Abstract
The objective of this study was to quantify the phytoextraction of the potentially toxic elements Al, As, Cd, Co, Cr, Cu, Mo, Ni, Pb, Se, V, and Zn by Indian mustard, rapeseed, and sunflower from a contaminated riparian soil. To achieve this goal, a greenhouse pot experiment was established using a highly contaminated grassland soil collected at the Wupper River (Germany). The impact of ethylene-diamine-tetra-acetic acid (EDTA), humate (HK), and phosphate potassium (PK) on the mobility and uptake of the elements by rapeseed also was investigated. Indian mustard showed the highest efficiency for phytoextraction of Al, Cr, Mo, Se, and V; sunflower for Cd, Ni, Pb, and Zn, and rapeseed for Cu. The bioconcentration ratios were higher than 1 for the elements (except As and Cu), indicating the suitability of the studied plants for phytoextraction. Application of EDTA to the soil increased significantly the solubility of Cd, Co, Cr, Ni, and Pb and decreased the solubility of Al, As, Se, V, and Mo. Humate potassium decreased significantly the concentrations of Al and As in rapeseed but increased the concentrations of Cu, Se, and Zn. We may conclude that HK can be used for immobilization of Al and As, while it can be used for enhancing the phytoextraction of Cu, Se, and Zn by rapeseed. Phosphate potassium immobilized Al, Cd, Pb, and Zn, but enhanced phytoextraction of As, Cr, Mo, and Se by rapeseed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
High concentrations of potentially toxic elements (PTEs) in soils and sediments may cause long-term risks to humans and ecosystems (Rinklebe et al. 2007; Paller and Knox 2010; Shaheen et al. 2014a). Many riparian and wetland soils worldwide and in Germany are highly contaminated with PTEs (Knox et al. 2006; Shaheen and Rinklebe 2014; Rinklebe and Shaheen 2014; Frohne et al. 2014). Due to the high concentrations of PTEs in riparian soils, these elements might be released and mobilized (Shaheen et al. 2014a, b, c), leading to soil and groundwater contamination what increase the possibility of entering into the grassland and food chain (Overesch et al. 2007; Licina et al. 2010). Thus, remediation of soils contaminated with PTEs has received increasing attention and is doubtless an important issue for an adequate environmental management (Ok et al. 2011a, b, c; Ahmad et al. 2012, 2014; Shaheen and Rinklebe 2015; Shaheen et al. 2015a, b).
Phytoremediation of soils contaminated with PTEs is a natural, environmental friendly, and a cost-effective technology based on the use of specially selected element-accumulating plants to remove these elements from soils and waters (Prasad et al. 2015). Phytoextraction is a subprocess of phytoremediation in which plants remove toxic elements from soil (Greipsson 2011; Ali et al. 2013; Sessitsch et al. 2013). Recently, using oil-producing crops for phytoremediation of PTE-contaminated soils is recommended because they are not for direct food consumption, and thus, bioaccessibility is hampered (Srikanth et al. 2013). Indian mustard (Brassica juncea L.), rapeseed (Brassica napus), and sunflower (Helianthus annuus) are traditional crops which can be used for the production of oil and also used in phytoremediation of soils polluted with PTEs (Chen and Cutright 2001; Grispen et al. 2006; Suthar et al. 2013).
Nowadays many farmers tend to focus a part of their production on technical crops and/or the utilization of classical crops as main products or byproducts for industrial as well as energy purposes. As for the biomass energy generation, oil crops are primarily used as a substitute for diesel engine fuels thanks to the high-energy content in their oils. Thus, cultivation of bioenergy crops, i.e., rapeseed, Indian mustard, and sunflower, in the contaminated soils has the potential to become a profitable enterprise when combined with biofuel production, especially in view of the increasing oil prices over the coming years (Grispen et al. 2006; Ali et al. 2013). Additionally, since phytoextraction is a long-term technology, fields undergoing phytoremediation need to be kept productive to achieve economically viable and socially acceptable decontamination. Moreover, the use of energy and/or biodiesel crops as PTEs phytoextraction plants would give contaminated soils a productive value and decrease remediation costs (Greipsson 2011; Rinklebe and Shaheen 2015).
Very little is known on PTE tolerance and accumulation of oil-producing crops such as Indian mustard, rapeseed, and sunflower growing in polluted riparian grassland soils. In addition, those plant species have not been investigated for this purpose in contaminated riparian soils. Moreover, no attempts have been made to study the effectiveness of some applicable materials, i.e., humate potassium (HK), phosphate potassium (PK), and the ethylene-diamine-tetra-acetic acid (EDTA), for the mobilization/immobilization of PTEs in heavily contaminated riparian soils. Therefore, the novelty of the current study is that we examine a variety of crops not tested as accumulators for PTEs in this soil and not restricted in a particular geographical locality. Additionally, the use of plants, which are not for direct food consumption and of high economic value, e.g., bioenergy crops such as rapeseed, Indian mustard, and sunflower, as test crops in toxic metal-contaminated soils, is an issue worth evaluating.
We hypothesized that the oil-producing and non-edible plants such as rapeseed, Indian mustard, and sunflower might be a possible trial for phytoremediation of these contaminated riparian sites. In addition, application of humate potassium and phosphate potassium might enhance the phytoextraction of PTEs by these plants. Therefore, the objectives of our study were (1) to examine the efficiency of Indian mustard, rapeseed, and sunflower for phytoextraction of Al, As, Cd, Co, Cr, Cu, Mo, Ni, Pb, Se, V, and Zn from a highly contaminated riparian soil and (2) to assess the impact of EDTA, humate potassium, and phosphate potassium on the phytoextraction of the elements by rapeseed.
