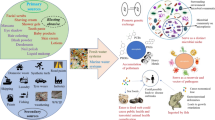
Abstract
Microplastics are one of the major contaminants of aquatic nature where they can interact with organic and inorganic pollutants, including trace metals, and adsorb them. At the same time, after the microplastics have entered the aquatic environments, they are quickly covered with a biofilm - microorganisms which are able to produce extracellular polymeric substances (EPS) that can facilitate sorption of trace metals from surrounding water. The microbial community of biofilm contains bacteria which synthesizes EPS with antimicrobial activity making them more competitive than other microbial inhabitants. The trace metal trapping by bacterial EPS can inhibit the development of certain microorganisms, therefore, a single microparticle participates in complex interactions of the diverse elements surrounding it. The presented review aims to consider the variety of interactions associated with the adsorption of trace metal ions on the surface of microplastics covered with biofilm, the fate of such microplastics and the ever-increasing risk to the environment caused by the combination of these large-scale pollutants - microplastics and trace metals. Since aquatic pollution problems affect the entire planet, strict regulation of the production, use, and disposal of plastic materials is needed to mitigate the effects of this emerging pollutant and its complexes could have on the environment and human health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
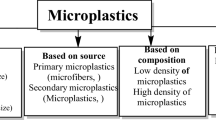
Microplastics (MPs) are particles with size less than 5 mm and are one of the major contaminants of the environment, especially the aquatic nature. The total amount of waste plastics getting into the marine environment is estimated as 8 million tons per year (Rodrigues et al. 2019), and the number of pieces of plastic in the ocean is between 15 and 51 trillion (Bowley et al. 2021). In aquatic environments, microplastic particles can interact with inorganic and organic pollutants adsorbing them on to the surface and potentially increasing the risks to environment, animals and humans. Bacteria exist in two different states in aquatic environments, namely, planktonic or free-living bacteria and sessile bacteria attached to biotic or abiotic surfaces. However, in the natural environment most bacteria live attached to different materials (Khatoon et al. 2018). Therefore, as microplastics enter into the natural water reservoirs, they serve as a convenient material for colonization and quickly become covered with biofilms. These biofilms can have impact on MPs modifying their physical properties, changing their distribution in the water column, participation in the adsorption of inorganic and organic substances on the surface of the microplastic-biofilm complex, and thus the trophic transfer and release of the adsorbed pollutants into environment (Rummel et al. 2017; Stabnikova et al. 2021; Vaseashta et al. 2021). There are studies indicating that biofilm could play a significant role in the ability of microplastics to be a vector for migration of such contaminants as trace metals (Guan et al. 2020; Liu et al. 2021b; Wang et al. 2021).
Currently, a number of studies on adsorption of trace metals on microplastics in the marine and freshwater environments have been conducted (Ahechtia et al. 2020; Fan et al. 2021; Liu et al. 2021b; Turner and Holmes 2015; Wang et al. 2020; Zou et al. 2020). Although most of the research on microplastics is being carried out by its own design and detected parameters (Rozman and Kalčíková 2022), it is still possible to identify basic patterns and trends in the adsorption processes of trace metals by microplastics and their interactions with microbial community of biofilm covered microplastics.
Accumulation of trace metals by microplastics
In most studies, dedicated to the interaction of microplastics and chemical elements, the term “heavy metals” is used. This term was proposed in 1817 by the German chemist Leopold Gmelin who divided the elements into nonmetals, light metals, and heavy metals. However, for present there is no strongly accepted definition of the term “heavy metals”. So, a list of heavy metals according to the different definitions could include different elements. For example, “heavy metals” could be defined to a group of elements that have an atomic number greater than 20 and an atomic mass higher than 23 (Koller and Saleh, 2018). Other definitions differentiate “heavy metals” from “light metals” by atomic density which should be above 5 g/cm3 (Raychaudhuri et al. 2021). Some of these elements, such as copper, zinc, and iron, are essential for plants, animals, and humans, but become toxic at high concentrations; other ones, such as arsenic, cadmium, chromium, lead, and mercury, are considered as persistent and toxic pollutants having carcinogenic and mutagenic effects, and their presence even at low concentration is dangerous for marine habitats (Edelstein and Ben-Hur 2018; Tchounwou et al. 2012). The use of the term “heavy metals” in the scientific literature is a subject of a long-term discussion and has both supporters of its use (Batley 2012; Ali and Khan2018) and those who consider its use inappropriate (Appenroth 2010; Chapman 2012; Nieboer and Richardson, 1980). According to the International Union of Pure and Applied Chemistry it is considered as meaningless (Duffus 2002), however, this term is increasingly used in the scientific literature (Pourret and Hursthouse 2019). Term “heavy metals” especially often is used in environmental studies including aquatic pollution with microplastic. Meanwhile, the terms like “metal”, “metalloid”, “trace metal elements” or “potentially toxic element” are now proposed instead of “heavy metals” (Pourret et al. 2021). In the present paper we use terms “trace metals” as the most accurate instead of “heavy metals.
Trace metals (TMs) together with microplastics are one of the major pollutants of the environment, the study of their interaction is important for prevention and reduction of the risks caused by their introduction into aquatic water systems (Guan et al. 2020; Khalid et al. 2021; Liu et al. 2021b; Richard et al. 2019; Torres et al. 2021). TMs adsorbed on microplastics increase its toxicity and, thus, the danger to the environment is escalated. The complexation of TMs and MPs can also increase bioavailability of trace metals, and these microplastic particles having the combined toxic effects can pose risks to human health (Cao et al. 2021).
Different TMs are detected on microplastic in natural water bodies, so MPs particles can be considered as carriers of trace metals. There is a lot of research devoted to the trace metal-microplastic interaction in water bodies. MPs have a hydrophobic surface and due to small size large surface area, making it a suitable material for adsorption of contaminants that increase its potential negative impact on the environment. Comparison of adsorption capacities of non-degradable and degradable plastics and natural materials has shown that there is no significant differences in the amounts of adsorbed TMs. It was interesting finding that the sorption capacity of trace metals of degradable microplastics such as polybutylene succinate (PBS), polycaprolactone (PCL), and polylactic acid (PLA) is similar or even higher than the ones of non-degradable MPs such as polyethylene (PE), polyethylene terephthalate (PET), polyamide (PA), polypropylene (PP), polyvinyl chloride (PVC), and polystyrene (PS) (Torres et al. 2021). In study by Guan et al. (2020) it was shown that natural substrates in the water body had greater adsorption capacity than PS particles. Richard et al. (2019) showed that glass pellets accumulated greater amounts of Al, Ba, Ca, Mg, Na, U and Zn than low-density polyethylene (LPE) microplastic. However, given the amount of non-degradable plastic entering the marine environment, the gravity of the problem of trace metal adsorption on microplastic surfaces becomes evident.
Metal adsorption depends on the type of microplastic particle
The surface physicochemical properties of MPs, including chemical structure, and electronegativity play an important role in metal sorption (Zou et al. 2020). In a study by Han with co-authors (2021) it was shown that PE and PET had higher Cu(II), Cr(III), and Pb(II) adsorption capacity than PP microplastics. Comparison of four virgin microplastics particles namely chlorinated polyethylene (CPE), PVC, LPE, and high-density polyethylene (HPE) for sorption of bivalent trace metals Cu(II), Cd(II), and Pb(II) showed that the sorption ability of studied MPs followed the sequence of CPE > PVC > HPE > LPE and Pb(II) had significantly stronger sorption than Cu(II) and Cd(II) (Zou et al. 2020). It has been shown that microplastics were in the following order in terms of Pb(II) sorption rate constants: PS > PE > PVC, and the maximum adsorption capacity at pH 6.0 and a temperature of 25 °C was, μg/g, 128.5; 416.7, and 483.1 respectively (Lin et al. 2021a, b). PP showed higher capacity of adsorption compared to PA, polyester (PL), PE, and PVC to trace metals in ground and surface water in India in the following order: (a) Cd > Mn > Pb > As and (b) Mg > Zn > As > Pb > Cu (Selvam et al. 2021). Trace metals Pb, Cu and Cd in seawater showed higher absorbance on PVC and PP particles compared with PA, PE, and polyformaldehyde (Gao et al. 2019). A significant adsorption of Pb, Cr and Zn on PE and PVC MPs and low adsorption on PET in different waters have been shown (Godoy et al. 2019).
Metal adsorption depends on metal species
Comparison of Cu(II), Cr(III), and Pb(II) adsorption on PE, PET and PP showed that amount of Pb(II) was the largest, especially on PET particles (Han et al. 2021). Comparison of Pb, Cu, Cd, and Zn adsorption on PP microplastics showed that the adsorption capacities of polypropylene for Pb and Cu were significantly higher than that of Cd and Zn (Fan et al. 2021). Comparison of the adsorption of different trace metals on PS microplastics showed that Pb had the greatest adsorption capacity on PS, followed by Cu, Zn, and Cd (Barus et al. 2021). According to the results of different authors, Pb had higher adsorption capability than other tested trace metals either in sea- and freshwater to such microplastics particles as PS (Barus et al. 2021), PE, PET (Han et al. 2021) and PP (Fan et al. 2021; Han et al. 2021). This indicates that Pb has the highest adsorption ability to various types of microplastics particles having physisorption onto the MPs as the main sorption mechanism (Lin et al. 2021a).
