Abstract
The present study was conducted to assess the impact of gibberellic acid on growth and yield of sunflower in hexavalent chromium [Cr(VI)]-contaminated soil in the presence as well as absence of pressmud. Seeds of sunflower were sown in potted soil amended with pressmud as an organic amendment and contaminated with different levels of Cr(VI) (12, 18, and 24 mg kg−1) by using K2Cr2O7 salt. Gibberellic acid (10−4 M) was applied at time of seedling emergence in the rhizosphere. The results showed that Cr(VI) stress significantly reduced the growth and yield of sunflower. However, application of gibberellic acid and pressmud reversed the toxic effects of Cr(VI) and improved the growth and yield of sunflower. Combined application of gibberellic acid and pressmud further improved growth and yield compared to their separate application in Cr(VI) stress. Moreover, gibberellic acid and pressmud decreased the uptake of Cr and stabilized it in the soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chromium (Cr) is one of the toxic metals widely used in leather tanning, alloy preparation, electroplating, drilling mud, refractory steel, and catalytic manufacture (Ozdemir et al. 2005). It exists in environment in various valence forms (Cr−2, Cr−1, Cr0, Cr+1, Cr+2, Cr+3, Cr+4, Cr+5, and Cr+6), but the most stable forms are Cr+6 and Cr+3. Chromium toxicity to life forms is highly dependent on its oxidation states, i.e., Cr(VI) is more soluble and toxic as compared to Cr(III) which is less toxic and less soluble (Boonyapookana et al. 2002). Extensive use of Cr compounds in metal plating, hide tanning, wood preservation, and water treatment is continuously deteriorating the quality of environment (Nriagu 1988). According to Agency for Toxic Substance and Disease Registry, Cr(VI) is genotoxic and carcinogenic for humans (ATSDR 2000). Besides its toxicity to humans, Cr(VI) also disturbs the soil ecology and plant growth due to its toxic nature even at low concentration (John et al. 2009). The phytotoxicity of Cr(VI) hampers the plant growth by reducing seed germination, decreasing growth of root and shoot, and reducing total production of dry matter (Andaleeb et al. 2008). This impaired growth and development of plant might be due to disruption of normal physiological processes, i.e., photosynthesis, water relation, mineral nutrition, and enzymatic activity by Cr(VI) (Shanker et al. 2005). Due to its highly oxidizing nature, Cr(VI) crosses the plasma membrane and produces reactive oxygen species such as hydroxyl radicals (OH−), superoxide radicals (O2 −), and hydrogen peroxide (H2O2) radicals which oxidize protein, lipids, and nucleic acid (Shanker et al. 2005). Growth of sunflower is also affected by toxicity of Cr(VI), due to reduced net photosynthetic rate attributable to decreased chlorophyll contents and impaired transpiration, which might be due to decreased stomatal conductance which results in reduced seed germination, seedling growth, root and shoot length, and yield (Andaleeb et al. 2008). Metal stress alters the normal balance of phytohormones and decreases the plant growth (Gangwar et al. 2011a). Phytohormones (plant growth regulators) are low-molecular-weight compounds which act as chemical messengers for regulation of normal plant physiological processes and promote plant growth, enhance plant resistance against biotic and abiotic stresses, and have the ability to minimize the toxic effect of heavy metals on plants (Halter et al. 2005). They are keys for plants to respond an ever-changing environment and external stimuli and mediate plant growth in their specific synergistic and/or antagonistic cross-talk (Curaba et al. 2014). In response to abiotic stresses, biosynthesis of abscisic acid (ABA) increases while the level of cytokinin (CK) and gibberellins (GA) decreases (Peleg and Blumwald 2011). However, exogenous application of phytohormones can modulate the toxicity of Cr(VI) on plants most probably by maintaining hormonal balance of plants under metal stress (Rademacher 