Abstract
Endophytic bacteria from roots and crude seed extracts of a Cu-tolerant population of Agrostis capillaris were inoculated to a sunflower metal-tolerant mutant line, and their influence on Cu tolerance and phytoextraction was assessed using a Cu-contaminated soil series. Ten endophytic bacterial strains isolated from surface-sterilized A. capillaris roots were mixed to prepare the root endophyte inoculant (RE). In parallel, surface-sterilized seeds of A. capillaris were crushed in MgSO4 to prepare a crude seed extract containing seed endophytes (SE). An aliquot of this seed extract was filtered at 0.2 μm to obtain a bacterial cell-free seed extract (SEF). After surface sterilization, germinated sunflower seeds were separately treated with one of five modalities: no treatment (C), immersion in MgSO4 (CMg) or SEF solutions and inoculation with RE or SE. All plants were cultivated on a Cu-contaminated soil series (13–1020 mg Cu kg−1). Cultivable RE strains were mostly members of the Pseudomonas genera, and one strain was closely related to Labrys sp. The cultivable SE strains belonged mainly to the Bacillus genera and some members of the Rhodococcus genera. The treatment effects depended on the soil Cu concentration. Both SE and SEF plants had a higher Cu tolerance in the 13–517 mg Cu kg−1 soil range as reflected by increased shoot and root DW yields compared to control plants. This was accompanied by a slight decrease in shoot Cu concentration and increase in root Cu concentration. Shoot and root DW yields were more promoted by SE than SEF in the 13–114 mg Cu kg−1 soil range, which could reflect the influence of seed-located bacterial endophytes. At intermediate soil Cu (416–818 mg Cu kg−1 soil), the RE and CMg plants had lower shoot Cu concentrations than the control, SE and SEF plants. At high total soil Cu (617–1020 mg Cu kg−1), root DW yield of RE plants slightly increased and their root Cu concentration rose by up to 1.9-fold. In terms of phytoextraction efficiency, shoot Cu removal was increased for sunflower plants inoculated with crude and bacterial cell-free seed extracts by 1.3- to 2.2-fold in the 13–416 mg Cu kg−1 soil range. Such increase was mainly driven by an enhanced shoot DW yield. The number and distribution of endophytic bacteria in the harvested sunflower tissues must be further examined.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background, aim, and scope
Increasing attention is devoted to phytoremediation options for metal(loid)-contaminated soils and engineered plants to improve their effectiveness (Vangronsveld et al. 2009; Mench et al. 2009, 2010). Some microorganisms, particularly beneficial bacteria and fungi, can improve plant performance under stressful environments (Lebeau et al. 2008; Compant et al. 2010; Cherian et al. 2012). One option to promote metal phytoextraction through increases in shoot biomass and/or shoot metal concentration is the use of plant growth-promoting bacteria (PGPB) associated with many plant species (Rajkumar et al. 2009; Glick 2010; Ma et al. 2011a; Luo et al. 2012). Many PGPB can thrive as endophytic bacteria in plant parts (Mastretta et al. 2009; Compant et al. 2010). The colonizing process may be initiated in the root zone, but these bacteria may also originate from the phyllosphere, the anthosphere and the spermosphere (Compant et al. 2005a). Compared to rhizosphere and phyllosphere microorganisms, endophytic bacteria are likely to interact more closely with their host (Sturz and Nowak 2000).
In pioneer studies, potential Cu-resistant plant growth-promoting Rhizobacteria (PGPR) have been isolated in various manners, notably from contaminated soils, to promote the phytoremediation of Cu-contaminated soils. Inoculation of Brassica juncea seeds with a Cu-resistant PGPR strain, Achromobacter xylosoxidans Ax10, isolated from a Cu mine soil increased the root and shoot biomasses of plants grown in a sterilized, Cu-spiked soil and improved their Cu uptake (Ma et al. 2009). The incorporation of Pseudomonas aspleni into the soil also facilitated Cu uptake in Brassica napus by increasing its biomass (Reed and Glick 2005). Pseudomonas jessenii increased biomass of Ricinus communis and was efficient at solubilizing Cu (Rajkumar et al. 2009). Seed inoculation with Proteus vulgaris increased germination, biomass and chlorophyll content and decreased root and shoot Cu accumulation of Cajanus cajan (Rani et al. 2008). A bacterial strain isolated from the rhizosphere of Elsholtzia splendens growing on Tonglu Mountain Cu mines increased soil water-soluble Cu, as well as root and shoot Cu accumulation (Chen et al. 2005).
Endophytic bacteria were defined as those bacteria that colonize the internal tissue of the plant showing no external sign of infection or negative effect on their host (Ryan et al. 2008). Their common functions and their application for bioaugmentation of metal-contaminated soils were reviewed elsewhere (Ryan et al. 2008; Sessitsch et al. 2013). Bioaugmentation with trace element (TE)-resistant endophytic bacteria can promote plant establishment and growth and influence both macronutrient and TE uptake by roots in contaminated soils under phytoremediation (Burd et al. 2000; Belimov et al. 2005; Madhaiyan et al. 2007; Weyens et al. 2009; Sessitsch et al. 2013). Shoot metal removal can increase in endophyte-inoculated plants due to an enhanced biomass production and/or TE uptake and accumulation in aerial plant parts (Lodewyckx et al. 2001; Sheng et al. 2008; Kuffner et al. 2010; Sun et al. 2010; Ma et al. 2011b; Luo et al. 2012; Sessitsch et al. 2013). Evidence of increased metal accumulation in plants inoculated with such endophytes has been obtained for Pb (Sheng et al. 2008), Zn (Kuffner et al. 2010) and Ni (Lodewyckx et al. 2001). Conversely, endophytic bacteria can decrease metal accumulation in host plants. Methylobacterium oryzae strain CBMB20 and Burkholderia sp. strain CBMB40 from tissues of Oryza sativa stimulated the growth of Lycopersicon esculentum but decreased shoot and root Ni and Cd concentrations (Madhaiyan et al. 2007). Metal-resistant endophytes can be isolated from seeds. From a collection of endophytic bacterial strains, obtained from seeds of tobacco plants grown on Cd/Zn-contaminated soils in Northern Europe, a Cd-resistant Sanguibacter sp., a Pseudomonas sp. and a consortium of Cd-resistant endophytes were found to increase 3-fold Cd accumulation in Nicotiana tabacum (Mastretta et al. 2009). Inoculation with consortia often resulted in more pronounced beneficial effects on plant biomass production as compared with inoculation with single strains, suggesting synergistic effects of the consortia members.
Little is known about the influence of endophytic bacteria on plant development and their interactions with plants exposed to Cu excess. A high diversity and specificity of endophytic bacteria associated with different plant parts of cuprophyte species has been found. Sun et al. (2010) identified 32 endophytic isolates from E. splendens and Commelina communis, living preferably in the leaves and stems. Their sequence analysis revealed α-, β- and γ-Proteobacteria, Firmicutes, Actinobacteria and Bacteroidetes. Kabagale et al. (2010) isolated 31 taxonomic units, belonging to 17 genera, mainly Proteobacteria, from two Cu hyperaccumulators, i.e. Haumaniastrum katangense and Crepidorhopalon tenuis, Katanga, Congo. Three Cu-resistant endophytes isolated from Cu-tolerant plants grown on Cu mine wasteland, i.e. Ralstonia sp. J1-22-2, Pantoea agglomerans Jp3-3 and Pseudomonas thivervalensis Y1-3-9, increased the biomass and above-ground tissue Cu contents of rape (Zhang et al. 2011). In metal-tolerant grasses such as Agrostis sp., numerous endophytic bacteria and fungi are present (Wang et al. 2005; Saikkonen et al. 2000; Bazely et al. 2007). Endophytic bacterial strains isolated from surface-sterilized roots of metallicolous and non-metallicolous Agrostis capillaris populations, sampled, respectively, at a wood preservation site and a forest edge in Southwest France, differed in their plant growth-promoting traits and Cu-resistance (Jaunatre, unpublished data). To date, researchers have concentrated on the cultivable members of these endophytic communities, whereas uncultivable strains represent between 95 and 99 % of the total bacteria and are rarely addressed. Moreover, studies investigating the influence of endophytes obtained from both seeds and roots on plant tolerance to Cu excess are scarce, especially for plants grown in non-spiked topsoils from Cu-contaminated sites.