Materials and methods
Sampling site and collection of the soil
The study site is located at the lower course of the Wupper River close to the confluence into the Rhine River, near the town Leverkusen, about 20 km to the north of Cologne, Germany (E 2570359, N 5661521; 51°4′0.48″N, 6°4′0.48″E). The site is used as grassland. The study site is flooded periodically by the Wupper River, usually in springtime. The soil is classified as Eutric Fluvisols according to IUSS-FAO (2014). The industrialization started very early in the Wupper River catchment and was intense during the last centuries. The main sources are discharges from metal, textile, and chemical industry during the last centuries. Particularly knife manufacturing, electroplating, and textile bleaching occurred frequently in this catchment. Thus, high concentrations of PTEs, particularly Cu, occur in soils of this area (Frohne et al. 2011, 2014).
Soil profile was excavated, described in detail and investigated. The particular soil horizon (26–40 cm) was selected for this study since it reveals the highest contamination of Cu (3041.9 mg kg−1). About 400 kg soil was collected from an area 4 m2. The sampling was performed in eight replicates of about 50 kg which were pooled to one composite sample. Soil material was homogenized, air-dried, and crushed handily. Soil properties and oxides content were determined according to standard methods (Blume et al. 2011). Total concentrations of the elements in the soil were extracted after its digestion in a microwave system (Milestone MLS 1200 Mega, Germany) (USEPA 2007). The available form from the studied elements was extracted by 1 M ammonium bicarbonate (NH4HCO3) and 0.005 M diethylene triamine pentaacetic acid (DTPA) according to Soltanpour and Schwab (1977). Chemical fractions of the elements were sequentially extracted based on the work of Tessier et al. (1979) and proposed by Sánchez-Martín et al. (2007). The method used discriminates the elements into soluble + exchangeable (F1: 1 M NH4OAc (pH 7.0), sorbed and bound to carbonate (F2: 1 M NH4OAc adjusted to pH 5 with HOAc), Fe–Mn oxide bound (F3: 0.175 M (NH4)2C2O4 and 0.1 M H2C2O4), and organically bound (F4: 0.1 M Na4P2O7). Separation between steps was by decantation of the supernatant after centrifugation at 5000 rpm for 20 min. The concentrations of Al, As, Cd, Co, Cr, Cu, Mo, Ni, Pb, Se, V, and Zn in the digested and extracted soil samples were measured by inductively coupled plasma-optical emission spectroscopy (ICP–OES) (Ultima 2, Horiba Jobin Yvon, Unterhaching, Germany). A 4-point calibration was performed with standard solutions (CertiPur, Merck) diluted in deionised water. Each sample was measured in three replications. The relative standard deviation of replicate analysis was below 5 %. The concentrations were calculated on the basis of dry weight of samples (105 °C, 24 h).
Pot experiments
The efficiency of Indian mustard, rapeseed, and sunflower to phytoextract Al, As, Cd, Co, Cr, Cu, Mo, Ni, Pb, Se, V, and Zn from the studied soil was quantified in a greenhouse pot experiment. A portion of 4 kg air-dried soil was placed into 25-cm-diameter and 30-cm-height pots. The influence of EDTA, HK, and PK on the phytoavailability of the elements to the rapeseed was investigated by mixing of these materials with the soil (0.5 %) 1 week before cultivation. The commercial HK (Hiuminova) was used in this study. It is potassium salt of humic acid, is manufactured commercially by alkaline extraction of lignite (brown coal) or peat, and is used as a soil conditioner. The HK is an alkaline (pH 8.76) and contain 10 % K2O. The HK2PO4 and EDTA (292.2 gmol−1) were obtained from Sigma-Aldrich (purity above 99 %).
A complete randomized block design was composed of three treatments, i.e., HK, PK, and EDTA as well as the control with three replicates each. The pots were irrigated with approximately 1 L deionized water to reach a moisture content of about 60 % of the field capacity and incubated for 1 week. Indian mustard, rapeseed, and sunflower were planted. Thirty seeds of rapeseed and Indian mustard (later thinned to 25) and fifteen seeds of sunflower were sown in each pot. During the germination period, soil moisture was maintained at 80 % of field capacity and after thinning was raised to field capacity. The pots were irrigated with deionized water. The moisture content was kept at field capacity by weighting the pots daily and adding the lost water. The pots were irrigated with 300 ml from a nutrient solution that contains 0.8 g NH4NO3 L−1 and 0.4 g HK2PO4 L−1 (solution pH 7.2) after 2 and 5 weeks from the germination. The plants were harvested 12 weeks after seeding. The above-ground portion of plants was harvested (2.5 cm from the soil).
Preparation and analyses of plant and soil samples
The harvested plants were thoroughly washed with 1 mM HCl, rinsed in distilled water, and dried to constant weight at 70 °C in a forced draft oven. The dry biomass of plants was recorded, and then the plant samples were grounded to fine powder in a stainless grinder and stored in plastic bags until analysis. One gram of plant material was dry-ashed in a muffle furnace at 450 °C for 5 h, extracted with 20 % hydrochloric acid (Jones et al. 1991). The soil samples were taken out of the pots, air-dried, passed through a 2-mm sieve, and analyzed for pH (1:1 H2O) and for available elements using AB-DTPA (Soltanpour and Schwab 1977). Concentrations of the elements in plant and soil samples were measured by ICP–OES (see above).