1990; Gangwar et al. 2011a, b). Previously, auxins (AUX) and CKs have been reported for their positive role in plant growth promotion under heavy metal stress (Gangwar and Singh 2011; Gangwar et al. 2011a; Ali et al. 2013; Farooq et al. 2015). GAs are the well-known physiological important phytohormones which increase the height of plant, and fresh and dry weights of root, shoot, and leaves in heavy metal stress (El-Shourbagy et al. 1990). Gibberellic acid (GA) improves the growth of plant in Cr stress by improving the antioxidants levels and sustaining the enzymatic activities for nitrogen assimilation thereby counteracting the phytotoxic effects of Cr(VI) (Gangwar et al. 2011b). Therefore, exogenous application of GA may regulate the internal hormonal balance of plants in Cr stressed conditions and could improve growth and yield of sunflower. Moreover, organic amendments can also be effectively used for reduction of higher valence Cr(VI) to immobile Cr(III) forms and thereby decreasing its mobilizations (Bolan and Duraisamy 2003; Eary and Rai 1991; Sharma and Forster 1993; Anderson et al. 1994; Kozuh et al. 2000; Bolan et al. 2003). Some organic amendments like sugarcane dregs compost, soybean meal, rice bran, biosolid compost, farm yard manure, fish manure, horse manure, spent mushroom, pig manure, and poultry manure have been reported for reduction of Cr(VI) and subsequent immobilization of Cr in soil (Bolan et al. 2003; Chiu et al. 2009). Organic amendments improve the organic matter status of heavy metal contaminated soils which buffers the soil pH and reduces the uptake of heavy metals by plants (Park et al. 2011). These amendments provide carboxyl, phenoxyl, and hydroxyl groups, which play important role in complexation and adsorption of heavy metals and their activities in the soil (Lee et al. 2004; Mahmood 2010). Pressmud (PrM) is a rich source of organic matter which improves the growth and yield of plants and reduces the uptake of heavy metals in plants (Sabir et al. 2013). However, if organic amendments are supplemented with exogenously applied phytohormones like gibberellic acid, growth of plant may be improved positively as compared to their separate use under Cr(VI) contamination. Keeping in view the possible role of GA and pressmud as an organic amendment to improve the growth of plants in soil contaminated with Cr(VI) and subsequent stabilization of Cr in soil due to its transformation from Cr(VI) to Cr(III), the present study was conducted to evaluate separate and combined effects of GA and pressmud on growth and yield of sunflower in Cr(VI)-contaminated soil.
Materials and methods
A pot experiment under ambient condition was conducted in the wire house to evaluate the separate and combined effects of GA and PrM on growth and yield of sunflower in a soil contaminated with Cr(VI). The earthen pots lined with polyethylene bags were filled with 10 kg of homogeneously mixed, air-dried soil with sandy clay loam texture having EC 1.41 dS m−1, pH 7.5, saturation percentage 37.5 %, organic matter 0.60 %, available phosphorous 7.30 mg kg−1, and extractable potassium 129 mg kg−1 while Cr(VI) was not detectable in that soil. Prior to filling pot, this soil was contaminated by using K2Cr2O7 as source of Cr(VI) and finally, three levels of Cr(VI) were maintained that were 12, 18, and 24 mg kg−1. The soil was allowed to equilibrate for 2 weeks after contamination with Cr(VI). GA and PrM were applied separately as well as in combination, and in order to segregate the effect of Cr(VI) contamination, one control treatment was maintained where neither contamination nor GA and PrM were applied. PrM was added as an organic amendment at the rate of 2.5 % organic matter on dry weight basis and mixed thoroughly with soil. PrM used in experiment had different physicochemical characteristics like organic matter 12 %, pH 6.4, EC 3.1 dS m−1, total phosphorous 0.8 %, and total potassium 0.9 %. Ten seeds of sunflower were sown in each pot, and after 20 days of germination, the plants were thinned to