Abstract
The toxic effect of microplastics (MPs) on living organisms is caused by the plastics and other pollutants attached to their surface. The interaction of MPs with hazardous toxic pollutants such as lead (Pb) is possible due to the large surface area of MPs and the high adsorption capacity of heavy metals such as Pb. When combined with toxic heavy metals, the interaction of MPs with aquatic environments and living organisms leads to environmental and biological problems. When MPs enter water, they form a biofilm under the influence of organic and inorganic substances, significantly altering the adsorption–desorption properties of the heavy metal. The current study aims to understand the effect of Pb-MP interaction on MPs by investigating biofilm formation in MPs. By reviewing the studies in the existing literature, the study analyses how biofilm formation affects the adsorption behavior of Pb heavy metal on the surface of MPs. Furthermore, future perspectives highlight potential research directions aiming to fill the knowledge gaps in this field. Addressing the challenges, it also highlights the need for a multidisciplinary approach to understanding microplastic and heavy metal interactions in aquatic ecosystems and to assess the long-term effects of these interactions on ecology and health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Environmental pollution, especially the spread of microplastics (MPs) in natural ecosystems and their capacity to adsorb various pollutants, is becoming an increasing threat to global environmental health and natural ecosystems (Jiang et al., 2020; Prokić et al., 2019; Wu et al., 2019). In this context, understanding the environmental impacts and propagation dynamics of Pb carried on microplastic (MP) surfaces has become a crucial research area for sustainable environmental management. MPs are prevalent in the environment, spreading through the atmosphere (Adebiyi & Kok, 2020), water (Alvim et al., 2020), sediment (Brandon et al., 2019), and soil (Alengebawy et al., 2021), negatively impacting the health of organisms in ecosystems (Cao et al., 2021). MPs can adsorb various harmful substances such as organochlorine pesticides, persistent organic pollutants, endocrine disruptors, toxic organic chemicals, and heavy metals onto their surfaces (Sajid et al., 2016), leading to the potential transfer of these substances to living organisms, resulting in bioaccumulation and biomagnification (Akhbarizadeh et al., 2019), which can cause disruptions in metabolic (Wang et al., 2023), neurological (Viana et al., 2020), reproductive (Afreen et al., 2023), and immune systems. (Sharifinia et al., 2020). MPs, vectors for toxic elements, can keep heavy metals such as Cd, Co, Cr, Cu, Ni, Pb and Zn on their surfaces (Godoy et al., 2019).
The adsorption of organic pollutants onto the MPs' surface is a multifaceted and complex process that depends on the characteristics of both the pollutants and the MPs, as well as the conditions of the solution and the environment (Wang et al., 2020). In this process, numerous factors, such as hydrophobic interactions, chemical interactions, surface properties, time and temperature, play crucial roles in specifying the type and degree of adsorption mechanisms (Munoz et al., 2021). Among the adsorption mechanisms are physical adsorption, chemical adsorption, ion exchange, complex formation, surface reaction, and pore filling (Abbas et al., 2018), which influence the attachment strength and pollutants' reversibility to the MP surface (Tourinho et al., 2019). Additionally, inorganic contaminants like metal ions can be adsorbed on MPs' surface (Fu et al., 2021a, 2021b). In such cases, different mechanisms can come into play, including surface oxidation, surface complexation, co-precipitation, and electrostatic interactions (Nagoya et al., 2019). Tang et al., (2021) conducted a study examining the adsorption capacities of nylon-MPs for Cu, Ni, and Zn. The study finding revealed that the sequence of ions adsorbed by MPs was Cu, Zn, and Ni. The results indicated that the primary mechanism governing adsorption is surface complexation. Environmental factors such as air humidity, pH, salinity, organic matter abundance, attached biofilms, and redox potential influence pollutants adsorbed on the MP surface (Tang et al., 2021). Lin et al., (2021) explored the sorption kinetics and mechanisms of Pb on polyvinyl chloride (PVC), polyethylene (PE), and polystyrene (PS)-MPs, which brought to light that the sorption capacities of MPs were subject to factors like pH, ionic strength, and MP type. The predominant factors influencing this phenomenon were primarily intraparticle diffusion and the ultimate equilibrium process. The highest sorption capacities recorded were 483.1 μg/g for PVC, 416.7 μg/g for PE, and 128.5 μg/g for PS, respectively. Advanced analyses using Fourier transform infrared spectroscopy (FTIR) and X-ray photoelectron spectroscopy (XPS) revealed no formation of new bonds Pb-MP, emphasizing physisorption as the primary force driving Pb sorption (Lin et al., 2021). According to Gu et al., (2018), Pb exhibits significant biotoxicity and proves resistant to biodegradation, exerting adverse effects on organisms even at lower concentrations (Gu et al., 2018).