Metal adsorption depends on size of microplastics
Generally, it is considered that microplastic particles with smaller size possess higher adsorption capacities for trace metals than larger ones because of higher surface area to volume ratio (Khalid et al. 2021). It was shown that the metal adsorption capacities of PE, PET and PP for Cu(II), Cr(III), and Pb(II) depended on the size of the plastic particles and increased with the decrease of particle size (Han et al. 2021). The metal adsorption capacity for particles with size less than 0.9 mm was greater than that of 0.9–2 mm and 2–5 mm microplastics. The decrease of microplastic size from 2–5 mm to less than 0.9 mm resulted in the increased amount of adsorbed Cu(II), Cr(III), and Pb(II) in 1.8–2.2, 1.3–1.5, and 1.94–2.83 times, respectively. This effect was most prominent for PE microplastic, however, for PP microplastic it was relatively low (Han et al. 2021). However for PS microparticles with diameters of 0.02, 0.05, 0.13, and 0.25 mm used for adsorption of lead, cadmium, copper, and zinc, the greater amounts of heavy metals were observed on the larger PS particles, and authors concluded that the particle with the larger size had the higher adsorption capacity (Barus et al. 2021).
Metal adsorption depends on microplastics aging
Aging of microplastics accelerates the process of trace metal adsorption. Prolonged weathering and abrasion in the natural environment lead to increase of specific surface and area available for adsorption (Khalid et al. 2021). Initially, PS were regular spheres with a smooth surface, but during aging treatment the surface became irregular, pores and voids appeared on the surface and the surface became rough. Similar changes of PS microplastics were reported by Hüffer et al. (2018). The changes in functional groups of PS were observed and the amount of oxygen-containing functional groups (C=O, C–OH, O–C=O) increased with increasing aging time (Mao et al. 2020). Meanwhile, due to photo-oxidation processes the surface on MPs becomes negatively charged and acquires the ability to adsorb trace metals. Ultraviolet light is considered an important factor for microplastic aging due to its oxidative degradation called photoaging. The increase of specific surface and formation of oxygen-containing functional groups resulted in an increase of the adsorption capacity of MPs for trace metals (Cheng et al. 2022). The adsorption of Cu in seawater on aged PVC microplastics was significantly higher than on virgin ones (Brennecke et al. 2016). Aged PE microplastics adsorbed silver ions more intensively than pristine ones (Kalčíková et al. 2020). Photo-aged PET using UV radiation showed higher adsorption capacity for metal ions (Cu(II) and Zn(II)) in aqueous solution than pristine PET. This effect increased with prolonged exposure to UV radiation (Wang et al. 2020). It was shown that trace metals, Ag, Cd, Co, Cr, Cu, Hg, Ni, Pb, and Zn, suspended in river water (pH 6.5) adsorbed better on aged, weathered plastic pellets than on virgin ones (Turner and Holmes 2015).
Adsorption of trace metals Pb2+, Cu2+, Cd2+, Ni2+ and Zn2+ by PS was significantly improved after microplastic particles were aged in pure and sea-water under UV irradiation, which changes the physicochemical properties of the aged PS, such as the roughening and the number of oxygen-containing groups on the surface. It was noted that environmental conditions could play a key role in the microplastics ageing. The degree of ageing was higher for UV irradiated seawater than pure water, which may be due to the influence of salinity (Mao et al. 2020). Treatment with UV irradiation in seawater resulted in generation of new alcohol, carboxylic acid and fatty ether functional groups: –OH, –COOH, and –C–O– on the surface of PE microplastic particles; only alcohol functional groups were produced on the surface of PP microparticles; and there was no significant change in the surface functional groups of PET microparticles (Han et al. 2021). Adsorption capacity of PP microplastics was increased 1.7–2.5 times after MP exposure to a Xenon lamp for 28 days. Due to the light irradiation percentage of oxygen-containing functional groups in MPs increased from 2.80 to 20.95 wt% (Lin et al. 2021a, b).
Metal adsorption on microplastics depends on environmental conditions
pH
External conditions such as pH have a significant influence on the adsorption of trace metals on microplastics, and a clear dependence of metal sorption onto the MPs was demonstrated (Davranche et al. 2019; Lin et al. 2021a, b; Wang et al. 2020; Zou et al. 2020). The increase of the adsorption percentages of Cu, Zn, Cd, and Pb on PP and PE microplastics in aquatic environments with pH increase was shown (Ahechti et al. 2020). The increase of pH in river water resulted in increased sorption of such trace metals as Cd, Co, Ni and Pb and decreased sorption of Cr on plastic pellets while sorption of Cu did not change (Holmes et al. 2014). In another study, increasing pH of river water resulted in an increase in adsorption of Ag, Cd, Co, Ni, Pb and Zn and decrease in adsorption of Cr on PP pellets (Turner and Holmes 2015). It was shown that the amount of adsorbed Pb and Cu on microplastics floating in Musi River, Indonesia, was higher at pH of water 6.5–6.6 than 6.2 (Purwiyanto et al. 2020).
Meanwhile, the zeta potential of the MPs (PVC, PS, and PE) was strongly dependent on the pH of the environment. All of these microplastics had pHpzc (the point of zero charge, meaning of the pH at which the charge of the particle is zero) around 3.0, so they had negative charge at pH from 3.0 to 11.0, and positive at pH lower than 3.0 (Lin et al. 2021a, b). The negative charge of the surface of the MPs was explained by the presence of negatively charged groups that are bonded chemically to the MPs during polymerization (Lu et al. 2018). pH has influence not only on charge of microplastics but also on forms in which trace metal is present in aquatic environments. In experiment with Pb sorption onto PS, PE, and PVC, adsorption on MPs increased with the increasing pH in the range of 2.0 to 6.0 when Pb was mainly presented as Pb2+ (Lin et al. 2021a, b). Meanwhile at high pH Pb is present in forms of Pb(OH)+, Pb(OH)2, and Pb(OH)3− (Lin et al. 2021a, b). Thus, an increase in Pb adsorption on microplastics was explained by the electrostatic attraction of positively charged Pb2+ to negatively charged MPs. So, the low zeta potential of MPs can enhance the adsorption of cationic ions due to electrostatic attraction and repel anionic ions. It is known that the pH of marine waters is close to 8.2, meanwhile most natural freshwaters have pH values in the range from 6.5 to 8.0, and so, MPs are more likely to have negative charge in the water environment.
Salinity
The increase of salinity of the trace metal solution (Pb, Cd, Cu, and Zn) resulted in an increase of time needed for their adsorption on PS microparticles, and lower amounts of the trace metals were adsorbed (Barus et al. 2021). The adsorption of Cu, Zn, Cd, and Pb on PP and PE microplastics in aquatic environments decreased with increase in salinity (Ahechti et al. 2020). At 0.15 and 30% salinity a gradual decrease of the adsorption rate for trace metals was shown (Barus et al. 2021). The authors suggest that this effect could be explained by cations competing for adsorption onto the surface of the MP (Purwiyanto et al. 2020; Yu et al. 2019). However, generally, the effect of salinity on metal ion adsorption on microplastics depends on the type of MPs and metal speciation (Godoy et al. 2019; Holmes et al. 2014; Yu et al. 2019).
Exposure time
Rochman et al. (2014) suggested that there is a positive correlation between the accumulation of trace metals on microplastics and the time of their contact, plastic debris can accumulate more metals the longer it remains in the sea. Contents of Cu and Zn on PVC in sea water gradually increased during 14 days of experiment (Brennecke et al. 2016).
Concentration of trace metals in the environment
It was shown for Pb and Mn that degree of adsorption on PP and PVC microplastics positively correlated with concentration of these metals in the seawater in a field experiment (Gao et al. 2019). However, no significant differences were found in adsorption of nine metals (Al, Cr, Mn, Fe, Co, Ni, Zn, Cd and Pb) on five plastic types (PET, HPE, PVC, LPE, and PP) in seawater after 12 months (Rochman et al. 2014). It is assumed that the influence of the concentration of metals in the environment on the amount of adsorbed metal on microplastics is especially evident in the initial period of contact, which can last for some days, and is levelled with prolonged contact, as in the study of Rochman with co-authors (2014).
The presence of organic substances in the surrounding water
It has been shown that dissolved organic substances from the surrounding natural waters can play a certain role in the process of metal adsorption on microplastics: (a) providing an additional area for metal binding; and/or (b) competing with metal ions in adsorption on the microplastic surface (Godoy et al. 2019).
Mechanism of adsorption of trace metals on microplastics particles
It is assumed the adsorption of trace metals on microplastics in aqueous medium can be generally described as the electrostatic interactions, van der Waals forces, and π–π interactions (Fu et al. 2021; Liu et al. 2021b; Torres et al. 2021). However, hydrophobic interactions, pore-filling, and hydrogen bonding also plays a certain role in the process of interaction for trace metals with MPs (Torres et al. 2021). Identified mechanisms of interaction support fast process and stable bonding of metals on the MPs covered with biofilm. In natural aquatic environments microplastic particles are usually negatively charged because their pHpzc is estimated as 3.0, meaning electrostatic interactions are of the greatest importance in the process of adsorption of trace metals on MPs (Lin et al. 2021a, b) and van der Waals forces and π–π interactions play a less significant role and its contribution to adsorption depends on the polymer type (Liu et al. 2021b). Godoy with co-authors studying adsorption of trace metals Cd, Co, Cr, Cu, Ni, Pb and Zn on five different types of microplastics found that the main interaction mechanism might be chemical adsorption (Godoy et al. 2019).
