Abstract
Background and aims
The potential use of a metal-tolerant sunflower mutant line for both biomonitoring and phytoremediating a Cu-contaminated soil series was investigated.
Methods
The soil series (21–1,170 mg Cu kg−1) was sampled in field plots at control and wood preservation sites. Sunflowers were cultivated 1 month in potted soils under controlled conditions.
Results
pH and dissolved organic matter influenced Cu concentration in the soil pore water. Leaf chlorophyll content and root growth decreased as Cu exposure rose. Their EC10 values corresponded to 104 and 118 μg Cu L−1 in the soil pore water, 138 and 155 mg Cu kg−1 for total soil Cu, and 16–18 mg Cu kg−1 DW shoot. Biomass of plant organs as well as leaf area, length and asymmetry were well correlated with Cu exposure, contrary to the maximum stem height and leaf water content.
Conclusions
Physiological parameters were more sensitive to soil Cu exposure than the morphological ones. Bioconcentration and translocation factors and distribution of mineral masses for Cu highlighted this mutant as a secondary Cu accumulator. Free Cu2+ concentration in soil pore water best predicted Cu phytoavailability. The usefulness of this sunflower mutant line for biomonitoring and Cu phytoextraction was discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anthropogenic soil contamination by Cu can result in serious negative consequences, such as damages on ecosystems, agricultural productivity, contamination of water resources and health risks for animals (Adriano 2001). In France, 838 Cu-contaminated sites are referenced by the national authority (Basol 2013). In many cases, the use of Cu-based preservatives (e.g. Cu sulphate and CCA) is involved. At wood preservation sites, wood washings often result in Cu-contaminated topsoils (Bes and Mench 2008; Mench and Bes 2009). Copper bioavailability is influenced by many factors, notably soil type, total soil Cu, composition of soil pore water (i.e. CuSPW, Cu2+, Ca2+, Mg2+, pH, DOM, ionic strength, and redox potential), and runoff parameters (Gunkel et al. 2003; Luo et al. 2006; Thakali et al. 2006; Chaignon et al. 2009; Forsberg et al. 2009; Bravin et al. 2010a). Copper phytotoxicity is mainly due to the existence of two readily interconvertible oxidation states that makes Cu highly reactive and a catalyst of the formation of free radicals through the Haber-Weiss reaction in plants with subsequent metal-induced oxidative signalling and damage to cells at level of lipids, membranes, nucleic acids, and proteins (Yruela 2009; Smeets et al. 2013). Excess Cu interferes with important cellular processes such as photosynthesis, pigment synthesis, plasma membrane permeability and other metabolic mechanisms, causing a strong inhibition of plant development (Kuepper et al. 2009; Palmer and Guerinot 2009; Jung et al. 2012; Smeets et al. 2013).
Biomonitoring is a part of the initial risk assessment of TE-contaminated soils. It is also used for evaluating the efficacy of remediation options implemented to either remove contaminants or minimize pollutant linkages of TE-contaminated soils (Adriano et al. 2004). Various bioindicators have been used for assessing ecotoxicity of Cu-contamination (da Silva et al. 2010; Fritsch et al. 2010; Leduc et al. 2008; Maderova et al. 2011; Marchand et al. 2011). In terrestrial ecosystems, plants are sensitive organisms to metal exposure and accumulation, due to a large contact surface with the soil and the atmosphere. Responses of vegetative structures are widely examined at various scales, ranging from molecular compounds to plant individuals, population, and community (Bes et al. 2010; Ernst and Peterson 1994; Hernandez and Pastor 2008; Korpe and Aras 2011). Phenotypic responses of plants to excess Cu can be divided into two main groups: (1) biometrical or structural (i.e. morphological and anatomical) and (2) physiochemical or functional (i.e. physiological and (bio)chemical) responses (Ernst and Peterson 1994; Lagadic et al. 1997). Sensitivity of parameters used to asses phenotypic responses into these groups differ according to soil contamination levels (Meers et al. 2006). In most cases, physiochemical biomarkers are more sensitive, e.g. primarily protein and DNA (genotoxic effects) damages are detected (Korpe and Aras 2011; Mendoza-Soto et al. 2012; Mocquot et al. 1996; Qi et al. 2006). In addition, morphological parameters alone are considered not sensitive enough to fully assess potential phytotoxic effects in moderately contaminated soils (Meers et al. 2006).
For metal phytoextraction, beside hyperaccumulators, secondary metal-accumulators, notably non-food crops, deserve attention due to their significant aerial biomass and financial opportunities from plant-based feedstock (Mench et al. 2010; Vamerali et al. 2010; Vangronsveld et al. 2009; Vassilev et al. 2004). Plants with high shoot biomass, shoot TE concentrations related to TE exposures in the growing media, and high shoot TE removals, are relevant candidates for both biomonitoring TE-contaminated soils and TE phytoextraction. Sunflower (Helianthus annuus L.) is a potential candidate for coupling both objectives as (1) its morphological and physiological traits, mainly at seedling stage, reflect TE exposure (Lin et al. 2003; Madejon et al. 2003; Nehnevajova et al. 2012), and (2) it provides financial returns through oilseed and biomass production and can be included in a sustainable crop rotation promoting soil functions, nutrient cycles, microbial community and other ecosystem services, with either no or acceptable residual pollutant linkages (Faessler et al. 2010; Nehnevajova et al. 2009; Tahsin and Yankov 2007). In contrast to transgenic plants, which use is restricted to laboratory scale in many countries, non-genetically modified plants can be tested for improving shoot TE removals under field conditions. Mutant lines, obtained by seeds exposure to EMS, have been selected for higher shoot metal concentrations (Nehnevajova et al. 2005, 2009, 2012). Such sunflower mutants accumulate more metals (Zn, Cd, Pb, and Cu) than their motherlines at high metal exposure in field conditions (Kolbas et al. 2011; Nehnevajova et al. 2009). Characterization of the seedling responses of such mutants to increasing exposure of metals such as Cu and comparison with field dataset could calibrate a plant test with a wider responsive range, able to better discriminate initial and residual soil phytotoxicities and the feasibility of metal phytoextraction.
This study aimed at assessing phenotypic seedling responses of a metal-tolerant sunflower mutant line growing on a soil series with increasing total soil Cu, sampled in field plots at a wood preservation site and a control site. Soil physico-chemical parameters and composition of soil pore water were measured to characterize soil Cu exposure. Relationships between biometrical and physiochemical parameters of 4-week-old seedlings and parameters of soils and soil pore water were investigated for determining relevant plant endpoints for biomonitoring. Dose-effect relationships were proposed for potential application in plant testing and predicting potential use of this mutant line for phytoextraction in Cu-contaminated soils.
Material and methods
Site and field trial
The wood preservation site (about 10 ha, Saint-Médard d’Eyrans, Gironde, SW France; N 44°43.353, W 000°30.938) has been used for over a century (Mench and Bes 2009). Topsoils (0–0.25 m, Fluviosol) are sandy, i.e. 85.8 % sand, 5.9 % clay, 8.3 % silt, 1.6 % OM, and C/N 17.2, with a low CEC (3.5 cmol kg−1), and display a high spatial variability for total soil Cu and Cu in soil pore water, mainly reflecting long-term used of Cu-based salts and washings of treated timbers (Bes et al. 2010; Mench and Bes 2009). Distribution of soil Cu in physical and operationally defined soil fractions as well as impacts on soil enzyme activities were reported by Lagomarsino et al. (2011).
The field trial dedicated to aided phytoextraction at the sub-site P1-3 consists in four blocks and 31 field plots (2 m2) with total soil Cu (CuTOT) in topsoil (0–25 cm) varying between 163 and 1,170 mg Cu kg−1 (Table 1; Kolbas et al. 2011). CuTOT exceeded the median and upper whisker background values (in mg Cu kg−1) of French sandy soils, i.e. 3.2 and 8.4, and of topsoils in the Aquitaine region, France, i.e. 13.9 and 55.8 (Kolbas et al. 2011). Total soil contents (in mg kg−1) varied between 4.8 and 8.6 for As, 4.7–6.0 for Ni, 15.8–22.5 for Cr, 35–98 for Zn, 17–23 for Pb, 0.1–0.2 for Cd, and 1.5–2.0 for Co. For these elements, background levels of French sandy soils were only slightly exceeded for Zn (48 mg kg−1) in 14 plots, but total soil As, Co, Cd, Cr, Ni, Pb, and Zn did not differ between the plots and globally Cu was the main contaminant in plot topsoils (Kolbas et al. 2011).
As sunflower cultivars were unable to grow in the untreated plots with high CuTOT and based on a pot experiment (Bes and Mench 2008), compost made from poultry manure and pine bark chips (5 % w/w, Orisol, Cestas, France) and dolomitic limestone (0.2 % w/w, Prodical Carmeuse, Orthez, France) were incorporated into the topsoils (0–25 cm) of three blocks (i.e. block #1: plots #1 to #10, block #2: plots #11 to #20, and block #3: plot #21 to #30, hereafter referred to OMDL plots) in March 2008 (Kolbas et al. 2011). Block #4 remained untreated and was considered as a single plot (UNT #31), whereas each amended block was divided in 10 plots (2 m2). An uncontaminated plot (2 m2, CTRL-plot #32) with similar alluvial sandy soil type was managed in a kitchen garden located at 18 km from the site (Gradignan, France). Across the 32 field plots, soil pH ranged from 7.0 (UNT) to 7.54 (CTRL), CEC from 31 (UNT) to 194 mmol kg−1 (CTRL), and total organic matter from 14.5 (UNT) to 78 g kg−1 (CTRL). Soil pH, CEC and total organic matter in OMDL plots varied in the ranges 7.18–7.29, 47–48 mmol kg−1, and 25.1–28.4 g kg−1, respectively.
