Abstract
Microplastics (MPs) can pose ecological risk to the environment and have the potential to negatively affect human health, raising serious public concerns. It is recognized that MPs could act as a vector for various environmental pollutants including heavy metals and potentially influencing their mobility, fate, and bioavailabilty in the environment. However, knowledge on the mechanisms underpinning the interaction processes between MPs and heavy metals is far from clear. This review discusses the effects of MPs on the adsorption/desorption, speciation and bioavailability, and toxicity of various heavy metals. The present review also systematically identifies the environmental factors (e.g., pH, ionic strength, and organic matters) that could affect their interaction processes. This work aims to establish a meaningful perspective for a comprehensive understanding of the indirect ecological risks of MPs as vectors for contaminants. The work also provides a reference for the development of better regulatory strategies in mitigating the negative effects caused by the co-existence of MPs and heavy metals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
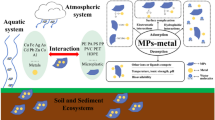
The favorable properties of plastics, including lightweight, durability, and low price, make them an inevitable part of our modern life, leading to an exponential increase in their production in the past decades. Generally, plastics with particle size smaller than 5 mm are defined as microplastics (MPs) (Alimi et al. 2018; Strungaru et al. 2019). Compared with larger plastics, MPs (including nanoplastics, NPs) are more difficult to be intercepted by specialized filter organs such as gastric filters. As a result, MPs and NPs are much easier to pass through epithelial tissues via transformation and be accumulated in the biological systems (e.g., circulatory system in mussels, gills and gut ) once they are ingested by organisms (Lu et al. 2016; Kim et al. 2021). Therefore, MPs or NPs with smaller size and larger specific surface area have higher potential to induce toxicity. In addition, several MPs have been shown to exhibit significant adsorption capacity for various contaminants, primarily due to their large specific surface area (Abbasi et al. 2020; Abdolahpur Monikh et al. 2020; Dong et al. 2020). The interactions between MPs and contaminants may alter their environmental behaviors such as chemical speciation, bioavailability, and toxicity, potentially generating new undefined risks to animals and ecosystem health. Recently, related studies have mainly focused on the interactions between hydrophobic organic contaminants and MPs because of their similar physiochemical properties (Fries and Zarfl 2012; Koelmans et al. 2013). However, relatively little information has been known about the influences of MPs on inorganic pollutants such as heavy metals.
Environmental behaviors and ecological risks of heavy metals could be affected by MPs from direct or indirect pathways. On one hand, MPs can directly alter physicochemical properties such as adsorption and desorption processes of heavy metals, resulting in changes to the bioaccumulation and toxic effects on organisms (Godoy et al. 2019; Lee et al. 2019; Lian et al. 2020; Liao and Yang 2020; Lu et al. 2020; Wakkaf et al. 2020; Zhou et al. 2020). On the other hand, environmental factors such as pH and dissolved organic matter can affect MP-metal interactions, resulting in changes in the speciation and bioavailability of metals (Yu et al. 2020, 2021). These physiolchemical changes would likely affect the environmental risks of MPs as well as metals. However, the interaction processes of different heavy metals to distinct MPs could be heterogeneous and complex, and the paramters governing MP-metal interactions have remained largely unknown. Currently, available reviews have mainly focused on analytical methods, ecotoxicological effects, microbial/chemical degradation, and detection and removal of MPs (Dey et al. 2021; Gao et al. 2020; Huang et al. 2021; Li et al. 2020a; Othman et al. 2021). However, reviews on the effects of MPs on the environmental behaviors of heavy metals is limited. Improving our understanding on MP-metal interactions is important for reducing the uncertainy in ecological risk assessment, particularly where elevated MPs and heavy metals co-exist in the environment.
Under this context, the present work aims to (1) provide a critical review of the influence of MPs on the adsorption, desorption, speciation, bioavailability, accumulation, and toxicity of heavy metals; (2) summarize the environmental parameters that could affect the abovementioned processes; and (3) critically assess the possible mechanisms that may govern their interactions in aquatic or terrestrial environments. The significance of this review is to provide a reference toward the development of better mitigation methods for the negative impacts of these contaminants.
Effects of MPs on the Environmental Behaviors of Heavy Metals
The unique physicochemical properties of MPs make them strong adsorbents/vectors for heavy metals, which can significantly alter the bioavailability and toxicity of metals (Table 1).
