Abstract
Silicon (Si) is as an important fertilizer element, which has been found effective in enhancing plant tolerance to variety of biotic and a-biotic stresses. This study investigates the Si potential to alleviate zinc (Zn) toxicity stress in cotton (Gossypium hirsutum L.). Cotton plants were grown in hydroponics and exposed to different Zn concentration, 0, 25, and 50 μM, alone and/or in combination with 1 mM Si. Incremental Zn concentration in growth media instigated the cellular oxidative damage that was evident from elevated levels of hydrogen peroxide (H2O2), electrolyte leakage, and malondialdehyde (MDA) and consequently inhibited cotton growth, biomass, chlorophyll pigments, and photosynthetic process. Application of Si significantly suppressed Zn accumulation in various plant parts, i.e., roots, stems, and leaves and thus promoted biomass, photosynthetic, growth parameters, and antioxidant enzymes activity of Zn-stressed as well unstressed plants. In addition, Si reduced the MDA and H2O2 production and electrolyte leakage suggesting its role in protecting cotton plants from Zn toxicity-induced oxidative damage. Thus, the study indicated that exogenous Si application could improve growth and development of cotton crop experiencing Zn toxicity stress by limiting Zn bioavailability and oxidative damage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The term heavy metal refers to the elements with an atomic density greater than 6 g cm−3 (Adriano 2001). These elements include copper (Cu), manganese (Mn), iron (Fe), cadmium (Cd), mercury (Hg), nickel (Ni), and lead (Pb). Some heavy metals such as Cu, Mn, Fe, and zinc (Zn) are essential for proper plant growth as micronutrients. Despite of their role in plant growth and multiple functions in daily human life, elevated levels of these elements pose serious negative environmental concerns (Ali et al. 2013a; Han et al. 2002). These heavy metals are naturally present in soils in low concentration but unrestrained geological and anthropogenic activities have increased the concentrations of some of these elements to unsafe levels for living organisms (Basta et al. 2001; Dembitsky and Rezanka 2003; Farid et al. 2013a). Higher concentrations of these heavy metals exert strong toxicological effects to all forms of life including microorganisms, plants, animals, and human, although degree of the toxicity varies for different organisms.
Zinc is widely used in different industrial processes for the production of variety of useful products. However, excessive release of Zn from industrial processes such as mine tailings, smelting, and waste disposal or leakage of wastes and use of these industrial effluents for agriculture purpose has tremendously increased the Zn concentration in agricultural soils (Luo et al. 2000; Zhao et al. 2003).
Zinc deficiency is one of the most widespread micronutrient deficiencies in plants and causes severe reductions in crop production. There are a number of physiological impairments in Zn-deficient cells causing inhibition of the growth, changes in metabolism stunted growth and chlorosis, and differentiation and development of plants (Falkengren-Grerup et al. 1987; Cakmak 2000). Above a certain optimum concentrations (300 mg kg−1), Zn becomes toxic to plant growth in soil (Ehsan et al. 2013; Marschner 1995). Elevated Zn concentrations in soil usually lead to physiological, morphological, and biochemical disturbances that eventually limits plant yield (Cuypers et al. 1999). Major symptoms of Zn toxicity include yield and growth inhibition, and leaf chlorosis (Broadley et al. 2007; Tewari et al. 2008). Elevated Zn levels in soil interfere with the uptake, translocation, and regularization of essential ions by changing their ionic homeostatic system. Nutrient deficiency, in turn, impairs various metabolic processes vital for plant growth such as photosynthesis, transpiration, and enzymatic activities (Ali et al. 2013b; Abbas et al. 2009; Broadley et al. 2007).
Silicon is the second most abundant element and fills up 28 % of the total earth crust (Epstein 1994; Gong et al. 2006; Ming et al. 2012). Its concentration in the soil solution ranges from 0.01 to 1.99 mM and is controlled by silicate minerals. Generally, silicon (Si) is not considered an essential micronutrient for growth of higher plants (Epstein 1999). However, it has been applied as an important fertilizer component which enhances plant tolerance to variety of biotic and a-biotic stresses (Hattori et al. 2005; Liang et al. 2005).
Silicon has been found effective to ameliorate heavy metal toxicity stress in many plant species (Rogalla and Romheld 2002; Shi et al. 2005) mainly by increasing solution pH and limiting metal phyto-availability (Cocker et al. 1998); therefore, Si could be the potential candidate for the alleviation of Zn toxicity and uptake.