Soils and soil pore waters
Three soil samples (3 kg soil FW) were collected in each plot (n = 32) with a stainless spade from the 0–25 cm soil layer and combined to make a composite soil sample, which was air-dried, sieved (<5 mm, nylon mesh), and manually homogenized. Trace element concentrations in soils were determined with standard methods and a quality scheme by INRA LAS (2013), Arras, France, i.e. inductively coupled plasma/atomic emission spectroscopy (ICP-AES) for metals after wet digestion in HF and HCIO4, and hydride-generation for As after wet digestion in H2SO4/HNO3 (2/1) with V2O5 at 100 °C (3 h). Two certified reference materials, i.e. BCR No. 141 (calcareous loam soil) and BCR No. 142 (light sandy soil) from the Bureau Communautaire de Référence, were used by INRA LAS in the quality scheme.
Soil samples (1 kg air-dried) were placed in plastic pots (1.3 L) (in triplicates), watered with deionised water, and daily maintained at 70 % of field capacity (10 % of air-dried soil mass). One Rhizon MOM moisture sampler (Eijkelkamp, The Netherlands) was inserted with a 45° angle into each potted soil. Soil pore water (10 mL) were collected three times with 1 week of interval and kept at 4 °C prior to analyse pH (Hanna instruments, pH 210, combined electrode Ag/AgCl - 34) and Al, Cu, B, Na, K, Fe, Mn, P, and Zn (ICP-AES, Varian Liberty 200). As total soil As and Cr were low in all sampled soils (Kolbas et al. 2011), these elements were analyzed in soil pore water only for several selected samples. Free copper (Cu2+) ions were determined by a Cu-ISE (Fischer Bioblock) after calibration (Buck and Cosofret 1993; Luo et al. 2006). During the measures, nitrogen bubbling was maintained into soil pore waters notably to remove carbon dioxide and oxygen and to reduce the oxidation of soluble compounds. The free Cu activity, i.e. the concentration of free Cu ions corrected with the Debye-Huckel theory activity coefficient—is reported in pCu2+ defined as the negative log10 of the molar quantity of Cu2+ ions activities and, therefore, pCu2+ are unitless. The content of dissolved organic matter (DOM) in soil pore waters was quantified by both spectrophotometry (spectrophotometer CARY 100 Scan, 340 nm, Baker et al. 2008) and analysis of total organic carbon (TOC) (Hiper TOC Analyzer Thermo scientific, HiPerTOC 2004).
Biotest and plant analysis
The sunflower mutant 1 line [M6 (6th generation), 1/67-35-190-04], obtained by chemical mutagenesis using EMS (Nehnevajova et al. 2005, 2009) and previously assessed at field scale vs. commercial cultivars (Kolbas et al. 2011), were sowed in each potted soil (four seeds per pot, in triplicates) in a climatic chamber, with the following conditions: 14 h light/10 h darkness regime, 150 μmol m−2 s−1, 25 °C/22 °C, 65 % relative humidity (ISO 2005). Pots were arranged in a fully randomized block design on a table and watered daily with deionized water (50 % water holding capacity). The soils were fertilized twice, i.e. just before starting plant culture and 2 weeks after with a modified Hoagland n°2 nutrient solution (Hewitt 1966) supplying no Fe and other trace metals.
Plants were collected after 1 month at growth stage 2.4 (CETIOM 1995) when the 2nd pair of leaves reached 4 cm length. Roots were carefully washed in deionized water and gently blotted. Shoots and roots were weighed (FW), rinsed in distilled water, oven-dried at 50 °C for 48 h, and then DW yield and WC were determined. Other biometrical parameters were measured, i.e. stem and leaf length, leaf area and asymmetry (scanner EPSON Expression 10000 XL, software WINFOLIA, Kryazheva et al. 1996). Root microstructures were observed (binocular PERFEXSCIENCES with camera moticam 2000 and lamp Motic MLC-150c). Chlorophyll a and b and total carotenoids were extracted from the 2nd leaf pair (1 cm2, in duplicates) with DMF and their foliar contents computed from measurements at 470, 647 and 664.5 nm of the extracts (spectrophotometer CARY 100 Scan, Lagriffoul et al. 1998). Plant samples were ground in a titanium mill (Retsch MM200). Weighed aliquots of plant materials (0.5 g DW) were wet digested under microwaves (Marsxpress, CEM) with 5 mL supra-pure 14 M HNO3 and 2 mL 30 % (v/v) H2O2 not stabilized by phosphates. Certified reference material (maize V463 BIPEA - Bureau InterProfessionnel d’Etudes Analytiques, France) and blank reagents were included in all series. Element concentrations in digests were determined by ICP-AES (Varian Liberty 200). All elements were recovered (>95 %) according to the standard values and standard deviation for replicates (n = 3) was <5 %.
To quantify the impact of excess Cu and efficiency of OMDL amendment on sunflower growth, several indexes were computed: (1) the Relative Treatment Efficiency Index (RTEI = (Pt − Pc)/(Pt + Pc), Marchand et al. 2010) to compare the OMDL soils with the UNT soil; (2) the Relative Parameter Index (RPI(%) = 100 × (Pt − Pmin)/(Pmax − Pmin), Bravin et al. 2010b) which determines the relative increase in values compared with the total amplitude of increase, and (3) the tolerance index (TI(%) = 100 × Pt/Pmax, Ke et al. 2007), where Pt - value of the plant parameter in a treatment ; Pc - value of the plant parameter in the control; Pmin and Pmax - minimum and maximum values of the plant parameter.
The translocation factor (TF = Cshoot / Croot), bioconcentration factor (BCF, BCFshoot = Cshoot / Csoil, BCFroot = Croot / Csoil), and shoot Cu removal, i.e. so-called also mineral mass (CuMM, CuMMshoot = Cshoot × DWshoot) computed with shoot Cu concentration (mg kg−1 DW) and shoot biomass (g DW ha−1), were calculated to assess the metal phytoextraction efficiency (Kolbas et al. 2011; Li et al. 2010); Cshoot and Croot are the metal concentrations in the sunflower shoots and roots, respectively, Csoil is the metal concentration in the soil, and DWshoot is the shoot DW yield.
Statistical analysis
Statistical analysis (one way ANOVAs) was performed to evaluate differences in (1) parameters of soils and soil pore waters and (2) biometrical plant parameters depending on blocks. Post hoc Tukey’s HSD tests were conducted to assess multi-comparison of mean values. Differences were considered statistically significant at p < 0.05. A maximum likelihood factor analysis was also conducted on soil, soil pore water, and plant parameters (biometrical and physiochemical). Pearson correlation coefficients (linear regression) between soil and plant parameters were calculated (significance level, p < 0.05). Stepwise regression AIC (Akaike Information Criterion), with subset regressions (r2 adjusted) on the parameters of soils and soil pore water was used to predict the relevant parameters for phytoextraction studies: pCu2+, shoot DW yield, CuSH and CuMM. Best combination of a minimum number of factors and the maximal r2 adjusted were expressed by multivariate equations (Eq. 1–5). Exposure concentrations (EC10 and EC50) of Cu respectively leading to 10 % and 50 % changes of plant parameters were calculated using linear regression and drc (dose–response curve) package (Knezevic et al. 2007). All statistical analyses were performed using R software (version 2.14.1, R Foundation for Statistical Computing, Vienna, Austria).
Results
Soils and soil pore waters (Table 1)
Total soil Cu (in mg kg−1) ranged from 21 (CTRL soil) to 1,170 (plot #30) and mean values were higher in blocks #3 and #4 than in blocks #1 and #2. Total Cu concentration in soil pore water (CuSPW, in mg Cu L−1) varied between 0.15 (CTRL soil) and 0.93 (plot #21), its mean values increasing from 0.22 ± 0.04 (block #2) to 0.76 ± 0.09 (block #3) in contaminated soils and being similar in blocks #1 and #2. The OMDL incorporation did not affect total soil Cu when blocks #3 and #4 (UNT) were compared, but it increased CuSPW. Free Cu ions (Cu2+) and CuSPW in blocks #1 and #2 showed similar trends. Concentrations for Cu2+ (in μM) were lower in block #1 and #2 soils than in block #3 soils and peaked in the UNT soil. Due to both high OM content and low total soil Cu in the CTRL soil, the Cu2+concentration in soil pore water was the lowest. Based on total soil Cu, the OMDL treatment reduced the Cu2+concentration of soil pore water in amended soils. This was particularly significant in block #3 compared with UNT. The free Cu ions:CuSPW ratio (in %) in soil pore water ranged from 0.94 to 4.92 (mean value: 2.37) in OMDL soils and peaked to 49.1 in the UNT soil. Most soil Cu parameters were correlated (Fig. 1, Circle 1). Soil parameters placed at the figure centre were relatively independent, i.e. DOM, TOC, Na, B, As, and P in soil pore water. pCu2+ (ISE) decreased from 7.52 to 5.38 (Table 1) and was better correlated with CuTOT and CuSPW than Cu2+ (Table 2, Fig. 1).
