, Naeem A, Ali S, Sabir M, Qayyum MF (2015) Effect of inorganic amendments for in situ stabilization of cadmium in contaminated soils and its phyto-availability to wheat and rice under rotation. Environ Sci Pollut Res 22:16897–16906. doi:10.1007/s11356-015-4883-y
Rivelli AR, Puschenreiter M, De Maria S (2014) Assessment of cadmium uptake and nutrient content in sunflower plants grown under Cd stress. Plant Soil Environ 60:80–86
Rizwan M, Ali S, Ibrahim M, Farid M, Adrees M, Bharwana SA, Zia-ur-Rehman M, Qayyum MF, Abbas F (2015) Mechanisms of silicon-mediated alleviation of drought and salt stress in plants: a review. Environ Sci Pollut Res 22:15416–15431. doi:10.1007/s11356-015-5305-x
Rizwan M, Ali S, Qayyum MF, Ok YS, Zia-ur-Rehman M, Abbas Z, Hannan F (2016a) Use of maize (Zea mays L.) for phytomanagement of Cd-contaminated soils: a critical review. Environ Geochem Health doi:10.1007/s10653-016-9826-0
Rizwan M, Ali S, Rizvi H, Rinklebe J, Tsang DC, Meers E, Ok YS, Ishaque W (2016b) Phytomanagement of heavy metals in contaminated soils using sunflower: a review. Crit Rev Environ Sci Technol 46:1498–1528
Rizwan M, Ali S, Abbas F, Adrees M, Zia-ur-Rehman M, Farid M, Gill RA, Ali B (2017) Role of organic and inorganic amendments in alleviating heavy metal stress in oil seed crops. In: Ahmad P (ed) Oil Seed Crops: Yield and Adaptations under Environmental Stress, 1st edn, vol. 12. John Wiley & Sons, Ltd., 224–235
Saleem M, Asghar HN, Khan MY, Zahir ZA (2015) Gibberellic acid in combination with pressmud enhances the growth of sunflower and stabilizes chromium(VI)-contaminated soil. Environ Sci Pollut Res 22:10610–10617. doi:10.1007/s11356-015-4275-3
Schützendübel A, Schwanz P, Teichmann T, Gross K, Langenfeld-Heyser R, Godbold DL, Polle A (2001) Cadmium-induced changes in antioxidative systems, hydrogen peroxide content, and differentiation in Scots pine roots. Plant Physiol 127:887–898
Shaheen SM, Rinklebe J (2015) Phytoextraction of potentially toxic elements by Indian mustard, rapeseed, and sunflower from a contaminated riparian soil. Environ Geochem Health 37:953–967. doi:10.1007/s10653-015-9718-8
Shakoor MB, Ali S, Hameed A, Farid M, Hussain S, Yasmeen T, Najeeb U, Bharwana SA, Abbasi GH (2014) Citric acid improves lead (Pb) phytoextraction in Brassica napus L. by mitigating Pb-induced morphological and biochemical damages. Ecotoxicol Environ Saf 109:38–47
Shamsi IH, Zhang GP, Hu HL, Xue QY, Hussain N, Ali E, Shen QF, Zheng W, Zhang QC, Liu XX, Jabeen Z (2014) Assessment of the hazardous effects of Cd on physiological and biochemical characteristics of soybean genotypes. Int J Agric Biol 16:41–48
Singh HP, Mahajan P, Kaur S, Batish DR, Kohli RK (2013) Chromium toxicity and tolerance in plants. Environ Chem Lett 11:229–254
Song NH, Yin XL, Chen GF, Yang H (2007) Biological responses of wheat (Triticum aestivum) plants to the herbicide chlorotoluron in soils. Chemosphere 68:1779–1787
SPSS I (2009) PASW statistics release 18. IBM Corp, Armonk
Tauqeer HM, Ali S, Rizwan M, Ali Q, Saeed R, Iftikhar U, Ahmad R, Farid M, Abbasi GH (2016) Phytoremediation of heavy metals by Alternanthera bettzickiana: growth and physiological response. Ecotoxicol Environ Saf 126:138–146
Wahid A, Ghani A, Javed F (2008) Effect of cadmium on photosynthesis, nutrition and growth of mung bean. Agron Sustain Dev 28:273–280
Wang C, Wang X, Tian Y, Xue Y, Xu X, Sui Y, Yu H (2008) Oxidative stress and potential biomarkers in tomato seedlings subjected to soil lead contamination. Ecotoxicol Environ Saf 71:685–691
Zaheer IE, Ali S, Muhammad R, Farid M, Shakoor MB, Gill RA, Najeeb U, Iqbal N, Ahmad R (2015) Citric acid assisted phytoremediation of copper by Brassica napus L. Ecotoxicol Environ Saf 120:310–317. doi:10.1016/j.ecoenv.2014.03.007
Zhang XZ (1992) The measurement and mechanism of lipid peroxidation and SOD, POD and CAT activities in biological system. In: Zhang XZ (ed) Research methodology of crop physiology. Agriculture Press, Beijing, pp 208–211
Zhang JX, Kirham MB (1994) Drought stress-induced changes in activities of superoxide dismutase, catalase, and peroxidase in wheat species. Plant Cell Physiol 35:785–791
Acknowledgments
This study is the part of PhD dissertation of Mr. Mujahid Farid. The authors are highly thankful to the Government College University, Faisalabad, Pakistan, and the Higher Education Commission of Pakistan for the financial and technical support during this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
Supplementary Fig. 1
(DOCX 42 kb)
Supplementary Fig. 2
(DOCX 50 kb)
Rights and permissions
About this article
Cite this article
Farid, M., Ali, S., Akram, N.A. et al. Phyto-management of Cr-contaminated soils by sunflower hybrids: physiological and biochemical response and metal extractability under Cr stress. Environ Sci Pollut Res 24, 16845–16859 (2017). https://doi.org/10.1007/s11356-017-9247-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9247-3