maintain three seedlings in each pot. GA was also applied at the rate of 10−4 M per pot at emergence stage in the roots of plants. The plants were irrigated with tap water when required. The experiment was conducted by following completely randomized design (CRD) with three replications. Plants at physiological maturity were harvested, and data regarding growth and yield parameters were recorded. After harvesting, the plants were separated into different parts, i.e., roots, stems, and achenes. These samples of plant were oven-dried at 80 °C till constant weight. These plant samples were ashed in muffle furnace at 600 °C for 6 h. The ashed samples were dissolved into a mixture of 2 M HCl and 1 M HNO3 and filtered, and final volume was made up to 50 mL. For determination of total chromium, this filtrate was run on atomic absorption spectrophotometer (Perkin Elmer AAnalyst 100, USA) and for Cr(VI) determination, filtrate was treated with 1,5-diphenylcarbazide as color developing agent. Purple color was obtained after 15 min due to formation of complex by Cr(VI) in the presence of 1,5-diphenylcarbazide. The absorbance was measured at 540 nm by using spectrophotometer (Nicolet Evolution 300, Thermo Electron Corporation, England) (Gheju et al. 2009; Khan et al. 2013; Maqbool et al. 2014). Data was analyzed statistically by using computer-based statistical software Statistix-8.1 (Analytical Software, Tallahassee, USA). Means were compared by applying Duncan’s new multiple range test (DMRT) (Steel and Torrie 1984).
Results and discussion
Chromium (Cr) is widespread toxic metal in the environment that can cause detrimental impacts on plants, animals, humans, as well as agricultural soils. Chromium in the form of Cr(VI) creates many nutritional and physiological disorders in plants. The study showed that Cr(VI) hampered the growth of sunflower and caused a significant decrease in length and fresh and dry weights of shoot and root at various levels (12, 18, and 24 mg kg−1) of Cr(VI) contamination as compared to noncontaminated control. However, at 24 mg kg−1 of Cr(VI) contamination, more severity of Cr(VI) toxicity was observed and sunflower growth was significantly decreased as compared to Cr(VI) contamination of 12 and 18 mg kg−1. The length and fresh and dry weights of shoot and root were significantly decreased up to 57, 57, 60, 63, 58, and 60 %, respectively, in soil contaminated with 24 mg kg−1 of Cr(VI) as compared to control (where no Cr(VI), PrM and GA were applied; Tables 1 and 2). Hexavalent chromium is known to be phytotoxic due to high electronegativity which makes it permeable to cellular membranes by the aid of essential ion carriers such as sulfate (Kaszycki et al. 2005), leading to cellular damage due to formation of various reactive intermediates which destroy cellular organelles, protein, and nucleic acid (Kaszycki et al. 2005). Hexavalent chromium also affects growth of plant indirectly because of impaired photosynthetic apparatus and mitochondrial electron transport chain due to oxidative damage by production of reactive oxygen species (ROS) (Vernay et al. 2007; Pandey et al. 2009). Hindrance in sunflower growth and development by Cr(VI) might be attributed to decreased stomatal conductance, chlorophyll contents, photosynthetic activity, and transpiration rates (Andaleeb et al. 2008; Ali et al. 2011). Moreover, Cr(VI) interferes in uptake and translocation of essential plant nutrients like P, Mn, Fe, Cu, and Zn (Liu et al. 2008). Therefore, reduction in growth of sunflower might be attributed to deficiency of these essential nutrients (Khan et al. 2001). Hexavalent chromium induces plasmolysis, affects leaf water potential badly, and thereby hampers the growth of plant (Vernay et al. 2007). Decreased root growth is much likely due to impairment in root functioning and physiology by Cr(VI) toxicity as reported by Terry and Banuelos (2000). The reduction in growth and yield of sunflower in a soil contaminated with Cr(VI) could possibly be due to one or more abovementioned factors.