Several crops are promising for phytoremediating metal-contaminated soils (Mench et al. 2010; Rajkumar et al. 2012). One of these, sunflower (Helianthus annuus L.) is a relevant species allowing both metal phytoextraction and financial opportunities from its biomass conversion, e.g. oilseed production for biodiesel and platform chemicals, methane production from oil cake and fiberboards (Vangronsveld et al. 2009; Ronda et al. 2011; Evon et al. 2012). Sunflower is fairly responsive to bacterial enrichment (Chen and Cutright 2003; Vangronsveld et al. 2009; Lyubun and Chernyshova 2010). Its use to cleanup inorganic and organic contaminants is developing (Meers et al. 2005; Vangronsveld et al. 2009; Adesodun et al. 2010; Faessler et al. 2010b; Rivelli et al. 2012; Herzig et al. 2014). Some commercial cultivars and mutant lines have a potential for both shoot Cu removal and oilseed production (Kolbas et al. 2011). The potential influence of Cu-resistant endophytic bacterial strains on the phenotypic traits and shoot Cu removal of these sunflowers is however not documented.
This paper aimed at evaluating whether or not inoculating a metal-tolerant sunflower mutant line grown in potted soils with increasing total soil Cu, with either root or seed endophytic bacteria, has a beneficial effect on plant phenotypic traits, Cu tolerance, mineral composition and shoot Cu removal. The inoculants used were a consortium of root endophytic strains representing the dominant Cu-tolerant isolates obtained from the roots of a Cu-tolerant A. capillaris population and the entire endogenous bacterial consortium (including the uncultivable bacteria) extracted from the seeds of the same Cu-tolerant A. capillaris population.
Materials and methods
Preparation of inoculants
Endophytic bacteria from A. capillaris seeds
Endophytic bacterial strains were extracted from seeds following a modified version of Mastretta et al. (2009) targeting the entire extracted endogenous seed bacterial consortium (including the uncultivable bacteria). Seeds (2 g) of a Cu-tolerant A. capillaris population, collected at a wood preservation site (St. Médard d’Eyrans, France, N 44° 43.353 W 000° 30.938; Bes et al. 2010), were submerged for 30 s in 70 % ethanol and rinsed in sterilized MilliQ water for 30 s. Seeds were thereafter placed for 15 min in a solution of 42 % sodium hypochlorite (1 % active chloride) supplemented with one droplet of Tween 80 per 100 mL of solution, rinsed three times with sterilized MilliQ water for 10 min and then recovered on a sterile nylon grid. Aliquots of the third rinsing solution and the seeds were both plated on 869 medium to ensure surface sterility (Mergeay et al. 1985). If no growth was observed after 7 days, the surface sterilization was considered to be successful. Surface-sterilized seeds were milled in a sterile mortar containing sterile Fontainebleau sand and 5 mL of 10 mM MgSO4 solution. This crude seed extract was halved: One part was directly used as an inoculant (SE), and for comparative purposes, the other part was filtered through a sterile Minisart (0.2 μm) which should retain almost all bacterial cells (this was named the bacterial cell-free extract, SEF). To test for the presence and also extraction of seed endophytes, aliquots of SE and SEF solutions were plated in duplicate onto 1/10 strength 869 agar medium (10.0 g tryptone, 5.0 g yeast extract, 5.0 g NaCl, 1.0 g glucose, 0.35 g CaCl2·2H2O in 1 L deionized water adjusted to pH 7.0; Mergeay et al. 1985) supplemented with 100 μg mL−1 of the fungicide cycloheximide. After 7 days incubation at 28 °C, colony-forming units (CFUs) were counted and the CFU per milliliter inoculum determined. Distinct morphotypes (5–10 colonies) were sub-cultured at least three times and cryo-preserved at −70 °C in culture medium supplemented with 15 % (v/v) glycerol. No bacterial colonies were observed after 7 days incubation in SEF plates. Purified strains were grown in liquid medium (1/10 strength 869), and genomic DNA was extracted from bacterial cell pellets. Briefly, the method consists of alkaline cell lysis followed by phenol/chloroform/isopropanol alcohol purification. DNA quality was checked by gel electrophoresis on a 0.8 % agarose gel. PCR amplification targeting the 16S rRNA gene was carried out using the primers 16S-27F (5′-AGAGTTTGATCMTGGCTCAG-3′) and 16S-1492R (5′-TACGGYTACCTTGTTA CGACTT-3′) (Lane 1991). PCR reactions were performed in a total volume of 50 μL containing: 1× Taq buffer (Invitrogen), 2.5 mM MgCl2, 0.1 mM of each dNTP, 1.75 U Taq polymerase (Invitrogen), 0.4 μM of each primer and 1 μL of extracted DNA. Thermocycling conditions were the following: 2 min at 94 °C, 30 cycles of 1 min at 94 °C, 1 min at 55 °C and 2 min at 72 °C and 1 cycle of 10 min at 72 °C. PCR products were partially sequenced (approximately 750 bases) using the primer 16S-27F (Lane 1991). Sequence data were checked using the Chromas v.1.45 software (Technelysium Pty. Ltd., Australia) and assessed for similarity with sequences of the Ribosomal Database Project (RDP; Cole et al. 2009). In parallel, strains were characterized for various plant growth-promoting traits (data not shown) as for root-located endophytic bacteria (Table 1).
Endophytic bacteria isolated from surface-sterilized A. capillaris roots
Ten strains of Cu-tolerant endophytic bacteria, the so-called M1 to M10, were previously isolated from surface-sterilized roots of the Cu-tolerant A. capillaris population described above and characterized for various plant growth-promoting traits (Table 1). Isolates were grouped according to their BOX-PCR profiles at a similarity level of 92 % (following methods of Becerra-Castro et al. 2011) into six groups. These isolates represented the dominant members of the cultivable population of root endophytes. Most of them were identified as members of the Pseudomonas genera, and one strain was closely related to Labrys sp. (99.8 % similarity). To prepare the root endophyte inoculant (RE), each strain was cultivated in liquid 869 medium for 3 days, harvested by centrifugation (4000g, 15 min) and re-suspended in 10 mM MgSO4 to an optical density of 0.7 at 660 nm (about 107 cells per mL). The final inoculum mixture contained an equal volume of the suspension of each strain. The same amount of sterile 10 mM MgSO4 was added to control seeds (CMg). In the control (C) treatment, sunflower seeds were untreated (Table 2).
Sunflower cultivation
Seeds (100 g) of the sunflower mutant line 1 [M6 (6th generation), 1/67-35-190-04] obtained by chemical mutagenesis using ethyl methane sulfonate (EMS) (Herzig et al. 2014) and harvested in 2009 at a non-contaminated site were surface-sterilized using the protocol described above for A. capillaris seeds and then germinated in axenic conditions on sterilized filter paper imbibed with 10 mM MgSO4 in a bacterial oven at 25 °C in the dark. This mutant line showed high shoot Cu removal in field plots at high total soil Cu (Kolbas et al. 2011). Germinated seeds (root length, 3–5 mm) were inoculated (100 μL added to the roots) with either the RE or SE inoculants or were exposed to 100 μL of either SEF or CMg under a vertical flux cabinet and maintained for 2 days in axenic conditions in a growth chamber [temperature 25 °C (day)/17 °C (night), relative humidity 60–65 % and a 12-h (day) photoperiod provided by Philips TDL 58WT33 fluorescent tubes, photosynthetic active radiation 160 μmol m−2 s−1] to optimize the endophytic bacteria penetration in theory via root hairs and the micro-cuts due to root growth (Bressan and Borges 2004).