Quality control and statistical analysis
In all measurements, blanks, triplicate measurements of PTEs in extracts, and analyses of certified reference materials for each element (Merck) were routinely included for quality control. Quality control of the extraction efficiency for the total element concentrations was performed using certified soil reference materials (BRM No. 13 and BRM No. 10a) obtained from the Federal Institute for Materials Research and Testing (BAM). The average recovery ranged from 85 to 104.5 % depending on the element. Maximum allowable relative standard deviation between replicates was set to 5 % for soil and 10 % for plant analyses. The detection limits were 28 µg L−1 for Al, 10 µg L−1 for As, 2.7 µg L−1 for Cd, 7.0 µg L−1 for Co, 7.1 µg L−1 for Cr, 5.4 µg L−1 for Cu, 12 µg L−1 for Mo, 10 µg L−1 for Ni, 8.0 µg L−1 for Pb, 1.5 µg L−1 for Se, 7.5 µg L−1 for V, and 1.8 µg L−1 for Zn. Values below the detection limit were set 1/8 of the detection limit for statistical analyses. Statistical analyses were performed using the analysis of variance (ANOVA) and Duncan’s multiple range tests to compare the means of the treatments at a level of significance of p < 0.05 using the SPSS 22 package.
Results and discussions
Soil characterization
Basic properties of the soil are presented in Table 1. The soil texture was dominated by silt. The soil was weakly acidic, contained high organic carbon, and showed a moderate cation exchange capacity (CEC). The concentrations of Fe oxides in the soil were higher than those of Mn oxides. The amorphous Fe and Mn (oxalate extractable) values were relatively low compared with the crystalline form (CBD extractable) suggesting that the majority of Fe and Mn existed in crystalline form. Consequently, the acidity, high organic carbon content, and the oxide forms were expected to affect the element mobility and their phytoavailability to the plants.
Element concentrations in the soil
Concentrations of total, available (AB-DTPA), and fractions of the elements in the soil are presented in Table 2. Concentrations of total As, Cd, Co, Cr, Cu, Ni, Pb, Se, and Zn exceeded the precautionary values of the German Federal Soil Protection and Contaminated Sites Ordinance (BBodSchV 1999). In addition, the values of As and Cu exceeded the action values (50 and 1300 mg kg−1 for As and Cu, respectively; BBodSchV 1999). The risk of Cu pollution in this riverine soil needs great concern because Cu can become mobilized at high concentrations leading to soil and groundwater contamination, which increases the possibility of Cu entering the food chain via vegetation (Shaheen et al. 2014a; Rinklebe and Shaheen 2015). High Cu concentrations can pose very high eco-toxicological and environmental risks and have significant agricultural relevance. Therefore, the German authorities must act per law to remediate these soils. Moreover, the values of As, Cr, Cu, Pb, Se, and Zn were higher than the upper limit of the trigger action values for PTEs in agricultural soils as reported by Kabata-Pendias (2011), implying harmful soil alterations which need soil remediation. The element availability as extracted using AB-DTPA was high for Cd, Cu, Ni, and Zn (1.7, 183.1, 2.04, and 28.5 mg kg−1, respectively). Higher total concentrations of the elements in the studied soil are very likely due to the anthropogenic activities (Frohne et al. 2011, 2014, 2015).
Distribution of the elements among their fractions differed widely based on the element and the fraction (Table 2). The Al, Mo, Se, and V were distributed in the residual fraction by a percent (% of the total) ranged from 61.7 to 86.0 %, while the other elements distributed in the non-residual fractions (potential mobile fraction; PMF = ∑F1–F4). The Mn showed the highest potential mobility (95 %) followed by Cr (78 % of total), Cd (75 %), Co (73 %), As (70 %), Pb (62 %), Ni (57 %), Zn (55 %), and Cu (53 %). Among the PMF, the oxide fraction (F3) was dominant for all elements [except Pb which are concentrated in the organic fraction (F4)]. Cadmium showed the highest mobility (25 %) (mobile fraction; MF = ∑F1–F2) followed by Cu (16 %), Zn (10 %), Pb (4 %), and Ni (3 %), while the other elements showed negligible concentrations in this fraction (Table 2).
Phytoextraction of the elements by Indian mustard, rapeseed, and sunflower
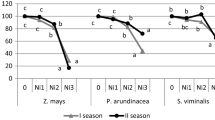
Concentrations of the elements in the plants and AB-DTPA extractable elements in the associated soil samples are given in Figs. 1 and 2, respectively. Indian mustard showed significant higher concentrations (mg kg−1) of Al (358.8), Cr (3.1), Mo (7.4), Se (1.6), and V (0.97) than sunflower and rapeseed (Fig. 1). Sunflower showed the highest concentrations of Cd (3.2), Ni (5.5), Pb (1.1), and Zn (534.0), while the highest concentrations of Cu (62.1) were recorded in rapeseed (Fig. 1). Nonsignificant differences were recorded between the concentrations of As and Co in the three plants (Fig. 1).
Concentrations of the elements in Indian mustard (IM), sunflower (SF), and rapeseed (RS) as well as in rapeseed plus the soil amendments. HK humate potassium; PK phosphate potassium. Values accompanied by different letters are significantly different within columns at the level (P < 0.05). Please notice the different scales
Concentrations of AB-DTPA-extractable elements (mg/kg) in the soil as affected by the three different plants as well as by rapeseed plus soil amendments. IM Indian mustard, SF sunflower, RS rapeseed, HK humate potassium, PK phosphate potassium. Values accompanied by different letters are significantly different within columns at the level (P < 0.05). Please notice the different scales
Critical concentrations of trace elements in sensitive plant tissues (mg kg−1) as reported by Kabata-Pendias (2011) were 1–10 for As, 5–10 for Cd, 10–20 for Co, 1–2 for Cr, 15–20 for Cu, 20–30 for Ni, 5–10 for Pb, and 150–200 for Zn. The plants showed higher Cr (except sunflower), Cu, and Zn concentrations than the critical levels. However, the As, Cd, Co, Ni, and Pb concentrations were lower than the critical levels. These results may indicate that Indian mustard, rapeseed, and sunflower can grow on soil contaminated with Cr, Cu, and Zn and therefore seem to tolerate these elements to the given levels. However, none of the studied plants revealed element concentrations >1000 mg kg−1; thus, none of them are hyperaccumulators (Baker and Brooks 1989).