Maximum likelihood factor analysis performed on soil, soil pore water and plant parameters. DOM dissolved organic matter, EL epicotyl length, HL hypocotyl length, LA leaf asymmetry, LL leaf length, LWC leaf water content, MM mineral mass of Cu in shoot, SL stem length, TLA total leaf area, TOC total organic carbon, * - total element in the soil, ** - total element in the soil pore water, without * - shoot concentration for each element. Root metal concentrations were no shown because they were highly correlated with total soil metals and may not necessarily reflect the plant physiological processes
The pH of soil pore water increased from 5.82 (UNT) to 7.39 (plot #5) in contaminated soils and leveled up to 7.9 in the CTRL soil. This parameter was in decreasing order CTRL > block #1 = block #2 > block #3 > UNT (Table 1) and highly correlated with the pCu2+ concentration (R = 0.78; Table 2). It was less relevant to predict CuSPW (R = −0.52). The ratio of free Cu2+ ions in the soil pore water exponentially decreased as its pH rose (Y = 2 10+11x−12.9, R2 = 0.59, Table 1). The DOM values in the soil pore water determined by both TOC and spectrophotometry methods were well correlated (R = 0.8; p < 0.001, Table 2), except a slight discrepancy for the CTRL soil with a higher value for the spectrophotometry method (Table 1). All amended blocks roughly had a 2.5–3 times higher DOM concentration (108–127 mg C L−1) than UNT (44 mg C L−1) (Table 1). Spectrophotometric DOM values were significantly negatively correlated with free Cu ions (R = −0.51, p = <0.01) and the Cu2+:CuSPW ratio (R=−0.49; p < 0.01) (Table 2). However, TOC values were less related to Cu2+ (R = −0.37; p < 0.05).
Modeling of Cu exposure based on soil parameters
Adjusted R2 for pCu2+ in simple regression with pH in soil pore water was 0.58 (Fig. 2a). It increased to 0.82 for pCu2+ in multiple regression with pH in soil pore water and CuSPW (Fig. 2a, line 1): pCu2+ = 0.84 + 0.78*pH - 1.11*CuSPW (Eq. 1, R2 = 0.82, p value < 0.0001). The CuTOT had less importance for predicting pCu2+ (R2 = 0.80, p < 0.0001). The best combination with three soil factors was achieved with pH, TOC and CuSPW (Fig. 2a, line 2): pCu2+ = 2.56 + 0.63*pH + 0.0033*TOC − 1.30*CuSPW, (Eq. 2, R2 = 0.86, p value < 0.0001).
Stepwise regression AIC (Akaike Information Criterion) with subset regressions (y-axis: adjusted r2, adjr2; the greyscale indicated the adjr2 levels) between a pCu2+, b shoot DW yield, c shoot Cu concentration, d shoot Cu removal, and the physico-chemical parameters of soil and soil pore water. Line 1 – for two variables in the regression; line 2 – for three variables in the regression. CaSPW, BSPW, FeSPW, MgSPW, PSPW, KSPW and NaSPW are respectively the total concentrations of Ca, B, Fe, Mg, P, K and Na in the soil pore water
Plant parameters and their relationships with soil parameters
Morphological and physiological parameters varied in response to Cu exposure (Table 3). Correlations of plant phenotypic traits with soil Cu parameters are listed in Table 4. The soil, soil pore water, and plant parameters were projected on a plane according to their respective correlations (Fig. 1). The majority of plant parameters grouped in Circle 2, and were correlated negatively with total soil Cu and positively with pCu2+, except stem, epicotyl and hypocotyl lengths, and leaf WC. Based on the whole dataset, pCu2+, free Cu2+ ions, and the free Cu ions:CuSPW ratio well correlated with many plant parameters, i.e. n = 13, 12, and 10 significant correlations with Rp ≥ |0.5|, respectively (Table 4, Fig. 3) demonstrating that these indicators of labile Cu pool can predict the phytotoxicity of studied Cu-contaminated soils. CuTOT, pH in soil pore water, and CuSPW were, in a lesser extent, also frequently correlated with phenotypic traits, i.e. n = 9, 9, and 8 significant correlations, respectively.
Root and shoot DW yields
Increase in total soil Cu reduced root growth and length, notably for the UNT plants, and induced changes in the structure of root system such as an increase in lateral root formation. Roots of block #3 plants and especially of UNT plants displayed a brownish color and were short, thick, and highly branched (‘barb-wire’ or ‘coralloid’ roots), with a reduced formation of root hairs. Excess Cu indicated by low pCu2+ in soil pore water reduced root DW yield, and thus whole plant biomass, before to negatively impact shoot DW yield (Table 3, Fig. 2). Root DW yield of UNT plants was seven folds lower than that of CTRL plants whereas this ratio was 5.7 folds for the shoot DW yield (Table 3). Correlations of root DW yield with indicators of soil Cu exposure (i.e. CuTOT, CuSPW, and pCu2+) were stronger than those of shoot DW yield (0.79 and 0.56; 0.81 and 0.51; 0.76 and 0.62, respectively, Table 4). Shoot DW yield is however more easy to determine in bioassay and useful to compute shoot Cu removal by seedlings, which might be an indicator of the phytoextraction potential at field scale. Beside pCu2+, Mg and Ca concentrations in soil pore water (MgSPW and CaSPW) influenced the modelling of shoot DW yield (Figs. 1 and 2b line 1): DWSH = 0.269 + 0.029 * CaSPW + 0.114 * MgSPW (R2 = 0.51; p value < 0.0001)
The root and shoot relative indexes varied with Cu exposure and amendment incorporation (Table 6). Blocks #3 and #4 (UNT) had similar CuTOT, but OMDL enhanced the tolerance index (TI) of shoots in average by 42 % with a 17 %–59 % range, whereas TI for the roots increased only by 20 % with a 13 %–33 % range (Table 6). The RTEI confirmed that the OMDL treatment promoted plant growth, notably shoots compared to roots in block #3 (0.54 and 0.41, respectively). Plants in blocks #1 and #2 showed higher TI than in block #3, reaching about 70 % for roots and shoots, that merely reflected both a lower total soil Cu and decreased Cu exposure induced by OMDL. Similar trends were observed for other indices (Table 3). Changes in the shoot DW:root DW ratio indicated which plant part was more affected by increasing Cu exposure. In control plants, this ratio was rather low (2.36 ± 0.97, Table 3). As total soil Cu increased, it peaked to 4.34 ± 0.79 for block #3 plants showing that roots were more impacted, but fell down in the UNT plants (2.94 ± 0.91) (Table 1).
Maximum stem height and leaf parameters
As free Cu ions increased in soil pore water, i.e. pCu2+ < 7, stem elongation and thinning occurred (Fig. 3), although maximum stem height was not linearly correlated with total soil Cu. Plants from block #3 were higher than control plants (12.2 ± 1.1 cm and 8.7 ± 2.9 cm, respectively), but plants from blocks #1 and #2 and the control had similar stem height (in cm, 9.9, 10.5, and 8.7, respectively) (Table 3). The epicotyl length was more correlated with Cu exposure than the hypocotyl length (0.31 and −0.19, respectively, Table 4). At high Cu2+ concentrations in soil pore water (pCu2+ < 6), the stem was shorter (Fig. 3) and presented a reddening (purple coloration). The shoot growth of plants cultivated on the UNT soil was stopped at the hypocotyl stage (3.5 ± 1.1 cm, Table 3).
Visual symptoms of Cu phytotoxicity, i.e. wilting, foliar chlorosis, bronzing, necrosis, and leaf asymmetry, were recorded as Cu exposure increased. Leaf length, area, and symmetry were significantly reduced as Cu2+ increased in the soil pore water (Table 3) and were better correlated with this parameter (−0.73, −0.61, and 0.74, respectively) than with CuTOT and CuSPW (Table 4; Figs. 1 and 2). In control plants, leaf water content (WC) remained close to 50 % (Table 3). Leaf water content slightly negatively correlated with pCu2+ (r = −0.53; Table 4; Fig. 1), and as Cu exposure rose, WC varied between 49 % and 57 % for plants cultivated in the OMDL soils and significantly peaked up to 74 % for the UNT plants (Table 3, Fig. 3).
ChlTOT widely ranged from 381 mg m−2 in control leaves to 7 mg m−2 in UNT leaves (Table 3). Chlorophylls significantly decreased (50 folds) more than carotenoids (7 folds) (Table 3; Fig. 3). Changes in photosynthetic pigments were highly correlated with soil Cu parameters, e.g. ChlTOT up to R = −0.91 with CuSPW (Table 4) and R = 0.86 with pCu2+, and well fitted by a power function (Fig. 3). They correlated with soil pH in a lesser extent, i.e. 0.63 for ChlTOT and 0.6 for Carot. The Chl a/Chl b ratio did not depend on CuTOT and CuSPW (Table 4). However, increases in Cu2+concentration and the Cu2+:CuSPW ratio in soil pore water greatly reduced foliar Chl b content (r = 0.74 and 0.76, respectively, Table 4, Fig. 2).
Copper concentrations in plant tissues and effective concentrations
Mean values of root Cu concentrations ranged from 355 to 1,233 mg kg−1 for the OMDL plants, with 177 (plot # 20) and 1,768 mg Cu kg−1 (plot # 22) as minimum and maximum values, and peaked up to 3,272 mg Cu kg−1 for the UNT plants (Table 5). Root Cu concentration and soil Cu parameters were strongly correlated, i.e. CuTOT (r = 0.77), CuSPW (r = 0.70), and pCu2+ (r = −0.93) (Table 4). Root Cu concentration increased according to a second degree polynomial function as pCu2+ diminished from 7.5 to 6.2 (Fig. 3). Mean values of shoot Cu concentrations for the OMDL plants varied between 16 and 36 mg kg−1, with 12.7 (plot #10) and 44.8 (plot #22) as minimum and maximum values, and were lower than for the UNT plants (154 mg kg−1, Table 5). Shoot Cu concentration less depended on CuTOT (r = 0.52), but well correlated with Cu2+ concentration in the soil pore water (r = 0.98, Table 4). Shoot Cu concentration rose and well fitted a second degree polynomial function as pCu2+ decreased from 7.5 to 6.2 (Fig. 3). Besides usual correlations with both pH and pCu2+ in soil pore water (Table 4), shoot Cu concentration depended also on Mg in soil pore water (Fig. 2c line 1, CuSH = 436 − 53.6 * pCu2+ + 3.98 * MgSPW (R2 = 0.81, p < 0.0001).