The present study was planned to explore the potential of Si in ameliorating negative effects of Zn stress on growth, biomass, antioxidant enzymes activities, photosynthetic pigments, electrolyte leakage, and gas exchange attributes of cotton (Gossypium hirsutum L.) seedlings.
Materials and methods
Experimental site
The complete set of experiments for study was conducted at the wire house of Ayub Agricultural Research Institute (AARI), Pakistan, and all biochemical analyses were performed in labs of Government College University, Faisalabad, Pakistan.
Plant material and growth conditions
Cotton leaf curl virus (CLCuV) is one of the major biotic constraints of cotton production (Akhtar et al. 2005). Healthy seeds of high-yielding and CLCuV-resistant cotton genotype (MNH 886) were immersed in the concentrated sulfuric acid solution for 15 min to remove the short fiber from the seed surface. The seeds were then thoroughly rinsed with distilled water and sown in 2-in. layers of sterilized quartz sand trays in a growth chamber under a photoperiod of 16-/8-h light/dark and light intensity of 400 ± 25 μmolm−2 s−1. The day/night temperature was set at 30/25 °C with relative humidity at 85 %. After 2 weeks of germination, the uniform seedlings were wrapped with foam at a root shoot junction and transplanted in thermo pore sheets having evenly spaced holes floating on 40-L capacity tubs, lined with polyethylene sheet containing modified Hoagland’s solution (Ehsan et al 2014). The basic nutrient medium had composition (Ca(NO3)2 2.5 mM, MgSO4 1 mM, KCL 0.5 mM, KH2PO4 0.5 mM, FeCl3 0.1 μM, CuSO4 0.2 μM, ZnSO4 1 μM, H3BO3 20 μM, H2MoO4 0.005 μM, MnSO4 2 μM). Continuous aeration to nutrient solution was supplied by air pump. The solution was changed every week. Plants were grown in a complete randomized design (CRD) with three replicates. Two weeks after transplanting, Zn levels (0, 25, and 50 μM) were developed by ZnCl2 application and Si was as sodium silicate (Na2SiO3) (0 and 1 mM). The pH of solution was maintained to 6.0 ± 0.1 by adding 1 M H2SO4 or NaOH.
Measurement of plant growth and biomass
After the 60 days of Zn and Si treatment, plants were harvested and various growth parameters such as plant height, root length, and number of leaves per plant were measured. Fresh biomass measurements were recorded immediately after harvesting, and same samples were used for biochemical analysis. For dry biomass measurements, the plant parts (leaf, stem, and root) were stored in oven with 70–80 °C for 72 h and then dry biomass was recorded and same samples were used for measurement of Zn content. Leaf area of individual plants was measured by leaf area meter (LI-2000, LI-COR, USA).
Soil-plant analysis development value
For the analysis of leaf greenness/soil-plant analysis development (SPAD) value, SPAD-502 (Zheijang Top Instruments Co., Ltd., China) meter was used.
Gas exchange parameters
At the end of experiment (60 days after treatment), various gas exchange parameters such as photosynthetic rate (A), stomatal conductance (Gs), transpiration rate (E), and water use efficiency (A/E) of second fully expanded leaves were determined using Infra-Red Gas Analyzer (IRGA) (Analytical Development Company, Hoddesdon, England).
Assessment of electrolyte leakage
At the end of experiment (after 6 weeks), the topmost fully expanded leaves were cut into 5-mm-long fragments and positioned in test tubes filled with 8-mL distilled water. The tubes were incubated in a water bath at 32 °C for 2 h, and initial electrical conductivity (EC) of the medium EC1 was noted. The samples were autoclaved at 121 °C for 20 min to discharge all electrolytes, and then cooled at 25 °C, and final EC2 was measured (Dionisio-Sese and Tobita, 1998). Electrolyte leakage (EL) was recorded by using pH/conductivity meter (model 720, INCO-LAB Company, Kuwait) and calculated by the following formula:
Measurement of chlorophyll contents and total carotenoids
Chlorophyll a, chlorophyll b, total chlorophyll, and total carotenoids of the topmost fully expanded cotton leaves were determined by spectrophotometer after the 60 days of treatment. Fresh weight (0.2 g) of leaves were extracted in 85 % (v/v) aqueous acetone for an overnight and the absorbance at of extract was measured at 452.5, 644, and 663 nm using a spectrophotometer (AA6300, Shimadzu, Kyoto, Japan). Chl a, b, total chlorophyll, and carotenoids contents were calculated using the following equations:
Finally, the pigment fractions were calculated as milligrams per gram leaf fresh biomass.