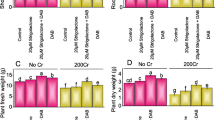
However, growth of sunflower was improved significantly due to application of GA as well as PrM at all levels of contamination. Moreover, the improvement in growth of sunflower was more prominent by the combined use of GA and PrM at all levels of contamination (12, 18, and 24 mg kg−1) of Cr(VI) as compared to their sole application. Sole application of GA and PrM significantly improved the length and fresh and dry weights of shoot and root of sunflower at all levels of contamination (12, 18, and 24 mg kg−1) of Cr(VI). Although the effect of sole application of both GA and PrM was statistically nonsignificant with each other for improving growth and yield of sunflower at all levels (12, 18, and 24 mg kg−1) of Cr(VI) contamination (Tables 1 and 2). However, phytotoxicity of Cr(VI) was further minimized due to combined application of GA and PrM and significant improvement in growth of sunflower was observed as compared to their sole use at all levels of contamination. At 24 mg kg−1 of Cr(VI) contamination, GA decreased the toxic effect of Cr(VI) and significantly improved the length and fresh and dry weights of shoot and root up to 29, 28, 44, 38, 49, and 42 %, respectively, at 24 mg kg−1 of Cr(VI) contamination as compared to same level of contamination alone. The results showed that application of PrM also improved the length and fresh and dry weights of shoot and root up to 29, 29, 51, 40, 55, and 45 %, respectively, at 24 mg kg−1 of Cr(VI) contamination at same level of Cr(VI) alone. No doubt, sole application of GA as well as PrM significantly improved the growth of sunflower at 24 mg kg−1 of Cr(VI) contamination level but effect of their combined application was more pronounced for improving its growth. Combined application of GA and PrM resulted in an increase in the length and fresh and dry weight of shoot and root up to 40, 35, 81, 67, 67, and 67 %, respectively, at 24 mg kg−1 of Cr(VI) contamination as compared to plants grown at 24 mg kg−1 of Cr(VI) alone (Tables 1 and 2). Of course, combined application of GA and PrM significantly enhanced the plant growth in all Cr(VI) contamination levels but the stress recovery was always less than 100 %. The results regarding yield of sunflower in Cr(VI) contamination presented in Fig. 1 clearly indicated that 12, 18, and 24 mg kg−1 of Cr(VI) contamination had variable but significant negative effect on the yield of sunflower. Yield of sunflower was decreased significantly up to 27, 33, and 42 % at 12, 18, and 24 mg kg−1 of Cr(VI) contamination levels, respectively, as compared to plant without metal stress. However, application of GA minimized the growth hampering effect of Cr(VI) and significantly increased yield up to 15, 17, and 12 % at 12, 18, and 24 mg kg−1 of Cr(VI), respectively, as compared to plants grown at contamination of 12, 18, and 24 mg kg−1 alone, respectively. PrM application also significantly increased the yield of sunflower up to 17, 20, and 13 % at 12, 18, and 24 mg kg−1 of Cr(VI) contamination, respectively, as compared to plants grown at 12, 18, and 24 mg kg−1 alone, respectively. Comparatively, the results of combined application of GA and PrM were better as compared to sole application of GA and PrM at all levels of Cr(VI) contamination. The combined application of GA and PrM increased the yield up to 27, 33, and 23 % at 12, 18, and 24 mg kg−1 of Cr(VI) contamination, respectively, as compared to plants grown at contamination of 12, 18, and 24 mg kg−1 alone, respectively.