Soil-to-plant transfer factor can be used to estimate a plant’s potential for phytoremediation purpose (Yoon et al. 2006; Tomovic et al. 2013). The above-ground plant-to-soil ratio (bioconcentration ratio; BCR), which represents the ratio of the concentration of an element in above-ground vegetation to the available concentration in the soil, was calculated according to Wang et al. (2006) as follows: BCR = mg element kg−1 plant/mg AB-DTPA-element kg−1 soil.
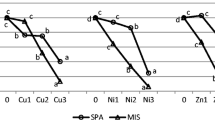
Results of BCR values are presented in Fig. 3. The ability of Indian mustard to absorb Al, Cr, Mo, and V was significantly higher than rapeseed and sunflower. The ability of sunflower to absorb Cd, Ni, Pb, and Zn was higher than Indian mustard and rapeseed, while rapeseed showed a higher ability to absorb Cu than Indian mustard and sunflower.
Bioconcentration ratio of the elements for the three different plants as affected by the plants and soil amendments. IM Indian mustard, SF sunflower, RS rapeseed, HK humate potassium, PK phosphate potassium. Values accompanied by different letters are significantly different within columns at the level (P < 0.05). Please notice the different scales
Aluminum showed the highest BCR values (505–1441) followed by Mo (16–68), Co (16–68), Se (12–51), Zn (7–17), Cr (1.3–5.2), Pb (2.4–4.1), V (1.7–3.4), Ni (0.97–1.9), Cd (0.47–1.7), As (0.46–0.59), and Cu (0.17–0.26) (Fig. 3). These results indicate that the BCR values differed widely among the elements and the plants and decreased with increasing AB-DTPA-extractable element. Copper showed the highest AB-DTPA-extractable concentrations and the lowest BCR values (Figs. 2, 3), indicating that the BCR values depend on available element concentration. This was probably due to the fact that the increases in Cu concentration in plants with high available Cu in the soil (i.e., the numerator in the BCR fraction) were disproportionally lower than AB-DTPA increases (i.e., the denominator in BCR) caused by high Cu concentrations in the soil. Contrasting to Cu, although concentrations of AB-DTPA-extractable Al were very low as compared to the other elements, their concentrations in plant and BCR values were higher than the other elements, which might mean that a significant part of the non-residual fractions (Table 2) is mobile and might be available to plants.
The process of phytoextraction generally requires the translocation of elements to easily harvestable plant parts, i.e., shoots. Tolerant plants tend to restrict soil–root and root–shoot transfers and therefore have much less accumulation in their biomass, while hyperaccumulators actively take up and translocate elements into their aboveground biomass. Plants exhibiting BCR values less than 1 are considered to be unsuitable for phytoextraction (Baker and Brooks 1989). Thus, the three studied plant species were capable of accumulating the elements in the above-ground biomass, and most of them had high BCR values (higher than 1 in most cases except for As and Cu for the three plants and for Cd with Indian mustard and rapeseed), which means a high ability of element accumulation by the plants. Thus, based on the BCR values, the studied plants were efficient in taking up the studied elements (except As and Cu). In addition, based on the results of BCR (Fig. 3), we may recommend Indian mustard for phytoremediation of soils contaminated with Al, As, Co, Cr, Mo, Se, and V, while sunflower is recommended for soils contaminated with Cd, Ni, Pb, and Zn, and rapeseed seems to be more suitable for Cu-contaminated soils.
Although the soil was never flooded during the pot experiment and, thus, very low redox potentials (E h) have obviously not occurred, however, it is known that low E h occur at field conditions at the riparian soil under study. Therefore, E h are also considered to affect solubility of elements in this particular soil under field conditions. Prevailing oxidizing conditions might facilitate the mobility of many elements in flooded soils, which may be attributed to dissolution of sulfides and the resulted release of elements (Du Laing et al. 2009; Frohne et al. 2011; Shaheen et al. 2014a, b, c). In opposite, several elements such as antimony, arsenic, chromium, and vanadium are known to be mobile under anoxic conditions (Frohne et al. 2011, 2015). Recently, Rinklebe et al. (2015) reported that the pattern of E h/pH and the release mechanisms of trace elements in this highly contaminated soil and in the same soil treated with biochar-based material are basically similar which is an important finding also with view to a sustainable management of these ecosystems.
Impact of EDTA, HK, and PK on phytoavailability of the elements to rapeseed
Mixing the soil with EDTA, HK, and PK changed significantly concentrations of AB-DTPA-extractable elements as compared to the non-treated soil (RS) (Fig. 2). The EDTA (RS + EDTA) increased significantly the concentrations of AB-DTPA extractable Cd, Co, Cr, Cu, Ni, Pb, and Zn, while it decreased significantly the concentrations of Al, As, Mo, Se, and V compared with the RS (Fig. 2). Therefore, application of EDTA inhibited the seed germination by 100 % because of the severe increasing of solubility of many toxic element especially Pb. The addition of chelating agents and the consequent formation of element-chelate complexes prevent precipitation and sorption of the elements in the soil, thereby maintaining their availability for plant uptake (Suthar et al. 2013). The addition of chelates to the soil can also bring elements into solution through desorption of sorbed species and dissolution of precipitated compounds (Freitas and Nascimento 2009). The EDTA increased solubility of Pb, Zn, and Cd and therefore might be used for enhancement of the phytoextraction of these elements by the plants. Despite the possible usefulness of this technology, some concerns have been expressed regarding the potential inherent risk of leaching of elements to groundwater (Marques et al. 2009). The addition of chelates to PTE-contaminated matrix can increase the levels of water-extractable elements. For example, the application of EDTA to PTE-contaminated soil has been reported to increase significantly the concentrations of Cd, Zn, and Pb in soil solution which could pose an environmental risk as groundwater contamination (Chen and Cutright 2001; Lai and Chen 2005). The results of EDTA treatment conclude that application of synthetic chelating agents such as EDTA to heavily metal-contaminated soil like in our soil increase the availability of some elements such as Pb, Co, Zn, Ni, Cr, and Cd, decrease the availability of some other elements, i.e., Al, As, Se, V, and Mo, and thus might be useful for immobilization of these elements.