Measurements of anti-oxidant enzymes activities
For evaluation of antioxidant enzymes, 0.5-g fresh leaf and root samples were extracted in a cold buffer solution. Different buffers solutions were employed for each enzyme. The extracted samples were centrifuged at 15,000 rpm for 20 min under 4 °C. The supernatant was collected in micro centrifuge tubes and used for determining the activities of antioxidant enzymes.
Superoxide dismutase (SOD, EC 1.15.1.1) activity was analyzed by nitroblue tetrazolium (NBT) method (Beauchamp and Fridovich 1971) by calculating the NBT photoreduction. The reaction mixture (3 mL) used for measuring absorbance at 560 nm was consisted of 50 mM sodium phosphate buffer (pH 7.8), 13 mM methionine, 75 μM NBT, 10 μM EDTA, 2 mM riboflavin, and enzyme extract (100 μL). Reaction in mixture was initiated by placing tubes under two 15-W fluorescent lamps for 10 min.
Guaiacol peroxidase (POD, EC 1.11.1.7) activity was assayed according to method of Putter and Becker (1974) with some modification. Reaction mixture (3 mL) consisted of 100 μL enzyme extract, 100 μL guaiacol (1.5 %, v/v), 100 μL hydrogen peroxide (H2O2) (300 mM), and 2.7 mL (25 mM) potassium phosphate buffer with 2 mM EDTA (pH 7.0) was used for measuring the change absorbance due to oxidation of guaiacol at 470 nm (ε = 26.6 mM−1 cm−1).
Catalase (CAT, EC 1.11.1.6) activity was determined by Aebi (1984) method. The assay mixture (3.0 mL) was comprised of 100 μL enzyme extract, 100 μL H2O2 (300 mM), and 2.8 mL 50 mM phosphate buffer with 2 mM EDTA (pH 7.0). The CAT activity was assayed by monitoring reduction in absorbance at 240 nm as a consequence of H2O2 disappearance (ε = 39.4 mM−1 cm−1).
Ascorbate peroxidase (APX, EC 1.11.1.11) activity was assayed according to Nakano and Asada (1981) method. The reaction mixture containing 100 μL enzyme extract, 100 μL ascorbate (7.5 mM), 100 μL H2O2 (300 mM), and 2.7 mL 25 mM potassium phosphate buffer with 2 mM EDTA (pH 7.0) was used for measuring APX activity. The oxidation of ascorbate was observed by monitoring change in absorbance at 290 nm (ε = 2.8 mM−1 cm−1).
Hydrogen peroxide contents
H2O2 was extracted by homogenizing 50 mg leaf or root tissues with 3 mL of phosphate buffer (50 mM, pH 6.5). Then, the homogenate was centrifuged at 6000×g for 25 min. To measure H2O2 content, 3 mL extracted solution was mixed with 1 mL 0.1 % titanium sulfate in 20 % (v/v) H2SO4, and mixture was centrifuged at 6,000×g for 15 min. The intensity of yellow color of the supernatant was measured at 410 nm. H2O2 content was computed using the extinction coefficient of 0.28 μmol−1 cm−1.
Malondialdehyde content
Lipid peroxidation of leaf and root tissues was measured in terms of malondialdehyde (MDA, a product of lipid peroxidation) contents by thiobarbituric acid (TBA) reaction method (Heath and Packer 1968), with minor modifications as described by Dhindsa et al. (1981) and Zhang and Kirham (1994).
Soluble protein content
Estimation of soluble protein contents was carried out according to Bradford (1976) method, using Coomassie Brilliant Blue G-250 as a dye and albumin as a standard.
Estimation of zinc concentration and accumulation in cotton
After 60 days treatments, cotton plants from each treatment were harvested and thoroughly washed with tap water, distilled water, and deionized water three times, respectively. Plants were then separated into roots, shoots, and leaves and dried at 80 °C in an oven for 48 h, and then ground into powder. Of each sample, 0.5 g were dry-ashed, extracted with and dissolved in 10 mL of concentrated H2SO4, and then centrifuged it at 400 rpm for 15 min. Zn concentrations in root, shoot, and leaf tissues were determined by flame atomic absorption spectrometry (Nov Aa 400 Analytik Jena, Germany).
The concentration of Zn in plant root, stem and leaf was measured by the following formula:
The accumulation of Zn in plant shoot and root was estimated by the following formula:
Statistical analysis
All values presented in this experiment are mean of three replicates. A statistical package, SPSS version 21.0 (SPSS, Chicago, IL), was used for analyzing the data. A three-way variance analysis (ANOVA) was carried out, followed by the Duncan’s multiple range tests to define the substantial difference among means of treatments.