Interaction between trace metals and biofilm covered microplastics
Microplastics released into natural water bodies, become a place of microorganisms’ collection, thus forming biofilms (Stabnikova et al. 2021). Microplastics serve as an ecological niche for microorganisms, transport for their moving, and in some cases be a carbon source for their growth. In turn, microorganisms of biofilms can change physico-chemical properties of microplastics and even biodegrade it, supporting appearance of new functional groups on the surface of microplastic particles, enabling more intensive interaction with metal ions. For example, significant changes in microplastic properties, such as, crystallinity for PE, stiffness for PP, and maximum compression for PS were observed as a result of exposure to ambient bacterioplankton from the Baltic Sea during 2 weeks (McGivney et al. 2020). When assessing the environmental risk of microplastics, an important point is to clarify the effect of the presence of biofilm on its surface on the adsorption of inorganic and organic substances. The role of biofilm in metal accumulation on microplastics was studied in few research articles (de Araújo and de Oliveira 2020; Richard et al. 2019; Rummel et al. 2017), however these studies demonstrate major changes of properties of microplastic particles in respect to trace metals. It is known that in an environment highly contaminated with trace metals, as a result of inherited biochemical, physiological and genetic changes, bacterial strains that are resistant to high concentrations of these pollutants appear (Fashola et al. 2016). Main factors hampering better understanding of the biofilm development include used methods for microplastics isolation, requiring destruction of biofilm as well as highly complex structure and properties of EPS.
Influence of trace metals on microbial communities of biofilm covered microplastics
Biofilm forming microorganisms participate in interactions of microplastics and trace metals (Liu et al. 2021a, b). Adsorption of trace metals on microplastics can cause changes in the structure and function of microbial communities of microplastic biofilms which can in turn lead to change of the further process of trace metal adsorption (de Araújo and de Oliveira 2020) (Fig. 1).
For example, a clear relation of Cu content in real sea water (Toulon Bay, NW Mediterranean Sea) on the diversity of biofilm community and Cu bioaccumulation onto MPs has been observed (Djaoudi et al. 2021). Diversity of prokaryotic as well as eukaryotic organisms were higher in the biofilms formed on microplastics incubated in the most Cu contaminated sea water (400 nM). The higher values of bioaccumulated Cu per dry weight of MPs were also determined in the most contaminated site. Such facts make it possible to assume an essential role of the microorganisms on the interactions between MPs and trace metals (Liu et al. 2021b).
It was shown that the presence of biofilms on microplastics had a special impact on the adsorption of some chemical substances, including trace metals. Presence of Cu in coastal areas has been observed worldwide (Corcoll et al. 2019). The addition of Cu as CuCl2⋅2H2O changed microbial composition of the biofilm communities in biofilms formed on polyethylene terephthalate glycol (PETG) slides placed in microcosm containing filtered natural sea water enriched with phosphate and nitrate. Cu was added in different concentrations from 0.01 to 10 μM. Periphyton from the outer layer of 50–60 stones and pebbles from seawater of the Gullmar fjord on the Swedish west coast was used as inoculum and the experiment lasted 18 days. It is known that addition of Cu may have a toxic effect on the formed periphyton microbial community but continuous exposures may lead to community adaptation with changes in composition of species (Serra et al. 2009). The highest tolerance was found in the Cyanobacteria phylum, especially in the Nostocophycideae and Oscilaltoriphycideae subclasses (Corcoll et al. 2019), which agreed with previously reported results (Giner-Lamia et al. 2016; Serra et al. 2009). However, representatives of subclass Synechococcophycideae showed sensitivity to Cu. It was found that the most sensitive taxa to Cu were from the phylum Proteobacteria, and significant changes of bacterial community composition were observed even at low concentration of Cu, 0.06 μM. Meanwhile, this concentration is even below the current environmental quality standard regarding surface water for Cu (0.07 μM) according to Swedish Marine and Water Authority's regulations. Under experimental conditions, authors noted Cu tolerance of Bacteroidetes presented in biofilms. Because a lot of species from the phylum Proteobacteria are involved in nitrification and denitrification processes, it was suggested that Cu pollution in marine areas could lead to impaired nitrogen cycles. Thus, the increase in tolerance to Cu of the biofilm microbial community was accompanied with the changes in its composition. However, no changes in the relative abundance of most classes and families of fungi were observed in case of Cu exposure in different studies (Corcoll et al. 2019; Gardham et al. 2014; Yang et al. 2018).
To protect cells from toxic impact of Cu, bacteria reduced Cu transport enhanced efflux of Cu ions and complexation with cell components (Cervantes and Gutierrezcorona 1994). It was found for the Gram-negative Escherichia coli that bacterial resistance to toxic activity of Cu/Ag depends on several factors including two intracellular proteins: membrane-bound sensor CusS located in the inner cell membrane and its response regulator protein CusR in the extracellular fluid. These proteins together regulate the transcription of the Cus operon that plays important roles in cells’ resistance to Cu/Ag toxicity mechanism. CusS in the presence of metal sends a signal to a regulatory protein CusR, which binds to DNA and activates a gene that generates transport proteins that remove the toxins from the cell (Fu et al. 2020). Meanwhile, Cu tolerance in fungi has been due to diverse mechanisms such as (1) Cu complexation by cell wall components; (2) changes in Cu uptake; (3) extracellular chelation or precipitation of Cu by secreted metabolites, and (4) synthesis of intracellular Cu-binding metallothioneins and phytochelatins (Cervantes and Gutierrezcorona 1994). These mechanisms allow fungi to survive in the presence of Cu.
Influence of biofilm on adsorption of trace metals on microplastics
In aquatic environments, a major part of microplastic particles are covered with biofilm. Formation of biofilm on the surface of microplastics could lead to the change of its properties (Fig. 1). Thus, it was noted that the formation of a biofilm on microparticles led to an increase in the number of hydrophilic groups on the MPs surface causing a decrease in the hydrophobicity of its surface, and increased the number of carboxyl and ketone groups thereby increasing the adsorption capacity of microplastics towards metal ions (Tu et al. 2020). Adsorption capacity of PS microplastics towards trace metals was enhanced by biofilms formed after being released in an eutrophic urban lake for 4 weeks (Guan et al. 2020). Similar results were shown for LPE pellets, submerged for 28 days in an estuary (San Francisco Bay, USA) to evaluate biofilm development: amount of accumulated metals on biofilm covered microplastics correlated positively with amount of biofilm (Richard et al. 2019).
It was shown for PLA and LPE plastic pellets that metal (Cu, Pb, Al, K, U, Co, Mg and Mn) concentrations positively correlated with the amount of biofilm formed on microplastics in estuarine waters (Richard et al. 2019). Similar results have been obtained for PS submerged into an urban lake: the presence of biofilms enhanced the adsorption capacity of Pb(II) onto microplastics (Qi et al. 2021). It was shown that PS particles with the size of 4 mm covered with biofilm of a model freshwater fungus Acremonium strictum strain KR21–2 had higher adsorption capacity towards Cu(II) and ability to reduce Cr(VI) than those without biofilm, which may be related to the functional groups of biofilm EPS (Wu et al. 2022). Thus, it is evident that biofilm developed on microplastics enhances the role of MPs as a vector for transportation of trace metals in natural water bodies. Furthermore, it was found that biofilm enhanced the combined toxicity of Pb(II) and microplastics, so, this combined action may increase environmental risk for freshwater bodies (Qi et al. 2021).
Extracellular polymeric substances (EPSs) produced by aquatic bacteria and their role in trace metal adsorption
Extracellular polymeric substances (EPSs) produced by aquatic bacteria with antimicrobial properties
The factor determining the participation of biofilms in the regulation of the interaction of trace metals and microplastics is the ability of certain types of microorganisms to synthesize extracellular polymeric substances (EPS) that are mostly composed of heteropolysaccharides (exopolysaccharides) which consist of such monomers as glucose, mannose, fructose, galactose, rhamnose; galacturonic, glucuronic, guluronic, mannuronic acids, N-acetyl-D-glucosamine, and N-acetyl-D-galactosamine, and also protein, but sometimes include also nucleic acids and lipids. In general, polysaccharides represent 40–95% of the EPS (Flemming and Wingender 2001). EPS play an important role in the formation of biofilms, and bacterial biofilms could be considered as microbial communities embedded in extracellular polymeric substances, which determine their physicochemical and biological properties and ecological survival (Flemming et al. 2016). Biofilm polysaccharides could provide a lot of benefits to the microbial cells such as surface adherence, environmental protection and resistance, and structural integrity (Limoli et al. 2015; Wingender et al. 1999). In marine environments microbial exopolysaccharides may be present in dissolved form and, being rich in organic carbon, are an important source of carbon for different marine habitats. They can also be in the form of aggregates in a gel-like slime matrix or as components of biofilms (de Carvalho and Fernandes 2010; Flemming and Wingender 2001). A lot of the microorganisms involved in biofilm formation can produce EPS (Casillo et al. 2018; Delbarre-Ladrat et al. 2014; Tu et al. 2020). The functions of these EPS include the production of microbial aggregates, adhesion to biotic or abiotic surfaces, colonization of surfaces, sequestering of nutrients from the water phase, protection of the bacterial cells from a stress caused by environmental conditions and ensuring of ecosystem stability (Delbarre-Ladrat et al. 2014; Fletcher and Floodgate 1973; Nichols et al. 2005). EPS produced by bacteria are negatively charged, so adsorption capacity of microplastic covered with biofilm containing EPS should increase (Fig. 1).
Some EPS synthesized by bacteria had strong antimicrobial activity, which could be a factor which causes domination of these strains in biofilm covered microplastics. Examples of aquatic bacteria isolated from natural water bodies as producers of EPS with antimicrobial properties are shown in Table 1.