Adsorption and Desorption of Heavy Metals
MPs can accumulate diverse heavy metals such as lead (Pb), cadmium (Cd), copper (Cu), chromium (Cr), nickel (Ni), zinc (Zn) etc., while adsorbed/additive heavy metals can also be released from the surface or inside of MPs (Godoy et al. 2019; Abbasi et al. 2020; Kern et al. 2021; Liao and Yang 2020). These processes can therefore change the environmental concentrations of metals. In fact, several studies have shown that concentrations of heavy metals have a positive or negative significant correlation with the amount of MPs in sediment/organisms (Akhbarizadeh et al. 2017, 2018; Yazdani Foshtomi et al. 2019; Zhu et al. 2020). In addition, MPs show different adsorption and desorption capacities for various heavy metals (Godoy et al. 2019; Wang et al. 2020c). For instance, the sorption strength of PS MPs with metals was found to follow the order of Pb2+ > Cu2+ > Cd2+ > Ni2+ (Yuan et al. 2020). Additionally, the release of Cr from MPs was found to be faster than Pb under the same conditions (Godoy et al. 2020). This may be attributed to the differences of distinct hydrated ionic radii and complexing capabilities/affinities of the divalent cations (Zou et al. 2020). The adsorption, release, and diffusion behavior of heavy metals can also be affected by the composition of MPs, primarily owing to the distinct properties of various MPs (e.g., specific surface area, porosity, morphology, surface charge and/or surface degradation). The experimental medium as well as the various characteristics of heavy metals may also contribute to the differences in the adsorption properties of metals onto MPs (Godoy et al. 2019; Li et al. 2019; Guo et al. 2020b; Liao and Yang 2020).
Speciation and Bioavailability of Heavy Metals
The existence of MPs can alter metal speciation in the environment. For instance, MPs increase the organic-bound fractions of heavy metals by direct adsorption and subsequently changing the soil physicochemical and biogeochemical properties such as dissolved organic carbon (DOC) and pH (Yu et al. 2020, 2021). Additionally, the presence of MPs in the soil can decrease the exchangeable, carbonate-bound, and Fe-Mn oxide-bound fractions of Cu, Cr, and Ni (Yu et al. 2020). On the other hand, a study showed that soil diethylenetriaminepentaacetic acid (DTPA)-extractable Cd concentrations increased due to the presence of HDPE, PE, PLA, and PS (Wang et al. 2020a, b). Speciation changes may ultimately alter the bioavailability of metals or metalloids in environmental matrices. For example, the presence of MPs in the soil was found to decrease Arsenic (As, metalloid) bioavailability due to As adsorption by MPs (Dong et al. 2021). In contrast, Cd bioaccessibility to earthworms was shown to increase in MP-contamainted soil (Zhou et al. 2020). In addition, it has been demonstrated that Cd exhibited a higher bioavailability when PLA is present in soil-plant systems, when compared to PE (Wang et al. 2020b). Collectively, these studies showed that the influence of MPs on metal speciation and bioavailability can be modified by a variety of factors, such as the types of MPs, soil chemistry, and environmental conditions (e.g., incubation time, experimental mediums, etc.) (Liao and Yang 2020; Yu et al. 2020, 2021).
Changes in the Accumulation and Toxicity of Heavy Metals by MPs
The interactions between MPs and heavy metals may modify their bioaccumulation and toxicity. For example, the adverse effects of MPs and/or heavy metals on organisms could be synergistically exacerbated in a dose- or size-dependent manner when these two pollutants coexist (Lee et al. 2019; Wang et al. 2020a). There is no toxicity of 1 mg L− 1 PS nanoscale plastic debris or 1 µg L− 1 Ag+ to Daphnia magna; however, toxicity was observed when the organisms were exposed to a mixture of these two contaminants (Abdolahpur Monikh et al. 2020). Another example is that of growth inhibition in animals, which has been observed in yellow seahorse and earthworms with higher accumulations of Cd (Lu et al. 2018; Banaee et al. 2019; Sun et al. 2019; Yan et al. 2020; Zhou et al. 2020; Wang et al. 2021). This may be attributed to the oxidative damage and the inflammatory responses caused by the coexistence of these two contaminants (Lu et al. 2018; Wen et al. 2018; Lee et al. 2019). Interestingly, several studies have also demonstrated some opposite phenomenon, i.e., organisms may be protected by the interactions between Cd and chelating MPs. For example, PVC was found to reduce the toxicity of Cd to nematodes, possibly due to the high chelating capacity of PVC and its polymers (Wakkaf et al. 2020). Moreover, the presence of MPs partially reduced Cd contents in leaves of Triticum aestivum L., and decreased the mercury (Hg) and Cd accumulation by Dicentrarchus labrax and Symphysodon aequifasciatus, respectively (Barboza et al. 2018; Wen et al. 2018; Lian et al. 2020). Furthermore, Hg elimination in the mussel, Mytilus galloprovincialis was promoted when MPs were present (Fernández et al. 2020). In addition, MPs were not found to enhance Cd bioconcentrations in plant tissues nor inhibit algal growth after the combined exposure with Cu (Bellingeri et al. 2019; Wang et al. 2020a, b). Moreover, results showed that the presence of organisms such as fish also influenced concentration of Hg in solution and the interaction processes between MPs and heavy metals (Barboza et al. 2018). These modifications would in turn affect the toxicity and bioaccumulation of metals in organisms.