Shoot DW yield responded to shoot Cu concentration with an exponential equation (y = 2.972 e− 0.01x, r = 0.7). Shoot DW yield was plotted with indicators of soil Cu exposure (Fig. 3) and the computed EC10 and EC50 values (Table 4) corresponded to 323 mg and 717 mg Cu kg−1 in soil. Changes in shoot DW yield for block #1 and #2 plants were also partly explained by changes in other soil parameters (i.e. pH, OM, etc.) after OMDL incorporation (data not shown). The indicators of Cu uptake (TF and BCFshoot) plotted with CuTOT were well fitted by a hyperbolic equation (r = 0.79 for BCFshoot and 0.69 for TF) but their significance decreased over 300 mg Cu kg−1 soil. The BCFshoot value varied between 0.04 (UNT) to 0.28 (CTRL) (Table 5). The TF value peaked in the control soil (0.26) and was the lowest in block #3 (0.029) according to increase in root Cu concentration (Table 5). The TF for Cu showed a 10-fold decrease when total soil Cu exceeded 500 mg kg−1, and then remained at a steady value for higher CuTOT in the OMDL plots (Table 5). For the UNT plants, TF value increased 1.6-fold compared to block #3 plants due to higher shoot Cu concentration.
Ionome of plant parts (Table 5)
Shoot Fe concentration varied in the 48–69 mg kg−1 range for the control and OMDL plants but peaked to 354 mg Fe kg−1 in the UNT plants due to their lower shoot DW yield (Table 3). As Cu exposure rose, shoot K concentration increased, whereas root K concentration decreased, except in the UNT plants for which both were reduced likely due to K leakage from highly Cu-stressed roots. Magnesium concentrations were enhanced in both shoot and roots in relation to Cu exposure (R = 0.93 for shoots). The correlation between shoot Cu and Zn concentrations (R = 0.75) resulted from a cluster effect, as the composition of UNT plants opposed to other ones, and it faded without the UNT data. Shoot B and Cu concentrations were positively correlated (R = 0.9) on all the pCu2+ range (Fig. 1). Shoot Ca concentration was lower at both low (UNT) and high (CTRL) pCu2+ in soil pore water (Tables 1 and 5).
Shoot Cu removal (Table 5)
Shoot Cu removal varied (in mg Cu plant−1) from 0.02 (CTRL) to 0.115 (UNT). Its value was more increased in amended soils (block #3) than shoot Cu concentration (1.6 for CuSH and 4.3 folds for CuMM, respectively). Shoot Cu removal highly paralleled soil Cu parameters (e.g. pCu2+) and pH in soil pore water (−0.82 and −0.71, respectively) (Table 4 and Fig. 1). As shoot DW yield, shoot Cu removal was multivariable and its modelling using only soil parameters showed a low significance (Fig. 2d). The best three-term equation included pCu2+, Mg in soil pore water, and total soil Ni (NiTOT): CuMM = 0.24 − 0.027 * pCu2+ + 0.0048 * MgSPW + 0.0099 * NiTOT (r2 = 0.54, p < 0.001).
Discussion
Cu exposure in the soil series
Sustainable phytomanagement of Cu-contaminated soils needs to assess ecological risks before, during and following their remediation, and to evaluate the potential of new phytoremediation options. In situ and ex situ (in potted soils) collection of soil pore water may be a routine, cost-effective way to monitor root exposure to metals and predict phytotoxic risks (Moreno-Jimenez et al. 2011) as well as impacts on microbes (Maderova et al. 2011). Concentrations in pore waters collected in field conditions ranged between 2 and 104 μg Cu L−1 in uncontaminated soils and 25–27,100 μg Cu L−1 in contaminated soils, and at two Cu-contaminated sites, corresponding to historic neighbouring industrial legacy, Cu in pore water reached 25–47 μg L−1 (Merton Bank, UK) and 49–1,190 μg L−1 (Prescot, UK) (Moreno-Jimenez et al. 2011). In S. Fergus sandy Cu-contaminated soils (total soil Cu: 43–2,710 mg kg−1; pH 5.2–6.2), Cu in pore water varied between 30 and 4,050 μg L−1 (Maderova et al. 2011). Data of our soil series (total soil Cu: 21–1,170 mg kg−1, CuSPW 150–837 μg Cu L−1, Table 1) fitted into these intervals, with wider ranges for Cu2+ in soil pore water and total soil Cu than for pH, TOC, and CuSPW (Table 1). The linear relationship between CuSPW and total soil Cu (Table 2) became weaker at high CuTOT, confirming previous findings (Inaba and Takenaka 2005; Kolbas et al. 2011). This may reflect the influence of other soil factors, e.g. soil pH, OM, other element contents, etc. Soils well-aged with respect to metals and organic residues are suitable for resolving questions of metal solubility dependence on OM and pH (Datta and Young 2005; Thakali et al. 2006). Here, soil Cu contamination has been built during more than 50 years, and compost has been added 2 years before our plant testing. Adding compost into the soil caused Cu complexation with OM, notably the coarse compost fraction, at the P1-3 sub-site (Lagomarsino et al. 2011), and it decreased CuSPW after 1 month into a potted Cu-contaminated soil (2,600 mg Cu kg−1; pH 6.25) from the same wood preservation site (Bes and Mench 2008). Liming close to neutrality would promote such complexation and increase Cu binding to (hydr)oxides and clays with subsequently a decrease in water-soluble Cu fraction (Lagomarsino et al. 2011). This was confirmed by roughly a 10-fold decrease of Cu2+ in pore water when comparing OMDL-treated soils of block #3 and the UNT soil, but CuSPW was enhanced despite increased soil pH (Table 1). This suggested Cu mobilisation by DOM (Beesley and Dickinson 2011). DOM in pore waters ranged between 30 and 125 mg C L−1 in an unpolluted urban soil amended with a green compost and reached 25–50 mg C L−1 in a brownfield soil (Moreno-Jimenez et al. 2011). Composted amendments increased DOM in soil pore water to 100–300 mg C L−1 and co-mobilised Cu (Beesley and Dickinson 2011). Our values are similar, i.e. 39.6 (UNT) - 172 mg C L−1 (block #1), highest values reflecting high OM content in the CTRL soil and compost addition into the OMDL soils (Table 1). Soil OM and DOM in the soil solution react with Cu, and their complexes modify Cu solubility, chemical species, and resupply from soil solid phases (Ashworth and Alloway 2007; Temminghoff et al. 1997). Based on DGT (diffusion gradients in thin film), the replenishment capacity for Cu was reduced in the OMDL soils of block #3 although the initial solubility was higher (Pang and Puschenreiter, 2013, personal communication) suggesting OM fractions may differently affect Cu bioavailability. The stability of Cu-OM complexes and their different dissociation rates related to the ligand functional groups may regulate Cu mobility and bioavailability from soil to roots (Thakali et al. 2006; Degryse et al. 2009). Soluble low molecular weight compounds, from mineralized OM and rhizodeposition, could increase Cu solubility, whereas high molecular weight compounds (e.g. humic acids, coarse compost fraction) may sorb Cu. Dissociation rates of soluble Cu-OM complexes may also influence Cu root uptake by Cu transporters of the COPT (COPper Transporter)/Ctr (Copper transporter) protein family (Jung et al. 2012). This may explain higher correlations of shoot DW yield and of shoot and root Cu concentrations with pCu2+ than CuTOT and CuSPW (Table 4).
Soil pH and OM are key-players mutually dependent for Cu in the processes of precipitation, sorption by and distribution in soil fractions, and operational mobility (Clemente et al. 2010; Maderova et al. 2011). At pH 6.6, CuSPW would be mostly in the Cu-DOM form (>99 %, Temminghoff et al. 1997). This was validated in both CTRL and OMDL soils but not in the UNT soil (Table 1). Exponential decrease of the ratio of free Cu2+ ions with increasing pH and DOM in the soil pore water (Table 1) likely reflected Cu binding by OM in the solid phase and DOM (Carrillo-Gonzalez et al. 2006; Luo et al. 2006; Sauvé et al. 1997). Modelling of pCu2+ as indicator of Cu exposure (Eq. 1 and 2) confirmed the influence of pH, OM and CuSPW as in previous models (McBride et al. 1997; Sauvé et al. 1997; Sauvé 2003).