Lipopeptide EPS synthesized by a marine bacteria Bacillus circulans possessed antimicrobial activity against Gram-positive and Gram-negative pathogenic and semi-pathogenic microbial strains including strains Escherichia coli NCIM, Micrococcus flavus NCIM 2376, Serratia marcescens NCIM 2397, Bacillus pumilis MTCC 2296, Proteus vulgaris NCIM 2857, Citrobacter freundii NCIM 2488, Proteus mirabilis NCIM 2300, Mycobacterium smegmatis NCIM 5138, Alcaligens faecalis NCIM 2105, Acetobacter calcoaceticus NCIM 2886, Bordetella bronchiseptica NCIM 2267, Klebsiella aerogenes NCIM 2098, and Enterobacter cloacae NCIM 2164 (Das et al. 2008). The biosurfactant was also active against methicillin-resistant Staphylococcus aureus and multidrug-resistant Escherichia coli and Klebsiella pneumoniae (Das et al. 2008). The glycolipid EPS produced by Brevibacterium casei MSA19 isolated from the marine sponge Dendrilla nigra had bacteriostatic activity against mixed pathogenic biofilm bacteria (Kiran et al. 2010b). Nocardiopsis lucentensis MSA04 isolated from marine sponge Dendrilla nigra synthesized EPS containing glycolipid with a hydrophobic non-polar hydrocarbon chain (nonanoic acid methyl ester) and hydrophilic sugar, 3-acetyl 2,5 dimethyl furan. It was suggested that this EPS could be used in bioremediation processes in the marine environment (Kiran et al. 2010c). Fructose and fucose rich exopolysaccharide produced by the thermophilic Bacillus licheniformis T14, isolated from a shallow hydrothermal vent of Panarea Island, Italy, was effective against biofilm formation by multiresistant clinical strains of Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Staphylococcus aureus (Spanò et al. 2016). Strain Rhodotorula mucilaginosa UANL-001 isolated from the water streams of the river in Mexico produced EPS with high content of carbon, hydrogen and oxygen (93%) and did not contain nitrogen and sulphur. The main monosaccharide was glucose, 82%, followed by mannose, galactose and fucose (Vazquez-Rodriguez et al. 2018). The exopolysaccharide effectively capped Zn and Ni producing biopolymer-metal nanoparticle biocomposites with antimicrobial properties against both wild-type and antibiotic-resistant strains of Staphylococcus aureus and Pseudomonas aeruginosa (Garza-Cervantes et al. 2019).
Marine bacteria living under extreme conditions, such as high or very low temperature, high pressure, or salinity, are often able to produce EPS which protects these microbial water inhabitants from stress, high salinity, and negative impact of trace metals (Casillo et al. 2018). Thus, it was shown that the exopolysaccharides produced by cyanobacteria in Synechocystis protected cells from death caused by TiO2 nanoparticles in natural and artificial waters and salt concentration (De Philippis et al. 2011; Jittawuttipoka et al. 2013; Planchon et al. 2013; Xu et al. 2021). The mesophilic strain Alteromonas macleodii subsp. fijiensis biovar deepsane isolated from deep-sea hydrothermal vents on the East Pacific Rise at 2600 m depth synthesized exopolysaccharide, containing different types of carbohydrates with sulphate, lactate and pyruvate substituents, named deepsane, which is used in cosmetics (Le Costaouëc et al. 2012). Marine bacterium Pseudoalteromonas tunicata produces extracellular compounds with antibacterial and antifungal activities that inhibit different fouling organisms, including marine algae, bacteria, and fungi (Egan et al. 2002). So, the presence of such bacteria in microbial biofilm covered microplastics should ensure their predominance in the microbial community.
Extracellular polymeric substances (EPSs) produced by aquatic bacteria for trace metal adsorption
The factor determining the participation of biofilms in the regulation of the interaction of trace metals and microplastics is the ability of certain types of microorganisms to synthesize EPS. The EPSs often contain different functional groups including uronic acid (up to 20–50%), hydroxyl, carboxyl and phosphate groups, sulphated units, glycerate, and pyruvate or succinate substituents which form a negative charge at the pH of seawater (pH ~ 8) allowing them to act as ligands toward dissolved cations including trace, and toxic metals (Banerjee et al. 2021; Escárcega-González et al. 2018; Gupta and Diwan 2017; Nichols et al. 2005). Thus, EPS have been proposed to be used for biosorption of trace metals in the processes of biotreatment (Banerjee et al. 2021; Li et al. 2015; Mohite et al. 2017; Raj et al. 2018) or as capping agents in production of numerous metallic nanoparticles which are now widely used in biomedical sciences and engineering (Sathiyanarayanan et al. 2017). Interaction between EPS and trace metals occurs due to the reaction between metal cations and the charged functional groups, such as hydroxyl, carboxyl, phosphoric, and amine groups, which determines the possibility of adsorption of metals on exopolysaccharides, so, EPS could reduce metal ions and cap them. Examples of application of EPS produced by bacteria isolated from different aquatic environments with trace metals are present in Table 2.
Different trace metals such as Pb, Cu and Co were adsorbed by anionic EPS produced by halophilic bacteria Halomonas almeriensis, 24.5; 12.2, and 10.0 mg/g, respectively (Llamas et al. 2012). The Bacillus cereus strain KMS3-1 isolated from polluted coastal sediment produced EPS containing functional groups (O‒H, CH, C=O, C‒O, and C‒C=O). This EPS could adsorb Cd(II), Cu(II), and Pb(II) in quantity of 54.05, 71.42, and 78.74 mg/g, respectively, and could be used as chelating agent for wastewater treatment (Mathivanan et al. 2021). Klebsiella spp. isolated from paper mill wastewater in China, produced EPS made up of 84.6% polysaccharides consisting of rhamnose, mannose, glucose and galactose at a molar ratio of 6.48:2.47:1:1.74, and 6.1% protein (Yin et al. 2014). The polysaccharides which have a large number of functional groups (hydroxyl, amide and carboxyl) were more likely to adsorb positively charged particles and showed potential for industrial application as bioflocculant. EPS produced by a photosynthetic bacterium Proteiniphilum acetatigenes PSB isolated from a water purifying agent was effective for removal of Cu and Pb from aquatic environments (Hu and Liu 2021). Bacillus megaterium strain PL8 synthesized acidic polysaccharide composed of galactose, galacturonic acid, glucose, glucuronic acid and mannose at a molar ratio of 45.1: 33.8:9.3:9.2:2.4, respectively, and displayed high adsorption ability of Pb, Zn, and Ni (Pu et al. 2020). The strain Alteromonas sp. JL2810, isolated from surface seawater from the South China Sea, produced exopolysaccharide containing rhamnose, mannose and galacturonic acid with such functional groups as O–H, C=O, and C–O–C. This EPS was able to adsorb such trace metals as Cu(II), Ni(II) and Cr(VI) with maximum biosorption capacities 140.8, 226.3, and 215.2 mg/g, respectively (Zhang et al. 2017). A halophilic, thermotolerant strain Bacillus licheniformis B3-15, isolated from water of a shallow submarine hot spring off the coast of the Eolian Islands, Italy, produced an exopolysaccharide with manno-pyranosidic configuration and repeating tetrasaccharide unit. The strain was resistant to Cd(II), Zn(II), As(II) and Hg(II) and could be used as a biosorbent in wastewater treatment (Maugeri et al. 2002). The EPS produced by Pseudoalteromonas sp. strain TG1 isolated from a seawater sample from the shores of Oban Bay, Scotland, was a glycoprotein composed of carbohydrates, 32.3%, and protein, 8.2%. EPS contained hexoses (rhamnose, fucose, galactose, glucose, and mannose), amino sugars (galactosamine, glucosamine, and muramic acid), uronic acids (galacturonic and glucuronic acid), and the pentose xylose. Major amino acids were aspartic acid, glutamic acid, glycine, and alanine (Gutierrez et al. 2008). EPS contained a high amount of uronic acid (28%), negative charges of the uronic acids, together with hydroxyl groups of monosaccharides provided the possibility for binding of metal ions: Na (154.5 mg/g polymer), Mg(II) (31.0 mg/g polymer), and K (10.6 mg/g polymer) (Gutierrez et al. 2008). The EPS produced by Pseudoalteromonas sp. MER144 isolated from Antarctic seawater contained 35% carbohydrates, 14% uronic acid, and 12% protein and could bind Hg and Cd (Caruso et al. 2018). Exopolysaccharide synthesized by Enterobacter A47 isolated from glycerol by-product aqueous solution mainly composed of neutral sugars, fucose, galactose and glucose, and the acidic sugar glucuronic acid revealing high ability for the biosorption of Pb, Co, Cu, and Zn cations, and has a great potential as biosorbent for treating waters and wastewaters (Concórdio-Reis et al. 2020a). Xanthate-functionalized EPS synthesized by marine Pseudomonas aeruginosa JP-11 formed nanoparticles of Cd sulphide (CdS) to remove Cd by adsorption from Cd-containing wastewater up to 88.7% (Raj et al. 2016).
A lot halophiles and halotolerant marine bacteria such as Halomonas eurihalina, H. maura, H. ventosae, H. anticariensis, Alteromonas hispanice, and Idiomarina rambicola are known as producers of exopolysaccharides which can be used as viscosifying, jellying, emulsifying and metal binding substances (Elsakhawy et al. 2017). Sulphated mauran (MR), one of the most studied extremophilic bacterial EPSs, due to its fascinating rheological properties, attracts metal ions to form stable nanomaterials because of the uronic acid contents (Sathiyanarayanan et al. 2017).