Influecne of Environmental Factors and Possible Mechanisms
Various environmental factors such as water pH, ionic strength, and organic matters can directly (adsorption, complexation effects) or indirectly influence the chemical speciation, transport, and fate of heavy metals on MPs (Table 2).
pH
pH can alter the zeta potential of MPs, or the precipitation of heavy metals, thus increasing or decreasing the adsorption amounts of some metals. In general, the MPs zeta potentials decrease with increasing pH value. If the point of zero charge of MPs is lower than water pH, the charge of MPs becomes negative; this would increase the electrostatic attraction between polymers and metals (i.e., cations). On the other hand, the precipitation of some metals is very likely to occur in environments when pH > 7. A study showed that the adsorption of some heavy metals on MPs is pH-dependent (Godoy et al. 2019), and the increase in pH value was found to enhance the adsorption amounts of Pb, Cd, Co, Ni, Cu, and Zn onto MPs (Holmes et al. 2014; Guo et al. 2020b; Lin et al. 2021a, b; Purwiyanto et al. 2020; Tang et al. 2020; Wang et al. 2020c; Zou et al. 2020). These increases were likely attributed to the increase of charged sites on MPs. In contrast, Cr6+ adsorption onto MPs was found to reduce as the pH was increased, possibly due to the relatively weak coulombic interactions between the oxyanionic form of Cr6+ and the MPs with reduced positive charge in their surface (Holmes et al. 2014). On the other hand, pH was shown to have no influence on Cu adsorption (Holmes et al. 2014). Overall, pH appears to affect the adsorption of heavy metals on MPs by modifying the electrostatic properties on the MP surface (Guo et al. 2020b; Lin et al. 2021a).
Ionic Strength
The surface charge, sorption site, aggregation of MPs, and activity of heavy metals, can be modified by ionic strength. This will in turn affect the adsorption behaviors between heavy metals and MPs. In contrast to the observation with pH, the adsorption of heavy metals (Cd, Cu, Co, Ni, and Pb etc.) on MPs is inhibited by increasing ionic strength (Holmes et al. 2014; Guo et al. 2020b; Lian et al. 2020; Tang et al. 2020; Lin et al. 2021a, b). This is because high ionic strength can increase the competition of heavy metals on the sorption sites of MPs while simultaneously decreasing the activity of heavy metals in solution (Zou et al. 2020). However, high ionic strength was shown to increase Cr6+ adsorption due to a reduction in the negative-negative (surface-chromate) repulsion between MP and Cr6+ (Holmes et al. 2014). On the other hand, because of the strong complexation of Cu2+ with CPE, the effect of increasing ionic strength on Cu2+ sorption to CPE is minimal (Zou et al. 2020). A few studies have demonstrated that salinity has no significant effects on MPs adsorption of Cu and Pb (Holmes et al. 2014; Purwiyanto et al. 2020).
Organic Matters
The presence of organic matter can alter the adsorption/desorption capacities, bioaccumulation, and toxicity of heavy metals via interfering with the interaction processes between MPs and heavy metals. The attachment of natural organic matter to the functionalized surfaces of MPs may facilitate their binding with heavy metals (Bradney et al. 2019; Li et al. 2019). For example, Cd sorption on MPs was found to increase with increasing humic acid levels (Guo et al. 2020b). The sorption of Ag, Pb, Cr, and Cu onto MPs was also found to be enhanced by organic matters (Godoy et al. 2019; Qiao et al. 2019; Abdolahpur Monikh et al. 2020). These enhanacements may be ascribed to the increased adsorption sites and the abundance of functional groups on the MP surfaces. Moreover, the bioaccumulation and toxicity of Cu in fish was found to be elevated by the coexistence of MPs and natural organic matters, likely because of Cu-ion transport inhibition in hepatocytes and oxidative stress enhancement (Qiao et al. 2019). Additionally, organic matter can compete with metals for the adsorption sites on the MPs, which reduces the available heavy metals for MPs sorption, resulting in the desorption of heavy metals (Abbasi et al. 2020). For example, Pb2+ adsorption is reduced with increased fulvic acid concentration, possibly due to the increased complexation between Pb2+ and fulvic acid (Tang et al. 2020). Moreover, the addition of dissolved organic matter inhibited the combined toxicity of Ag+ and nanoscale plastic debris to Daphnia magna (Abdolahpur Monikh et al. 2020). These results indicated the modulatory role of organic matters on the adsorption/desorption, accumulation, and toxicity of heavy metals in relation to MPs.