Plant parameters
In overall, the increase of free Cu ions in soil pore water was stressing the physiology of sunflower seedlings, which influenced their morphological parameters (Table 4; Fig. 3). Oxidative stress and Cu-induced changes in chloroplast ultrastructure and nutrient homeostasis at sub-cellular level explained leaf chlorosis and necrosis (Mocquot et al. 1996; Palmer and Guerinot 2009; Smeets et al. 2013). Across the tested plant parameters, photosynthetic pigment contents were the most sensitive to excess Cu in the soil and soil pore water, e.g. EC10 of ChlTOT and Carot corresponded to 7.35 and 7.33 for pCu2+, 104 and 114 μg Cu L−1 for CuSPW, and 138 and 151 mg Cu kg−1 soil for CuTOT, respectively (Table 4). The ratio Chla:Chlb had also an early response (7.29 for pCu2+). Total chlorophyll content and Chla:Chlb ratio were changed in Rousos et al. (1989), but net photosynthesis and aboveground biomass were not affected. Arellano et al. (1995) and Luna et al. (1994) reported depressed growth, the breakdown of chlorophyll and carotenoids, and a reduced photosynthetic capacity at high Cu exposures. High Cu concentrations may destroy thylakoid membranes via lipid peroxidation and especially affect photosystem II (Patsikka et al. 2002; Yruela 2009). Leaf chlorosis can block the activity of ribulose, 1–5, bisphosphate carboxylase-oxygenase and CO2 fixation and interfere at the photosystem level, inducing a higher sensitivity to photoinhibition (Cook et al. 1997; Cuypers et al. 2000; Patsikka et al. 2002). The reduction of the grana structure is consistent with the increased Chla:Chlb ratio and may indicate that the synthesis of the photosystem cores takes metabolic preference over the synthesis of the light-harvesting complex II (Patsikka et al. 2002; Rivelli et al. 2012). A reduction of photosynthetic surface area and content of photosynthetic pigments generally decrease the intensity of photosynthesis and the carbohydrate accumulation, and finally the plant biomass, as confirmed in Table 3 and Fig. 3.
Morphological parameters, which are integrative responses, were less sensitive than physiological parameters to excess Cu based on EC values (Table 4). Changes in the root system of Cu-stressed sunflower confirmed previous reports (Bravin et al. 2010a; Lequeux et al. 2010). Root biomass was the most sensitive (Table 4 and Fig. 3), its EC10 value corresponding to 7.3 for pCu2+, 155 mg Cu kg−1 soil for CuTOT and 118 μg Cu L−1 for CuSPW, whereas shoot yield was reduced over 323 mg Cu kg−1 soil (CuTOT) and 261 μg L−1 (CuSPW) (Table 4). This may be due to Cu retention in roots and the influence of seed reserves. The ratio (shoot DW:root DW) had also higher EC10 values than roots, i.e. CuTOT: 193 mg Cu kg−1 soil, CuSPW: 143 μg L−1, and pCu2+ 7.28 (Table 4). The root system is plastic and excess Cu inhibits primary root growth and simultaneously stimulates lateral root formation due to changes in mineral profile, hormonal status, mitotic activity, cell membrane viability, H2O2 concentration, and lignin deposition (Jiang et al. 2000; Lequeux et al. 2010). Damages on roots likely reduce nutrient and water uptake, causing shoot growth reduction and changes in ionome of plant parts (Lequeux et al. 2010).
The RTEI, RPI and TI values (Table 6) confirmed that free Cu ions preferentially influence root biomass, and less shoot yield (Song et al. 2004). At similar total soil Cu, Cu in soil pore water was more bound by DOM in the OMDL soils of block #3 than in the UNT soil (Table 1), root better developed (Table 6), and root physiological activity, notably Cu-induced peroxidase activity (Mocquot et al. 1996), would be less impacted by Cu. This may partly explain, higher shoot Cu removal by sunflower at field scale after incorporation of OMDL into Cu-contaminated soils (Kolbas et al. 2011).
Moderate shoot Cu concentrations in plants of block #1 and #2 caused stem elongation and etiolation with a hormesis effect as defined by Calabrese and Blain (2009) (EC50 corresponding to 671 μg L−1 for CuSPW). Stem reddening at pCu2+ below 6 suggested an increase in anthocyanins and the antioxidant status of plants (Posmyk et al. 2009). High shoot Cu concentrations contribute to disturb the mitosis (Jiang et al. 2004; Liu et al. 2009). Leaf asymmetry (LA) integrates this breakdown affecting leaf and individual development (Parsons 1992). Our data (Table 4 and Fig. 3) supported LA as a relatively early routine biomarker to detect chemically stressed plant and to assess the environment quality, even though its relationship with contaminant exposure may be influenced by other limiting factors (Kryazheva et al. 1996; Ambo-Rappe et al. 2008).
Water stress damages photosynthetic apparatus, inhibits plant photosynthesis, influences enzyme activities, and induces oxidative stress damaging proteins, membrane lipids and other cellular components (Rivelli et al. 2012; Waraich et al. 2011). In uncontrolled water regime, water content (WC) decreases in metal-stressed plants (Barcelo and Poschenrieder 1990). Depending on plant metal content and water regime, WC may be affected by reduced water movement and stomatal closure (Alaoui-Sosse et al. 2004). Excess Cu reduces the water potential and transpiration rates and enhances diffusive resistance and relative WC of cauliflower (Chatterjee and Chatterjee 2000). On the correlation map (Fig. 1), WC was rather independent of other plant parameters and its EC10 value corresponding to 620 mg kg−1 for CuTOT and 538 μg L−1 for CuSPW reflected a low sensitivity to Cu excess (Table 4).
Plant composition and shoot Cu removal
Concentration of Cu in plant tissues generally mirrors root Cu exposure, but the relationship depends on plant species and plant parts (Poschenrieder et al. 2001). As Cu increases in the substrate, the relative Cu concentration rises in roots, is reduced in aerial parts, and remains constant in the hypocotyl of sunflower (Forsberg et al. 2009; Lin et al. 2003). Relationships between root and shoot Cu concentrations (data log-transformed) and pCu2+ were well fitted by quadratic curves (Fig. 3), which agreed with Panou-Filotheou and Bosabalidis (2004) and suggested to use this sunflower mutant as bioindicator for assessing Cu exposure and phytotoxicity of Cu-contaminated soils. As sunflower cultivars and most plant species, this sunflower mutant displayed higher Cu concentrations in roots than in shoots and TF values below 1 (Table 5) (Alaoui-Sosse et al. 2004; Navari-Izzo et al. 2006; Song et al. 2004). Root Cu concentrations may however reflect Cu co-precipitation on the Fe-root plaque, incomplete desorption during the washing procedure and storage in the root ultrastructure, and must be used with caution as indicator of soil Cu exposure (Dickinson et al. 2009; Panou-Filotheou and Bosabalidis 2004). Fellet et al. (2007) reported higher TF (0.124) and BCFshoot (0.19) values in sunflower than our data (Table 5), likely due to higher total soil Cu (1,589–1,943 mg Cu kg−1). Elevated TF values for some elements (i.e. P, K, Mg, and Ca) in Cu-stressed plants compared with controls (Table 5) confirmed their relationships with the shoot yield, but reports suggested their role in controlling oxidative stress in plant cells (Cook et al. 1997; Thakali et al. 2006). Functional responses of plants to Cu exposure generally are earlier indicators than structural ones (Meers et al. 2006; Mocquot et al. 1996). This was confirmed by our results (Table 4). The EC10 values of the most sensitive parameters, i.e. chlorophyll and carotenoid contents (Table 4), corresponded to 16–18 mg Cu kg−1 DW in shoots. Such values matched with the upper critical threshold values (20–30 mg Cu kg−1 DW) reported in leaves and shoots of many plants (Macnicol and Beckett 1985; Mocquot et al. 1996).
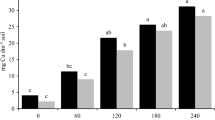
Secondary metal accumulators with high biomass production for feedstock can be used for metal phytoextraction and providing financial opportunities from the biomass valorization (Mench et al. 2010; Vassilev et al. 2004). Here, similar RTEI values showed that shoot Cu removals in this bioassay predicted those obtained in the field plots in 2008–2009 (Fig. 4). This will be useful to figure potential shoot Cu removal by this sunflower mutant at field scale, even though it may depend on annual climatic conditions and further OM incorporations into the Cu-contaminated soil.
Comparison of RTEI index for shoot Cu removal in field and pot experiments. (data for shoot Cu removals in the field experiment obtained in Kolbas et al. 2011)
Sunflower accumulates metals mainly in roots (Madejon et al. 2003; Singh et al. 2004), even though some reports claimed its ability to translocate Pb, Cd, and Zn in aerial parts (Adesodun et al. 2010; Faessler et al. 2010). Shoot Cu and Zn concentrations were more correlated accounting for the UNT soil (Table 5), which agreed with Nehnevajova et al. (2009). The synergy between shoot B and Cu concentrations in relation with soil Cu contamination confirmed previous studies (Santra et al. 1989).
Potted plant test is a routine way to assess the phytotoxicity of contaminated soils and amendment effectiveness for improving soils (Bes and Mench 2008). Dissonances can however occur between controlled and field conditions (Bravin et al. 2010b; Kidd et al. 2009; Warne et al. 2008). For this soil series and compared to field conditions (Kolbas et al. 2011), discrepancies concerned shoot WC, shoot Cu concentration (e.g. for this sunflower grown in block #3 soils, 36 mg and 85 mg kg−1 in pots and plots, respectively), and soil Cu exposure leading to plant mortality, likely because water supply and evapotranspiration were not limited in pots. Exposure time and influence of environmental factors were also lower in controlled conditions.
Total soil Cu in this soil series was mainly distributed in the 21–400 mg kg−1 (n = 20) and the 800–1,170 mg kg−1 ranges (n = 10), with less values (n = 2) in the 400–800 mg kg−1 one. Consequently, dose-effect relationships were a bit weakened by a clustering effect (Fig. 3), leading to a slight uncertainty for the EC10 and EC50 values of several plant parameters (e.g. SL, WC, and TLA, Table 4). An option to further improve such modeling of dose-effect relationships requested for a routine way will be to work on an aged soil series with a better distribution of soil Cu exposure, which can be obtained notably with the so-called fading method (Japenga et al. 2007).