The improvement in the growth and yield attributes of plants in Cr(VI) contamination by GA application might be correlated with various functions of GA in plants as it increases total protein content, total nitrogen content, nitrate reductase activity, ammonium assimilation, glutathione reductase and dehydroascarbate activities. Glutathione reductase and dehydroascarbate enhances glutathione and ascorbate (Gangwar et al. 2011b). Glutathione and ascorbate are antioxidants that play important role in scavenging of reactive oxygen species and prevent oxidative stress in plants (Noctor and Foyer 1998). Moreover, GA increases root biomass, shoot biomass, photosynthesis, water uptake, chlorophyll content, nutrient uptake, and root length (Gangwar et al. 2011b). The abiotic stresses cause perturbation in the normal hormonal balance of plants (Iqbal and Ashraf 2013), and most probably, application of GA improved the hormonal balance of sunflower in Cr(VI) contamination which ultimately might have been resulted in improved growth and yield. Moreover, increase in growth and yield of sunflower in Cr(VI) contamination due to application of PrM might be result of decreased phytotoxic effects of Cr(VI) because PrM is a rich source of OM which provides the electrons for reduction of Cr(VI) into Cr(III) that is less toxic and immobile in soil (Bolan et al. 2003; Hsu et al. 2009; Chiu et al. 2009). Furthermore, PrM also supplies nutrients that promote the growth of plants in stress conditions (Sabir et al. 2013) and it is much likely that mineralization of PrM might provide the additional nutrients, increase the nutrient cycling, and enhance the buffering capacity of soil (Stewart et al. 2000; Clemente et al. 2007) and thus increase the growth and yield of plant in Cr(VI) contamination. While, better plant growth and yield due to combined use of GA and PrM in Cr(VI) contamination might also be due to cumulative effect of both GA and PrM. PrM might have increased the efficiency of GA by minimizing the uptake of chromium, increasing the nutrient and water uptake, thereby facilitating the plants with better nutrition under stress condition (Sabir et al. 2013; Singh et al. 2007).
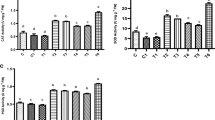
The results revealed that concentration of Cr(VI) and total Cr in different tissues of sunflower were significantly increased by increasing concentration of Cr(VI) in soil from 12 to 24 mg kg−1. However, application of GA and PrM alone as well as in their combination significantly decreased the total Cr and Cr(VI) contents in root, shoot, and achenes of sunflower at all Cr(VI) contamination levels. Maximum total Cr and Cr(VI) contents in root, shoot, and achenes were recorded at 24 mg kg−1 of Cr(VI) contamination level. GA significantly decreased the Cr(VI) contents in root, shoot, and achene by 10, 17, and 93 % at 24 mg kg−1 of Cr(VI) contamination level, respectively, as compared to plants grown at same level of Cr (VI) alone without any amendment. Application of PrM decreased the Cr(VI) contents up to 48, 42 and 47 % in root, shoot and achene at 24 mg kg-1 of Cr(VI) stress respectively, as compared to 24 mg kg−1 of Cr(VI) contamination alone. However, combined application of GA and PrM was more effective for decreasing Cr(VI) contents in all parts of plant as compared to their separate use (Tables 3 and 4). Cr(VI) was decreased up to 53, 55, and 53 % in root, shoot, and achene, respectively, by combined application of GA and PrM at 24 mg kg−1 of Cr(VI) contamination as compared to plants grown at same level of contamination alone. As far as total Cr is concerned, GA significantly decreased the total Cr contents in root, shoot, and achene up to 17, 12, and 23 % at 24 mg kg−1 of Cr(VI), respectively, as compared to same level of contamination alone. PrM decreased the total Cr contents up to 43, 40, and 47 % in root, shoot, and achene at 24 mg kg−1 of Cr(VI) contamination as compared to 24 mg kg−1 of Cr(VI) stress alone. However, combined use of GA and PrM further decreased the total Cr contents in root, shoot, and achene up to 54, 54, and 50 % at 24 mg kg−1 Cr(VI) as compared to plants grown at same level of contamination alone. In our study, application of GA decreased the concentration of total Cr and Cr (VI) in plant tissue, i.e., root, shoot, and achene. This decrease in uptake of Cr(VI) might be due to involvement of GA in membrane transport processes, as GA alters membrane permeability (Erdei et al. 1989). Therefore, it might decrease the Cr(VI) uptake. GA also increases the biomass of plants. Due to increase in biomass of plants, more organic acids are released by plants. These organic acids form complexes with Cr(VI) and make it unavailable to plants. It might be possible that GA involved indirectly in enhancing activity of rhizospheric microorganisms due more release of root exudates which might ultimately result in conversion of Cr(VI) to Cr(III) by microbial transformation (Khan et al. 2013). Decrease in concentration of chromium in root, shoot, and achene of sunflower in chromium-contaminated soil by application of PrM might be due to the formation of chelate with chromium and reduction of the Cr(VI) into Cr(III) (Farrell et al. 2010; Sabir et al. 2013). PrM (organic matter) immobilizes chromium by the formation of chromium–organic matter complexes (Bolan and Duraisamy 2003). PrM also plays a pivotal role, especially in the reduction of chromium by providing a source of electron donor and carbon substrate for microorganisms (Bolan et al. 2003; Hsu et al. 2009; Chiu et al. 2009). More decrease in concentration of chromium in root, shoot, and achene by combined application of GA and PrM as compared to individual application of GA and PrM might be due to synergistic effect of both (Sabir et al. 2013; Gangwar et al. 2011b). This indicated that if GA is applied in the presence of PrM, the efficiency of stabilization may be increased.