Results
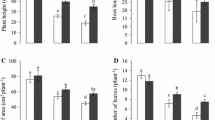
Increasing Zn concentrations in the growth media significantly inhibited cotton growth. Although, effects of lower Zn concentration were not significantly different in comparison to Zn 50 μM. Induction of Si enhanced ability of these plants to cope the Zn toxicity stress and significantly increased the plant growth in term of plant height, root length, number of leaves per plant, and leaf area in each respective treatment. The effect of exogenous Si on biomass of different plant parts under Zn stress is shown in Table 1.
In addition, Si-treated cotton plants contained significantly higher biomass compared with control treatment; illustrating the multidimensional ability of Si to mitigate environmental stresses. Higher Zn concentration (50 μM) significantly reduced fresh and dry weight of leaf, stem, and root compared with control. Exogenous Si application significantly recovered the biomass production in Zn (25 and 50 μM) toxicity-stressed cotton plants.
Toxic Zn concentrations in the growth media significantly inhibited various leaf gas exchange parameters such as net photosynthetic rate (E), transpiration rate (A), stomatal conductance (Gs), and water use efficiency (A/E) of cotton plants (Fig. 1a–c). Application of higher Zn concentration (Zn 50 μM) alone caused maximum reduction in all the gas exchange parameters compared with any other treatment. Silicon significantly recovered the gas exchange parameters of Zn toxicity-stressed plants; it provided assistance to Zn50 + Si and Zn25 + Si, up to 32 and 25 %, respectively. In addition, Si treatment to non-stressed caused 20 % increase in all gas exchange.
Effect of Zn and Si on transpiration rate, stomatal conductance (a), net photosynthetic rate (b), and water use efficiency (c) of cotton seedlings grown in solution culture with different Zn treatments (0, 25, and 50 μM) treated or not with 1 mM Si. Values are mean ± SD (n = 3). Different letters indicate that values are significantly different at P < 0.05
Effect of Zn toxicity on chlorophyll a, b, total chlorophyll, and total carotenoids contents of cotton plants with supplementation of Si is illustrated in the in Fig. 2a–d. Chlorophyll contents (a, b, and total) significantly decreased with the increasing Zn concentrations in growth media Chlorophyll contents of cotton leaves showed a decreasing trend under various treatment, i.e., Zn50 > Zn50 + Si > Zn25 > Zn25 + Si > Si > control (from maximum to minimum reduction). Zn50 treatment caused 66 and 60 % reduction in chlorophyll a and chlorophyll b contents, respectively, compared with control, while 45 % reduction was recorded in each of chlorophyll a and b contents under Zn25. Adding Si to growth media caused significant increase in the chlorophyll a, 23.2 % for Zn25, 26.2 % for Zn50 than the respective Zn treatments alone. In addition, it caused 10 % increase in chlorophyll a content in comparison to control, while addition of Si created accommodating condition for growth and alleviated the effect of Zn more than 21 and 31 % for Zn50 and Zn25, respectively. Similar trend was followed by the protein content and SPAD value in cotton plant (Fig. 2c, d). The protein content was higher in leaves than roots while the maximum protein content was measured in treatment having Si alone while the minimum was measured observed in Zn50.
Effect of Zn and Si on chlorophyll a and b, total chlorophyll, and total carotenoids (a), electrolyte leakage (b), protein content (c), and SPAD value (d) in leaf and roots of cotton seedlings grown in solution culture with different Zn treatments (0, 25, and 50 μM) treated or not with 1 mM Si. Values are mean ± SD (n = 3). Different letters indicate that values are significantly different at P < 0.05
Zn-induced solute leakage in cotton leaves and roots is presented in Fig. 2c. A significant increase in the electrolyte leakage was recorded under Zn stress (25 and 50 μM). Silicon application to Zn-stressed or Zn-unstressed cotton plants significantly lowered the solute leakages in leaf and root tissues.
Modification in antioxidant enzymes such as SOD, POD, CAT, and APX of cotton leaves and roots under various treatments of Zn and Si as alone or in combination is described in Fig. 3a, b. Control cotton plants showed the higher values of antioxidant enzymes. Under Zn toxicity stress at 25 μM, all four tested antioxidant enzymes showed different behavior as compared to control. While, as the concentration of Zn increased, the activities of enzymes decreased but exogenous application of silicon to the zinc-treated plants had synergetic effect on the anti-oxidant enzyme activities on both Zn (25 and 50 μM).