Soil series with increasing total soil Cu (13–1020 mg kg−1) were obtained by mixing two similar air-dried alluvial sandy soils (Fluviosoil), i.e. a Cu-contaminated soil sampled (0–25 cm) in the plot #31 of the BIOGECO phytoremediation platform (Kolbas et al. 2011) and an uncontaminated soil sampled (0–25 cm) at the INRA Couhins experimental farm previously cropped with maize, in a ratio increasing from 0:100 to 100:0 % (Table 2). For all plant treatments, one seedling was transplanted into each potted soil (in triplicates).
Pots were placed in a climatic chamber with the following conditions: 14 h light/10 h darkness regime, 150 μmol m−2 s−1, 25 °C/22 °C and 65 % relative humidity (ISO 2005). Pots were arranged in a fully randomized block design on a table and watered daily with deionized water (50 % of water-holding capacity). The soils were fertilized twice, i.e. before starting the plant culture and 2 weeks after transplantation, with a modified Hoagland no. 2 nutrient solution (Hewitt 1966) deprived of Fe and other trace metals.
Plants were collected after 1 month at growth stage B4 (CETIOM 1995) when the 2nd pair leaves reached the 4-cm length. Shoots and roots were harvested, weighed (FW), rinsed in distilled water, oven-dried at 50 °C for 48 h and DW yield was determined. Other biometrical parameters were measured, i.e. root, stem and leaf lengths. The photosynthetic pigments were extracted from the 2nd pair of leaves (L2, 1 cm2, duplicates) with N,N-dimethylformamide (DMF) and contents of total chlorophyll (Chl TOT), Chl a, Chl b and total carotenoids were computed from measurements at 470, 647 and 664.5 nm of the extracts (spectrophotometer CARY 100 Scan, Lagriffoul et al. 1998).
Mineral composition of plant samples
Plant samples were ground in a titanium mill (Retsch MM200). Weighed aliquots of plant material (0.5 g DW) were wet digested in a laboratory microwave (Marsxpress, CEM) at 180 °C with 5 mL supra-pure 14 M HNO3 and 2 mL 30 % (v/v) H2O2 not stabilized by phosphates. Certified reference material (maize V463 BIPEA, Bureau InterProfessionnel d’Etudes Analytiques, France) and blank reagents were included in all series. Element concentrations in digests were determined by ICP-AES (Varian Liberty 200). All elements were recovered (>95 %) according to the standard values and standard deviation for replicates (n = 3) was <5 %. In the text, all element concentrations in plant parts are expressed based on DW.
Statistical analysis
All statistical analyses were performed using R software (version 2.12.0, R Foundation for Statistical Computing, Vienna, Austria). A two-way ANOVA test was used to analyze the differences in plant parameters across the soil series and plant treatments. Normality and homoscedasticity of residuals were met for all tests. Post hoc and Tukey HSD tests were performed to assess multi-comparison of means. A principal component analysis (PCA) was performed on all soil and plant parameters after having centered and scaled the values with the package Ade4. The degree of co-linearity of the soil properties was determined using the Pearson correlation coefficient test. Differences were considered significant if the p value was p < 0.05. Stars represent differences using pairwise t test on all datasets. Letters determined with the Scott-Knott package discriminate data using a SNK test for each data group.
Results and discussion
Plant parameters
None of the five treatments produced additional symptoms in shoots apart from those induced by Cu.
Stem length
Stem length of all treated plants was generally shorter than that of control (C) plants (Fig. 1a, b). Significant differences across the soil series occurred up to 516 mg Cu kg−1 in the soil. The C and CMg plants displayed a hormesis response (i.e. the stimulated phase in growth response curves that is induced by low toxic concentrations of metal ions without evidence of the underlying mechanisms; Poschenrieder et al. 2013) which peaked at 415 mg Cu kg−1 soil. Compared to C plants, this hormesis was less expressed for CMg plants and did not occur for the RE, SE and SEF plants. In most cases, the correlation between the stem length and the shoot biomass was weak because inoculated plants had short but thick stems and bigger leaves. This confirmed that stem length is a weak indicator of phytotoxicity for sunflower plants exposed to Cu excess (Kolbas et al. 2014).
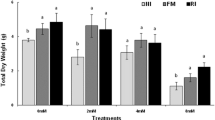
Plant morphological responses to increasing soil Cu exposure (mg kg−1 soil) using CMg and SEF modalities and SE and RE inoculants: a, b stem length (SL, cm), c, d shoot DW yield (DW SH, g DW plant−1), e, f root DW yield (DW RT, g DW plant−1) and g, h total DW yield (DW TOT, g DW plant−1). t test indicates significant differences between inoculated and control (C) plants; *P < 0.05
Shoot DW yield
In the 13–517 mg Cu kg−1 soil range, the SE and SEF modalities stimulated shoot DW yield whereas the RE inoculant or control treatments did not influence this parameter (except for CMg at 13–214 Cu kg−1 and RE at 416 Cu kg−1, but differences were not significant) (Fig. 1c). In this Cu range, the SE inoculant increased shoot biomass between 1.6- and 2-fold compared to the control plants. The shoot DW yield of SE plants was also significantly higher than that of SEF plants but only at the lowest Cu exposures (13–114 mg Cu kg−1 soil). In addition, the SE inoculant significantly reduced the number of visual symptoms of Cu phytotoxicity induced by Cu excess (i.e. chlorosis and necrosis) at moderate Cu level. These data suggest a beneficial effect of seed endophytes on shoot yield as previously reported for several metal(loid)-stressed plants (Glick 2010; Lyubun and Chernyshova 2010; Sessitsch et al. 2013; Wang et al. 2013), but showed that this beneficial effect is dependent on the Cu exposure level. Here, the seed endophytic strains present in the SE inoculant belonged mainly to the genera Bacillus sp. identified as Bacillus atrophaeus (when possible to species level) and also some members of the genera Rhodococcus (identified as Rhodococcus erythropolis). The bacterial density of the inoculum was low (105 CFUs mL−1 inoculum) but comparable with what has been found for different plant species, e.g. seed inoculum of N. tabacum (Mastretta et al., 2009). After filtration to 0.2 μm, no cultivable endophytic bacteria were found in the SEF extract; however, the presence of uncultivable strains cannot be completely ruled out (Rylo Sona Janarthine and Eganathan 2012). Additional experiments such as denaturing gradient gel electrophoresis technology (PCR-DGGE), fluorescence in situ hybridization (FISH) and scanning electronic microscopy (SEM) observations are needed to address their presence.
The beneficial effects of endophytic bacteria are generally attributed to 1-aminocyclopropane-1-carboxylate (ACC) utilization, the production of indoleacetic acid (IAA) and siderophores and solubilization of phosphates (Reed and Glick 2005; Ma et al. 2009; Rajkumar et al. 2012). To maintain constant levels of ACC in the extracellular medium, plants must exude larger ACC amounts that cannot be converted into ethylene (Glick 2010; Sessitsch et al. 2013). The presence of growth stimulation factors depends on endophytic bacteria: Within these microbial communities, nearly 20 to 80 % of the strains produce IAA, 7 to 36 % have the ACC deaminase and 40 to 95 % produce siderophores (Burd et al. 2000; Idris et al. 2004; Kuffner et al. 2008; Sziderics et al. 2007). All of the root endophytic strains included in the root endophyte consortium (RE) were able to produce siderophores, and some of them have ACC deaminase and can solubilize inorganic phosphorus (Table 1). The phenotypic characterization of collections of bacterial strains is a routine procedure when selecting for interesting bacterial inoculants for phytoremediation purposes. However, the plant growth-promoting traits which are studied in vitro frequently do not correlate with the actual effects of the inoculants when used in a plant bacterial system (Becerra-Castro et al. 2012). It is therefore not wholly unsurprising that the RE inoculants did not influence shoot DW yield (Fig. 1).