Capability of bacterial EPS to trap metal ions is now widely used for the production of nanoparticles that possess antimicrobial activity (Table 3).
Table 3 summarizes examples of bacterial EPS used for synthesis of antimicrobial agents. Aldehyde groups in rhamnose and pyranose sugars and hemiacetal groups of rhamnose sugars present in the exopolysaccharides produced by Escherichia coli actively participated in the reduction of Ag+ to form silver nanoparticles (AgNPs) that were immobilized within the EPS matrix. It was demonstrated that EPS produced by E. coli serves as a permeability barrier which protects cells from antibacterial activity of silver ions (Kang et al. 2013). EPS, a glyco-lipoprotein synthesized by Ochrobactrum rhizosphaerae, was used for the production of silver nanoparticles AgNPs as antimicrobial agents against Vibrio cholera. It was concluded that the free –CH2OH groups of GLP molecules were oxidized to carboxyl groups (COO–) which was accompanied with reduction of Ag+ to Ag0 and production of nanoparticles (Gahlawat et al. 2016). Thus, the presence of toxic trace metals on microplastics could be a factor which determines the domination of microorganisms which could survive due to their ability to synthesize EPS.
Bioavailability and potential toxicity of trace metals adsorbed on microplastics
The adsorption of trace metals on microplastics increases its ecotoxicity and the risk of accumulation in the environment and organisms including humans. Microplastics are found everywhere in aquatic ecosystems. In the case when there are adsorbed trace metals on biofilm covered microplastics, this can lead to synergistic toxicities of these two contaminants together with an increase in bioavailability of trace metals (Ding et al. 2022; Sleight et al. 2017). Some authors draw attention to the need to regulate plastic production in order to reduce environmental pollution, as well as to focus on the reuse or recycling of used plastic materials to decrease their accumulation in nature (Alimba and Faggio 2019; Kumar et al. 2021; Liu et al. 2021a).
Conclusions
Adsorption of trace metals on biofilm covered microplastics is multifaceted process: (a) trace metals can be adsorbed on microplastics changing its properties and influencing attachment of microorganisms to the MPs surface; (b) microplastics in aquatic environments are covered with biofilm; (c) trace metals adsorbing on microplastics covered with biofilm changes the microbial composition of biofilm; (d) biofilm covered microplastics changes its surface properties and influences trace metal adsorption; (e) microorganisms of biofilm very often produce exopolysaccharides which can trap trace metals; (g) EPS produced by microorganisms from biofilm covered MPs can have antimicrobial effect which change the composition of microbial community of biofilm and, in turn, adsorption capacity for trace metals; (h) there are a lot factors influencing the process of trace metal adsorption on MPs such as pH, salinity, time of exposure, concentration of TMs in surrounding medium, ageing, UV-effect. Processes of microplastics covered with biofilm altogether with adsorption of trace metals on MPS or trapping them with EPS synthesized by microorganisms of biofilm are spontaneously introduced in natural water bodies. Dangers and threats to the global environment provided by the microplastics serving as carriers for microorganisms of biofilm and adsorbed trace metals is increasing. The numerous studies on the interaction of microplastics with trace metals indicate that there is an ever-increasing danger to the environment of the combination of these two large-scale pollutants, and today there is only one real way to deal with this worldwide problem: Since this problem affects the entire planet, laws must be developed and adopted to regulate the production, use, and disposal of plastic materials as well as new water treatment solutions must be put in place to eliminate microplastic and trace metal discharge into the environment.
References
Ahechti M, Benomar M, El Alami M, Mendiguchía C (2020) Metal adsorption by microplastics in aquatic environments under controlled conditions: exposure time, pH and salinity. Int J Environ Anal Chem 102:1118–1125. https://doi.org/10.1080/03067319.2020.1733546
Ali H, Khan E (2018) What are heavy metals? Long-standing controversy over the scientific use of the term ‘heavy metals’—Proposal of a comprehensive definition. Toxicol Environ Chem 100:6–19. https://doi.org/10.1080/02772248.2017.1413652
Alimba CG, Faggio C (2019) Microplastics in the marine environment: current trends in environmental pollution and mechanisms of toxicological profile. Environ Toxicol Pharmacol 68:61–74. https://doi.org/10.1016/j.etap.2019.03.001
Appenroth KJ (2010) What are ‘“heavy metals”’ in plant sciences? Acta Physiol Plant 32:615–619. https://doi.org/10.1007/s11738-009-0455-4
Arias S, Del Moral A, Ferrer MR, Tallon R, Quesada E, Bejar V (2003) Mauran, an exopolysaccharide produced by the halophilic bacterium Halomonas maura, with a novel composition and interesting properties for biotechnology. Extremophiles 7:319–326. https://doi.org/10.1007/s00792-003-0325-8
Banerjee A, Sarkar S, Govil T, González-Faune P, Cabrera-Barjas G, Bandopadhyay R, Salem DR, Sani RK (2021) Extremophilic exopolysaccharides: biotechnologies and wastewater remediation. Front Microbiol 12:721365. https://doi.org/10.3389/fmicb.2021.721365
Barus BS, Chen K, Cai M, Li R, Chen H, Li C, Wang J, Cheng SY (2021) Heavy metal adsorption and release on polystyrene particles at various salinities. Front Mar Sci. https://doi.org/10.3389/fmars.2021.671802
Batley GE (2012) “Heavy metal”: a useful term. Integr Environ Assess Manag 8:215. https://doi.org/10.1002/ieam.1290
Bowley J, Baker-Austin C, Porter A, Hartnell R, Lewis C (2021) Oceanic hitchhikers: assessing pathogen risks from marine microplastic. Trends Microbiol 29:107–116. https://doi.org/10.1016/j.tim.2020.06.011
Brennecke D, Duarte B, Paiva F, Caçador I, Canning-Clode J (2016) Microplastics as vector for heavy metal contamination from the marine environment. Estuar Coast Shelf Sci 178:189–195. https://doi.org/10.1016/j.ecss.2015.12.003
Cao Y, Zhao M, Ma X, Song Y, Zuo S, Li H, Deng W (2021) A critical review on the interactions of microplastics with heavy metals: mechanism and their combined effect on organisms and humans. Sci Total Environ 788:147620. https://doi.org/10.1016/j.scitotenv.2021.147620
Caruso C, Rizzo C, Mangano S, Poli A, Di Donato P, Nicolaus B, Di Marco G, Michaud L, Lo Giudice A (2018) Extracellular polymeric substances with metal adsorption capacity produced by Pseudoalteromonas sp. MER144 from Antarctic seawater. Environ Sci Pollut Res 25:4667–4677. https://doi.org/10.1007/s11356-017-0851-z
Casillo A, Lanzetta R, Parrilli M, Corsaro MM (2018) Exopolysaccharides from marine and marine extremophilic bacteria: structures, properties, ecological roles and applications. Mar Drugs 16:69. https://doi.org/10.3390/md16020069
Cervantes C, Gutierrezcorona F (1994) Copper resistance mechanisms in bacteria and fungi. FEMS Microbiol Rev 14:121–137. https://doi.org/10.1111/j.1574-6976.1994.tb00083.x
Chapman PM (2012) “Heavy metal”: cacophony, not symphony. Integr Environ Assess Manag 8:216. https://doi.org/10.1002/ieam.1289
Cheng F, Zhang T, Liu Y, Zhang Y, Qu J (2022) Non-negligible effects of UV irradiation on transformation and environmental risks of microplastics in the water environment. J Xenobiot 12:1–12. https://doi.org/10.3390/jox12010001
Chikkanna A, Ghosh D, Kishore A (2018) Expression and characterization of a potential exopolysaccharide from a newly isolated halophilic thermotolerant bacteria Halomonas nitroreducens strain WB1. PeerJ 6:e4684. https://doi.org/10.7717/peerj.4684
Concórdio-Reis P, Reis MAM, Freitas F (2020a) Biosorption of heavy metals by the bacterial exopolysaccharide FucoPol. Appl Sci 10:6708. https://doi.org/10.3390/app10196708
Concórdio-Reis P, Pereira CV, Batista MP, Sevrin C, Grandfils C, Marques AC, Fortunato E, Gaspar FB, Matias AA, Freitas F, Reis MAM (2020b) Silver nanocomposites based on the bacterial fucose-rich polysaccharide secreted by Enterobacter A47 for wound dressing applications: synthesis, characterization and in vitro bioactivity. Int J Biol Macromol 163:959–969. https://doi.org/10.1016/j.ijbiomac.2020.07.072