The Existence of Other Contaminants
In the study of the behaviors of heavy metals in relation to MPs, antagonistic or synergistic effects are observed when other elements or organic pollutants coexist with metals. For example, when Cd, Zn, or Pb was presented individually, increased adsorption of Cd on PET was observed (Abbasi et al. 2020). In contrast, PET was found to adsorb higher amounts of Zn when the above mentioned heavy metals all co-existed in the testing solutions (Abbasi et al. 2020). Notably, desorption of metals occurs more easily when multiple heavy metals are present (Abbasi et al. 2020). This is because of the saturation of the adsorption sites on the MP surfaces; different affinities between heavy metals and MPs and the competition between different heavy metals may affect the adsorption process. In addition, the co-existence of organic contaminants can affect adsorption of heavy metals onto MPs (Lin et al. 2021a). For example, prothioconazole (fungicide) was shown to have no effect on Sn adsorption but could reduce the adsorption of Cr, As, Pb, and Ba onto MPs (Li et al. 2020b). In contrast, prothioconazole was found to enhance Cu adsorption onto MPs (Li et al. 2020b). The increased adsorption of Cu by prothioconazole could be due to the formation of triazole thiolates with Cu. In summary, the presence of other contaminants, the speciation of metal ions, and their relative concentrations, could modify the adsorption and the release of heavy metals from MPs (Gao et al. 2019; Li et al. 2020b; Tang et al. 2021).
Physiochemical Characteristics of MPs
Adsorption of heavy metals onto MPs is a spontaneous process governed by the various characteristics of MPs, such as sizes and composition, aging degree, and degradability (Bayo et al. 2017; Gao et al. 2019; Abdolahpur Monikh et al. 2020; Lu et al. 2020; Naqash et al. 2020; Tang et al. 2020). For instance, the extent of Ag+ sorption onto nanoscale plastic debris was found to follow: 600 nm PS > 300 nm PS > 300 nm PE (Abdolahpur Monikh et al. 2020). Similarly, Cd2+ release and sorption were also found to be dependent on the sizes and types of MPs (Qiao et al. 2019; Liu et al. 2020; Guo et al. 2020b). In addition, different MPs exhibit different affinities to heavy metals (Godoy et al. 2019). For example, compared to PS, PVC showed a higher affinity to Pb2+ and Cu2+ because of its higher surface area and higher polarity (Brennecke et al. 2016; Lin et al. 2021b). Furthermore, the sorption affinity of Cu2+, Cd2+, and Pb2+ to MPs was shown to follow the order of CPE > PVC > HPE > LPE, likely owing to the differences in chemical structure and electronegativity of the MPs (Zou et al. 2020). On the other hand, the adsorption of As, Ti, Ni, and Cd was enhanced by the increasing degree of aging, oxidation, and biodegradability of plastics (Lee et al. 2019; Li et al. 2020b; Wang et al. 2020c). Compared with original/newly generated MPs, aged/weathered MPs have higher adsorption capacities for heavy metals (Vedolin et al. 2018; Lee et al. 2019; Gao et al. 2020; Lu et al. 2020; Mao et al. 2020; Tang et al. 2020; Wang et al. 2020c; Aghilinasrollahabadi et al. 2021). This could be ascribed to the increased surface area and the diverse functional groups (e.g., oxygen containing function groups) present on the surface of aged MPs (Guo et al. 2020b; Tang et al. 2020, 2021). Furthermore, degradable MPs exhibit a higher oral bioaccessibility and greater release of Cr6+ than nondegradable MPs in the whole digestive system in-vitro method, primarily due to possible destruction of surface chemical structures in degradable MPs (Liao and Yang 2020). Overall, the distinct physicochemical properties of MPs (e.g., composition, size, aging degree, polarity, and degradability) are key factors influencing MP-metals interactions in the environment.