Pb-MP interaction adversely affects soil, plants, water, and human health (Kumar et al., 2022). Pb can be disseminated from various sources that pose harm to the environment and human health (Karbalaei et al., 2018). Sources contributing to the accumulation of Pb on the surface of MPs include industrial activities releasing Pb into the environment, wastewater, agricultural pesticides, waste batteries, lead-based paints, toys, Pb pellets from ammunition, leaded-gasoline, cosmetic products (Hettiarachchi et al., 2024). Also, its sources are associated with landfills, leachates, mines, petrochemical industry and agricultural runoff (Obeng-Gyasi, 2019). These sources release Pb into water, soil, and air, allowing it to adsorb onto or penetrate the surface of MPs (Adji et al., 2022; Sharma et al., 2021). By carrying Pb along the food chain, MPs adversely affect living organisms' health (Kumar et al., 2020). Pb exposure is associated with severe health problems (Obasi & Akudinobi, 2020; Tong et al., 2000; Wang et al., 2009). Pb can access the body through inhaling, swallowing, or contact with the skin, amassing in the bloodstream, skeletal structure, cerebral region, renal system, hepatic tissue, neural system, and additional bodily structures (Collin et al., 2022; Pandey & Madhuri, 2014). This accumulation can result in damage to brain functions, DNA, and chromosomes, leading to allergic reactions, fatigue, headaches, and adverse reproductive effects (Bhargava et al., 2017; Engwa et al., 2019; Jaishankar et al., 2014; Singh et al., 2018).
Several investigations have highlighted the prevalent association of MPs with Pb in freshwater environments. Investigating the adsorption of Pb on MPs and their synergistic impacts is crucial for comprehending the coexistence of MPs with various pollutants. Therefore, this study focuses on Pb as a representative contaminant. This study aims to evaluate Pb-MP interactions deposited on the surface of MPs to understand the propagation dynamics and environmental exposure of these interactions. This study also helps to grasp the current limitations of Pb-MP interactions and highlights important perspectives and challenges for future studies.
2 Influence of Biofilm Formation on the Adsorption of Heavy Metals in MPs
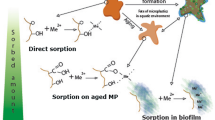
The biofilm can readily establish its presence on the surface of MPs (He et al., 2022). Following immersion in aqueous settings, a layer composed of organic and inorganic substances termed the conditioning membrane (Rummel et al., 2017) occurs on the MPs' surface, playing an important role in contributing to the subsequent development of the biofilm (Guan et al., 2020) (Fig. 1).
As seen in Fig. 1, biofilm is a cluster of microorganisms where cells adhere to each other or to the surface they are on (Qiang et al., 2021), which arises from the conditioning membrane formed on the surface of MPs after they enter the aquatic environment, composed of inorganic and organic substances (Feng et al., 2020). This layer facilitates microorganisms' attachment to MPs and biofilm formation (Tu et al., 2020a, 2020b). Weak bonds are formed between microorganisms and MPs through physical forces (van der Waals force, electrostatic forces, and hydrophobic interactions), which can be fragile or strengthened depending on environmental conditions (Fu et al., 2021a, 2021b; Prajapati et al., 2022). However, strong bonds can also form between microorganisms and MPs through chemical forces (hydrogen bond, ionic bond, and covalent bond), which are independent of environmental conditions and allow microorganisms to adhere permanently to the surface of MPs (Moyal et al., 2023).
The secretion of extracellular polymeric substance (EPS) is a crucial step in the process of microorganisms adhering to MPs' surface (Deng et al., 2021). This process begins with the initial attachment of pioneer microorganisms to the surface of MPs (Debroy et al., 2022). Attachment to MPs typically occurs in reversible and irreversible stages (Dennehy & Abedon, 2021). On the surface they adhere to, microorganisms release extracellular polysaccharides, referred to as EPS (Liu et al., 2021a, 2021b), containing polysaccharides, proteins, nucleic acids, and lipids (Izadi et al., 2021), which facilitates a tighter binding of microorganisms to the surface of MPs (Stabnikova et al., 2022). The role of EPS is to create an adhesive matrix covering the surface of MPs, thereby enhancing the resilience of microorganisms and facilitating the formation of a biofilm (Tu et al., 2020a, 2020b). This process is a significant ecological phenomenon, influencing the interaction of microorganisms with MPs and enhancing the resistance of MPs to environmental conditions (Deng et al., 2021).