Ant-oxidative enzyme activities POD and CAT (a), SOD and APX (b), MDA (c), and H2O2 (d) in leaves and roots of cotton seedlings grown in solution culture with different Zn treatments (0, 25, and 50 μM) treated or not with 1 mM Si. Values are mean ± SD (n = 3). Different letters indicate that values are significantly different at P < 0.05
Increasing Zn concentration significantly elevated the MDA and H2O2 levels in both cotton root and leaf tissues. (Fig. 3c, d). Si application significantly inhibited the Zn toxicity-induced oxidative damage to cotton plants by lowering MDA and H2O2 levels.
Increasing levels of Zn in growth media significantly increased the leaf Zn contents, i.e., Zn accumulation was 352 mg kg−1 DW under Zn25 that almost doubled under Zn50 treatments (653 mg kg−1 DW) (Fig. 4). Silicon treatments helped plant to limit Zn uptake to under no Zn toxic conditions. Thirty-four and 19 % reduction in Zn uptake was observed under Zn50 + Si and Zn25 + Si, respectively, compared with Zn only treatments. Zn uptake increased in order of control < Si < Zn25 + Si < Zn25 < Zn50 + Si < Zn50.
Zinc accumulation in cotton stem tissues was to 1055 mg kg−1 DW under Zn50, while Si treatment caused up to 23 % reduction in Zn uptake.
Cotton roots accumulated maximum Zn concentration compared with leaves and stem. Under Zn50 treatment, Zn concentrations in root tissues reached as high as 3600 mg kg−1 DW that was approximately 20 times higher compared with control. Zn uptake under Zn25 concentration was 2177 mg kg−1 DW that was significantly higher compared with control plants and significantly lowers than the Zn50 treatment. Addition of Si significantly decreased metal accumulation in cotton root tissues under various Zn levels, i.e., 30 and 48 % reduction in Zn50 and Zn25 treatments, respectively.
Discussion
This study investigated the Zn toxicity effects on morphological, physiological, and biochemical properties of cotton plants and role of Si in alleviating Zn-induced damage. Zn toxicity negatively influenced the various plant growth parameters such as fresh and dry biomass and length of root, stem, and leaves suggesting that toxic levels of Zn in the environments could potentially limit the cotton yield. Previous studies suggested growth and yield reduction of various crop species under Zn toxicity (Morina et al. 2010; Kaya et al. 2009). Cotton growth reduction observed in the present study could be the result of Zn-induced oxidative damage to membranes which are caused by upregulation of H2O2 and MDA levels under Zn toxicity. Zinc toxicity-induced lipid membrane peroxidation impaired plant biomass producing machinery (chlorophyll pigments) and photosynthetic process leading towards growth inhibition. Reduction in leaf chlorophyll contents could be associated with peroxidation of chloroplast membranes and inhibited chlorophyll biosynthesis and protochlorophyllide reductase activity through Zn-induced activation of chlorophyll degrading species chlorophyllase (Drazkiewice 1994; Rao et al. 2007).
Increasing Zn concentrations in growth media significantly increased Zn concentrations in all plant tissues (root, stem, and leaf) which results in decreasing of plant biomass, chlorophyll content, and gas exchange characteristics along with antioxidant enzymes activities. In addition, elevated Zn concentration in growth media competed with the uptake of micro and macro nutrients and the damage was exacerbated by root growth inhibition. This idea was supported by Cuypers et al. (2001), who observed Zn toxicity evoked visible damage and physiological disorders in common bean (Phaseolus vulgaris L.) by disturbing cell division.
In response to elevated levels of tissue Zn concentrations and oxidative stress, cotton plants exhibited modification in anti-oxidant enzyme activity. Environmental adverse effects such as metal stresses usually leads to unbalanced hyper generation of ROS while antioxidant enzymes are known to have important roles in plant stress tolerance (Mittler et al. 2004). Upregulation of SOD and POD activity under lower Zn concentration, i.e., Zn25 and reduction under Zn50 suggested limited capacity of cotton to defy Zn toxicity-induced oxidative burst.
Modifications in activities of antioxidative enzymes are related to Zn toxicity that causes redox imbalances (Morin et al. 2010). Superoxide dismutase principally plays an important role in ROS detoxification by catalyzing of free O2 − to O2 and H2O2 in (Bonnet et al. 2000). Increased SOD activities in both root and leaf tissues were recorded in (plant name) under Zn toxicity (Morin et al. 2010), while increased POD activity was discussed by Luo et al. (2010) in jatropha. The enzymes act as scavengers of H2O2 and other ROS and are actively involved in the biosynthesis of lignin (Gonzalez et al. 1999). Degenhardt and Gimmler (2000) and Küpper et al. (2000) also discovered that Zn prefers to accumulate at root parts; in cell walls, of the xylem parenchyma and in endodermal layer more than any other part of plant. Morina et al. (2010) referred Zn toxicity-induced oxidative stress is due to high affinity of Zn to root cell walls in Verbascum thapsus L. plants.