Possible Mechanisms on the Interactions Between MPs and Heavy Metals
The Mechanisms Associated with the Physiochemical Characteristics of MPs or Heavy Metals
The physiochemical characteristics of MPs and heavy metals determine their interaction mechanisms [e.g., physisorption (simple process and weak bonds) and chemisorption (electrostatic interaction, surface complexation, and intraparticle diffusion etc.)] which ultimately affect their sorption processes (Bayo et al. 2017; Godoy et al. 2019; Guo et al. 2020a; Lin et al. 2021a, b; Purwiyanto et al. 2020; Zou et al. 2020; Tang et al. 2020, 2021). For instance, the oxygen-containing functional groups such as the carboxyl group on the surface of nylon MPs were identified to promote the adsorption processes of metal ions, which faciliated the surface complexation mechanism (Tang et al. 2020, 2021). On the other hand, the mechanism of electrostatic interaction could be related to the hydrated ionic radius of heavy metals. For example, Pb2+ was demonstrated to exhibit a remarkable higher sorption than Cu2+ and Cd2+, which was suggested to be owing to a lower hydrated ionic radius of Pb2+ and the larger electrostatic interaction between Pb2+ and MPs (Zou et al. 2020).
Other Potential Mechanisms
The effect of salinity on the adsorption process of Pb2+ onto hexabromocyclododecane-PS MPs demonstrated that electrostatic interaction could be the predominant adsorption mechanism (Lin et al. 2021a). However, mechanisms involved in the adsorption process of metals and plastics, which are likely to be varied and complex, remain largely unknown. Other potential or related mechanisms dominated the interactions should be further explored under different environmental conditions (Brennecke et al. 2016). At present, some studies simply adopted several kinds of models (e.g., Langmuir model and Freundlich model) to assess the mechanisms between heavy metals and MPs (Godoy et al. 2019; Purwiyanto et al. 2020). This could inevitably caused some limitations such as results uncertainty, variability and incomparability, thus hindering our understanding of their environmental fate. Further development in analytical technologies or new characterization methods could be considered to unveil the complex interaction mechanisms between MPs and heavy metals.
Conclusions and Perspectives
In conclusion, there is increasing evidence for the effects of MPs on the mobility, bioavailability, and toxicity of heavy metals. The physicochemical properties of MPs and heavy metals, as well as various environmental factors, have been shown to influence their interaction processes. As a result, assessment of the ecological risks of MPs should take environmental parameters and the presence of metals (as well as other contaminants) into account. In a real world setting, complex environmental factors and variations, and the presence of different contaminants, would affect the fate, transport, and bioavailiby of metals. Further investigation is required to understand the mechanisms of these interactions and to elucidate how their interactions modulate the effects of MPs and metals on animal and ecosystem health.
Abbreviations
- PE:
-
Polyethylene
- PLA:
-
Polylactic acid
- PS:
-
Polystyrene
- PVC:
-
Polyvinyl chloride
- PET:
-
Polyethylene terephthalate
- HDPE:
-
High-density polyethylene
- HPE:
-
High crystallinity polyethylene
- LPE:
-
Low crystallinity polyethylene
- CPE:
-
Chlorinated polyethylene
References
Abbasi S, Moore F, Keshavarzi B, Hopke PK, Naidu R, Rahman MM, Oleszczuk P, Karimi J (2020) PET-microplastics as a vector for heavy metals in a simulated plant rhizosphere zone. Sci Total Environ 744:140984
Abdolahpur Monikh F, Vijver MG, Guo Z, Zhang P, Darbha GK, Peijnenburg WJGM (2020) Metal sorption onto nanoscale plastic debris and trojan horse effects in Daphnia magna: Role of dissolved organic matter. Water Res 186:116410
Aghilinasrollahabadi K, Salehi M, Fujiwara T (2021) Investigate the influence of microplastics weathering on their heavy metals uptake in stormwater. J Hazard Mater 408:124439
Akhbarizadeh R, Moore F, Keshavarzi B, Moeinpour A (2017) Microplastics and potentially toxic elements in coastal sediments of Iran’s main oil terminal (Khark Island). Environ Pollut 220:720–731