Conclusion
A metal-tolerant sunflower mutant line was grown in pots on a Cu-contaminated soil series collected in field plots, with pCu2+ ranging from 5.38 to 7.52 in the soil pore water. Chlorophyll and carotenoid contents, root DW yield, and leaf asymmetry had the lowest EC10 values to detect an adverse effect of excess Cu. Strong relationships between phenotypic traits of sunflower and indicators of labile Cu pool in the soil such as Cu2+ in pore water suggested to use this mutant line as bioindicator for assessing soil Cu exposure, phytotoxicity of Cu-contaminated soils, and potential shoot Cu removal. The relevance of soil pore water to assess the phytotoxicity of Cu-contaminated soils and of seedling responses to orient their phytomanagement at field scale deserves further attention based on additional soil series.
Abbreviations
- BCF:
-
Bioconcentration factor
- Carot:
-
Carotenoid content
- CCA:
-
Chromated copper arsenate
- CEC:
-
Cation exchange capacity
- Chl:
-
Chlorophyll
- CTRL:
-
Control soil
- Cu-ISE:
-
Copper ion selective electrode
- ChlTOT:
-
Total chlorophyll content
- CuMM:
-
Shoot Cu removal
- CuRT:
-
Root Cu concentration
- CuSH:
-
Shoot Cu concentration
- CuSPW:
-
Total Cu concentration in the soil pore water
- CuTOT:
-
Total soil Cu
- DL:
-
Dolomitic limestone
- DMF:
-
N,N-dimethylformamide
- DOM:
-
Dissolved organic matter
- DW:
-
Dry weight
- DWSH:
-
Shoot DW yield
- EC10 :
-
in a graded dose response curve, the concentration of a compound where 10 % of its maximal effect is observed
- EMS:
-
Ethyl methanesulphonate
- INRA LAS:
-
French National Institute for Agricultural Research - Laboratory of soil analysis Arras France
- ISE:
-
Ion selective electrode
- LA:
-
Leaf asymmetry
- OM:
-
Organic matter
- RPI:
-
Relative parameter index
- RTEI:
-
Relative treatment efficiency index
- SL:
-
Stem length
- TE:
-
Trace element
- TF:
-
Translocation factor
- TI:
-
Tolerance index
- TLA:
-
Total leaf area
- TOC:
-
Total organic carbon
- UNT:
-
Untreated soil
- WC:
-
Water content
References
Adesodun JK, Atayese MO, Agbaje TA, Osadiaye BA, Mafe OF, Soretire AA (2010) Phytoremediation potentials of sunflowers (Tithonia diversifolia and Helianthus annuus) for metals in soils contaminated with zinc and lead nitrates. Water Air Soil Pollut 207:195–201. doi:10.1007/s11270-009-0128-3
Adriano DC (2001) Trace elements in terrestrial environments: biogeochemistry, bioavailability, and risks of metals, 2nd edn. Springer Verlag, New-York, 871 p
Adriano DC, Wenzel WW, Vangronsveld J, Bolan NS (2004) Role of assisted natural remediation in environmental cleanup. Geoderma 122:121–142. doi:10.1016/j.geoderma.2004.01.003
Alaoui-Sosse B, Genet P, Vinit-Dunand F, Toussaint ML, Epron D, Badot PM (2004) Effect of copper on growth in cucumber plants (Cucumis sativus) and its relationships with carbohydrate accumulation and changes in ion contents. Plant Sci 166:1213–1218. doi:10.1016/j.plantsci.2003.12.032
Ambo-Rappe R, Lajus DL, Schreider MJ (2008) Increased heavy metal and nutrient contamination does not increase fluctuating asymmetry in the seagrass Halophila ovalis. Ecol Indic 8:100–103. doi:10.1016/j.ecolind.2006.12.004
Arellano JB, Lazaro JJ, Lopez-Gorgé J, Baron M (1995) The donor side of photosystem-II as the copper-inhibitory binding-site - fluorescence and polarografic studies. Photosynth Res 45:127–134. doi:10.1007/bf00032584
Ashworth DJ, Alloway BJ (2007) Complexation of copper by sewage sludge-derived dissolved organic matter: Effects on soil sorption behaviour and plant uptake. Water Air Soil Pollut 182:187–196. doi:10.1007/s11270-006-9331-7
Baker A, Tipping E, Thacker SA, Gondar D (2008) Relating dissolved organic matter fluorescence and functional properties. Chemosphere 73:1765–1772. doi:10.1016/j.chemosphere.2008.09.018
Barcelo J, Poschenrieder C (1990) Plant water relations as affected by heavy-metal stress - a review. J Plant Nutr 13:1–37. doi:10.1080/01904169009364057
Basol (2013) Base de données Basol sur les sites et sols pollués ou potentiellement pollués appelant une action des pouvoirs publics, à titre préventif ou curatif. http://basol.environnement.gouv.fr/. Accessed 11 March 2013
Beesley L, Dickinson N (2011) Carbon and trace element fluxes in the pore water of an urban soil following greenwaste compost, woody and biochar amendments, inoculated with the earthworm Lumbricus terrestris. Soil Biol Biochem 43:188–196. doi:10.1016/j.soilbio.2010.09.035
Bes C, Mench M (2008) Remediation of copper-contaminated topsoils from a wood treatment facility using in situ stabilisation. Environ Pollut 156:1128–1138. doi:10.1016/j.envpol.2008.04.006
Bes CM, Mench M, Aulen M, Gaste H, Taberly J (2010) Spatial variation of plant communities and shoot Cu concentrations of plant species at a timber treatment site. Plant Soil 330:267–280. doi:10.1007/s11104-009-0198-4
Bravin MN, Michaud AM, Larabi B, Hinsinger P (2010a) RHIZOtest: a plant-based biotest to account for rhizosphere processes when assessing copper bioavailability. Environ Pollut 158:3330–3337. doi:10.1016/j.envpol.2010.07.029
Bravin MN, Le Merrer B, Denaix L, Schneider A, Hinsinger P (2010b) Copper uptake kinetics in hydroponically-grown durum wheat (Triticum turgidum durum L.) as compared with soil’s ability to supply copper. Plant Soil 331:91–104. doi:10.1007/s11104-009-0235-3
Buck RP, Cosofret VV (1993) Recommended procedures for calibration of ion-selective electrodes. Pure Appl Chem 65:1849–1858. doi:10.1351/pac199365081849
Calabrese EJ, Blain RB (2009) Hormesis and plant biology. Environ Pollut 157:42–48. doi:10.1016/j.envpol.2008.07.028
Carrillo-Gonzalez R, Simunek J, Sauvé S, Adriano D (2006) Mechanisms and pathways of trace element mobility in soils. Adv Agron 91:111–178
CETIOM (1995) Les stades repères du tournesol (détails). Available at http://www.cetiom.fr/tournesol/cultiver-du-tournesol/atouts-points-cles/stades-reperes/stades-reperes-detailles/?print=1. Access on 12 March 2013
Chaignon V, Quesnoit M, Hinsinger P (2009) Copper availability and bioavailability are controlled by rhizosphere pH in rape grown in an acidic Cu-contaminated soil. Environ Pollut 157:3363–3369. doi:10.1016/j.envpol.2009.06.032
Chatterjee J, Chatterjee C (2000) Phytotoxicity of cobalt, chromium and copper in cauliflower. Environ Pollut 109:69–74. doi:10.1016/s0269-7491(99)00238-9
Clemente R, Hartley W, Riby P, Dickinson NM, Lepp NW (2010) Trace element mobility in a contaminated soil two years after field-amendment with a greenwaste compost mulch. Environ Pollut 158:1644–1651. doi:10.1016/j.envpol.2009.12.006
Cook CM, Vardaka E, Lanaras T (1997) Concentrations of Cu, growth and chlorophyll content of field-cultivated wheat growing in naturally enriched Cu soil. Bull Environ Contam Toxicol 58:248–253, Available at http://springerlink.bibliotecabuap.elogim.com/article/10.1007%2Fs001289900327#page-1. Access on July 4, 2013
Cuypers A, Vangronsveld J, Clijsters H (2000) Biphasic effect of copper on the ascorbate-glutathione pathway in primary leaves of Phaseolus vulgaris seedlings during the early stages of metal assimilation. Physiol Plant 110:512–517. doi:10.1111/j.1399-3054.2000.1100413.x
da Silva RF, Antoniolli ZI, Lupatini M, Trindade LL, da Silva AS (2010) Tolerance of canafistula (Peltophorum dubium (Spreng) Taub.) seedlings inoculated with Pisolithus microcarpus to copper contaminated soil. Ciencia Florestal 20:147–156, Available at http://www.researchgate.net/publication/44188992_Tolerncia_De_Mudas_De_Canafstula_(_Peltophorum_dubium_(Spreng.)_Taub.)_Inoculada_Com_Pisolithus_microcarpus_A_Solo_Com_Excesso_De_Cobre/file/9fcfd50b34e54a967f.pdf. Access on July 4, 2013
Datta SP, Young SD (2005) Predicting metal uptake and risk to the human food chain from leaf vegetables grown on soils amended by long-term application of sewage sludge. Water Air Soil Pollut 163:119–136. doi:10.1007/s11270-005-0006-6
Degryse F, Smolders E, Zhang H, Davison W (2009) Predicting availability of mineral elements to plants with the DGT technique: a review of experimental data and interpretation by modelling. Environ Chem 6:198–218. doi:10.1071/EN09010
Dickinson NM, Baker AJM, Doronila A, Laidlaw S, Reeves RD (2009) Phytoremediation of inorganics: realism and synergies. Int J Phytorem 11:97–114. doi:10.1080/15226510802378368
Ernst WHO, Peterson PJ (1994) The role of biomarkers in environmental assessment. 4. Terrestrial plants. Ecotoxicology 3:180–192. doi:10.1007/bf00117083