An additional beneficial effect might be caused by the presence of either Mg2+ and SO4 2− ions or seed and bran compounds in the small volume of seed macerate, notably to explain the responses of SEF plants (Kinraide et al. 2004; Lequeux et al. 2010; Zagorchev et al. 2013). As the shoot yield of CMg plants was not, or was only slightly, increased and remained lower than that of SE and SEF plants (Fig. 1c, d), this does not support a single biological action of MgSO4. The higher effect of the SE inoculant compared to SEF suggests the influence of endophytic bacteria present in the SE inoculant and not only that of soluble bioactive compounds in seed extracts. The effect of the SEF modality could be due to antimicrobial and antioxidant compounds, polyphenols such as resveratrol oligomers, allelochemicals or flavonoids such as procyanidins from endophyte-infected grasses (Sarkar et al. 2009; Li et al. 2009; Kiran et al. 2011; Wood et al. 2002; Wu et al. 2011).
Root DW yield
The root DW yield was significantly increased by CMg, SE and SEF modalities in plants grown between 13 and 517 mg Cu kg−1 soil. Compared to C plants, the root DW yield of CMg plants was enhanced by 1.3- to 2.2-fold in the 13–315 mg Cu kg−1 soil range. Root DW yield was promoted by 1.2- to 3.2-fold for SE and SEF plants between 13 and 517 mg Cu kg−1 soil. At lower soil Cu concentrations, the increase in root DW yield was more pronounced with SE compared to SEF. However, at higher total soil Cu concentrations, SE and SEF modalities had no effect on root yields. A hormesis effect, which is rare for roots, was found for the SEF and CMg plants, respectively, at 114 and 214 mg Cu kg−1 soil. These values were lower than those for stem length. The RE inoculant had a weak but significant beneficial effect at 416 and 617 mg Cu kg−1 soil.
Both RE and CMg modalities contained MgSO4, but the RE plants had a lower root DW yield than the CMg and SE plants at 13 and 214 mg Cu kg−1 soil range. This may indicate a negative influence of the Cu-tolerant, cultivable root endophytes obtained from A. capillaris. Not all root endophytic strains may have the same effect. Since we used a consortium of root endophytes as an inoculant, we could possibly have introduced a mix of both beneficial and pathogenic strains leading to an overall negative effect of the combination. Similarly, the inoculation of Lupinus luteus with endophytic bacteria extracted from roots of another species decreased its biomass because exogenous endophytic bacteria differing in biochemical behaviour from endogenous endophytes may induce defence reactions and have a negative effect on the plant growth (Barac et al. 2004). Compant et al. (2005b) reported the release of phenolic compounds after inoculation with endophytic bacteria, which is a typical defence response of plants against pathogenic bacteria. Bazely et al. (2007) suggested a cost for the host plant to support the endophyte presence. Only 1–5 % of the total bacterial community is thought to be cultivable (De la Iglesia et al. 2006), and it is possible that potential pathogenic entities for the plant are selected. We combined ten root endophytic strains which were the dominant isolates observed. They were isolated on the basis of morphological traits and functional responses, but several of them were in fact the same strain when BOX profiles were then compared (Table 1). In our final RE inoculant, some strains were therefore added at a much higher rate than others. No attempt was made to re-isolate the inoculated RE strains, so we do not know which of them survived or their competitiveness. Nonetheless, the RE inoculant stimulated plant growth at 416 and 617 mg Cu kg−1 soil, suggesting that the activity and influence of these inoculants may also depend on the plant exposure to Cu.
Chlorophyll content
All modalities showed a higher total chlorophyll content (Chl TOT) at low Cu level, a gradual decrease as soil Cu concentration increased and a bronzing effect at high Cu exposure(Fig. 2a, b). RE plants showed a significant increase at 416 mg Cu kg−1 soil compared with C and CMg plants. At high Cu exposure (1020 mg Cu kg−1), ChlTOT of RE plants was lower than that of control plants (Fig. 2a). In general, visible chlorosis occurred somewhat earlier (416 mg Cu kg−1 soil) for C and CMg plants than for SE and SEF plants (617 mg Cu kg−1 soil), which showed a significant increase in chlorophyll content and the weakening hormesis effect in the range between 13 and 617 mg Cu kg−1 soil, except at 114 and 214 mg Cu kg−1. The maximum improving effect (2.9-fold) was recorded for SE at 517 mg Cu kg−1 soil, but at this Cu exposure, difference between the SE and SEF modalities was not significant. The lack of significant differences between the SE and SEF modalities may be due to the low bacterial density of the SE inoculant. Similarly, the chlorophyll content of plants exposed to metals (Ni, Zn and Pb) increased when either the wild type or a bacterium mutant of Kluyvera ascorbata was present (Burd et al. 2000). Increase in chlorophyll content was also reported for Cajanus cajan after inoculation by Proteus vulgaris (+38 %, Rani et al. 2008) and for Alnus firma seedlings growing in a polymetallic contaminated soil after inoculation of endophytic bacteria (Babu et al. 2013).
Plant functional responses to increasing soil Cu exposure using CMg and SEF modalities and SE and RE inoculants: a, b total chlorophyll content (Chl TOT, mg m−2), c, d shoot Cu concentration (Cu SH, mg kg−1 DW), e, f root Cu concentration (Cu RT, mg kg−1 DW) and g, h shoot Cu removal (Cu MM, μg plant−1). t test indicates significant differences between inoculated and control (C) plants; *P < 0.05
Copper in plant tissues and shoot Cu removal
Shoot Cu concentration
In the 13–315 mg Cu kg−1 soil range, shoot Cu concentrations of control and all treated plants increased in a similar manner (except for CMg at 13 mg Cu kg−1 soil, which was significantly higher than others) and levelled off around 20 mg Cu kg−1 without any differences between the treatments (Fig. 2c, d). When total soil Cu reached 416 mg kg−1, shoot Cu concentration of control plants exceeded the upper critical threshold value (i.e. 25 mg kg−1, Kolbas et al. 2014), then it peaked to 40 mg kg−1 in plants grown in 718 mg Cu kg−1 soil and decreased thereafter. Between 416 and 819 mg Cu kg−1 soil, the RE and CMg plants had lower shoot Cu concentrations than the C (Fig. 2d), SE and SEF plants (Fig. 2c). Compared to control plants, both SE and SEF modalities decreased shoot Cu concentrations at 517 mg Cu kg−1 soil, while above this level of Cu exposure, shoot concentrations were only reduced by the SE inoculant. Shoot Cu concentration of RE plants increased progressively and reached the upper critical threshold value only at the highest Cu exposures (819–1020 mg Cu kg−1 soil). Overall, inoculated plants did not display higher shoot Cu concentration than the control plants.
Root Cu concentration
In the 13–315 mg Cu kg−1 soil range, all plants showed a gradual linear increase in root Cu concentration (Fig. 2e, f). Increased Cu concentration in roots compared to shoots reflects the preferential accumulation of this metal in sunflower roots (Alaoui-Sosse et al. 2004; Navari-Izzo et al. 2006). At 416 mg Cu kg−1 soil, root Cu concentration was higher in both SE and SEF plants (Fig. 2f). Above this soil Cu exposure, control root concentrations levelled up to 1000 mg Cu kg−1. In contrast, root Cu concentration of inoculated plants exceeded this value at 617 mg Cu kg−1 soil and then levelled up to 1500 Cu kg−1, except that of RE plants which continued to increase and reached 2000 Cu kg−1. In the 416–617 mg Cu kg−1 soil range, the SE and SEF modalities increased root Cu concentration and simultaneously presented higher root and shoot DW values (Fig. 1d, f and h); therefore, this was not a dilution effect. At high Cu exposure, the RE inoculant and Mg supply increased root Cu concentration (Fig. 2e), which may promote Cu phytostabilization (Dickinson et al. 2009). Magnesium may promote Cu compartmentalization and defence mechanisms (Shaul 2002). Root endophytic bacteria may enhance Cu exposure and storage in roots. For instance, microbes from the rhizosphere of E. splendens are likely key players in facilitating Cu solubility in contaminated soil and Cu accumulation in roots (2.5-fold) (Chen et al. 2005).