Microbial proliferation represents the final stage of biofilm formation, a process that encompasses the development of a dynamic microbial community on the surface of MPs (Sooriyakumar et al., 2022). In the concluding phase of biofilm formation, additional microorganisms continuously settle on the surface of MPs. This occurs through the proliferation of previously attached microorganisms in earlier stages and the arrival of new microorganisms (Battulga et al., 2022). Alongside the proliferation of microorganisms, organic substances, cell residues, and other biological remnants generated during biofilm formation accumulate on the surface of MPs (Hale et al., 2020). Simultaneously, depending on environmental conditions, inorganic residues may also become part of this biofilm matrix (Flemming et al., 2023), initiating a process of accumulation and diversification within the biofilm. The biofilm on the surface of MPs becomes increasingly enriched and diversified through the proliferation of microorganisms and the combination of organic/inorganic residues, supporting intricate interactions among microorganisms (Arias-Andres et al., 2019). Factors such as relationships between different species, competition, and cooperation play an important role in this phase of the microbial community (Ghoul & Mitri, 2016; Gralka et al., 2020). In the final stage of microbial proliferation and biofilm formation, a complex and dynamic microbial ecosystem emerges on the surface of MPs, which shape biological interactions in the MP environment, influencing resistance against environmental factors (Sooriyakumar et al., 2022). Li et al., (2022a, 2022b) examined how Pb behaves in terms of adsorption on biodegradable poly(butylene succinate) (PBS) MPs during the process of biodegradation. The study findings suggest that the amount of Pb adsorbed by biofilm-colonized, biodegraded PBS-MPs is approximately 10 times greater than that observed with pristine PBS (647.09 μg/g compared to 64.13 μg/g). This notable increase is attributed to both the presence of biofilm colonization and the degradation of PBS (Li et al., 2022a, 2022b). Ahamed et al., (2020) noted a significant increase in the adsorption of Pb on low-density polyethylene (LDPE) surfaces in the presence of biofilm. This led to a 13-fold rise in the equilibrium adsorption capacity, reaching 1602 g/m2, compared to the absence of biofilm, which was only 124 g/m2 (Ahamed et al., 2020). The investigation conducted by Fan et al., (2021) revealed notable differences in the adsorption capacities of polypropylene (PP)-MPs for various heavy metals. Notably, the adsorption capacities for Pb and Cu were notably higher when compared to those for Cd and Zn (Fan et al., 2021). This suggests that Pb exhibits the highest adsorption capability across diverse MP particles, with physisorption onto the MPs identified as the primary sorption mechanism (Lin et al., 2021).
3 Selecting the Impact of Pb on the MPs
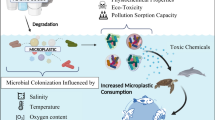
Heavy metals may be of natural origin or occur due to human activities (Vareda et al., 2019). Numerous compounds containing heavy metals exhibit high solubility in aqueous environments (Cánovas et al., 2023; Mariana et al., 2021). Heavy metal concentrations can vary due to the impact of human activities, and their discharge into the receiving environment is rapidly increasing (Hembrom et al., 2020). Heavy metal substances, which originate from the wastewater of different industries, may mix with the receiving environment due to human influence and natural factors (Vardhan et al., 2019). If heavy metals are discharged into the aquatic environment without adequate treatment, it can lead to severe problems for both the environment and living organisms (Mishra et al., 2019) (Fig. 2). Heavy metals are transported through complex processes such as dissolution, precipitation, complex formation, adsorption, and bioaccumulation in aquatic environments (Liu et al., 2022a, 2022b, 2022c), altering the physicochemical properties of water (Qiu et al., 2021), which disrupt vital functions in living organisms (Fu & Xi, 2020), including growth (Witkowska et al., 2021), development (Bharti & Sharma, 2022), reproduction (Massányi et al., 2020), respiration (Nowicka, 2022), photosynthesis (Souri et al., 2019), enzyme activity (Witkowska et al., 2021), protein synthesis (Engwa et al., 2019), and the structure of DNA and RNA (Bharti & Sharma, 2022), which can adversely affect human health.
Heavy metals cause long-term environmental pollution, and recent research has revealed the interaction between MP and heavy metals (Cao et al., 2021; Liu et al., 2022a, 2022b, 2022c; Zhang et al., 2022). Pb can be incorporated into the polymer matrix as a heavy metal, increasing the toxicity of the polymer. Pb might exist in the polymer matrix of MPs as functional additives or residues from recycling or reactions (Nguyen et al., 2023). Functional additives are substances used to modify polymers' processing and performance properties (Campanale et al., 2020). Recycling or reaction residues, on the other hand, are waste or leftover materials generated during the production, processing, or use of polymers (Khoo et al., 2021; Moumakwa, 2023). These materials can mix with the polymer matrix in unwanted or unknown ways, affecting the polymer's physical, chemical, or biological properties (Inamdar, 2022). The primary source of Pb is the smoke from vehicle exhaust (Heal et al., 2005; Negahban & Mokarram, 2021; Sassykova et al., 2019). Soils along roadsides are places where Pb accumulates heavily. MPs in the environment can adsorb Pb onto their surfaces, increasing their toxicity. MPs, widespread in aquatic and terrestrial ecosystems, can lead to the biological accumulation of Pb on their surfaces (Elgarahy et al., 2021), allowing Pb to be transported through atmospheric processes such as wind, rain, hail, and snow, facilitating the biological breakdown of MPs and their entry into the food chain (Özgenç et al., 2023). This can negatively impact organisms' growth, reproduction, behavior, and health (Padha et al., 2022). The tendency of Pb to bioaccumulate in the body and be stored in bone tissue is also concerning, leading to toxic effects in cases of long-term exposure (Collin et al., 2022). It can induce genetic disorders and developmental problems by affecting genetic material. Biomedical interactions can occur due to various toxicity mechanisms inside and outside cells (Paz-Sabillón et al., 2023). In contrast to other environmental materials, MP particles display heavy metal concentrations 10–100 times higher, primarily attributable to their smaller particle size and an increased surface area-to-volume ratio (Acosta-Coley et al., 2019).