Application of HK (RS + HK) improved dry biomass production compared with the non-amended soil (RS) (data not shown). Additionally, the HK treatment (RS + HK) changed significantly concentrations of the elements in the rapeseed as compared to the non-amended soil (RS). Humate potassium (RS + HK) decreased significantly the concentrations of Al and As, while increased the concentrations of Cu, Se, and Zn, in rapeseed as compared to RS (Fig. 1). Nonsignificant differences of concentrations of Cd, Co, Cr, Mo, Ni, Pb, and V in rapeseed between the RS and RS + HK treatments were recorded (Fig. 1). These results suggest that the HK might be used as an immobilizing agent for Al and As in the soil. However, the HK might be used for enhancing phytoextraction of Cu, Se, and Zn from the soil by rapeseed. Element immobilization by HK might be explained by its high alkalinity (pH 8.8) which increased soil pH and thus might increase element sorption and decrease element solubility and phytoavailability. Additionally, the high content of exchangeable functional groups in HK can absorb elements and decrease their solubility (Janos et al. 2010).
The impact of HK on element solubility differs based on soil pH, organic structure, and active groups as well as the molecular weight of organo-element complexes (Janos et al. 2010). Due to a great affinity of soil PTEs like Cu for organic complexing, soluble element-organic forms appear to comprise most of the element solution (Kabata-Pendias 2011). Wu et al. (2001) reported that Cu humate complexes are mobile in both acidic and alkaline conditions. The net effect of humate is to increase cupric ion mobility especially under alkaline conditions.
Additionally, we hypothesized that the increased uptake of Cu, Se, and Zn with the HK might be due to the dissolution of precipitated form that originates by the alkaline HK in the rhizosphere zone and release of these elements to soil solution and thus being available for plants. In particular, HK increased significantly the biomass yield compared with the control, which enhance the phytoextraction of these elements by these high biomass roots. These changes can be explained by the interaction of root exudates with the surrounding solids in the rhizosphere. As well, the HK might lead to an intense microbiological and biochemical activity in the rhizosphere which enables roots to mobilize certain precipitated elements by acidification and redox changes. This may increase the soluble fraction and decrease the sorbed and carbonate fraction of these elements in the rhizosphere (Malandrino et al. 2011).
Application of PK (RS + PK) improved seed germination, plant growth, and dry biomass yield by about 60 % (data not given). Additionally, the PK increased significantly the concentrations of As, Cr, Mo, and Se in rapeseed, while it decreased significantly concentrations of Al, Cd, Pb, and Zn as compared to rapeseed grown in the non-amended soil (RS) (Fig. 1). The role of phosphates in influencing the mobilization and bioavailability of As has been reported by Bolan et al. (2013) who found that addition of phosphate increased the mobility and bioavailability of As by 4.3-fold compared with the non-amended soil. Phosphate addition to soil influences uptake of As by plants through its effects on sorption, root absorption, and translocation from root to shoot. Additionally, uptake of As by plants is associated with the mechanism of uptake of P, where presumably As is taken up as a P analogue (Xu et al. 2007). Therefore, there is a growing interest in using P fertilizer to enhance plant uptake of As in contaminated sites, aiming at facilitating phytoremediation.
The PK decreased significantly the plant tissue concentrations of Pb in RS + PK as compared to RS (Fig. 1), which might mean that phosphates converted significant amounts of Pb from the mobile fractions to the residual fraction (Chen et al. 2007), and thus decreased the phytoavailability of Pb to rapeseed. The role of phosphates in decreasing the mobilization and bioavailability of Pb has been reported previously (Scheckel et al. 2005; Shaheen and Rinklebe 2015).
There are some reports that application of Cd-containing natural reactive phosphate rock can significantly increase the concentrations of Cd in the shoots of Zea mays (Rochayati et al. 2011). However, we found a high ability of phosphates in reducing Cd solubility. In this respect, Bolan et al. (2003) reported that the application of phosphate is effective in reducing Cd in contaminated soils. Thus, decreasing the plant tissue concentration of Cd and Pb in the PR-treated soil (RS + PK) compared with the control (RS; Fig. 1) suggested that PK amendment could significantly immobilize Cd and reduce their mobilization and phytoavailability in contaminated soils. There is conclusive evidence for the mitigated value of both water-soluble and water-insoluble phosphates to immobilize several metals in soils, thereby reducing their bioavailability for plant uptake (Brown et al. 1995). In addition, reducing the uptake of Al, Cd, Pb, and Zn in PK treatment might be due to the antagonistic interaction between P and three elements (Kabata-Pendias 2011). In general, phosphate reduced the metal mobility and used for immobilization of metals in soils through various processes, including direct metal adsorption, antagonistic interactions, phosphate anion-induced metal adsorption, and precipitation of metals with solution phosphate as metal phosphates (Adriano et al. 2004; Kabata-Pendias 2011).