Significantly higher Zn concentrations in roots compared with leaf tissues suggested cotton roots restrict Zn mobility, it was also evident from little or no change in Zn translocation from roots to leaves by raising Zn concentrations from 25 to 50 μM in the growth media which leads to release of electrolytes in root and leaf tissues. However, increase in Zn contents in cotton root tissues to toxic level (20-fold) under higher Zn levels inhibited plant growth. Although Zn is an essential micronutrient for plant growth and physiological processes, its concentration above a certain limit could impair physiological function.
Zinc-induced significant increase in leaf chlorophyll and gas exchange parameters of Zn-stressed plants improved biomass accumulation process. Previous studies suggested effectiveness of Si in plant against biotic and a-biotic stresses (Gurmani et al. 2013; Currie and Perry 2007; Liang et al. 2007). It is likely that the high plant biomass in Si-treated soil provoked a dilution effect on Zn concentration, whereby minimizing Zn-induced damages to plants. Positive effect of Si on Zn-stressed maize and tobacco plants were result of increased Fe concentration in leaf tissues stimulating chlorophyll synthesis (Szlek et al. 1990; Miller et al. 1995; Pei et al. 2010).
In the present study, Si greatly reduced Zn accumulation in cotton suggesting its role in reducing Zn bioavailability by changing the solution pH (Chen et al. 2000; Liang et al. 2005, 2007). Siliconmediated detrimental effects of Zn toxicity on cotton by limiting Zn uptake and translocation and thus the cellular oxidative damage. Under lower Zn levels, Si was relatively more effective in controlling Zn uptake possibly through reducing Zn bioavailability and exclusion from root tissues, as was evident from 48 % reduction in root Zn concentration. However, Si induced a parallel reduction in root and leaf Zn under Zn50 suggested Si was effective to suppress Zn bioavailability up to certain levels, and higher Zn influx into root tissues could be expected under increased Zn concentration. Inside the plant cells, Si improves tolerance to Zn toxicity by forming Zn-silicate in cytoplasm (Neumann and Nieden 2001; Gong et al. 2005). Silicon-induced inhibited Zn translocation to cotton shoots was result of Si deposition on cell wall forming a barrier to apoplastic route (Shi et al. 2005; Gong et al. 2008; Farid et al. 2013b). In addition to limiting Zn uptake and translocation, Si upregulated the activities of anti-oxidant enzymes (SOD and POD) assisting plant to defy Zn-induced oxidative damage.
Conclusion
In present study, Si application significantly reduced Zn uptake and toxicity to cotton and improved plant growth, biomass, chlorophyll content, photosynthetic parameters, and gas exchange attributes. Since higher translocation of metals to the aboveground plant parts invokes the risk of metal entry into food chain. Si-induced reduced metal bioavailability and uptake, and its translocation suggests its potential use for plants, especially vegetable crops growing under metal-polluted soils or irrigated with contaminated waters.
References
Abbas G, Khan MQ, Jamil M, Tahir M, Hussain F (2009) Nutrient uptake, growth and yield of wheat (Triticum aestivum) as affected by zinc application rates. Int J Agric Biol 11:389–396
Adriano D (2001) Trace elements in terrestrial environments: biogeochemistry, bioavailability, and risks of metals. Springer Verlag, New York
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Akhtar KP, Aslam M, Haq MA, Jamil FF, Khan AI, Elahi MT (2005) Plant pathology and nematology. J Cott Sci 9:175–181
Ali S, Farooq MA, Jahangir MM, Abbas F, Bharwana SA, Zhang GP (2013a) Effect of chromium and nitrogen form on photosynthesis and anti-oxidative system in barley. Biol Plant 57:785–791
Ali S, Farooq MA, Yasmeen T, Hussain S, Arif MS, Abbas F, Bharwana SA, Zhang GP (2013b) The influence of silicon on barley growth, photosynthesis and ultra-structure under chromium stress. Ecotoxicol Environ Saf 89:66–72
Basta NT, Gradwohl R, Snethen KL, Schroder JL (2001) Chemical immobilization of lead, zinc and cadmium in smelter-contaminated soils using bio-solids and rock phosphate. J Environ Qual 30:1222–1230