Influence of endophytic bacteria on metal(loid) uptake depends on the plant species and origin of the bacterial strain, i.e. inoculation increased Zn, Cd and Pb in Salix caprea (Kuffner et al. 2008) and Cu in B. juncea (Ma et al. 2009) but decreased As in sunflower (Lyubun and Chernyshova 2010). In S. caprea, a rhizosphere soil isolate reduced root metal concentrations, whereas the endophytic bacterial strain enhanced foliar metal concentrations but not plant growth. Root endophytes may promote root functions and TE uptake through the release of protons, siderophores, organic acids, phenolic compounds and polyamines (Rajkumar et al. 2009). Other mechanisms such as metal-binding peptides produced by bacterial strains may be involved in the enhancement of metal uptake by plants. Phytochelatins, metallothioneins and metallohistins are produced by certain bacteria in response to trace element stress (Sessitsch et al. 2013). Moreover, some metal-resistant PGPB such as Mesorhizobium amorphae CCNWGS0123 contains metal transporters from P-type ATPase, cation diffusion facilitator (CDF), hydrogenase/urease accessory proteins (HupE/UreJ) and chromate ion transporter (CHR) family involved in Cu, Zn, Ni as well as chromate resistance and homeostasis (Xie et al. 2014). Here, all root endophytic strains were siderophore producers, and several strains were able to produce organic acids to solubilize inorganic P and presented ACC deaminase activity (Table 1). Solubilization of P may indirectly increase the plant mineral nutrition (Malinowski et al. 2004; Glick and Stearns 2011).
Shoot Cu removal
In all treatments, shoot Cu removal, i.e. shoot DW yield × shoot Cu concentration, peaked between 114 and 214 mg Cu kg−1 soil (Fig. 2g, h). Differences were mainly induced by changes in shoot DW yield related to root growth and plant metabolism. In the 13–517 mg Cu kg−1 soil range, both SE and SEF modalities promoted shoot Cu removal by 30 and 120 % compared with control plants. In comparison, shoot Cu removal was increased by 8 % in A. firma seedlings (Babu et al. 2013) and 100 % in B. juncea (Ma et al. 2009). The abiotic CMg solution had a weak, insignificant positive effect at low total soil Cu (13–114 mg kg−1 soil). In contrast, RE did not enhance shoot Cu removal. Bacterial cell-free seed extract and seed endophytic bacteria showed the most promising influence for promoting Cu phytoextraction and, in particular, at low and moderate Cu exposures (Fig. 2h). In the 13–517 mg Cu kg−1 soil range, shoot Cu concentrations remained similar to the control, SE and SEF plants (Fig. 2d), meaning that the higher shoot DW yields for SE and SEF plants did not have a dilutive effect. Consequently, the main drivers for increasing shoot Cu removal were likely root development and functioning (Fig. 1f), maintenance of root-to-shoot Cu translocation (Fig. 2d) and shoot production (Fig. 1 d, h).
Endophytic bacteria may improve Cu tolerance and plant growth through several biological mechanisms. Bacterial ACC deaminase can limit ethylene production in stressed plant (Hardoim et al. 2008; Sun et al. 2010; Glick and Stearns 2011; Sessitsch et al. 2013). Four root endophytic strains had this activity (Table 1), but the RE inoculant did not promote shoot Cu removal (Fig. 2g) and influenced root Cu concentration at high soil Cu exposure (Fig. 2e). Secretion of phytohormones, especially of IAA, can lead to the formation of ACC, its root exudation and absorption by endophytic bacteria, which in turn convert it into ammonium and α-ketobutyrate. Here, no root endophytic strains were characterized as IAA producers (Table 1). Soluble bioactive compounds in seeds and bran, e.g. those listed above with antimicrobial and antioxidant properties, as well as the metal tolerance and phenotypic traits of alleged endophytic bacteria in A. capillaris seeds deserve more attention (Truyens et al. 2014).
To gain more information for data interpretation, further studies are pending: (1) identification of soluble bioactive compounds, notably elicitors, in the bacterial cell-free seed extract; (2) characterization of potential role of each seed endophyte and testing of the most effective consortium for bioaugmentation; (3) attempt to re-isolate our inoculants in the tissues of sunflower and use of improved tracking methods of bacterial inoculants to confirm their presence; (4) field testing of inoculated commercial cultivars and mutant lines of sunflower, potentially suitable for phytoextraction (Kolbas et al. 2011), with efficient endophytic bacterial consortia; and (5) examination of the number and distribution of endophytic bacteria in the tissues and seeds of sunflower.
Conclusions
Across this soil series, the inoculation of germinated, surface-sterilized sunflower seeds with bacterial cell-free and crude extracts of Cu-tolerant A. capillaris seeds similarly improved shoot Cu removal by sunflower when total soil Cu ranged from 14 to 517 mg kg−1. Over 517 mg Cu kg−1 soil, both seed extracts had no effect on plant parameters and Cu concentrations in sunflower shoots and roots. In the 13–114 mg Cu kg−1 soil range, shoot and root DW yields were more promoted by crude seed extracts, which contained endophytic bacteria belonging mainly to the genera Bacillus sp. and some members of the genera Rhodococcus. This suggested a beneficial seed-located endophytic bacterial influence in addition to soluble bioactive compounds in the bacterial cell-free extract. In contrast, cultivable endophytes from surface-sterilized roots of Cu-tolerant A. capillaris increased shoot and root DW yields of sunflower at high total soil Cu (416–617 mg kg−1), enhanced root Cu concentration as total soil Cu reached 819–1020 mg kg−1, but did not promote shoot Cu removal. The root- and seed-located endophytic strains and composition of bacterial cell-free seed extracts must be further investigated to explain such inoculated plant responses and induced molecular mechanisms.