Conclusion
Concentrations of toxic elements in the soil exceeded the precautionary value of Cd, Cr, Ni, Pb, and Zn and the action values of As and Cu set by the German Federal Soil Protection and Contaminated Sites Ordinance (BBodSchV 1999) as well as the maximum allowable soil concentrations and the trigger action values (except for V) for PTEs in agricultural soils as reported by Kabata-Pendias (2011). Therefore, assessing the phytoextraction of Al, As, Cd, Co, Cr, Cu, Mo, Ni, Pb, Se, V, and Zn from this soil by non-edible plants was an approach worth to be investigated. The plants revealed high BCR (>>1), which indicates the high efficiency of these plants for phytoextraction of the elements (except As and Cu) from the soil. We may conclude that Indian mustard can be recommended for phytoremediation of soils contaminated by Al, As, Co, Cr, Mo, Se, and V, while sunflower may be recommended for soils contaminated by Cd, Ni, Pb, and Zn, and rapeseed seems to be more suitable for Cu-contaminated soils. Thus, a combination of these three plants on such multi-element contaminated soils might be an option for future.
The results additionally indicate that application of synthetic chelating agents like EDTA to soils with high metal concentrations like our soil might increase the solubility of elements to toxic levels for seed germination and plant growth. Application of HK and PK improved the plant growth compared with control. The HK might be used as an immobilizing agent for Al and As, while PK might be recommended as an immobilizing agent for Al, Cd, Pb, and Zn. On the other hand, HK showed high efficiency in enhancement of the phytoextraction of Cu, Se, and Zn, while PK is recommended for enhancement of the phytoextraction of As, Cr, Mo, and Se from the soil by rapeseed. The results suggest that element-tolerant plant species with high BCR in combination with application of HK and PK can be considered to use for remediation and phytostabilization of toxic elements in this multi-contaminated riparian soil. However, verification of such remediation approaches of those contaminated sites in the field should be a challenge for the near future aiming to minimize the potential risk to humans and to the environment.
References
Adriano, D. C., Wenzel, W. W., Vangronsveld, J., & Bolan, N. S. (2004). Role of assisted natural remediation in environmental cleanup. Geoderma, 122, 121–142.
Ahmad, M., Hashimoto, Y., Moon, D. H., Lee, S. S., & Ok, Y. S. (2012). Immobilization of lead in a Korean military shooting range soil using eggshell waste: An integrated mechanistic approach. Journal of Hazardous Materials, 209–210, 392–401.
Ahmad, M., Rajapaksha, A. U., Lim, J. E., Zhang, M., Bolan, N., Mohan, D., et al. (2014). Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere, 99, 19–33.
Ali, H., Khan, E., & Sajad, M. A. (2013). Phytoremediation of heavy metals—Concepts and applications: A Review. Chemosphere, 91, 869–881.
Baker, A. J. M., & Brooks, R. R. (1989). Terrestrial higher plants which hyperaccumulate metallic elements. A review of their distribution, ecology and phytochemistry. Biorecovery, 1, 81–126.
BBodSchV. (1999). Bundes-Bodenschutz-und Altlastenverordnung (BBodSchV) vom 12. Juli 1999. [Federal Soil Protection and Contaminated Sites Ordinance dated 12 July 1999].
Blume, H.-P., Stahr, K., & Leinweber, P. (2011). Bodenkundliches Praktikum. Heidelberg: Springer Spektrum, Akademischer Verlag, 3. Aufl.
Bolan, N. D., Adriano, D. C., & Naidu, R. (2003). Role of phosphorous in (im)mobilization and bioavailability of heavy metals in the soil-plant system. Reviews of Environmental Contamination and Toxicology, 177, 1–44.
Bolan, N., Mahimairaja, S., Kunhikrishnan, A., & Naidu, R. (2013). Sorption–bioavailability nexus of arsenic and cadmium in variable-charge soils. Journal of Hazardous Materials, 261, 725–732.
Brown, S. L., Chaney, R. L., Angle, J. S., & Baker, A. J. M. (1995). Zinc and cadmium uptake by hyperaccumulator Thlaspi caerulescens and metal tolerant Silene vulgaris grown on sludge amended soils. Environmental Science and Technology, 29, 1581–1585.
Chen, H., & Cutright, T. (2001). EDTA and HEDTA effects on Cd, Cr, and Ni uptake by Helianthus annuus. Chemosphere, 45, 21–28.
Chen, S., Xu, M., Ma, Y., & Yang, J. (2007). Evaluation of different phosphate amendments on vailability of metals in contaminated soil. Ecotoxicology and Environmental Safty, 67, 278–285.
Du Laing, G., Rinklebe, J., Vandecasteele, B., Meers, E., & Tack, F. M. G. (2009). Trace metal behaviour in estuarine and riverine floodplain soils and sediments: A review. Science of the Total Environment, 407, 3972–3985.
Freitas, E. V., & Nascimento, C. W. (2009). The use of NTA for lead phytoextraction from soil from a battery recycling site. Journal of Hazardous Materials, 171, 833–837.
Frohne, T., Rinklebe, J., Diaz-Bone, R. A., & Du Laing, G. (2011). Controlled variation of redox conditions in a floodplain soil: Impact on metal mobilization and biomethylation of arsenic and antimony. Geoderma, 160, 414–424.
Frohne, T., Rinklebe, J., & Diaz-Bone, R. A. (2014). Contamination of floodplain soils along the Wupper River, Germany, with As Co, Cu, Ni, Sb, and Zn and the impact of pre-definite redox variations on the mobility of these elements. Soil Sediment Contamination: An International Journal, 23, 779–799.
Frohne, T., Diaz-Bone, R. A., Du Laing, G., & Rinklebe, J. (2015). Impact of systematic change of redox potential on the leaching of Ba, Cr, Sr, and V from a riverine soil into water. Journal of Soils and Sediments, 15, 623–633.