In this context, Pb-MP interaction enhances bioaccumulation, which is important in transferring environmental pollutants to organisms. The adsorption of Pb on MP surfaces contributes to the accumulation of these tiny particles in organisms in both aquatic and terrestrial environments. This creates a scenario where environmental toxins can ascend the food chain, presenting a potential threat and causing harm to ecosystems. Therefore, the Pb-MP interaction represents a significant research area for comprehending and managing environmental impacts.
4 Pb-MP Interactions in the Receiving Environment
The contamination of aquatic environments with heavy metals is a substantial global concern, given its persistence and enduring impact on ecosystems (Lin et al., 2016). When ingested from the environment, MPs can also bind to heavy metals, accumulating in organisms' bodies and potentially causing biological issues. Digestion of MPs allows heavy metals to be transported into organisms (Zhu et al., 2018). Heavy metals endanger human health when they enter the food chain. MPs bind heavy metals to their surfaces, harming living organisms in the sea, freshwater and land (Wen et al., 2018). As seen in Table 1, some laboratory experiments have proved how MPs affect heavy metals, which factors influence adsorption or desorption and how MPs transport heavy metals (Table 1). Similarly, recent investigations have indicated that MPs may be crucial carriers of toxic heavy metals (Godoy et al., 2019). A study conducted by Massos and Turner (2017) revealed that the analysis of the MP sample (n = 924) from two beaches showed the presence of Cd in 6.9% and Pb in 7.5% of all MPs, respectively. The concentration of both metals exceeded 1 mg/g. Maršić-Lučić et al., (2018) detected the presence of some metal ions in MP samples from beach sediments on the Croatian island of Vis. Compared to samples from the nearby ocean, the study showed that MPs had higher levels of trace metals such as Fe, Cr, Mn, Cu, Ni, Zn, Pb and Cd, confirming that MPs enrich the marine ecosystem in metals. In the study conducted by Lin et al., (2021), the adsorption characteristics of Pb ions were examined using PVC, PE, and PS. It was found that PVC exhibited the highest maximum adsorption capacity, reaching 483.1 μg/g for Pb ions. Davranche et al., (2019) revealed that 97% of lead Pb was detected on nanoparticles isolated from MPs gathered from the North Atlantic region. The research illustrates the ability of nanoparticles to bind with both organic pollutants and heavy metals, affirming that organic and inorganic origin pollutants exhibit an affinity for plastic. Similarly, the study by Fu et al., (2021a, 2021b) confirmed that naturally aged MPs exhibit a heightened adsorption capacity for Pb, with the primary mechanism of this process likely determined by the electrostatic force between the oxygen-containing functional groups present in MPs and Pb. The diffusivity of MPs allows Pb to diffuse into MPs over a larger area, leading to environmental contamination affecting a larger area, which means that Pb contamination in water sources can be widespread. This process affects the environmental behavior, bioavailability and toxicity of both MPs and Pb. Thus, Pb contamination in water bodies can affect a specific region where MPs are present and a large area where MPs are transported or dispersed.
As seen in Table 1, research on the Pb-MP interaction covers an important area for an in-depth understanding of the role of MPs in environmental systems (Cao et al., 2021). The Pb-MP interaction has many consequences in the aquatic environment, highlighting significant effects on microbial communities and biofilm formation. The diversity of MP species is an important factor in determining the absorption of Pb ions (Wang et al., 2022). Studies reveal that different MP types have different absorption properties and that Pb ions exhibit a high affinity for MP surfaces (Liu et al., 2021a, 2021b). High Pb concentrations in water systems and oxygen-containing functional groups increase Pb adsorption to the surface of MPs, contributing to a more efficient transfer of the heavy metal through MPs into water systems (Shen et al., 2021). It was also observed that the degree of oxidation, MP type and environmental conditions (pH, ion strength, dissolved organic matter (DOM)) affect Pb adsorption, and various factors regulate the Pb-MP interaction. In particular, the degree of oxidation determines the surface properties of MPs (Liu et al., 2022a, 2022b, 2022c). This reveals that Pb adsorption is enhanced with the increase of oxygen-containing groups on the MP surface (Zhou et al., 2020). The Pb-MP interaction is mostly characterized by physical adsorption, which is characterized by a direct process and weak binding (Li et al., 2022a, 2022b). In this context, Pb ions are physically adsorbed on the MP surface, meaning weak bonds bind them and can be released (Fu et al., 2021a, 2021b).