Abbreviations
- ACC:
-
1-Aminocyclopropane-1-carboxylate
- C:
-
Untreated plants
- Chl TOT:
-
Total chlorophyll content
- CMg:
-
Control plants supplemented with a solution of MgSO4
- CuTOT:
-
Total soil Cu
- DMF:
-
N,N-Dimethylformamide
- DW SH:
-
Shoot dry weight yield
- DW RT:
-
Root dry weight yield
- IAA:
-
Indoleacetic acid
- PGPB:
-
Plant growth-promoting bacteria
- RE:
-
Inoculant with endophytic bacteria from the surface-sterilized A. capillaris roots
- SE:
-
Inoculant with endophytic bacteria from the A. capillaris seeds
- SEF:
-
Bacterial cell-free seed extract obtained by filtering a SE aliquot at 0.2 μm
- SL:
-
Maximum stem length
- TE:
-
Trace element
References
Adesodun JK, Atayese MO, Agbaje TA, Osadiaye BA, Mafe OF, Soretire AA (2010) Phytoremediation potentials of sunflowers (Tithonia diversifolia and Helianthus annuus) for metals in soils contaminated with zinc and lead nitrates. Water Air Soil Pollut 207(1–4):195–201. doi:10.1007/s11270-009-0128-3
Alaoui-Sosse B, Genet P, Vinit-Dunand F, Toussaint ML, Epron D, Badot PM (2004) Effect of copper on growth in cucumber plants (Cucumis sativus) and its relationships with carbohydrate accumulation and changes in ion contents. Plant Sci 166(5):1213–1218. doi:10.1016/j.plantsci.2003.12.032
Babu AG, Kim J-D, Oh B-T (2013) Enhancement of heavy metal phytoremediation by Alnus firma with endophytic Bacillus thuringiensis GDB-1. J Hazard Mater 250:477–483. doi:10.1016/j.jhazmat.2013.02.014
Barac T, Taghavi S, Borremans B, Provoost A, Oeyen L, Colpaert JV, Vangronsveld J, van der Lelie D (2004) Engineered endophytic bacteria improve phytoremediation of water-soluble, volatile, organic pollutants. Nat Biotechnol 22(5):583–588. doi:10.1038/nbt960
Bazely DR, Ball JP, Vicari M, Tanentzap AJ, Berenger M, Rakocevic T, Koh S (2007) Broad-scale geographic patterns in the distribution of vertically-transmitted, asexual endophytes in four naturally-occurring grasses in Sweden. Ecography 30(3):367–374. doi:10.1111/j.2007.0906-7590.04985.x
Becerra-Castro C, Kidd PS, Prieto-Fernandez A, Weyens N, Acea M-J, Vangronsveld J (2011) Endophytic and rhizoplane bacteria associated with Cytisus striatus growing on hexachlorocyclohexane-contaminated soil: isolation and characterisation. Plant Soil 340(1–2):413–433. doi:10.1007/s11104-010-0613-x
Becerra-Castro C, Monterroso C, Prieto-Fernández A, Rodríguez-Lamas L, Loureiro-Viñas M, Acea MJ, Kidd PS (2012) Pseudometallophytes colonising Pb/Zn mine tailings: a description of the plant–microorganism–rhizosphere soil system and isolation of metal-tolerant bacteria. J Hazard Mater 217–218:350–359. doi:10.1016/j.jhazmat.2012.03.039
Belimov AA, Hontzeas N, Safronova VI, Demchinskaya SV, Piluzza G, Bullitta S, Glick BR (2005) Cadmium-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard (Brassica juncea L. Czern.). Soil Biol Biochem 37(2):241–250. doi:10.1016/j.soilbio.2004.07.033
Bes C, Mench M, Aulen M, Gasté H, Taberly J (2010) Spatial variation of plant communities and shoot Cu concentrations of plant species at a timber treatment site. Plant Soil 330:267–280. doi:10.1007/s11104-009-0198-4
Bressan W, Borges MT (2004) Delivery methods for introducing endophytic bacteria into maize. Biocontrol 49(3):315–322. doi:10.1023/b:bico.0000025372.51658.93
Burd GI, Dixon DG, Glick BR (2000) Plant growth-promoting bacteria that decrease heavy metal toxicity in plants. Can J Microbiol 46(3):237–245. doi:10.1139/cjm-46-3-237
CETIOM (1995) Les stades repères du tournesol (détails). Available at http://www.cetiom.fr/tournesol/cultiver-du-tournesol/atouts-points-cles/stades-reperes/stades-reperes-detailles/?print=1. Access on 21 May 2014
Chen H, Cutright TJ (2003) Preliminary evaluation of microbially mediated precipitation of cadmium, chromium, and nickel by rhizosphere consortium. J Environ Eng 129(1):4–9. doi:10.1061/(asce)0733-9372(2003)129:1(4)
Chen YX, Wang YP, Lin Q, Luo YM (2005) Effect of copper-tolerant rhizosphere bacteria on mobility of copper in soil and copper accumulation by Elsholtzia splendens. Environ Int 31(6):861–866. doi:10.1016/j.envint.2005.05.044
Cherian S, Weyens N, Lindberg S, Vangronsveld J (2012) Phytoremediation of trace element-contaminated environments and the potential of endophytic bacteria for improving this process. Crit Rev Environ Sci Technol 42(21):2215–2260. doi:10.1080/10643389.2011.574106
Cole JR, Wang Q, Cardenas E, Fish J, Chai B, Farris RJ, Kulam-Syed-Mohideen AS, McGarrell DM, Marsh T, Garrity GM, Tiedje JM (2009) The ribosomal database project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res 37(Database issue):D141–D145. doi:10.1093/nar/gkn879PMCID, PMC2686447
Compant S, Duffy B, Nowak J, Clement C, Barka EA (2005a) Use of plant growth-promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71(9):4951–4959. doi:10.1128/aem. 71.9.4951-4959.2005
Compant S, Reiter B, Sessitsch A, Nowak J, Clement C, Barka EA (2005b) Endophytic colonization of Vitis vinifera L. by plant growth promoting bacterium Burkholderia sp strain PsJN. Appl Environ Microbiol 71(4):1685–1693. doi:10.1128/aem. 71.4.1685-1693.2005
Compant S, Clement C, Sessitsch A (2010) Plant growth-promoting bacteria in the rhizo- and endosphere of plants: their role, colonization, mechanisms involved and prospects for utilization. Soil Biol Biochem 42(5):669–678. doi:10.1016/j.soilbio.2009.11.024
De la Iglesia R, Castro D, Ginocchio R, van der Lelie D, Gonzalez B (2006) Factors influencing the composition of bacterial communities found at abandoned copper-tailings dumps. J Appl Microbiol 100(3):537–544. doi:10.1111/j.1365-2672.2005.02793.x
Dickinson NM, Baker AJM, Doronila A, Laidlaw S, Reeves RD (2009) Phytoremediation of inorganics: realism and synergies. Int J Phytoremediation 11(2):97–114. doi:10.1080/15226510802378368
Evon P, Vandenbossche V, Rigal L (2012) Manufacturing of renewable and biodegradable fiberboards from cake generated during biorefinery of sunflower whole plant in twin-screw extruder: Influence of thermopressing conditions. Polymer Degradation and Stability 97(10):1940–1947. doi:10.1016/j.polymdegradstab.2012.01.025
Faessler E, Robinson BH, Stauffer W, Gupta SK, Papritz A, Schulin R (2010) Phytomanagement of metal-contaminated agricultural land using sunflower, maize and tobacco. Agric Ecosyst Environ 136(1–2):49–58. doi:10.1016/j.agee.2009.11.007
Glick BR (2010) Using soil bacteria to facilitate phytoremediation. Biotechnol Adv 28(3):367–374. doi:10.1016/j.biotechadv.2010.02.001
Glick BR, Stearns JC (2011) Making phytoremediation work better: maximizing a plant’s growth potential in the midst of adversity. Int J Phytoremediation 13(Suppl 1):4–16
Hardoim PR, van Overbeek LS, van Elsas JD (2008) Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol 16(10):463–471. doi:10.1016/j.tim.2008.07.008
Herzig R, Nehnevajova E, Pfistner C, Schwitzguébel JP, Ricci A, Keller C (2014) Feasibility of labile Zn phytoextraction using enhanced tobacco and sunflower: results of five- and one-year field-scale experiments in Switzerland. Int J Phytoremediation 16(7–8):735–754. doi:10.1080/15226514.2013.856846