Corcoll N, Yang J, Backhaus T, Zhang X, Eriksson KM (2019) Copper affects composition and functioning of microbial communities in marine biofilms at environmentally relevant concentrations. Front Microbiol 9:3248. https://doi.org/10.3389/fmicb.2018.03248
Das P, Mukherjee S, Sen R (2008) Antimicrobial potential of a lipopeptide biosurfactant derived from a marine Bacillus circulans. J Appl Microbiol 104:1675–1684. https://doi.org/10.1111/j.1365-2672.2007.03701.x
Davranche M, Veclin C, Pierson-Wickmann AC, El Hadri H, Grassl B, Rowenczyk L, Dia A, Ter Halle A, Blancho F, Reynaud S, Gigault J (2019) Are nanoplastics able to bind significant amount of metals? The lead example. Environ Pollut 249:940–948. https://doi.org/10.1016/j.envpol.2019.03.087
de Araújo LCA, de Oliveira MBM (2020) Effect of heavy metals on the biofilm formed by microorganisms from impacted aquatic environments. In: Dincer S, Özdenefe MS, Arkut A (eds) Bacterial biofilms. IntechOpen, London
de Carvalho CCCR, Fernandes P (2010) Production of metabolites as bacterial responses to the marine environment. Mar Drugs 8:705–727. https://doi.org/10.3390/md8030705
De Philippis R, Colica G, Micheletti E (2011) Exopolysaccharide-producing cyanobacteria in heavy metal removal from water: molecular basis and practical applicability of the biosorption process. Appl Microbiol Biotechnol 92:697–708. https://doi.org/10.1007/s00253-011-3601-z
Delbarre-Ladrat C, Sinquin C, Lebellenger L, Zykwinska A, Colliec-Jouault S (2014) Exopolysaccharides produced by marine bacteria and their applications as glycosaminoglycan-like molecules. Front Chem 2:85. https://doi.org/10.3389/fchem.2014
Ding T, Wei L, Hou Z, Li J, Zhang C, Lin D (2022) Microplastics altered contaminant behaviour and toxicity in natural waters. J Hazard Mater 425:127908. https://doi.org/10.1016/j.jhazmat.2021.127908
Djaoudi K, Angel J, Onrubia T, Boukra A, Guesnay L, Portas A, Barry-Martinet R, Angeletti B, Mounier S, Lenoble V, Briand JF (2021) Seawater copper content controls biofilm bioaccumulation and microbial community on microplastics. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2021.152278
Duffus JH (2002) Heavy metals" a meaningless term? (IUPAC Technical Report). Pure Appl Chem 74:793–807. https://doi.org/10.1351/pac200274050793
Edelstein M, Ben-Hur M (2018) Heavy metals and metalloids: sources, risks and strategies to reduce their accumulation in horticultural crops. Sci Hortic 234:431–444. https://doi.org/10.1016/j.scienta.2017.12.039
Egan S, James S, Kjelleberg S (2002) Identification and characterization of a putative transcriptional regulator controlling the expression of fouling inhibitors in Pseudoalteromonas tunicata. Appl Environ Microbiol 68:372–378. https://doi.org/10.1128/AEM.68.1.372-378.2002
Elsakhawy TA, Sherief FA, Abd-EL-Kodoos RY (2017) Marine microbial polysaccharides: environmental role and applications (An overview). Environ Biodivers Soil Secur 1:61–70. https://doi.org/10.21608/jenvbs.2017.1053.1004
Escárcega-González CE, Garza-Cervantes JA, Vázquez-Rodríguez A, Morones-Ramírez JR (2018) Bacterial exopolysaccharides as reducing and/or stabilizing agents during synthesis of metal nanoparticles with biomedical applications. Int J Polym Sci. https://doi.org/10.1155/2018/7045852
Fan T, Zhao J, Chen Y, Wang M, Wang X, Wang S, Chen X, Lu A, Zha S (2021) Coexistence and adsorption properties of heavy metals by polypropylene microplastics. Adsorp Sci Technol. https://doi.org/10.1155/2021/4938749
Fashola MO, Ngole-Jeme VM, Babalola OO (2016) Heavy metal pollution from gold mines: environmental effects and bacterial strategies for resistance. Int J Environ Res Public Health 13:1047. https://doi.org/10.3390/ijerph13111047
Flemming HC, Wingender J (2001) Relevance of microbial extracellular polymeric substances (EPSs)–Part I: structural and ecological aspects. Water Sci Technol 43:1–8. https://doi.org/10.2166/wst.2001.0326
Flemming H, Wingender J, Szewzyk U (2016) Biofilms: an emergent form of bacterial life. Nat Rev Microbiol 14:563–575. https://doi.org/10.1038/nrmicro.2016.94
Fletcher M, Floodgate GD (1973) An electron microscopic demonstration of an acidic polysaccharide involved in adhesion of a marine bacterium to solid surfaces. Microbiol 74:325–334. https://doi.org/10.1099/00221287-74-2-325
Fu B, Sengupta K, Genova LA, Santiago AG, Jung W, Krzemiński Ł, Chakraborty UK, Zhang W, Chen P (2020) Metal-induced sensor mobilization turns on affinity to activate regulator for metal detoxification in live bacteria. Proc Natl Acad Sci 117:13248–13255. https://doi.org/10.1073/pnas.1919816117
Fu Q, Tan X, Ye S, Ma L, Gu Y, Zhang P, Chen Q, Yang Y, Tang Y (2021) Mechanism analysis of heavy metal lead captured by natural-aged microplastics. Chemosphere 270:128624. https://doi.org/10.1016/j.chemosphere.2020.128624
Gahlawat G, Shikha S, Chaddha BS, Chaudhuri SR, Mayilraj S, Choudhury AR (2016) Microbial glycolipoprotein-capped silver nanoparticles as emerging antibacterial agents against cholera. Microb Cell Fact 15:25. https://doi.org/10.1186/s12934-016-0422-x
Gao F, Li J, Sun C, Zhang L, Jiang F, Cao W, Zheng L (2019) Study on the capability and characteristics of heavy metals enriched on microplastics in marine environment. Mar Pollut Bull 144:61–67. https://doi.org/10.1016/j.marpolbul.2019.04.039
Gardham S, Hose GC, Stephenson S, Chariton AA (2014) DNA metabarcoding meets experimental ecotoxicology: advancing knowledge on the ecological effects of copper in freshwater ecosystems. Adv Ecol Res 51:79–104. https://doi.org/10.1016/B978-0-08-099970-8.00007-5
Garza-Cervantes JA, Escárcega-González CE, Díaz Barriga Castro E, Mendiola-Garza G, Marichal-Cancino BA, López-Vázquez MA, Morones-Ramirez JR (2019) Antimicrobial and antibiofilm activity of biopolymer-Ni, Zn nanoparticle biocomposites synthesized using R. mucilaginosa UANL-001L exopolysaccharide as a capping agent. Int J Nanomed 14:2557–2571. https://doi.org/10.2147/IJN.S196470
Giner-Lamia J, Pereira SB, Bovea-Marco M, Futschik ME, Tamagnini P, Oliveira P (2016) Extracellular proteins: novel key components of metal resistance in cyanobacteria? Front Microbiol 7:878. https://doi.org/10.3389/fmicb.2016.00878
Godoy V, Blázquez G, Calero M, Quesada L, Martín-Lara MA (2019) The potential of microplastics as carriers of metals. Environ Pollut 255:113363. https://doi.org/10.1016/j.envpol.2019.113363
Guan J, Qi K, Wang J, Wang W, Wang Z, Lu N, Qu J (2020) Microplastics as an emerging anthropogenic vector of trace metals in freshwater: significance of biofilms and comparison with natural substrates. Water Res 184:116205. https://doi.org/10.1016/j.watres.2020.116205
Gupta P, Diwan B (2017) Bacterial exopolysaccharide mediated heavy metal removal: A Review on biosynthesis, mechanism and remediation strategies. Biotechnol Rep 13:58–71. https://doi.org/10.1016/j.btre.2016.12.006
Gutierrez T, Shimmield T, Haidon C, Black K, Green DH (2008) Emulsifying and metal ion binding activity of a glycoprotein exopolymer produced by Pseudoalteromonas sp. strain TG12. Appl Environ Microbiol 74:4867–4876. https://doi.org/10.1128/AEM.00316-08
Han X, Wang S, Yu X, Vogt RD, Feng J, Zhai L, Ma W, Zhu L, Lu X (2021) Kinetics and size effects on adsorption of Cu(II), Cr(III), and Pb(II) onto polyethylene, polypropylene, and polyethylene terephthalate microplastic particles. Front Mar Sci. https://doi.org/10.3389/fmars.2021.785146
Holmes LA, Turner A, Thompson RC (2014) Interactions between trace metals and plastic production pellets under estuarine conditions. Mar Chem 167:25–32. https://doi.org/10.1016/j.marchem.2014.06.001
Hu QP, Liu XQ (2021) Study on the adsorption of heavy metal by extracellular polymeric substances extracted from Proteiniphilum acetatigenes PSB-W. OALibJ 8:1–9. https://doi.org/10.4236/oalib.1107228
Hüffer T, Weniger AK, Hofmann T (2018) Sorption of organic compounds by aged polystyrene microplastic particles. Environ Pollut 236:218–225. https://doi.org/10.1016/j.dib.2018.03.053
Jittawuttipoka T, Planchon M, Spalla O, Benzerara K, Guyot F, Cassier-Chauvat C, Chauvat F (2013) Multidisciplinary evidences that Synechocystis PCC6803 exopolysaccharides operate in cell sedimentation and protection against salt and metal stresses. PLoS ONE 8:e55564. https://doi.org/10.1371/journal.pone.0055564