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44(1):276–287
Bonnet M, Camares O, Veisseire P (2000) Effects of zinc and influence of Acremonium lolii on growth parameters, chlorophyll fluorescence and antioxidant enzyme activities of ryegrass (Lolium perenne L. cv Apollo). J Exp Bot 51:945–953
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1):248–254
Broadley MR, White PJ, Hammond JP, Zelko I, Lux A (2007) Zinc in plant. New Phytol 173:677–702
Cakmak I (2000) Tansley review no. 111. Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol 146:185–205
Chen HM, Zheng CR, Tu C, She ZG (2000) Chemical methods and phytoremediation of soil contaminated with heavy metals. Chemosphere 41:229–234
Cocker KM, Evans DE, Hodson MJ (1998) The amelioration of aluminum toxicity by silicon in higher plants: solution chemistry or an in planta mechanism. Physiol Planta 104:608–614
Currie HA, Perry C (2007) Silica in plants: biological, biochemical and chemical studies. Ann Bot 23:1–7
Cuypers A, Vangronsveld J, Clijsters H (1999) The chemical behavior of heavy metals plays a prominent role in the induction of oxidative stress. Free Radic Res 31:839–843
Cuypers A, Vangronsveld J, Clijsters H (2001) The redox status of the plant cells (AsA and GSH) is sensitive to zinc imposed oxidative stress in roots and primary leaves of Phaseolus vulgaris. Plant Physiol Biochem 39:657–664
Degenhardt B, Gimmler H (2000) Cell wall adaptations to multiple environmental stresses in maize roots. J Exp Bot 51:595–603
Dembitsky VM, Rezanka T (2003) Natural occurrence of arseno compounds in plants, lichens, fungi, algal species, and microorganisms. Plant Sci 165:1177–1192
Dhindsa RS, Dhindsa PP, Thorpe TA (1981) Leaf senescence, correlated with increased level of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Dionisio-Sese ML, Tobita S, (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9
Drazkiewice M (1994) Chlorophyll-occurrence, function and mechanism of action. Environ Exp Bot 42:1–10
Ehsan S, Ali S, Noureen S, Farid M, Shakoor MB, Aslam A, Bharwana SA, Tauqeer HM (2013) Comparative assessment of different heavy metals in urban soil and vegetables irrigated with sewage/industrial waste water. Ecoterra 35:37–53
Ehsan S, Ali S, Noureen S, Mehmood K, Farid M, Ishaque W, Shakoor MB, Rizwan M (2014) Citric acid assisted phytoremediation of Cd by Brassica napus L. Ecotoxicol Environ Saf 106:164–172
Epstein E (1994) The anomaly of silicon in plant biology. Proc Nat Acad Sci USA 91:11–17
Epstein E (1999) Silicon. Annu Rev Plant Physiol Mol Biol 50:641–664
Falkengren-Grerup U, Linnermark N, Tyler G (1987) Chemosphere 16:2239
Farid M, Ali S, Shakoor MB, Bharwana SA, Rizvi H, Ehsan S, Tauqeer HM, Iftikhar U, Hannan F (2013a) EDTA assisted phytoremediation of cadmium, lead and zinc. Int J Agron Plant Prod 4(11):2833–2846
Farid M, Shakoor MB, Ehsan S, Ali S, Zubair M, Hanif MS (2013b) Morpho- logical, physiological and biochemical responses of different plant species to Cd stress. Int J Chem Biochem Sci 3:53–60
Gong H, Zhu X, Chen K, Wang S, Zhang C (2005) Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Sci 169:313–321
Gong HJ, Randall DP, Flowers TJ (2006) Silicon deposition in root reduces sodium uptake in rice (Oryza sativa L.) seedlings by reducing bypass flow. Plant Cell Environ 29:1970–1979
Gong HJ, Chen KM, Zhao ZG, Chen GC, Zhou WJ (2008) Effects of silicon on defense of wheat against oxidative stress under drought at different developmental stages. Biol Plant 52:592–596
Gonzalez LF, Rojas MC, Perez FJ (1999) Diferulate and lignin formation is related to biochemical differences of wall-bound peroxidases. Phytochem 50:711–717
Gurmani AR, Bano A, Najeeb U, Zhang JL, Khan SU, Flowers TJ (2013) Exogenously applied silicate and abscisic acid ameliorate the growth of salinity stressed wheat (Triticum aestivum L) seedlings through Na+ exclusion. Aus J Crop Sci 7(8):1123–1130
Han FX, Banin A, Su Y, Monts DL, Plodinec MJ, Kingery WL, Triplett GE (2002) Industrial age anthropogenic inputs of heavy metals into the pedosphere. Naturwissenschaften 89:497–504