Greipsson, S. (2011). Phytoremediation. Natural Education Knowledge, 2, 7–18.
Grispen, V., Hans, J. M., Nelissen, J., & Verkleij, A. C. (2006). Phytoextraction with Brassica napus L.: A tool for sustainable management of trace element contaminated soils. Environmental Pollution, 144, 77–83.
IUSS-FAO. (Ed.). (2014). World reference base for soil resources (Vol. 106). Rome: World Soil Resources Reports, FAO.
Janos, P., Vávrová, J., Herzogová, L., & Pilařová, V. (2010). Effects of inorganic and organic amendments on the mobility (leachability) of heavy metals in contaminated soil: A sequential extraction study. Geoderma, 159, 335–341.
Jones, J., Wolf, J. B., & Mills, H. A. (1991). Plant analysis handbook: A practical sampling, preparation, analysis, and interpretation guide. Athens: Micro-Macro Publishing, Inc.
Kabata-Pendias, A. (2011). Trace Elements in Soils and Plants (4th ed.). Boca Raton: CRC Press.
Knox, A. S., Paller, M. H., Nelson, E. A., Specht, W. L., Halverson, N. V., & Gladden, J. B. (2006). Metal distribution and stability in constructed wetland sediment. Journal of Environmental Quality, 35, 1948–1959.
Lai, H. Y., & Chen, Z. S. (2005). The EDTA effect on phytoextraction of single and combined metals-contaminated soils using rainbow pink (Dianthus chinensis). Chemosphere, 60, 1062–1071.
Licina, V., Antic-Mladenovic, S., Kresovic, M., & Rinklebe, J. (2010). Effect of high nickel and chromium background levels in serpentine soil on their accumulation in organs of a perennial plant. Communications in Soil Science and Plant Analysis, 41, 1–15.
Malandrino, M., Abollino, O., Buoso, S., Giacomino, A., Gioia, C., & Mentasti, E. (2011). Accumulation of heavy metals from contaminated soil to plants and evaluation of soil remediation by vermiculite. Chemosphere, 82, 169–178.
Marques, A. C., Rangel, A. S., & Castro, P. L. (2009). Remediation of heavy metal contaminated soils: Phytoremediation as a potentially promising clean-up technology. Critical Reviews in Environmental Science and Technology, 39, 622–654.
Ok, Y. S., Kim, S. C., Kim, D. K., Skousen, J. G., Lee, J. S., Cheong, Y. W., et al. (2011a). Ameliorants to immobilize Cd in rice paddy soils contaminated by abandoned metal mines in Korea. Environmental Geochemistry and Health, 33, 23–30.
Ok, Y. S., Lee, S. S., Jeon, W. T., Oh, S. E., Usman, A. R. A., & Moon, D. H. (2011b). Application of eggshell waste for the immobilization of cadmium and lead in a contaminated soil. Environmental Geochemistry and Health, 33, 31–39.
Ok, Y. S., Lim, J. E., & Moon, D. H. (2011c). Stabilization of Pb and Cd contaminated soils and soil quality improvements using waste oyster shells. Environmental Geochemistry and Health, 33, 83–91.
Overesch, M., Rinklebe, J., Broll, G., & Neue, H. U. (2007). Metals and arsenic in soils and corresponding vegetation at Central Elbe river floodplains (Germany). Environmental Pollution, 145, 800–812.
Paller, M. H., & Knox, A. S. (2010). Amendments for the in situ remediation of contaminated sediments: Evaluation of potential environmental impacts. Science of the Total Environment, 408, 4894–4900.
Prasad, M. N. V., Nakbanpote, W., Sebastian, A., Panitlertumpai, N., & Phadermrod, C. (2015). Phytomanagement of padaeng zinc mine waste, Mae Sot District, Tak Province, Thailand. In Soil remediation and plants: Prospects and challenges (Chap 23, pp. 661–687). http://www.sciencedirect.com/science/article/pii/B9780127999371000231.
Rinklebe, J., Franke, C., & Neue, H. U. (2007). Aggregation of floodplain soils as an instrument for predicting concentrations of nutrients and pollutants. Geoderma, 141, 210–223.
Rinklebe, J., & Shaheen, S. M. (2014). Assessing the mobilization of cadmium, lead, and nickel using a seven-step sequential extraction technique in contaminated floodplain soil profiles along the central Elbe River, Germany. Water, Air, and Soil pollution, 225, 2039. doi:10.1007/s11270-014-2039-1.
Rinklebe, J., & Shaheen, S. M. (2015). Miscellaneous additives can enhance plant uptake and affect geochemical fractions of copper in a heavily polluted riparian grassland soil. Ecotoxicology and Environmental Safety,. doi:10.1016/j.ecoenv.2015.04.046.
Rinklebe, J., Shaheen, S. M., & Frohne, T. (2015). Amendment of biochar reduces the release of toxic elements under dynamic redox conditions in a contaminated floodplain soil. Chemosphere,. doi:10.1016/j.chemosphere.2015.03.067.
Rochayati, S., Du Laing, G., Rinklebe, J., Meissner, R., & Verloo, M. (2011). Use of reactive phosphate rocks as fertilizer on Indonesian acid upland soils: Accumulation of cadmium and zinc in soils and shoots of maize plants. Journal of Plant Nutrition and Soil Science, 174, 186–194.
Sánchez-Martín, M., García-Delgado, M., Lorenzo, L., Rodríguez-Cruz, M., & Arienzo, M. (2007). Heavy metals in sewage sludge amended soils determined by sequential extractions as a function of incubation time of soils. Geoderma, 142, 262–273.