Akhbarizadeh R, Moore F, Keshavarzi B (2018) Investigating a probable relationship between microplastics and potentially toxic elements in fish muscles from northeast of Persian Gulf. Environ Pollut 232:154–163
Alimi OS, Budarz JF, Hernandez LM, Tufenkji N (2018) Microplastics and nanoplastics in aquatic environments: aggregation, deposition, and enhanced contaminant transport. Environ Sci Technol 52(4):1704–1724
Banaee M, Soltanian S, Sureda A, Gholamhosseini A, Haghi BN, Akhlaghi M, Derikvandy A (2019) Evaluation of single and combined effects of cadmium and micro-plastic particles on biochemical and immunological parameters of common carp (Cyprinus carpio). Chemosphere 236:124335
Barboza LGA, Vieira LR, Branco V, Figueiredo N, Carvalho F, Carvalho C, Guilhermino L (2018) Microplastics cause neurotoxicity, oxidative damage and energy-related changes and interact with the bioaccumulation of mercury in the European seabass, Dicentrarchus labrax (Linnaeus, 1758). Aquat Toxicol 195:49–57
Bayo J, Martinez A, Guillen M, Olmos S, Roca MJ, Alcolea A (2017) Microbeads in commercial facial cleansers: threatening the environment. Clean Soil Air Water 45(7):1600683
Bellingeri A, Bergami E, Grassi G, Faleri C, Redondo-Hasselerharm P, Koelmans AA, Corsi I (2019) Combined effects of nanoplastics and copper on the freshwater alga Raphidocelis subcapitata. Aquat Toxicol 210:179–187
Bradney L, Wijesekara H, Palansooriya KN, Obadamudalige N, Bolan NS, Ok YS, Rinklebe J, Kim K-H, Kirkham MB (2019) Particulate plastics as a vector for toxic trace-element uptake by aquatic and terrestrial organisms and human health risk. Environ Int 131:104937
Brennecke D, Duarte B, Paiva F, Caçador I, Canning-Clode J (2016) Microplastics as vector for heavy metal contamination from the marine environment. Estuar Coast Shelf Sci 178:189–195
Dey TK, Uddin ME, Jamal M (2021) Detection and removal of microplastics in wastewater: evolution and impact. Environ Sci Pollut Res 28(14):16925–16947
Dong YM, Gao ML, Qiu WW, Song ZG (2020) Adsorption of arsenite to polystyrene microplastics in the presence of humus. Environ Sci Proc Imp 22(12):2388–2397
Dong YM, Gao ML, Qiu WW, Song ZG (2021) Effect of microplastics and arsenic on nutrients and microorganisms in rice rhizosphere soil. Ecotoxicol Environ Saf 211:111899
Fernández B, Santos-Echeandía J, Rivera-Hernández JR, Garrido S, Albentosa M (2020) Mercury interactions with algal and plastic microparticles: Comparative role as vectors of metals for the mussel, Mytilus galloprovincialis. J Hazard Mater 396:122739
Fries E, Zarfl C (2012) Sorption of polycyclic aromatic hydrocarbons (PAHs) to low and high density polyethylene (PE). Environ Sci Pollut Res 19(4):1296–1304
Gao FL, Li JX, Sun CJ, Zhang LT, Jiang FH, Cao W, Zheng L (2019) Study on the capability and characteristics of heavy metals enriched on microplastics in marine environment. Mar Pollut Bull 144:61–67
Gao D, Li XY, Liu HT (2020) Source, occurrence, migration and potential environmental risk of microplastics in sewage sludge and during sludge amendment to soil. Sci Total Environ 742:140355
Godoy V, Blázquez G, Calero M, Quesada L, Martín-Lara MA (2019) The potential of microplastics as carriers of metals. Environ Pollut 255:113363
Godoy V, Martínez-Férez A, Martín-Lara M, Vellido-Pérez JA, Calero M, Blázquez G (2020) Microplastics as vectors of chromium and lead during dynamic simulation of the human gastrointestinal tract. Sustainability 12(11):4792
Guo X, Liu Y, Wang JL (2020a) Equilibrium, kinetics and molecular dynamic modeling of Sr2+ sorption onto microplastics. J Hazard Mater 400:123324
Guo XT, Hu GL, Fan XY, Jia HZ (2020) Sorption properties of cadmium on microplastics: the common practice experiment and a two-dimensional correlation spectroscopic study. Ecotoxicol Environ Saf 190:110118
Holmes LA, Turner A, Thompson RC (2012) Adsorption of trace metals to plastic resin pellets in the marine environment. Environ Pollut 160:42–48
Holmes LA, Turner A, Thompson RC (2014) Interactions between trace metals and plastic production pellets under estuarine conditions. Mar Chem 167:25–32