Overall, Pb-MP interaction is a complex process that can increase the environmental impacts of MPs and extend the pollution spread of the heavy metal in aquatic systems, which means that it can lead to long-term environmental contamination of Pb. Based on this information, it is clear that Pb-MP interaction is a severe problem in the aquatic environment. To solve this problem, taking measures such as reducing the sources of MPs and Pb, removing MPs and Pb from the aquatic environment, and monitoring the toxic effects of Pb-MP interaction can be an effective factor in the success of long-term solutions to environmental problems.
5 Future Perspectives and Challenges
Future research on heavy metal adsorption–desorption properties of biofilm-affected MPs should cover both fundamental and applied aspects. Fundamental aspects should aim to understand better biofilm's effects on heavy metal adsorption–desorption capacity, kinetics, isotherms, thermodynamics, and mechanisms of MPs. It should also systematically investigate the factors affecting the heavy metal adsorption–desorption properties of biofilm on MPs, such as biofilm thickness, structure, composition, age, type, shape, size, surface area, degree of contamination, concentration of heavy metal ions, pH, temperature, salinity, redox potential, etc. Studying them under long-term and realistic conditions to improve fundamental knowledge and applied solutions is important to better understand how heavy metal adsorption–desorption properties change with time. The applied aspects should aim to develop new methods and materials to modify biofilm-affected MPs' heavy metal adsorption–desorption properties and design new technologies and strategies to remove or recover them from the environment.
The current studies focusing on metal ions are generally concentrated on Pb and Cu. However, other metal ions should also be considered to understand biofilms' impact on the heavy metal adsorption mechanism by plastic residues. Particularly, as plastic pollution is increasing in aquatic environments, there is a need for further research in this area. Although the studies are mostly carried out in the laboratory, they should be simultaneously combined with in situ experiments to reveal the biofilm-enhanced adsorption mechanisms of metals. This biofilm may reflect realistic environmental factors that affect MPs' heavy metal adsorption–desorption properties. Additionally, while studies have mainly focused on adsorption isotherms and spectroscopic methods, there is a highlighted necessity for more work on mathematical modeling and dynamic analysis confirming the contribution of environmental conditions and biofilms to metal adsorption. Nevertheless, there still exists a knowledge gap in the current research literature:
-
(1)
The hydraulic parameters influencing the movement, accumulation, and resuspension of different MP particles in freshwater environments have not yet been sufficiently investigated. These parameters are important in transporting, settling, and remixing MPs in receiving environments. Therefore, it is important to examine the hydrodynamic behaviors of MPs and the factors influencing them to understand their fate and transport in freshwater environments.
-
(2)
Data on the age, degradation levels, and biofilm accumulation of MPs detected in the studied aquatic environment is limited. The age of MPs can help determine their sources and pathways of entry. The degradation levels of MPs vary depending on plastic types, environmental conditions, and microbial activity. The biofilm accumulation on MPs influences their density, hydrophobicity, toxicity, and biological interactions. Therefore, determining the age, degradation levels, and biofilm accumulations of MPs is necessary to understand the characteristics and impacts of MPs in freshwater environments.
-
(3)
The clear impact of biofilm accumulation on different MP density changes has not yet been fully understood. Biofilm accumulation can either decrease or increase the density of MPs, thereby affecting the probability of floating, which can influence MPs' transport, accumulation, and resuspension. Additionally, biofilm accumulation can alter the chemical composition, surface properties, and the release or absorption of toxic substances in MPs, affecting MPs’ environmental risk and toxicology. Therefore, investigating the open effect of biofilm accumulation on different MP density changes is important to understand the behavior and outcomes of MPs in freshwater environments.
-
(4)
Quantitatively determining the dominant process, such as release or absorption, for MPs with different characteristics is challenging. MPs exhibit diversity in terms of plastic types, shapes, sizes, surface properties, and biofilm accumulations, affecting MPs' release or absorption capacity and rate. MPs can release or absorb toxic substances in their environment, determining their and other organisms' toxic effects. Therefore, quantitatively determining the dominant process for MPs with different characteristics, such as release or absorption, is necessary to understand the toxicology of MPs in freshwater environments.