Kalčíková G, Skalar T, Marolt G, Kokalj AJ (2020) An environmental concentration of aged microplastics with adsorbed silver significantly affects aquatic organisms. Water Res 175:115644. https://doi.org/10.1016/j.watres.2020.115644
Kang F, Alvarez PJ, Zhu D (2013) Microbial extracellular polymeric substances reduce Ag+ to silver nanoparticles and antagonize bactericidal activity. Environ Sci Technol 48:316–322. https://doi.org/10.1021/es403796x
Khalid N, Aqeel M, Noman A, Khan SM, Akhter N (2021) Interactions and effects of microplastics with heavy metals in aquatic and terrestrial environments. Environ Pollut 290:118104. https://doi.org/10.1016/j.envpol.2021.118104
Khatoon Z, McTiernan CD, Suuronen EJ, Mah TF, Alarcon EI (2018) Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 4:e01067. https://doi.org/10.1016/j.heliyon.2018.e01067
Kiran GS, Sabu A, Selvin J (2010a) Synthesis of silver nanoparticles by glycolipid biosurfactant produced from marine Brevibacterium casei MSA19. J Biotechnol 148:221–225. https://doi.org/10.1016/j.jbiotec.2010.06.012
Kiran GS, Sabarathnam B, Selvin J (2010b) Biofilm disruption potential of a glycolipid biosurfactant from marine Brevibacterium casei. FEMS Immunol Med Microbiol 59:432–438. https://doi.org/10.1111/j.1574-695X.2010.00698.x
Kiran GS, Thomas TA, Selvin J (2010c) Production of a new glycolipid biosurfactant from marine Nocardiopsis lucentensis MSA04 in solid-state cultivation. Coll Surf b: Biointerfaces 78:8–16. https://doi.org/10.1016/j.colsurfb.2010.01.028
Koller M, Saleh HM (2018) Introductory chapter: introducing heavy metals. In: Saleh HM, Aglan RF (eds) Heavy metals. InTech, London
Kumar R, Sharma P, Manna C, Jain M (2021) Abundance, interaction, ingestion, ecological concerns, and mitigation policies of microplastic pollution in riverine ecosystem: a review. Sci Total Environ 782:146695. https://doi.org/10.1016/j.scitotenv.2021.146695
Le Costaouëc T, Cérantola S, Ropartz D, Ratiskol J, Sinquin C, Colliec-Jouault S, Boisset C (2012) Structural data on a bacterial exopolysaccharide produced by a deep-sea Alteromonas macleodii strain. Carbohydr Polym 90:49–59. https://doi.org/10.1016/j.carbpol.2012.04.059
Li Y, Li Q, Fengying Y, Bao J, Hu Z, Zhu W, Zhao Y, Lin Z, Dong Q (2015) Chromium (VI) detoxification by oxidation and flocculation of exopolysaccharides from Arthrobacter sp. B4. Int J Biol Macromol 81:235–240. https://doi.org/10.1016/j.ijbiomac.2015.07.013
Li Y, Li Q, Bao J (2017) Rapid biosynthesis of silver nanoparticles based on flocculation and reduction of an exopolysaccharide from Arthrobacter sp. B4: its antimicrobial activity and phytotoxicity. J Nanomater 2017:1–8. https://doi.org/10.1155/2017/9703614
Limoli DH, Jones CJ, Wozniak DJ (2015) Bacterial extracellular polysaccharides in biofilm formation and function. Microbiol Spectr. https://doi.org/10.1128/microbiolspec.MB-0011-2014
Lin Z, Hu Y, Yuan Y, Hu B, Wang B (2021a) Comparative analysis of kinetics and mechanisms for Pb(II) sorption onto three kinds of microplastics. Ecotoxicol Environ Saf 208:111451. https://doi.org/10.1016/j.ecoenv.2020.111451
Lin WH, Kuo J, Lo SL (2021b) Effect of light irradiation on heavy metal adsorption onto microplastics. Chemosphere 285:131457. https://doi.org/10.1016/j.chemosphere.2021.131457
Liu G, Dave PH, Kwong RWM, Wu M, Zhong H (2021a) Influence of microplastics on the mobility, bioavailability, and toxicity of heavy metals: a review. Bull Environ Contam Toxicol 107:710–721. https://doi.org/10.1007/s00128-021-03339-9
Liu S, Shi J, Wang J, Dai Y, Li H, Li J, Liu X, Chen X, Wang Z, Zhang P (2021b) Interactions between microplastics and heavy metals in aquatic environments: a review. Front Microbiol 12:652520. https://doi.org/10.3389/fmicb.2021.652520
Llamas I, Amjres H, Mata JA, Quesada E, Béjar V (2012) The potential biotechnological applications of the exopolysaccharide produced by the halophilic bacterium Halomonas almeriensis. Molecules 17:7103–7120. https://doi.org/10.3390/molecules17067103
Lu S, Zhu K, Song W, Song G, Chen D, Hayat T, Alharbi N, Chen C, Sun Y (2018) Impact of water chemistry on surface charge and aggregation of polystyrene microspheres suspensions. Sci Total Environ 630:951–959. https://doi.org/10.1016/j.scitotenv.2018.02.296
Mao R, Lang M, Yu X, Wu R, Yang X, Guo X (2020) Aging mechanism of microplastics with UV irradiation and its effects on the adsorption. J Hazard Mater 393:122515. https://doi.org/10.1016/j.jhazmat.2020.122515
Mathivanan K, Chandirika JU, Mathimani T, Rajaram R, Annadurai G, Yin H (2021) Production and functionality of exopolysaccharides in bacteria exposed to a toxic metal environment. Ecotoxicol Environ Saf 208:111567. https://doi.org/10.1016/j.ecoenv.2020.111567
Maugeri TL, Gugliandolo C, Caccamo D, Panico A, Lama L, Gambacorta A, Nicolaus B (2002) A halophilic thermotolerant Bacillus isolated from a marine hot spring able to produce a new exopolysaccharide. Biotechnol Lett 24:515–519. https://doi.org/10.1023/A:1014891431233
McGivney E, Cederholm L, Barth A, Hakkarainen M, Hamacher-Barth E, Ogonowski M, Gorokhova E (2020) Rapid physicochemical changes in microplastic induced by biofilm formation. Front Bioeng Biotechnol 8:205. https://doi.org/10.3389/fbioe.2020.00205
Mohite BV, Koli SH, Narkhede CP, Patil SN, Patil SV (2017) Prospective of microbial exopolysaccharide for heavy metal exclusion. Appl Biochem Biotechnol 183:582–600. https://doi.org/10.1007/s12010-017-2591-4
Nichols CM, Guezennec J, Bowman JP (2005) Bacterial exopolysaccharides from extreme marine environments with special consideration of the southern ocean, sea ice, and deep-sea hydrothermal vents: a review. Mar Biotechnol 7:253–271. https://doi.org/10.1007/s10126-004-5118-2
Nieboer E, Richardson DHS (1980) The replacement of the nondescript term “heavy metals” by a biologically and chemically significant classification of metal ions. Environ Pollut B 1:3–26. https://doi.org/10.1016/0143-148X(80)90017-8
Nouha K, Kumar RS, Tyagi RD (2016) Heavy metals removal from wastewater using extracellular polymeric substances produced by Cloacibacterium normanense in wastewater sludge supplemented with crude glycerol and study of extracellular polymeric substances extraction by different methods. Bioresour Technol 212:120–129. https://doi.org/10.1016/j.biortech.2016.04.021
Ozturk S, Kaya T, Aslim B, Tan S (2012) Removal and reduction of chromium by Pseudomonas spp. and their correlation to rhamnolipid production. J Hazard Mater 231–232:64–69. https://doi.org/10.1016/j.jhazmat.2012.06.038
Parmar P, Shukla A, Goswami D, Gaur S, Patel B, Saraf M (2020) Comprehensive depiction of novel heavy metal tolerant and EPS producing bioluminescent Vibrio alginolyticus PBR1 and V. rotiferianus PBL1 confined from marine organisms. Microbiol Res 238:154. https://doi.org/10.1016/j.mi:126526cres.2020.126526
Planchon M, Jittawuttipoka T, Cassier-Chauvat C, Guyot F, Gelabert A, Benedetti MF, Chauvat F, Spalla O (2013) Exopolysaccharides protect Synechocystis against the deleterious effects of titanium dioxide nanoparticles in natural and artificial waters. J Coll Interface Sci 405:35–43. https://doi.org/10.1016/j.jcis.2013.05.061
Pourret O, Hursthouse A (2019) It’s time to replace the term “heavy metals” with “potentially toxic elements” when reporting environmental research. Int J Environ Res Public Health 16:4446. https://doi.org/10.3390/ijerph16224446
Pourret O, Bollinger JC, Hursthouse A (2021) Heavy metal: a misused term? Acta Geochimica 40:466–471. https://doi.org/10.1007/s11631-021-00468-0
Pu L, Zeng YJ, Xu P, Li FZ, Zong MH, Yang JG, Lou WY (2020) Using a novel polysaccharide BM2 produced by Bacillus megaterium strain PL8 as an efficient bioflocculant for wastewater treatment. Int J Biol Macromol 162:374–384. https://doi.org/10.1016/j.ijbiomac.2020.06.167
Purwiyanto AIS, Suteja Y, Trisno NPS, Putri WAE, Rozirwan AF, Fauziyah CMR, Koropitan AF (2020) Concentration and adsorption of Pb and Cu in microplastics: case study in aquatic environment. Mar Pollut Bull 158:111380. https://doi.org/10.1016/j.marpolbul.2020.111380
Qi K, Lu N, Zhang S, Wang W, Wang Z, Guan J (2021) Uptake of Pb(II) onto microplastic-associated biofilms in freshwater: adsorption and combined toxicity in comparison to natural solid substrates. J Hazard Mater 411:125115. https://doi.org/10.1016/j.jhazmat.2021.125115
Raj R, Dalei K, Chakraborty J, Das S (2016) Extracellular polymeric substances of a marine bacterium mediated synthesis of CdS nanoparticles for removal of cadmium from aqueous solution. J Coll Interface Sci 462:166–175. https://doi.org/10.1016/j.jcis.2015.10.004