Huang W, Song B, Liang J, Niu QY, Zeng GM, Shen MC, Deng JQ, Luo Y, Wen XF, Zhang YF (2021) Microplastics and associated contaminants in the aquatic environment: a review on their ecotoxicological effects, trophic transfer, and potential impacts to human health. J Hazard Mater 405:124187
Kern S, Kern C, Pradja MM, Düring R-A, Rohnke M (2021) Spatially resolved indiffusion behavior of Cu2+ and Ni2+ in polypropylene. J Appl Polym Sci 138(2):49655
Kim J-H, Yu Y-B, Choi J-H (2021) Toxic effects on bioaccumulation, hematological parameters, oxidative stress, immune responses and neurotoxicity in fish exposed to microplastics: a review. J Hazard Mater 413:125423
Koelmans AA, Besseling E, Wegner A, Foekema EM (2013) Correction to plastic as a carrier of POPs to aquatic organisms: a model analysis. Environ Sci Technol 47(15):8992–8993
Lee WS, Cho HJ, Kim E, Huh YH, Kim HJ, Kim B, Kang T, Lee JS, Jeong J (2019) Bioaccumulation of polystyrene nanoplastics and their effect on the toxicity of Au ions in zebrafish embryos. Nanoscale 11(7):3173–3185
Li XW, Mei QQ, Chen LB, Zhang HY, Dong B, Dai XH, He CQ, Zhou J (2019) Enhancement in adsorption potential of microplastics in sewage sludge for metal pollutants after the wastewater treatment process. Water Res 157:228–237
Li P, Li QC, Hao ZN, Yu SJ, Liu JF (2020) Analytical methods and environmental processes of nanoplastics. J Environ Sci 94:88–99
Li RJ, Liu Y, Sheng YF, Xiang QQ, Zhou Y, Cizdziel JV (2020) Effect of prothioconazole on the degradation of microplastics derived from mulching plastic film: apparent change and interaction with heavy metals in soil. Environ Pollut 260:113988
Lian JP, Wu JN, Zeb A, Zheng SN, Ma T, Peng FH, Tang JC, Liu WT (2020) Do polystyrene nanoplastics affect the toxicity of cadmium to wheat (Triticum aestivum L.)? Environ Pollut 263:114498
Liao YL, Yang JY (2020) Microplastic serves as a potential vector for Cr in an in-vitro human digestive model. Sci Total Environ 703:134805
Lin LJ, Tang S, Wang XS, Sun X, Yu AQ (2021) Hexabromocyclododecane alters malachite green and lead(II) adsorption behaviors onto polystyrene microplastics: interaction mechanism and competitive effect. Chemosphere 265:129079
Lin Z, Hu YW, Yuan YJ, Hu BW, Wang BL (2021b) Comparative analysis of kinetics and mechanisms for Pb(II) sorption onto three kinds of microplastics. Ecotoxicol Environ Saf 208:111451
Liu HT, Liu K, Fu HY, Ji R, Qu XL (2020) Sunlight mediated cadmium release from colored microplastics containing cadmium pigment in aqueous phase. Environ Pollut 263:114484
Lu YF, Zhang Y, Deng YF, Jiang W, Zhao YP, Geng JJ, Ding LL, Ren HQ (2016) Uptake and accumulation of polystyrene microplastics in Zebrafish (Danio rerio) and toxic effects in liver. Environ Sci Technol 50(7):4054–4060
Lu K, Qiao RX, An H, Zhang Y (2018) Influence of microplastics on the accumulation and chronic toxic effects of cadmium in zebrafish (Danio rerio). Chemosphere 202:514–520
Lu XM, Lu PZ, Liu XP (2020) Fate and abundance of antibiotic resistance genes on microplastics in facility vegetable soil. Sci Total Environ 709:136276
Mao RF, Lang MF, Yu XQ, Wu RR, Yang XM, Guo XT (2020) Aging mechanism of microplastics with UV irradiation and its effects on the adsorption of heavy metals. J Hazard Mater 393:122515
Naqash N, Prakash S, Kapoor D, Singh R (2020) Interaction of freshwater microplastics with biota and heavy metals: a review. Environ Chem Lett 18(6):1813–1824
Othman AR, Abu Hasan H, Muhamad MH, Ismail NI, Abdullah SRS (2021) Microbial degradation of microplastics by enzymatic processes: a review. Environ Chem Lett. https://doi.org/10.1007/s10311-021-01197-9
Purwiyanto AIS, Suteja Y, Trisno, Ningrum PS, Putri WAE, Rozirwan, Agustriani F, Fauziyah, Cordova MR, Koropitan AF (2020) Concentration and adsorption of Pb and Cu in microplastics: case study in aquatic environment. Mar Pollut Bull 158:111380
Qiao RX, Lu K, Deng YF, Ren HQ, Zhang Y (2019) Combined effects of polystyrene microplastics and natural organic matter on the accumulation and toxicity of copper in zebrafish. Sci Total Environ 682:128–137
Strungaru S-A, Jijie R, Nicoara M, Plavan G, Faggio C (2019) Micro- (nano) plastics in freshwater ecosystems: abundance, toxicological impact and quantification methodology. Trac Trend Anal Chem 110:116–128