Conclusions
It might be concluded from the study that contamination of Cr(VI) negatively affected the growth and yield of sunflower. However, sole application of GA and PrM reversed the toxic effects of Cr(VI) stress and improved the growth and yield of sunflower plants. Combined application of GA and PrM was more effective to mitigate negative effect and improved more growth and yield as compared to their individual application in a Cr(VI)-contaminated soil. The study also revealed that application of GA and PrM either separately or in combination was unable to completely reverse the toxic effect of contamination.
References
Ali S, Zeng F, Cai S, Qiu B, Zhang G (2011) The interaction of salinity and chromium in the influence of barley growth and oxidative stress. Plant Soil Environ 57:153–159
Ali T, Mahmood S, Khan MY, Aslam A, Hussain MB, Asghar HN, Akhtar MJ (2013) Phytoremedaition of cadmium contaminated soil by auxin assisted bacterial inoculation. Asian J Agric Biol 1:79–84
Andaleeb F, Zia MA, Ashraf M, Khalid ZM (2008) Effect of chromium on growth attributes in sunflower (Helianthus annuus L). J Environ Sci 20:1475–1480
Anderson AJ, Kent DB, Davis JA (1994) Batch experiments characterizing the reduction of Cr(VI) using sub toxic material from a mildly reducing sand and gravel aquifer. Environ Sci Technol 28:175–185
ATSDR (2000) Toxicological profile for chromium. Agency for toxic substances and disease registry. http://www.atsdr.cdc.gov/toprofiles/tp7.html
Bolan NS, Duraisamy VP (2003) Role of inorganic and organic soil amendments on immobilisation and phytoavailability of heavy metals: a review involving specific case studies. Aust J Soil Res 41:533–556
Bolan NS, Adriano DC, Natesan R (2003) Effects of organic amendments on the reduction and phytoavailability of chromate in mineral soil. J Environ Qual 32:120–128
Boonyapookana B, Upatham ES, Kruatrachue M, Pokethitiyook P, Singhakaew S (2002) Phytoaccumulation and phytotoxicity of cadmium and chromium in duckweed Wolffia globosa. Int J Phytoremediat 4:87–100
Chiu CC, Cheng CJ, Lin TH, Juang KW, Lee DY (2009) The effectiveness of four organic matter amendments for decreasing resin-extractable Cr (VI) in Cr (VI)-contaminated soils. J Hazard Mater 161:1239–1244
Clemente R, Paredes C, Bernal MP (2007) A field experiment investigating the effects of olive husk and cow manure on heavy metal availability in contaminated calcareous soils from Mucia (Spain). Agri Ecosyst Environ 118:319–326
Curaba J, Singh MB, Bhalla PL (2014) miRNAs in the crosstalk between phytohormone signaling pathways. J Exp Bot 65:1425–1438
Eary LE, Rai D (1991) Chromate reduction by subsurface soils under acidic conditions. Soil Sci Soc Am J 55:676–683
El-Shourbagy MN, Ghaffar BA, El-Naggar RA (1990) Effect of IAA and GA3 on growth and mineral element contents of flax (Linum usitatissimum L.). Egypt J Bot 33:269–282
Erdei L, Msller I, Jensen P (1989) The effects of energy supply and growth regulators on K+ uptake into plant roots. Biochem Physiol Pllanz 184:345–361
Farooq H, Asghar HN, Khan MY, Saleem M, Zahir ZA (2015) Auxin mediated growth of rice in cadmium contaminated soil. Turk J Agric For 39. doi:10.3906/tar-1405-54