Challenges related to heavy metal adsorption–desorption properties of MPs exist at both experimental and theoretical levels. At the experimental level, there is a lack of standardized, sensitive, and reliable methods to measure heavy metal adsorption–desorption properties of biofilm-affected MPs. At the theoretical level, there is the complexity and diversity of numerous factors influencing the heavy metal adsorption–desorption properties of MPs. Furthermore, the lack of sufficient data and models to assess the environmental impacts and health risks of heavy metal adsorption–desorption properties of MPs is also a challenge. There are also technical challenges to improving or utilizing MPs' heavy metal adsorption–desorption properties, such as high cost, low efficiency, and low selectivity. Combating these challenges requires experimental and theoretical method development, data, model and technical improvements, and multidisciplinary collaboration, which can help better understand MP's heavy metal adsorption–desorption properties and is important for environmental risk assessment and waste management.
6 Conclusion
This study provides valuable insights into the complex relationships between the dynamic interactions of biofilm formation on MPs and Pb adsorption. Biofilm formation was found to be an important influence on the adsorption of heavy metals on MPs. Biofilms act as dynamic interfaces, altering MPs' surface properties and significantly changing heavy metals' adsorption–desorption capacity, adding a new dimension to MP pollution's environmental and biological problems. Selected effects on Pb-MP interactions have also been studied, revealing that these interactions are physical coupling and involve specific mechanisms based on factors such as surface chemistry, particle size and environmental conditions. The study of Pb-MP interactions in receiving environments has revealed a complex interaction influenced by various environmental factors. Therefore, dynamic interactions between environmental compartments play a critical role in understanding the interactions of MPs with potentially toxic elements, allowing for more effective environmental policies and, management strategies and interventions to minimize environmental risks.
Specific regulations, standards, and protocols are required to understand and effectively manage MPs' environmental impacts fully. These regulations should include a comprehensive approach across the various stages of MP production, transportation, use, disposal, and biotoxicity. At this point, future research and regulations are expected to contribute to environmental sustainability goals by establishing an effective framework to combat MP pollution. Scientific research can shed light on a better understanding of environmental impacts and the development of more effective strategies to address this problem. At the same time, establishing regulations on MPs at national and international levels can encourage various sectors and societies to take responsibility for this issue. By understanding these dynamics, researchers and policymakers can make informed decisions to reduce the environmental impacts of MPs and heavy metals in aquatic ecosystems. Future studies can further investigate the long-term effects of these interactions and develop targeted interventions and strategies to minimize ecological consequences.
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
Abbas, Z., Ali, S., Rizwan, M., Zaheer, I. E., Malik, A., Riaz, M. A., Shahid, M. R., Rehman, M. Z. U., & Al-Wabel, M. I. (2018). A critical review of mechanisms involved in the adsorption of organic and inorganic contaminants through biochar. Arabian Journal of Geosciences, 11, 1–23.
Acosta-Coley, I., Mendez-Cuadro, D., Rodriguez-Cavallo, E., de la Rosa, J., & Olivero-Verbel, J. (2019). Trace elements in microplastics in Cartagena: A hotspot for plastic pollution at the Caribbean. Marine Pollution Bulletin, 139, 402–411.
Adebiyi, A. A., & Kok, J. F. (2020). Climate models miss most of the coarse dust in the atmosphere. Science advances, 6(15), eaaz507.
Adji, B. K., Octodhiyanto, I., Rahmayanti, R., & Nugroho, A. P. (2022). Microplastic pollution in Rawa Jombor Reservoir, Klaten, Central Java, Indonesia: Accumulation in aquatic fauna, heavy metal interactions, and health risk assessment. Water, Air, & Soil Pollution, 233(4), 112.
Afreen, V., Hashmi, K., Nasir, R., Saleem, A., Khan, M. I., & Akhtar, M. F. (2023). Adverse health effects and mechanisms of microplastics on female reproductive system: A descriptive review. Environmental Science and Pollution Research, 30(31), 76283–76296.
Ahamed, T., Brown, S. P., & Salehi, M. (2020). Investigate the role of biofilm and water chemistry on lead deposition onto and release from polyethylene: An implication for potable water pipes. Journal of Hazardous Materials, 400, 123253.
Akhbarizadeh, R., Moore, F., & Keshavarzi, B. (2019). Investigating microplastics bioaccumulation and biomagnification in seafood from the Persian Gulf: A threat to human health? Food Additives & Contaminants: Part A, 36(11), 1696–1708.
Alengebawy, A., Abdelkhalek, S. T., Qureshi, S. R., & Wang, M.-Q. (2021). Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics, 9(3), 42.
Alvim, C. B., Mendoza-Roca, J. A., & Bes-Piá, A. (2020). Wastewater treatment plant as microplastics release source–quantification and identification techniques. Journal of Environmental Management, 255, 109739.
Arias-Andres, M., Rojas-Jimenez, K., & Grossart, H.-P. (2019). Collateral effects of microplastic pollution on aquatic microorganisms: An ecological perspective. TrAC Trends in Analytical Chemistry, 112, 234–240.
Battulga, B., Kawahigashi, M., & Oyuntsetseg, B. (2022). Characterization of biofilms formed on polystyrene microplastics (PS-MPs) on the shore of the Tuul River. Mongolia. Environmental Research, 212, 113329.