Hewitt E (1966) Sand and water culture methods used in the study of plant nutrition. The Eastern press Ltd, London
Idris R, Trifonova R, Puschenreiter M, Wenzel WW, Sessitsch A (2004) Bacterial communities associated with flowering plants of the Ni hyperaccumulator Thlaspi goesingense. Appl Environ Microbiol 70(5):2667–2677. doi:10.1128/a-em.70.5.2667-2677.2004
ISO (2005) Soil quality—determination of the effects of pollutants on soil flora Part 2: effects of chemicals on the emergence and growth of higher plants, vol ISO 11269–2. Geneva
Kabagale AC, Cornu B, van Vliet F, Meyer C-L, Mergeay M, Simbi J-BL, Droogmans L, Vander Wauven C, Verbruggen N (2010) Diversity of endophytic bacteria from the cuprophytes Haumaniastrum katangense and Crepidorhopalon tenuis. Plant Soil 334(1–2):461–474. doi:10.1007/s11104-010-0396-0
Kinraide TB, Pedler JF, Parker DR (2004) Relative effectiveness of calcium and magnesium in the alleviation of rhizotoxicity in wheat induced by copper, zinc, aluminum, sodium, and low pH. Plant Soil 259(1–2):201–208. doi:10.1023/b:plso.0000020972.18777.99
Kiran B, Lalitha V, Raveesha KA (2011) Antifungal and growth promoting potentiality of seeds of Psoralea corylifolia L. Res J Pharm Biol Chem Sci 2(3):564–573, Available at http://www.rjpbcs.com/pdf/2011_2(3)/68.pdf. Access on November 21, 2014
Kolbas A, Mench M, Herzig R, Nehnevajova E, Bes CM (2011) Copper phytoextraction in tandem with oilseed production using commercial cultivars and mutant lines of sunflower. Int J Phytoremediation 13(Suppl 1):55–76. doi:10.1080/15226514.2011.568536
Kolbas A, Mench M, Marchand L, Herzig R, Nehnevajova E (2014) Phenotypic seedling responses of a metal-tolerant mutant line of sunflower growing on a Cu-contaminated soil series: potential uses for biomonitoring of Cu exposure and phytoremediation. Plant Soil 376(1–2):377–397. doi:10.1007/s11104-013-1974-8
Kuffner M, Puschenreiter M, Wieshammer G, Gorfer M, Sessitsch A (2008) Rhizosphere bacteria affect growth and metal uptake of heavy metal accumulating willows. Plant Soil 304(1–2):35–44. doi:10.1007/s11104-007-9517-9
Kuffner M, De Maria S, Puschenreiter M, Fallmann K, Wieshammer G, Gorfer M, Strauss J, Rivelli AR, Sessitsch A (2010) Culturable bacteria from Zn- and Cd-accumulating Salix caprea with differential effects on plant growth and heavy metal availability. J Appl Microbiol 108(4):1471–1484. doi:10.1111/j.1365-2672.2010.04670.x
Lagriffoul A, Mocquot B, Mench M, Vangronsveld J (1998) Cadmium toxicity effects on growth, mineral and chlorophyll contents, and activities of stress related enzymes in young maize plants (Zea mays L.). Plant Soil 200(2):241–250. doi:10.1023/a:1004346905592
Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. Wiley, New York, pp 115–175
Lebeau T, Braud A, Jezequel K (2008) Performance of bioaugmentation-assisted phytoextraction applied to metal contaminated soils: a review. Environ Pollut 153(3):497–522. doi:10.1016/j.envpol.2007.09.015
Lequeux H, Hermans C, Lutts S, Verbruggen N (2010) Response to copper excess in Arabidopsis thaliana: impact on the root system architecture, hormone distribution, lignin accumulation and mineral profile. Plant Physiol Biochem 48(8):673–682. doi:10.1016/j.plaphy.2010.05.005
Li L, Henry GE, Seeram NP (2009) Identification and bioactivities of resveratrol oligomers and flavonoids from Carex folliculata seeds. J Agric Food Chem 57(16):7282–7287. doi:10.1021/jf901716j
Lodewyckx C, Taghavi S, Mergeay M, Vangronsveld J, Clijsters H, van der Lelie D (2001) The effect of recombinant heavy metal-resistant endophytic bacteria on heavy metal uptake by their host plant. Int J Phytoremediation 3(2):173–187. doi:10.1080/15226510108500055
Luo S, Xu T, Chen L, Chen J, Rao C, Xiao X, Wan Y, Zeng G, Long F, Liu C, Liu Y (2012) Endophyte-assisted promotion of biomass production and metal-uptake of energy crop sweet sorghum by plant-growth-promoting endophyte Bacillus sp SLS18. Appl Microbiol Biotechnol 93(4):1745–1753. doi:10.1007/s00253-011-3483-0
Lyubun Y, Chernyshova M (2010) Use of rhizobacteria to inoculate agricultural crops grown on arsenic-polluted soil. J Biotechnol 150:S247–S247. doi:10.1016/j.jbiotec.2010.09.118
Ma Y, Rajkumar M, Freitas H (2009) Inoculation of plant growth promoting bacterium Achromobacter xylosoxidans strain Ax10 for the improvement of copper phytoextraction by Brassica juncea. J Environ Manag 90(2):831–837. doi:10.1016/j.jenvman.2008.01.014
Ma Y, Prasad MNV, Rajkumar M, Freitas H (2011a) Plant growth promoting rhizobacteria and endophytes accelerate phytoremediation of metalliferous soils. Biotechnol Adv 29(2):248–258. doi:10.1016/j.biotechadv.2010.12.001
Ma Y, Rajkumar M, Luo Y, Freitas H (2011b) Inoculation of endophytic bacteria on host and non-host plants—effects on plant growth and Ni uptake. J Hazard Mater 195:230–237. doi:10.1016/j.jhazmat.2011.08.034
Madhaiyan M, Poonguzhali S, Sa T (2007) Metal tolerating methylotrophic bacteria reduces nickel and cadmium toxicity and promotes plant growth of tomato (Lycopersicon esculentum L.). Chemosphere 69(2):220–228. doi:10.1016/j.chemosphere.2007.04.017
Malinowski DP, Zuo H, Belesky DP, Alloush GA (2004) Evidence for copper binding by extracellular root exudates of tall fescue but not perennial ryegrass infected with Neotyphodium spp. endophytes. Plant Soil 267(1–2):1–12. doi:10.1007/s11104-005-2575-y
Mastretta C, Taghavi S, van der Lelie D, Mengoni A, Galardi F, Gonnelli C, Barac T, Boulet J, Weyens N, Vangronsveld J (2009) Endophytic bacteria from seeds of Nicotiana tabacum can reduce cadmium phytotoxicity. Int J Phytoremediation 11(3):251–267. doi:10.1080/15226510802432678
Meers E, Ruttens A, Hopgood M, Lesage E, Tack FMG (2005) Potential of Brassica rapa, Cannabis sativa, Helianthus annuus and Zea mays for phytoextraction of heavy metals from calcareous dredged sediment derived soils. Chemosphere 61(4):561–572. doi:10.1016/j.chemosphere.2005.02.026
Mench M, Schwitzguebel J-P, Schroeder P, Bert V, Gawronski S, Gupta S (2009) Assessment of successful experiments and limitations of phytotechnologies: contaminant uptake, detoxification and sequestration, and consequences for food safety. Environ Sci Pollut Res 16(7):876–900. doi:10.1007/s11356-009-0252-z
Mench M, Lepp N, Bert V, Schwitzguebel J-P, Gawronski SW, Schroeder P, Vangronsveld J (2010) Successes and limitations of phytotechnologies at field scale: outcomes, assessment and outlook from COST Action 859. J Soils Sediments 10(6):1039–1070. doi:10.1007/s11368-010-0190-x
Mergeay M, Nies D, Schlegel HG, Gerits J, Charles P, Vangijsegem F (1985) Alcaligenes-eutrophus CH34 is a facultative chemolithotroph with plasmid-bound resistance to heavy-metals. J Bacteriol 162(1):328–334
Navari-Izzo F, Cestone B, Cavallini A, Natali L, Giordani T, Quartacci MF (2006) Copper excess triggers phospholipase D activity in wheat roots. Phytochemistry 67(12):1232–1242. doi:10.1016/j.phytochem.2006.04.006