Scheckel, K. G., Ryan, J. A., Allen, D., & Lescano, N. V. (2005). Determining speciation of Pb in phosphate-amended soils: Method limitations. Science of the Total Environment, 350, 261–272.
Sessitsch, A., Kuffner, M., Kidd, P., Vangronsveld, J., Wenzel, W. W., Fallmann, K., & Puschenreiter, M. (2013). The role of plant-associated bacteria in the mobilization and phytoextraction of trace elements in contaminated soils: Review. Soil Biology & Biochemistry, 60, 182–194.
Shaheen, S. M., & Rinklebe, J. (2014). Geochemical fractions of chromium, copper, and zinc and their vertical distribution in soil profiles along the Central Elbe River, Germany. Geoderma, 228–229, 142–159.
Shaheen, S. M., & Rinklebe, J. (2015). Impact of emerging and low cost alternative amendments on the (im)mobilization and phytoavailability of Cd and Pb in a contaminated floodplain soil. Ecological Engneering, 74, 319–326.
Shaheen, S. M., Rinklebe, J., Frohne, T., White, J. R., & DeLaune, R. D. (2014a). Biogeochemical factors governing cobalt, nickel, selenium, and vanadium dynamics in periodically flooded Egyptian North Nile Delta rice soils. Soil Science Society of America Journal, 78, 1065–1078.
Shaheen, S. M., Rinklebe, J., Rupp, H., & Meissner, R. (2014b). Temporal dynamics of soluble Cd Co, Cu, Ni, and Zn and their controlling factor in a contaminated floodplain soil using undisturbed groundwater lysimeter. Environmental Pollution, 191, 223–231.
Shaheen, S. M., Rinklebe, J., Rupp, H., & Meissner, R. (2014c). Lysimeter trials to assess the impact of different flood-dry-cycles the dynamics of pore water concentrations of As, Cr, Mo, and V in a contaminated floodplain soil. Geoderma, 228–229, 5–13.
Shaheen, S. M., Rinklebe, J., & Selim, H. M. (2015a). Impact of various amendments on the bioavailability and immobilization of Ni and Zn in a contaminated floodplain soil. International Journal of Environmental Science and Technology,. doi:10.1007/s13762-014-0713-x.
Shaheen, S. M., Tsadilas, C. D., & Rinklebe, J. (2015b). Immobilization of soil copper using organic and inorganic amendments. Journal of Plant Nutrition and Soil Science, 178, 112–117.
Soltanpour, P. N., & Schwab, A. P. (1977). A new soil test for simultaneous extraction of macro- and micro-nutrients in alkaline soils. Communications in Soil Science and Plant Analysis, 8, 195–207.
Srikanth, Lavu R. V., Prasad, M. N., Pratti, V. L., Meißner, R., Rinklebe, J., Van De Wiele, T., et al. (2013). Trace metals accumulation in Bacopa monnieri and their bioaccessibility. Planta Medica, 79, 1081–1083.
Suthar, V., Mahmood-ul-Hassan, M., Memon, K. S., & Rafique, E. (2013). Heavy-metal phytoextraction potential of spinach and mustard grown in contaminated calcareous soils. Communications in Soil Science and Plant Analysis, 44, 2757–2770.
Tessier, A., Campbell, P. G., & Bisson, M. (1979). Sequential extraction procedure for the speciation of particulate trace metals. Analytical Chemistry, 51, 844–851.
Tomovic, G. M., Mihalovic, N. L., Tumi, A. F., Gajic, B. A., Misljenovic, T. D., & Niketic, M. S. (2013). Trace metals in soils and several Brassicaceae plant species from serpentine sites of Serbia. Archives of Environmental Protection, 39, 29–49.
U.S. Environmental Protection Agency. (2007). Microwave assisted acid digestion of sediments, sludges, soils, and oils, vol. 3051A. In Test methods for evaluating solid waste, physical/chemical methods (3rd ed.) Publ SW- 846, Rev 6. Washington, DC: US Environmental Protection Agency. http://www.epa.gov/epawaste/hazard/testmethods/sw846/pdfs/3051a.pdf. Accessed 14 Oct 14.
Wang, G., Su, M., Chen, Y., Lin, F., Luo, D., & Gao, S. (2006). Transfer characteristics of cadmium and lead from soil to the edible parts of six vegetable species in southeastern China. Environmental Pollution, 144, 127–135.
Wu, J., West, L. J., & Stewart, D. I. (2001). Copper(II) humate mobility in kaolinite soil. Engineering Geology, 60, 275–284.
Xu, X. Y., McGrath, S. P., & Zhao, F. J. (2007). Rapid reduction of arsenate in the medium mediated by plant roots. New Phytologist, 176, 590–599.
Yoon, J., Cao, X., Zhou, Q., & Ma, L. Q. (2006). Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Science of the Total Environment, 368, 456–464.
Acknowledgments
We thank the German Academic Exchange Foundation (Deutscher Akademischer Austauschdienst, DAAD) (GERSS Program; Code Number A1291166 and WAP program; Code Number A/14/05113 and the WAP program; Code Number A/14/05113) and the Egyptian Ministry of Higher Education, and the Egyptian Ministry of Scientific Research, Science and Technology Development Fund (STDF-STF; Project ID: 5333) for financial support of the postdoctoral scholarship of the first author at the University of Wuppertal, Germany. The authors thank C. Vandenhirtz, F. Seufzer, D. Theiss, and M. Langerhans-Muhlack for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shaheen, S.M., Rinklebe, J. Phytoextraction of potentially toxic elements by Indian mustard, rapeseed, and sunflower from a contaminated riparian soil. Environ Geochem Health 37, 953–967 (2015). https://doi.org/10.1007/s10653-015-9718-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-015-9718-8