Farrell M, Perkins WT, Hobbs PJ, Grith GW, Jones DL (2010) Migration of heavy metals in soil as influenced by compost amendments. Environ Pollut 158(1):55–64
Gangwar S, Singh VP (2011) Indole acetic acid differently changes growth and nitrogen metabolism in Pisum sativum L. seedlings under chromium (VI) phytotoxicity: implication of oxidative stress. Sci Hortic 129:321–328
Gangwar S, Singh VP, Garg SK, Prasad SM, Maurya JN (2011a) Kinetin supplementation modifies chromium (VI) induced alterations in growth and ammonium assimilation in pea seedlings. Biol Trace Elem Res 144:1327–1343
Gangwar S, Singh VP, Srivastava PK, Maurya JN (2011b) Modification of chromium (VI) phytotoxicity by exogenous application of Gibberellic acid in Pisum sativum (L) seedlings. Acta Physiol Plant 33:1385–1397
Gheju M, Balcu I, Ciopec M (2009) Analysis of hexavalent chromium uptake by plants in polluted soils. Ovidius Univ Annal Chem 20:127–131
Halter L, Hebbegger R, Schnitzler WH (2005) Gibberellic acid on artichokes (Cynara scolymus L.) cultivated in Germany to promote earliness and to increase productivity. Acta Hortic 681:75–82
Hsu NH, Wang SL, Lin YC, Sheng GD, Lee JF (2009) Reduction of Cr (VI) by crop-residue-derived black carbon. Environ Sci Technol 43:8801–8806
Iqbal M, Ashraf M (2013) Gibberellic acid mediated induction of salt tolerance in wheat plants: growth, ionic partitioning, photosynthesis, yield and hormonal homeostasis. Environ Exp Bot 86:76–85
John R, Ahmad P, Gadgil K, Sharma S (2009) Heavy metal toxicity: effect on plant growth, biochemical parameters and metal accumulation by Brassica juncea L. Int J Plant Prod 3:65–76
Kaszycki P, Gabrys H, Appenroth KJ, Jaglarz A, Sedziwy S, Walczak T, Koloczek H (2005) Exogenously applied sulphate as a tool to investigate transport and reduction of chromate in the duckweed Spirodelapolyrhiza. Plant Cell Environ 28:260–268
Khan S, Ullah SM, Sarwar KS (2001) Interaction of chromium and copper with nutrient elements in rice (Oryza sativa cv BR-11). Bull Inst Trop Agric Kyushu Univ 23:35–39
Khan MY, Asghar HN, Jamshaid MU, Akhtar MJ, Zahir ZA (2013) Effect of microbial inoculation on wheat growth and phyto-stabilization of chromium contaminated soil. Pak J Bot 45:27–34
Kozuh N, Stupar J, Gorenc B (2000) Reduction and oxidation processes of chromium in soils. Environ Sci Technol 34:112–119
Lee TM, Lai HY, Chen ZS (2004) Effect of chemical amendments on the concentration of cadmium and lead in long term contaminated soil. Chemosphere 57:1459–1471
Liu D, Zou J, Wang M, Jiang W (2008) Hexavalent chromium uptake and its effects on mineral uptake, antioxidant defence system and photosynthesis in Amaranthus viridis L. Bioresour Technol 99:262
Mahmood M (2010) Phytoextraction of heavy metals—the process and scope for remediation of contaminated soils. Soil Environ 29:91–109
Maqbool Z, Asghar HN, Shahzad T, Hussain S, Riaz M, Ali S, Arif MS, Maqsood M (2014) Isolating, screening and applying chromium reducing bacteria to promote growth and yield of okra (Hibiscus esculentus L.) in chromium contaminated soils. Ecotoxicol Environ Saf. doi:10.1016/j.ecoenv.2014.07.007i