Poschenrieder P, Cabot C, Martos S, Gallego B, Barceló J (2013) Do toxic ions induce hormesis in plants? Plant Sci 212:15–25. doi:10.1016/j.plantsci.2013.07.012
Rajkumar M, Ae N, Freitas H (2009) Endophytic bacteria and their potential to enhance heavy metal phytoextraction. Chemosphere 77(2):153–160. doi:10.1016/j.chemosphere.2009.06.047
Rajkumar M, Sandhya S, Prasad MNV, Freitas H (2012) Perspectives of plant-associated microbes in heavy metal phytoremediation. Biotechnol Adv 30(6):1562–1574. doi:10.1016/j.biotechadv.2012.04.011
Rani A, Shouche YS, Goel R (2008) Declination of copper toxicity in pigeon pea and soil system by growth-promoting Proteus vulgaris KNP3 strain. Curr Microbiol 57(1):78–82. doi:10.1007/s00284-008-9156-2
Reed MLE, Glick BR (2005) Growth of canola (Brassica napus) in the presence of plant growth-promoting bacteria and either copper or polycyclic aromatic hydrocarbons. Can J Microbiol 51(12):1061–1069. doi:10.1139/w05-094
Rivelli AR, Sd M, Puschenreiter M, Gherbin P, de Maria S (2012) Accumulation of cadmium, zinc, and copper by Helianthus annuus L.: impact on plant growth and uptake of nutritional elements. Int J Phytoremediation 14(4):320–334. doi:10.1080/15226514.2011.620649
Ronda JC, Lligadas G, Galia M, Cadiz V (2011) Vegetable oils as platform chemicals for polymer synthesis. European Journal of Lipid Science and Technology 113(1):46–58. doi:10.1002/ejlt.201000103
Ryan RP, Germaine K, Franks A, Ryan DJ, Dowling DN (2008) Bacterial endophytes: recent developments and applications. FEMS Microbiol Lett 278(1):1–9. doi:10.1111/j.1574-6968.2007.00918.x
Rylo Sona Janarthine S, Eganathan P (2012) Plant growth promoting of endophytic Sporosarcina aquimarina SJAM16103 isolated from the pneumatophores of Avicennia marina L. Int J Microbiol 2012(ID 532060):1–10. doi:10.1155/2012/532060
Saikkonen K, Ahlholm J, Helander M, Lehtimaki S, Niemelainen O (2000) Endophytic fungi in wild and cultivated grasses in Finland. Ecography 23(3):360–366. doi:10.1034/j.1600-0587.2000.d01-1645.x
Sarkar D, Bhowmik PC, Kwon Y-I, Shetty K (2009) Clonal response to cold tolerance in creeping bentgrass and role of proline-associated pentose phosphate pathway. Bioresour Technol 100(21):5332–5339. doi:10.1016/j.biortech.2009.03.086
Sessitsch A, Kuffner M, Kidd P, Vangronsveld J, Wenzel WW, Fallmann K, Puschenreiter M (2013) The role of plant-associated bacteria in the mobilization and phytoextraction of trace elements in contaminated soils. Soil Biol Biochem 60:182–194. doi:10.1016/j.soilbio.2013.01.012
Shaul O (2002) Magnesium transport and function in plants: the tip of the iceberg. Biometals 15(3):309–323
Sheng X, Xia J, Jiang C, He L, Qian M, Sheng XF, Xia JJ, Jiang CY, He LY, Qian M (2008) Characterization of heavy metal-resistant endophytic bacteria from rape (Brassica napus) roots and their potential in promoting the growth and lead accumulation of rape. Environ Pollut 156(3):1164–1170. doi:10.1016/j.envpol.2008.04.007
Sturz AV, Nowak J (2000) Endophytic communities of rhizobacteria and the strategies required to create yield enhancing associations with crops. Appl Soil Ecol 15(2):183–190. doi:10.1016/s0929-1393(00)00094-9
Sun L-N, Zhang Y-F, He L-Y, Chen Z-J, Wang Q-Y, Qian M, Sheng X-F (2010) Genetic diversity and characterization of heavy metal-resistant-endophytic bacteria from two copper-tolerant plant species on copper mine wasteland. Bioresour Technol 101(2):501–509. doi:10.1016/j.biortech.2009.08.011
Sziderics AH, Rasche F, Trognitz F, Sessitsch A, Wilhelm E (2007) Bacterial endophytes contribute to abiotic stress adaptation in pepper plants (Capsicum annuum L.). Can J Microbiol 53(11):1195–1202. doi:10.1139/w07-082
Truyens S, Jambon I, Croes S, Janssen J, Weyens N, Mench M, Carleer R, Cuypers A, Vangronsveld J (2014) The effect of long-term Cd and Ni exposure on seed endophytes of Agrostis capillaris and their potential application in phytoremediation of metal-contaminated soils. Int J Phytoremediation 16(7–8):643–659. doi:10.1080/15226514.2013.837027
Vangronsveld J, Herzig R, Weyens N, Boulet J, Adriaensen K, Ruttens A, Thewys T, Vassilev A, Meers E, Nehnevajova E, van der Lelie D, Mench M (2009) Phytoremediation of contaminated soils and groundwater: lessons from the field. Environ Sci Pollut Res 16(7):765–794. doi:10.1007/s11356-009-0213-6
Wang ZW, Wang SM, Ji YL, Zhao MW, Yu HS (2005) Plant endophyte research-detection and distribution of endophytic fungi in poaceous plants in saline-alkali areas in Dongying, Shandong, China. Pratacultural Sci 22(2):60–64
Wang W, Deng Z, Tan H, Cao L (2013) Effects of Cd, Pb, Zn, Cu-resistant endophytic enterobacter sp CBSB1 and Rhodotorula sp CBSB79 on the growth and phytoextraction of Brassica plants in multimetal contaminated soils. Int J Phytoremediation 15(5):488–497. doi:10.1080/15226514.2012.716101
Weyens N, van der Lelie D, Taghavi S, Vangronsveld J (2009) Phytoremediation: plant-endophyte partnerships take the challenge. Curr Opin Biotechnol 20(2):248–254. doi:10.1016/j.copbio.2009.02.012
Wood JE, Senthilmohan ST, Peskin AV (2002) Antioxidant activity of procyanidin-containing plant extracts at different pHs. Food Chem 77(2):155–161
Wu L, Huang Z, Qin P, Yao Y, Meng X, Zou J, Zhu K, Ren G (2011) Chemical characterization of a procyanidin-rich extract from sorghum bran and its effect on oxidative stress and tumor inhibition in vivo. J Agric Food Chem 59(16):8609–8615. doi:10.1021/jf2015528
Xie P, HaoX, Herzberg M, Luo Y, Nies DH, Wei G (2014) Genomic analyses of metal resistance genes in three plant growth promoting bacteria of legume plants in Northwest mine tailings, China. Journal of Environmental Sciences (in press). doi:10.1016/j.jes.2014.07.017
Y-f Z, L-y H, Z-j C, Q-y W, Qian M, X-f S (2011) Characterization of ACC deaminase-producing endophytic bacteria isolated from copper-tolerant plants and their potential in promoting the growth and copper accumulation of Brassica napus. Chemosphere 83(1):57–62. doi:10.1016/j.chemosphere.2011.01.041
Zagorchev L, Seal CE, Kranner I, Odjakova M (2013) A central role for thiols in plant tolerance to abiotic stress. Int J Mol Sci 14(4):7405–7432. doi:10.3390/ijms14047405
Acknowledgments
This work was financially supported by ADEME, Department of Urban Brownfields and Polluted Sites, Angers, France, and the European Commission under the Seventh Framework Programme for Research (FP7-KBBE-266124, GREENLAND). This study has been carried out in the framework of the Cluster of Excellence COTE. The authors thank Dr. Jean-Paul Maalouf for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Kolbas, A., Kidd, P., Guinberteau, J. et al. Endophytic bacteria take the challenge to improve Cu phytoextraction by sunflower. Environ Sci Pollut Res 22, 5370–5382 (2015). https://doi.org/10.1007/s11356-014-4006-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-4006-1