Faessler E, Robinson BH, Stauffer W, Gupta SK, Papritz A, Schulin R (2010) Phytomanagement of metal-contaminated agricultural land using sunflower, maize and tobacco. Agric Ecosyst Environ 136:49–58. doi:10.1016/j.agee.2009.11.007
Fellet G, Marchiol L, Perosa D, Zerbi G (2007) The application of phytoremediation technology in a soil contaminated by pyrite cinders. Ecol Eng 31:207–214. doi:10.1016/j.ecoleng.2007.06.011
Forsberg LS, Kleja DB, Greger M, Ledin S (2009) Effects of sewage sludge on solution chemistry and plant uptake of Cu in sulphide mine tailings at different weathering stages. Appl Geochem 24:475–482. doi:10.1016/j.apgeochem.2008.12.030
Fritsch C, Cosson RP, Coeurdassier M, Raoul F, Giraudoux P, Crini N, de Vaufleury A, Scheifler R (2010) Responses of wild small mammals to a pollution gradient: host factors influence metal and metallothionein levels. Environ Pollut 158:827–840. doi:10.1016/j.envpol.2009.09.027
Gunkel P, Roth E, Fabre B (2003) Copper distribution in chemical soil fractions and relationships with maize crop yield. Environ Chem Lett 1:92–97. doi:10.1007/s10311-002-0003-6
Hernandez AJ, Pastor J (2008) Relationship between plant biodiversity and heavy metal bioavailability in grasslands overlying an abandoned mine. Environ Geochem Health 30:127–133. doi:10.1007/s10653-008-9150-4
Hewitt E (1966) Sand and water culture methods used in the study of plant nutrition. The Eastern press Ltd, London
HiPerTOC (2004) Total organic carbon analyzer. Available at http://www.ankersmid-lab.be/AutoFiles/doc/4266_HiPerTOC_specsheet__PS42033[1].pdf. Accessed on 12 March 2013
Inaba S, Takenaka C (2005) Effects of dissolved organic matter on toxicity and bioavailability of copper for lettuce sprouts. Environ Int 31:603–608. doi:10.1016/S1001-0742(09)60346-6
INRA LAS (2013) Méthodes applicables aux sols. http://www5.lille.inra.fr/las/methodes_d_analyse/Sols. Accessed on 12 March 2013
ISO (2005) Soil quality - determination of the effects of pollutants on soil flora in part 2: effects of chemicals on the emergence and growth of higher plants, Geneva
Japenga J, Koopmans GF, Song J, Romkens PFAM (2007) A feasibility test to estimate the duration of phytoextraction of heavy metals from polluted soils. Int J Phytorem 9:115–132. doi:10.1080/15226510701232773
Jiang WS, Liu DH, Li HF (2000) Effects of Cu2+ on root growth, cell division, and nucleolus of Helianthus annuus L. Sci Total Environ 256:59–65
Jiang LY, Yang XE, He ZL (2004) Growth response and phytoextraction of copper at different levels in soils by Elsholtzia splendens. Chemosphere 55:1179–1187
Jung HI, Gayomba SR, Rutzke MA, Craft E, Kochian LV, Vatamaniuk OK (2012) COPT6 Is a plasma membrane transporter that functions in copper homeostasis in Arabidopsis and is a novel target of SQUAMOSA promoter-binding protein-like 7. J Biol Chem 287:33252–33267. doi:10.1074/jbc.M112.397810
Ke W, Xiong Z-T, Chen S, Chen J (2007) Effects of copper and mineral nutrition on growth, copper accumulation and mineral element uptake in two Rumex japonicus populations from a copper mine and an uncontaminated field sites. Environ Exp Bot 59:59–67. doi:10.1016/j.envexpbot.2005.10.007
Kidd P, Barcelo J, Pilar Bernal M, Navari-Izzo F, Poschenrieder C, Shilev S, Clemente R, Monterroso C (2009) Trace element behaviour at the root-soil interface: implications in phytoremediation. Environ Exp Bot 67:243–259. doi:10.1016/j.envexpbot.2009.06.013
Knezevic SZ, Streibig JC, Ritz C (2007) Utilizing R software package for dose–response studies: the concept and data analysis. Weed Technol 21:840–848. doi:10.1614/wt-06-161.1
Kolbas A, Mench M, Herzig R, Nehnevajova E, Bes CM (2011) Copper phytoextraction in tandem with oilseed production using commercial cultivars and mutant lines of sunflower. Int J Phytorem 13(Suppl 1):55–76. doi:10.1080/15226514.2011.568536
Korpe DA, Aras S (2011) Evaluation of copper-induced stress on eggplant (Solanum melongena L.) seedlings at the molecular and population levels by use of various biomarkers. Muta Res Gen Tox En 719:29–34. doi:10.1016/j.mrgentox.2010.10.003
Kryazheva NG, Chistyakova EK, Zakharov VM (1996) Analysis of development stability of Betula pendula under conditions of chemical pollution. Russ J Ecol 27:422–424
Kuepper H, Goetz B, Mijovilovich A, Kuepper FC, Meyer-Klaucke W (2009) Complexation and toxicity of copper in higher plants. I. Characterization of copper accumulation, speciation, and toxicity in Crassula helmsii as a new copper accumulator. Plant Physiol 151:702–714. doi:10.1104/pp. 109.139717
Lagadic L, Caquet T, Amiard JC, Ramade F (1997) Biomarqueurs en écotoxicologie. Aspects fondamentaux. Masson, Paris, p 419
Lagomarsino A, Mench M, Marabottini R, Pignataro A, Grego S, Renella G, Stazi SR (2011) Copper distribution and hydrolase activities in a contaminated soil amended with dolomitic limestone and compost. Ecotoxicol Environ Saf 74:2013–2019. doi:10.1016/j.ecoenv.2011.06.013
Lagriffoul A, Mocquot B, Mench M, Vangronsveld J (1998) Cadmium toxicity effects on growth, mineral and chlorophyll contents, and activities of stress related enzymes in young maize plants (Zea mays L.). Plant Soil 200:241–250. doi:10.1023/a:1004346905592
Leduc F, Whalen JK, Sunahara GI (2008) Growth and reproduction of the earthworm Eisenia fetida after exposure to leachate from wood preservatives. Ecotoxicol Environ Saf 69:219–226. doi:10.1016/j.ecoenv.2007.01.006
Lequeux H, Hermans C, Lutts S, Verbruggen N (2010) Response to copper excess in Arabidopsis thaliana: impact on the root system architecture, hormone distribution, lignin accumulation and mineral profile. Plant Physiol Biochem 48:673–682. doi:10.1016/j.plaphy.2010.05.005
Li Z, Tang S, Deng X, Wang R, Song Z (2010) Contrasting effects of elevated CO2 on Cu and Cd uptake by different rice varieties grown on contaminated soils with two levels of metals: implication for phytoextraction and food safety. J Hazard Mater 177:352–361. doi:10.1016/j.jhazmat.2009.12.039
Lin JX, Jiang WS, Liu DH (2003) Accumulation of copper by roots, hypocotyls, cotyledons and leaves of sunflower (Helianthus annuus L.). Bioresour Technol 86:151–155. doi:10.1016/s0960-8524(02)00152-9
Liu D, Xue P, Meng Q, Zou J, Gu J, Jiang W (2009) Pb/Cu effects on the organization of microtubule cytoskeleton in interphase and mitotic cells of Allium sativum L. Plant Cell Rep 28:695–702. doi:10.1007/s00299-009-0669-3
Luna CM, Gonzalez CA, Trippi VS (1994) Oxidative damage caused by an excess of copper in oat leaves. Plant Cell Physiol 35:11–15
Luo XS, Zhou DM, Wang YJ (2006) Free cupric ions in contaminated agricultural soils around a copper mine in eastern Nanjing City, China. J Environ Sci (China) 18:927–931. doi:10.1016/s1001-0742(06)60016-8
Macnicol RD, Beckett PHT (1985) Critical tissue concentrations of potentially toxic elements. Plant Soil 85:107–129. doi:10.1007/bf02197805
Madejon P, Murillo JM, Maranon T, Cabrera F, Soriano MA (2003) Trace element and nutrient accumulation in sunflower plants two years after the Aznalcollar mine spill. Sci Total Environ 307:239–257. doi:10.1016/s0048-9697(02)00609-5
Maderova L, Watson M, Paton GI (2011) Bioavailability and toxicity of copper in soils: Integrating chemical approaches with responses of microbial biosensors. Soil Biol Biochem 43:1162–1168. doi:10.1016/j.soilbio.2011.02.004
Marchand L, Mench M, Jacob DL, Otte ML (2010) Metal and metalloid removal in constructed wetlands, with emphasis on the importance of plants and standardized measurements: a review. Environ Pollut 158:3447–3461. doi:10.1016/j.envpol.2010.08.018
Marchand L, Mench M, Marchand C, Le Coustumer P, Kolbas A, Maalouf JP (2011) Phytotoxicity testing of lysimeter leachates from aided phytostabilized Cu-contaminated soils using duckweed (Lemna minor L.). Sci Total Environ 410:146–153. doi:10.1016/j.scitotenv.2011.09.049
McBride M, Sauvé S, Hendershot W (1997) Solubility control of Cu, Zn, Cd and Pb in contaminated soils. Eur J Soil Sci 48:337–346. doi:10.1111/j.1365-2389.1997.tb00554.x
Meers E, Ruttens A, Geebelen W, Vangronsveld J, Samson R, Vanbroekhoven K, Vandegehuchte M, Diels L, Tack FMG (2006) Potential use of the plant antioxidant network for environmental exposure assessment of heavy metals in soils. Environ Monit Assess 120:243–267. doi:10.1007/s10661-005-9059-7