Sun JH, Xia SD, Ning Y, Pan X, Qu JH, Xu YJ (2019) Effects of microplastics and attached heavy metals on growth, immunity, and heavy metal accumulation in the yellow seahorse, Hippocampus kuda Bleeker. Mar Pollut Bull 149:110510
Tang S, Lin LJ, Wang XS, Feng AX, Yu AQ (2020) Pb(II) uptake onto nylon microplastics: interaction mechanism and adsorption performance. J Hazard Mater 386:121960
Tang S, Lin LJ, Wang XS, Yu AQ, Sun X (2021) Interfacial interactions between collected nylon microplastics and three divalent metal ions (Cu(II), Ni(II), Zn(II)) in aqueous solutions. J Hazard Mater 403:123548
Vedolin MC, Teophilo CYS, Turra A, Figueira RCL (2018) Spatial variability in the concentrations of metals in beached microplastics. Mar Pollut Bull 129(2):487–493
Wakkaf T, Allouche M, Harrath AH, Mansour L, Alwasel S, Mohamed Thameemul Ansari KG, Beyrem H, Sellami B, Boufahja F (2020) The individual and combined effects of cadmium, polyvinyl chloride (PVC) microplastics and their polyalkylamines modified forms on meiobenthic features in a microcosm. Environ Pollut 266:115263
Wang FY, Zhang XQ, Zhang SQ, Zhang SW, Adams CA, Sun YH (2020a) Effects of co-contamination of microplastics and Cd on plant growth and Cd accumulation. Toxics 8(2):36
Wang FY, Zhang XQ, Zhang SQ, Zhang SW, Sun YH (2020b) Interactions of microplastics and cadmium on plant growth and arbuscular mycorrhizal fungal communities in an agricultural soil. Chemosphere 254:126791
Wang QJ, Zhang Y, Wangjin XX, Wang YL, Meng GH, Chen YH (2020) The adsorption behavior of metals in aqueous solution by microplastics effected by UV radiation. J Environ Sci 87:272–280
Wang L, Gao YX, Jiang W, Chen JX, Chen YS, Zhang XH, Wang GX (2021) Microplastics with cadmium inhibit the growth of Vallisneria natans (Lour.) Hara rather than reduce cadmium toxicity. Chemosphere 266:128979
Wen B, Jin SR, Chen ZZ, Gao JZ, Liu YN, Liu JH, Feng XS (2018) Single and combined effects of microplastics and cadmium on the cadmium accumulation, antioxidant defence and innate immunity of the discus fish (Symphysodon aequifasciatus). Environ Pollut 243:462–471
Wijesekara H, Bolan NS, Bradney L, Obadamudalige N, Seshadri B, Kunhikrishnan A, Dharmarajan R, Ok YS, Rinklebe J, Kirkham MB, Vithanage M (2018) Trace element dynamics of biosolids-derived microbeads. Chemosphere 199:331–339
Yan W, Hamid N, Deng S, Jia PP, Pei DS (2020) Individual and combined toxicogenetic effects of microplastics and heavy metals (Cd, Pb, and Zn) perturb gut microbiota homeostasis and gonadal development in marine medaka (Oryzias melastigma). J Hazard Mater 397:122795
Yazdani Foshtomi M, Oryan S, Taheri M, Darvish Bastami K, Zahed MA (2019) Composition and abundance of microplastics in surface sediments and their interaction with sedimentary heavy metals, PAHs and TPH (total petroleum hydrocarbons). Mar Pollut Bull 149:110655
Yu H, Hou JH, Dang QL, Cui DY, Xi BD, Tan WB (2020) Decrease in bioavailability of soil heavy metals caused by the presence of microplastics varies across aggregate levels. J Hazard Mater 395:122690
Yu H, Zhang Z, Zhang Y, Fan P, Xi BD, Tan WB (2021) Metal type and aggregate microenvironment govern the response sequence of speciation transformation of different heavy metals to microplastics in soil. Sci Total Environ 752:141956
Yuan WK, Zhou YF, Chen YL, Liu XN, Wang J (2020) Toxicological effects of microplastics and heavy metals on the Daphnia magna. Sci Total Environ 746:141254
Zhou YF, Liu XN, Wang J (2020) Ecotoxicological effects of microplastics and cadmium on the earthworm Eisenia foetida. J Hazard Mater 392:122273
Zhu XT, Qiang LY, Shi HH, Cheng JP (2020) Bioaccumulation of microplastics and its in vivo interactions with trace metals in edible oysters. Mar Pollut Bull 154:111079
Zou JY, Liu XP, Zhang DM, Yuan X (2020) Adsorption of three bivalent metals by four chemical distinct microplastics. Chemosphere 248:126064
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 41907309, U2032201).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, G., Dave, P.H., Kwong, R.W.M. et al. Influence of Microplastics on the Mobility, Bioavailability, and Toxicity of Heavy Metals: A Review. Bull Environ Contam Toxicol 107, 710–721 (2021). https://doi.org/10.1007/s00128-021-03339-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-021-03339-9