Raj K, Sardar UR, Bhargavi E, Devi I, Bhunia B, Tiwari ON (2018) Advances in exopolysaccharides based bioremediation of heavy metals in soil and water: a critical review. Carbohydr Polym 199:353–364. https://doi.org/10.1016/j.carbpol.2018.07.037
Raychaudhuri SS, Pramanick P, Talukder P, Basak A (2021) Chapter 6-Polyamines, metallothioneins, and phytochelatins: natural defense of plants to mitigate heavy metals. Stud Nat Prod Chem 69:227–261. https://doi.org/10.1016/B978-0-12-819487-4.00006-9
Richard H, Carpenter EJ, Komada T, Palmer PT, Rochman CM (2019) Biofilm facilitates metal accumulation onto microplastics in estuarine waters. Sci Total Environ 683:600–608. https://doi.org/10.1016/j.scitotenv.2019.04.331
Rochman CM, Hentschel BT, Teh SJ (2014) Long-term sorption of metals is similar among plastic types: implications for plastic debris in aquatic environments. PLoS ONE 9:e85433. https://doi.org/10.1371/journal.pone.0085433
Rodrigues JP, Duarte AC, Santos-Echeandía J, Rocha-Santos T (2019) Significance of interactions between microplastics and POPs in the marine environment: a critical overview. Trends Anal Chem 111:252–260. https://doi.org/10.1016/j.trac.2018.11.038
Rozman U, Kalčíková G (2022) Seeking for a perfect (non-spherical) microplastic particle: the most comprehensive review on microplastic laboratory research. J Hazard Mater 424:127529. https://doi.org/10.1016/j.jhazmat.2021.127529
Rummel CD, Jahnke A, Gorokhova E, Kühnel D, Schmitt M (2017) Impacts of biofilm formation on the fate and potential effects of microplastic in the aquatic environment. Environ Sci Technol Lett 4:258–267. https://doi.org/10.1021/acs.estlett.7b00164
Sathiyanarayanan G, Kiran SG, Selvin J (2013) Synthesis of silver nanoparticles by polysaccharide bioflocculant produced from marine Bacillus subtilis MSBN17. Coll Surf b: Biointerfaces 102:13–20. https://doi.org/10.1016/j.colsurfb.2012.07.032
Sathiyanarayanan G, Dineshkumar K, Yang YH (2017) Microbial exopolysaccharide-mediated synthesis and stabilization of metal nanoparticles. Crit Rev Microbiol 43:731–752. https://doi.org/10.1080/1040841X.2017.1306689
Selvam S, Jesuraja K, Venkatramanan S, Roy PD, Kumari VJ (2021) Hazardous microplastic characteristics and its role as a vector of heavy metal in groundwater and surface water of coastal south India. J Hazard Mater 402:123786. https://doi.org/10.1016/j.jhazmat.2020.123786
Serra A, Guasch H, Martí E, Geiszinger A (2009) Measuring in-stream retention of copper by means of constant-rate additions. Sci Total Environ 407:3847–3854. https://doi.org/10.1016/j.scitotenv.2009.01.056
Shah V, Ray A, Garg N, Madamwar D (2000) Characterization of the extracellular polysaccharide produced by a marine cyanobacterium, Cyanothece sp. ATCC 51142, and its exploitation toward metal removal from solutions. Curr Microbiol 40:274–278. https://doi.org/10.1007/s002849910054
Sleight VA, Bakir A, Thompson RC, Henry TB (2017) Assessment of microplastic-sorbed contaminant bioavailability through analysis of biomarker gene expression in larval zebrafish. Mar Pollut Bull 116:291–297. https://doi.org/10.1016/j.marpolbul.2016.12.055
Spanò A, Laganà P, Visalli G, Maugeri TL, Gugliandolo C (2016) In vitro antibiofilm activity of an exopolysaccharide from the marine thermophilic Bacillus licheniformis T14. Curr Microbiol 72:518–528. https://doi.org/10.1007/s00284-015-0981-9
Stabnikova O, Stabnikov V, Marinin A, Klavins M, Klavins L, Vaseashta A (2021) Microbial life on the surface of microplastics in natural waters. Appl Sci (switzerland) 11:11692. https://doi.org/10.3390/app112411692
Swapna TH, Papathoti NK, Khan MY, Reddy G, Hameeda B (2016) Bioreduction of Cr (VI) by biosurfactant producing marine bacterium Bacillus subtilis SHB 13. Int J Sci Res 75:432–438
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. Exp Suppl 101:133–164. https://doi.org/10.1007/978-3-7643-8340-4_6
Torres FG, Dioses-Salinas DC, Pizarro-Ortega CI, De-la-Torre GE (2021) Sorption of chemical contaminants on degradable and non-degradable microplastics: recent progress and research trends. Sci Total Environ 757:143875. https://doi.org/10.1016/j.scitotenv.2020.143875
Tu C, Chen T, Zhou Q, Liu Y, Wei J, Waniek J, Luo Y (2020) Biofilm formation and its influences on the properties of microplastics as affected by exposure time and depth in the seawater. Sci Total Environ 734:139237. https://doi.org/10.1016/j.scitotenv.2020.139237
Turner A, Holmes LA (2015) Adsorption of trace metals by microplastic pellets in fresh water. Environ Chem 12:600–610. https://doi.org/10.1071/EN14143
Vaseashta A, Ivanov V, Stabnikov V, Marinin A (2021) Environmental safety and security investigations of neustonic microplastic aggregates near water-air interphase. Pol J Environ Stud 30:3457–3469. https://doi.org/10.15244/pjoes/131947
Vazquez-Rodriguez A, Vasto-Anzaldo XG, Barboza Perez D, Vázquez-Garza E, Chapoy-Villanueva H, García-Rivas G, Garza-Cervantes JA, Gómez-Lugo JJ, Gomez-Loredo AE, Garza Gonzalez MT, Zarate X, Morones-Ramirez JR (2018) Microbial competition of Rhodotorula mucilaginosa UANL-001L and E. coli increase biosynthesis of non-toxic exopolysaccharide with applications as a wide-spectrum antimicrobial. Sci Rep 8:798. https://doi.org/10.1038/s41598-017-17908-8
Wang Q, Zhang Y, Wangjin X, Wang Y, Meng G, Chen Y (2020) The adsorption behaviour of metals in aqueous solution by microplastics effected by UV radiation. J Environ Sci 87:272–280. https://doi.org/10.1016/j.jes.2019.07.006
Wang J, Guo X, Xue J (2021) Biofilm-developed microplastics as vectors of pollutants in aquatic environments. Environ Sci Technol 55:12780–12790. https://doi.org/10.1021/acs.est.1c04466
Wingender J, Neu TR, Flemming HC (1999) What are bacterial extracellular polymeric substances? In: Wingender J, Neu TR, Flemming HC (eds) Microbial extracellular polymeric substances. Springer, Berlin, Heidelberg, pp 1–19
Wu C, Tanaka K, Tani Y, Bi X, Liu J, Yu Q (2022) Effect of particle size on the colonization of biofilms and the potential of biofilm-covered microplastics as metal carriers. Sci Total Environ 821:153265. https://doi.org/10.1016/j.scitotenv.2022.153265
Xu K, Li Z, Juneau P, Xiao F, Lian Y, Zhang W, Shu L, Jiang H, Zhang K, Wang C, Wang S, Yan Q, He Z (2021) Toxic and protective mechanisms of cyanobacterium Synechocystis sp in response to titanium dioxide nanoparticles. Environ Pollut 274:116508. https://doi.org/10.1016/j.envpol.2021.116508
Yang J, Wei W, Pi S, Ma F, Li A, Wu D, Xing J (2015) Competitive adsorption of heavy metals by extracellular polymeric substances extracted from Klebsiella sp. J1. Bioresour Technol 196:533–539. https://doi.org/10.1016/j.biortech.2015.08.011
Yang J, Xie Y, Jeppe K, Long S, Pettigrove V, Zhang X (2018) Sensitive community responses of microbiota to copper in sediment toxicity test. Environ Toxicol Chem 37:599–608. https://doi.org/10.1002/etc.3980
Yin YJ, Tian ZM, Tang W, Li L, Song LY, McElmurry SP (2014) Production and characterization of high efficiency bioflocculant isolated from Klebsiella sp. ZZ-3. Bioresour Technol 171:336–342. https://doi.org/10.1016/j.biortech.2014.08.094
Yu F, Yang C, Zhu Z, Bai X, Ma J (2019) Adsorption behaviour of organic pollutants and metals on micro/nanoplastics in the aquatic environment. Sci Total Environ 694:133643. https://doi.org/10.1016/j.scitotenv.2019.133643
Zhang Z, Cai R, Zhang W, Fu Y, Jiao N (2017) A novel exopolysaccharide with metal adsorption capacity produced by a marine bacterium Alteromonas sp. JL2810. Mar Drugs 15:175. https://doi.org/10.3390/md15060175
Zou J, Liu X, Zhang D, Yuan X (2020) Adsorption of three bivalent metals by four chemical distinct microplastics. Chemosphere 248:126064. https://doi.org/10.1016/j.chemosphere.2020.126064
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no known competing financial interests or personal relationships that could have appeared to influence the work reported in this review.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Stabnikova, O., Stabnikov, V., Marinin, A. et al. The role of microplastics biofilm in accumulation of trace metals in aquatic environments. World J Microbiol Biotechnol 38, 117 (2022). https://doi.org/10.1007/s11274-022-03293-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-022-03293-6