Mench M, Bes C (2009) Assessment of ecotoxicity of topsoils from a wood treatment site. Pedosphere 19:143–155. doi:10.1016/S1002-0160(09)60104-1
Mench M, Lepp N, Bert V, Schwitzguebel JP, Gawronski SW, Schroeder P, Vangronsveld J (2010) Successes and limitations of phytotechnologies at field scale: outcomes, assessment and outlook from COST Action 859. J Soil Sediment 10:1039–1070. doi:10.1007/s11368-010-0190-x
Mendoza-Soto AB, Sanchez F, Hernandez G (2012) MicroRNAs as regulators in plant metal toxicity response. Front Plant Sci 3:105. doi:10.3389/fpls.2012.00105
Mocquot B, Vangronsveld J, Clijsters H, Mench M (1996) Copper toxicity in young maize (Zea mays L) plants: effects on growth, mineral and chlorophyll contents, and enzyme activities. Plant Soil 182:287–300
Moreno-Jimenez E, Beesley L, Lepp NW, Dickinson NM, Hartley W, Clemente R (2011) Field sampling of soil pore water to evaluate trace element mobility and associated environmental risk. Environ Pollut 159:3078–3085. doi:10.1016/j.envpol.2011.04.004
Navari-Izzo F, Cestone B, Cavallini A, Natali L, Giordani T, Quartacci MF (2006) Copper excess triggers phospholipase D activity in wheat roots. Phytochemistry 67:1232–1242. doi:10.1016/j.phytochem.2006.04.006
Nehnevajova E, Herzig R, Federer G, Erismann KH, Schwitzguébel JP (2005) Screening of sunflower cultivars for metal phytoextraction in a contaminated field prior to mutagenesis. Int J Phytorem 7:337–349. doi:10.1080/16226510500327210
Nehnevajova E, Herzig R, Bourigault C, Bangerter S, Schwitzguebel JP (2009) Stability of enhanced yield and metal uptake by sunflower mutants for improved phytoremediation. Int J Phytorem 11:329–346. doi:10.1080/15226510802565394
Nehnevajova E, Lyubenova L, Herzig R, Schroeder P, Schwitzguébel JP, Schmuelling T (2012) Metal accumulation and response of antioxidant enzymes in seedlings and adult sunflower mutants with improved metal removal traits on a metal-contaminated soil. Environ Exp Bot 76:39–48. doi:10.1016/j.envexpbot.2011.10.005
Palmer CM, Guerinot ML (2009) Facing the challenges of Cu, Fe and Zn homeostasis in plants. Nat Chem Biol 5:333–340. doi:10.1038/nchembio.166
Panou-Filotheou H, Bosabalidis AM (2004) Root structural aspects associated with copper toxicity in oregano (Origanum vulgare subsp hirtum). Plant Sci 166:1497–1504. doi:10.1016/j.plantsci.2004.01.026
Parsons PA (1992) Fluctuating asymmetry - a biological monitor of environmental and genomic stress. Heredity 68:361–364
Patsikka E, Kairavuo M, Sersen F, Aro EM, Tyystjarvi E (2002) Excess copper predisposes photosystem II to photoinhibition in vivo by outcompeting iron and causing decrease in leaf chlorophyll. Plant Physiol 129:1359–1367. doi:10.1104/pp. 004788
Poschenrieder C, Bech J, Llugany M, Pace A, Fenes E, Barcelo J (2001) Copper in plant species in a copper gradient in Catalonia (North East Spain) and their potential for phytoremediation. Plant Soil 230:247–256. doi:10.1023/a:1010374732486
Posmyk MM, Kontek R, Janas KM (2009) Antioxidant enzymes activity and phenolic compounds content in red cabbage seedlings exposed to copper stress. Ecotoxicol Environ Saf 72:596–602. doi:10.1016/j.ecoenv.2008.04.024
Qi XM, Li PJ, Liu W, Xie LJ (2006) Multiple biomarkers response in maize (Zea mays L.) during exposure to copper. J Environ Sci (China) 18:1182–1188. doi:10.1016/s1001-0742(06)60059-4
Rivelli AR, de Maria S, Puschenreiter M, Gherbin P (2012) Accumulation of cadmium, zinc, and copper by Helianthus annuus L.: impact on plant growth and uptake of nutritional elements. Int J Phytorem 14:320–334. doi:10.1080/15226514.2011.620649
Rousos PA, Harrison HC, Steffen KL (1989) Physiological-responses of cabbage to incipient copper toxicity. J Am Soc Hortic Sci 114:149–152
Santra GH, Das DK, Mandal BK (1989) Relationship of boron with iron, manganese, copper and zinc with respect to their availability in rice soil. Environ Ecol 7:874–877
Sauvé S (2003) Modelling trace element exposure and effects on plants. In: Mench M, Mocquot B (eds) Risk assessment and sustainable land management using plants in trace element-contaminated soils. Centre INRA Bordeaux-Aquitaine, Villenave d’Ornon, pp 69–70, Available at http://w3.gre.ac.uk/cost859/book/Livre_COST837_2003_session_2.pdf. Access on July 4, 2013
Sauvé S, McBride MB, Norvell WA, Hendershot WH (1997) Copper solubility and speciation of in situ contaminated soils: effects of copper level, pH and organic matter. Water Air Soil Pollut 100:133–149. doi:10.1023/a:1018312109677
Singh S, Saxena R, Pandey K, Bhatt K, Sinha S (2004) Response of antioxidants in sunflower (Helianthus annuus L.) grown on different amendments of tannery sludge: its metal accumulation potential. Chemosphere 57:1663–1673. doi:10.1016/j.chemosphere.2004.07.049
Smeets K, Opdenakker K, Remans T, Forzani C, Hirt H, Vangronsveld J, Cuypers A (2013) The role of the kinase OXI1 in cadmium- and copper-induced molecular responses in Arabidopsis thaliana. Plant Cell Environ 36:1228–1238. doi:10.1111/pce.12056
Song J, Zhao FJ, Luo YM, McGrath SP, Zhang H (2004) Copper uptake by Elsholtzia splendens and Silene vulgaris and assessment of copper phytoavailability in contaminated soils. Environ Pollut 128:307–315. doi:10.1016/j.envpol.2003.09.019
Tahsin N, Yankov B (2007) Research on accumulation of zinc (Zn) and cadmium (Cd) in sunflower oil. J Tekirdag Agric Fac 4:109–112
Temminghoff EJM, Van der Zee S, deHaan FAM (1997) Copper mobility in a copper-contaminated sandy soil as affected by pH and solid and dissolved organic matter. Environ Sci Technol 31:1109–1115. doi:10.1021/es9606236
Thakali S, Allen HE, Di Toro DM, Ponizovsky AA, Rooney CP, Zhao FJ, McGrath SP (2006) A terrestrial biotic ligand model. 1. Development and application to Cu and Ni toxicities to barley root elongation in soils. Environ Sci Technol 40:7085–7093. doi:10.1021/es061171s
Vamerali T, Bandiera M, Mosca G (2010) Field crops for phytoremediation of metal-contaminated land. A review. Environ Chem Lett 8:1–17. doi:10.1007/s10311-009-0268-0
Vangronsveld J, Herzig R, Weyens N, Boulet J, Adriaensen K, Ruttens A, Thewys T, Vassilev A, Meers E, Nehnevajova E, van der Lelie D, Mench M (2009) Phytoremediation of contaminated soils and groundwater: lessons from the field. Environ Sci Pollut Res 16:765–794. doi:10.1007/s11356-009-0213-6
Vassilev A, Schwitzguebel JP, Thewys T, Van Der Lelie D, Vangronsveld J (2004) The use of plants for remediation of metal-contaminated soils. Sci World J 4:9–34
Waraich EA, Ahmad R, Saifullah, Ashraf MY, Ehsanullah (2011) Role of mineral nutrition in alleviation of drought stress in plants. Aust J Crop Sci 5:764–777
Warne MSJ, Heemsbergen D, McLaughlin M, Bell M, Broos K, Whatmuff M, Barry G, Nash D, Pritchard D, Penney N (2008) Models for the field-based toxicity of copper and zinc salts to wheat in 11 Australian soils and comparison to laboratory-based models. Environ Pollut 156:707–714. doi:10.1016/j.envpol.2008.06.012
Yruela I (2009) Copper in plants: acquisition, transport and interactions. Funct Plant Biol 36:409–430. doi:10.1071/fp08288
Acknowledgments
This work was financially supported by ADEME, Department of Urban Brownfields and Polluted Sites, Angers, France (Mrs F. Cadière as supervisor), the University of Bordeaux 1, Department of International Relationships, through European Commission Erasmus Mundus Lot 6, and the European Commission under the Seventh Framework Programme for Research (FP7-KBBE-266124, GREENLAND). Thanks to Dr. Christophe Barnier (Institut EGID University Bordeaux 3, France), Dr. Anne Serani-Loppinet (ICMCB, University Bordeaux 1, France), and Pr. Mikael Motelica (University of Orléans, France) for their help in the analysis of soil pore waters. Special thanks to Dr. Jean-Paul Maalouf and Galina Brutcova for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Juan Barcelo.
Highlights
A metal-tolerant sunflower mutant can be used for monitoring and phytoremediating soil Cu contamination at a wood preservation site.
Rights and permissions
About this article
Cite this article
Kolbas, A., Marchand, L., Herzig, R. et al. Phenotypic seedling responses of a metal-tolerant mutant line of sunflower growing on a Cu-contaminated soil series: potential uses for biomonitoring of Cu exposure and phytoremediation. Plant Soil 376, 377–397 (2014). https://doi.org/10.1007/s11104-013-1974-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-013-1974-8