Bhargava, P., Gupta, N., Vats, S., & Goel, R. (2017). Health issues and heavy metals. Austin J Environ Toxicol, 3(1), 3018.
Bharti, R., & Sharma, R. (2022). Effect of heavy metals: An overview. Materials Today: Proceedings, 51, 880–885.
Brandon, J. A., Jones, W., & Ohman, M. D. (2019). Multidecadal increase in plastic particles in coastal ocean sediments. Science advances, 5(9), eaax0587.
Campanale, C., Massarelli, C., Savino, I., Locaputo, V., & Uricchio, V. F. (2020). A detailed review study on potential effects of microplastics and additives of concern on human health. International Journal of Environmental Research and Public Health, 17(4), 1212.
Cánovas, C. R., González, R. M., Vieira, B. J., Waerenborgh, J. C., Marques, R., Macías, F., Basallote, M. D., Olias, M., & Prudencio, M. I. (2023). Metal mobility and bioaccessibility from cyanide leaching heaps in a historical mine site. Journal of Hazardous Materials, 448, 130948.
Cao, Y., Zhao, M., Ma, X., Song, Y., Zuo, S., Li, H., & Deng, W. (2021). A critical review on the interactions of microplastics with heavy metals: Mechanism and their combined effect on organisms and humans. Science of the Total Environment, 788, 147620.
Collin, M. S., Venkatraman, S. K., Vijayakumar, N., Kanimozhi, V., Arbaaz, S. M., Stacey, R. S., Anusha, J., Choudhary, R., Lvov, V., & Tovar, G. I. (2022). Bioaccumulation of lead (Pb) and its effects on human: A review. Journal of Hazardous Materials Advances, 7, 100094.
Davranche, M., Veclin, C., Pierson-Wickmann, A.-C., El Hadri, H., Grassl, B., Rowenczyk, L., Dia, A., Ter Halle, A., Blancho, F., & Reynaud, S. (2019). Are nanoplastics able to bind significant amount of metals? The lead example. Environmental Pollution, 249, 940–948.
Debroy, A., George, N., & Mukherjee, G. (2022). Role of biofilms in the degradation of microplastics in aquatic environments. Journal of Chemical Technology & Biotechnology, 97(12), 3271–3282.
Deng, H., Fu, Q., Li, D., Zhang, Y., He, J., Feng, D., Zhao, Y., Du, G., Yu, H., & Ge, C. (2021). Microplastic-associated biofilm in an intensive mariculture pond: Temporal dynamics of microbial communities, extracellular polymeric substances and impacts on microplastics properties. Journal of Cleaner Production, 319, 128774.
Dennehy, J. J., & Abedon, S. T. (2021). Adsorption: Phage acquisition of bacteria. In D. R. Harper, S. T. Abedon, B. H. Burrowes, & M. L. McConville (Eds.), Bacteriophages: Biology, technology, therapy (pp. 93–117). Springer International Publishing.
Elgarahy, A. M., Akhdhar, A., & Elwakeel, K. Z. (2021). Microplastics prevalence, interactions, and remediation in the aquatic environment: A critical review. Journal of Environmental Chemical Engineering, 9(5), 106224.
Engwa, G. A., Ferdinand, P. U., Nwalo, F. N., & Unachukwu, M. N. (2019). Mechanism and health effects of heavy metal toxicity in humans. Poisoning in the Modern World-New Tricks for an Old Dog, 10, 70–90.
Fan, T., Zhao, J., Chen, Y., Wang, M., Wang, X., Wang, S., Chen, X., Lu, A., & Zha, S. (2021). Coexistence and adsorption properties of heavy metals by polypropylene microplastics. Adsorption Science & Technology, 2021, 1–12.
Feng, L., He, L., Jiang, S., Chen, J., Zhou, C., Qian, Z.-J., Hong, P., Sun, S., & Li, C. (2020). Investigating the composition and distribution of microplastics surface biofilms in coral areas. Chemosphere, 252, 126565.
Flemming, H.-C., van Hullebusch, E. D., Neu, T. R., Nielsen, P. H., Seviour, T., Stoodley, P., Wingender, J., & Wuertz, S. (2023). The biofilm matrix: Multitasking in a shared space. Nature Reviews Microbiology, 21(2), 70–86.
Fu, Z., & Xi, S. (2020). The effects of heavy metals on human metabolism. Toxicology Mechanisms and Methods, 30(3), 167–176.
Fu, L., Li, J., Wang, G., Luan, Y., & Dai, W. (2021). Adsorption behavior of organic pollutants on microplastics. Ecotoxicology and Environmental Safety, 217, 112207.