Hattori T, Inanaga S, Araki H, An P, Morita S, Luxov AM, Lux A (2005) Application of silicon enhanced drought tolerance in Sorghum bicolor. Physiol Plant 123:459–466
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125(1):189–198
Kaya C, Tuna AL, Sonmez O, Ince F, Higgs D (2009) Mitigation effects of silicon on maize plants grown at high zinc. J Plant Nutr 32:1788–1798
Küpper H, Lombi E, Zhao FJ, McGrath SP (2000) Cellular compartmentation of cadmium and zinc in relation to other elements in the hyperaccumulator Arabidopsis halleri. Planta 212:75–84
Liang Y, Si J, R¨Omheld V (2005) Silicon uptake and transport is an active process in Cucumis sativus. New Phytol 167:797–804
Liang Y, Sun W, Zhu YG, Christie P (2007) Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: a review. Environ Pollut 147:422–428
Luo YM, Christie P, Baker AJM (2000) Soil solution Zn and pH dynamics in non- rhizosphere soil and in the rhizosphere of Thlaspi caerulescens grown in a Zn/Cd contaminated soil. Chemosphere 41:161–164
Luo ZB, He XJ, Chen L, Tang S, Chen F (2010) Effects of zinc on growth and antioxidant responses in Jatropha curcas seedlings. Int J Agric Biol 12:119–124
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic, London, pp 411–430
Miller GW, Huang IJ, Welkie GW, Pushnik JC (1995) Function of iron in plants with special emphasis on chloroplasts and photosynthetic activity. In Iron Nutrition in Soils and Plants. Springer Netherlands, pp 19–28
Ming DF, Pei ZF, Naeem MS, Gong HJ, Zhou WJ (2012) Silicon alleviates PEG-induced water-deficit stress in upland rice seedlings by enhancing osmotic adjustment. J Agron Crop Sci 198:14–26
Mittler R, Vanderauwera S, Gollery M, Breusegem FB (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498
Morina F, Jovanovic LJ, Mojovic M, Vidovic M, Pankovic D, Veljovic-Jovanovic S (2010) Zinc-induced oxidative stress in Verbascum thapsus is caused by an accumulation of reactive oxygen species and quinhydrone in the cell wall. Physiol Plant 140:209–224
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate- specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Neumann D, Nieden UZ (2001) Silicon and heavy metal tolerance of higher plants. Phytochem 56:685–692
Pei ZF, Ming DF, Liu D, Wan GL, Geng XX, Gong HJ, Zhou WJ (2010) Silicon improves the tolerance to water-deficit stress induced by polyethylene glycol in wheat (Triticum aestivum L.) seedlings. J Plant Growth Regul 29:106–115
Putter J, Becker R (1974) Methods of enzymatic analysis. Academic Press, Chicago
Rao AR, Dayananda C, Sarada R, Shamala TR, Ravishankar GA (2007) Effect of salinity on growth of green alga Botryococcus braunii and its constituents. Bioresour Technol 98:560–564
Rogalla H, Romheld V (2002) Role of leaf apoplast in silicon-mediated manganese tolerance of Cucumic sativus L. Plant Cell Environ 25:549–555
Shi XH, Zhang CC, Wang H (2005) Effect of Si on the distribution of Cd in rice seedlings. Plant Soil 272:53–60
Szlek M, Miller GW, Welkie GW (1990) Potassium effect of iron stress in tomato plants. 1. The effect on pH, Fe-reductase and chlorophyll. J Plant Nutr 13:215–219
Tewari RK, Kumar P, Sharma PN (2008) Morphology and physiology of zinc-stressed mulberry plants. J Plant Nutr Soil Sci 171:286–294
Zhang J, Kirkham MB (1994) Drought-stress-induced changes in activities of superoxide dismutase, catalase, and peroxidase in wheat species. Plant Cell Physiol 35(5):785–791
Zhao FJ, Lombi E, Mcgrath SP (2003) Assessing the potential for zinc and cadmium phytoremediation with the hyperaccumulator Thlaspi caerulescens. Plant Soil 249:37–43
Acknowledgments
The authors thank the Higher Education Commission of Pakistan for the financial support. The results presented in this paper are a part of M. Phil studies of Shad Ali Anwaar.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Anwaar, S.A., Ali, S., Ali, S. et al. Silicon (Si) alleviates cotton (Gossypium hirsutum L.) from zinc (Zn) toxicity stress by limiting Zn uptake and oxidative damage. Environ Sci Pollut Res 22, 3441–3450 (2015). https://doi.org/10.1007/s11356-014-3938-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3938-9