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
Nriagu JO (1988) Production and uses of chromium. Chromium in natural and human environment. Wiley, New York, pp 81–105
Ozdemir C, Karatas M, Dursun S, Argun ME, Dogan S (2005) Effect of MnSO4 on the chromium removal from the leather industry waste water. Environ Technol 26:397–400
Pandey V, Dixit V, Shyam R (2009) Chromium effect on ROS generation and detoxification in pea (Pisum sativum) leaf chloroplasts. Protoplasma 236:85–95
Park JH, Lamb D, Paneerselvam P, Choppala G, Bolan N, Chung JW (2011) Role of organic amendments on enhanced bioremediation of heavy metal(loid) contaminated soils. J Hazard Mater 185:549–574
Peleg Z, Blumwald E (2011) Hormone balance and abiotic stress tolerance in crop plants. Curr Opin Plant Biol 14:290–295
Rademacher W (1990) New types of plant growth retardants: additional perspectives for practical applications in agriculture and horticulture. In: Pharis RP, And Rood SB (eds) Plant growth substances. Springer, Berlin, pp 611–618
Sabir M, Hanafi MM, Aziz T, Ahmad HR, Rehman ZU, Saifullah M, Murtaza G, Hakeem KR (2013) Comparative effect of activated carbon, pressmud and poultry manure on immobilization and concentration of metals in maize (Zea mays) grown on contaminated soil. Int J Agric Biol 15:559–564
Shanker AK, Cervantes C, Loza-Tavera H, Avudainayagam S (2005) Chromium toxicity in plants. A review. Environ Int 31:739–753
Sharma DC, Forster CF (1993) Removal of hexavalent chromium using sphagnum peat moss. Water Res 27:1201–1208
Singh G, Brar MS, Malhi SS (2007) Decontamination of chromium by farm yard manure application in spinach grown in two texturally different Cr-contaminated soils. J Plant Nutr 30:289–308
Steel RGD, Torrie JH (1984) Principles and procedures of statistics. McGraw-Hill Pub, New York
Stewart BA, Robinson CA, Parker DB (2000) Examples and case studies of beneficial reuse of beef cattle byproducts. In: Dick WA (ed) Land application of agricultural, industrial, and municipal by-products. Soil Science Society of America Inc, Madison, pp 387–407
Terry N, Banuelos G (2000) Phytoremediation of contaminated soil and water. Lewis, New York, p 389
Vernay P, Gauthier-Moussard C, Hitmi A (2007) Interaction of bioaccumulation of heavy metal chromium with water relation, mineral nutrition and photosynthesis in developed leaves of Lolium perenne L. Chemosphere 68:1563–1575
Acknowledgments
Facilities for this study were provided by the Institute of Soil and Environmental Sciences, University of Agriculture, Faisalabad. We are thankful to the Department of Social Sciences and Humanities, Faculty of Sciences, University of Agriculture, Faisalabad, for providing services of editing this manuscript for English language.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Saleem, M., Asghar, H.N., Khan, M.Y. et al. Gibberellic acid in combination with pressmud enhances the growth of sunflower and stabilizes chromium(VI)-contaminated soil. Environ Sci Pollut Res 22, 10610–10617 (2015). https://doi.org/10.1007/s11356-015-4275-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4275-3