Fu, Q., Tan, X., Ye, S., Ma, L., Gu, Y., Zhang, P., Chen, Q., Yang, Y., & Tang, Y. (2021). Mechanism analysis of heavy metal lead captured by natural-aged microplastics. Chemosphere, 270, 128624.
Gao, F., Li, J., Sun, C., Zhang, L., Jiang, F., Cao, W., & Zheng, L. (2019). Study on the capability and characteristics of heavy metals enriched on microplastics in marine environment. Marine Pollution Bulletin, 144, 61–67.
Ghoul, M., & Mitri, S. (2016). The ecology and evolution of microbial competition. Trends in Microbiology, 24(10), 833–845.
Godoy, V., Blázquez, G., Calero, M., Quesada, L., & Martín-Lara, M. (2019). The potential of microplastics as carriers of metals. Environmental Pollution, 255, 113363.
Gralka, M., Szabo, R., Stocker, R., & Cordero, O. X. (2020). Trophic interactions and the drivers of microbial community assembly. Current Biology, 30(19), R1176–R1188.
Gu, Z., Song, W., Yang, Z., & Zhou, R. (2018). Metal–organic framework as an efficient filter for the removal of heavy metal cations in water. Physical Chemistry Chemical Physics, 20(48), 30384–30391.
Guan, J., Qi, K., Wang, J., Wang, W., Wang, Z., Lu, N., & Qu, J. (2020). Microplastics as an emerging anthropogenic vector of trace metals in freshwater: Significance of biofilms and comparison with natural substrates. Water Research, 184, 116205.
Hale, R. C., Seeley, M. E., La Guardia, M. J., Mai, L., & Zeng, E. Y. (2020). A global perspective on microplastics. Journal of Geophysical Research: Oceans, 125(1), e2018JC014719.
He, S., Jia, M., Xiang, Y., Song, B., Xiong, W., Cao, J., Peng, H., Yang, Y., Wang, W., & Yang, Z. (2022). Biofilm on microplastics in aqueous environment: Physicochemical properties and environmental implications. Journal of Hazardous Materials, 424, 127286.
Heal, M. R., Hibbs, L. R., Agius, R. M., & Beverland, I. J. (2005). Total and water-soluble trace metal content of urban background PM10, PM2. 5 and black smoke in Edinburgh. UK. Atmospheric Environment, 39(8), 1417–1430.
Hembrom, S., Singh, B., Gupta, S. K., & Nema, A. K. (2020). A comprehensive evaluation of heavy metal contamination in foodstuff and associated human health risk: A global perspective. In P. Singh, R. P. Singh, & V. Srivastava (Eds.), Contemporary environmental issues and challenges in era of climate change (pp. 33–63). Springer Singapore.
Hettiarachchi, G. M., Betts, A. R., Chandima Wekumbura, W. G., Lake, L., Mayer, M. M., Scheckel, K. G., & Basta, N. T. (2024). Chapter 6 - Lead: The most extensively spread toxic environmental contaminant. In R. Naidu (Ed.), Inorganic contaminants and radionuclides (pp. 113–150). Elsevier.
Inamdar, I. (2022). Recycling of plastic wastes generated from COVID-19: A comprehensive illustration of type and properties of plastics with remedial options. Science of the Total Environment, 838, 155895.
Izadi, P., Izadi, P., & Eldyasti, A. (2021). Holistic insights into extracellular polymeric substance (EPS) in anammosx bacterial matrix and the potential sustainable biopolymer recovery: A review. Chemosphere, 274, 129703.
Jaishankar, M., Tseten, T., Anbalagan, N., Mathew, B. B., & Beeregowda, K. N. (2014). Toxicity, mechanism and health effects of some heavy metals. Interdisciplinary Toxicology, 7(2), 60.
Jiang, X., Chang, Y., Zhang, T., Qiao, Y., Klobučar, G., & Li, M. (2020). Toxicological effects of polystyrene microplastics on earthworm (Eisenia fetida). Environmental Pollution, 259, 113896.
Karbalaei, S., Hanachi, P., Walker, T. R., & Cole, M. (2018). Occurrence, sources, human health impacts and mitigation of microplastic pollution. Environmental Science and Pollution Research, 25, 36046–36063.
Khoo, K. S., Ho, L. Y., Lim, H. R., Leong, H. Y., & Chew, K. W. (2021). Plastic waste associated with the COVID-19 pandemic: Crisis or opportunity? Journal of Hazardous Materials, 417, 126108.
Kumar, R., Ivy, N., Bhattacharya, S., Dey, A., & Sharma, P. (2022). Coupled effects of microplastics and heavy metals on plants: Uptake, bioaccumulation, and environmental health perspectives. Science of the Total Environment, 836, 155619.
Kumar, A., Kumar, A., Cabral-Pinto, M., Chaturvedi, A. K., Shabnam, A. A., Subrahmanyam, G., Mondal, R., Gupta, D. K., Malyan, S. K., Kumar, S. S., Khan, S. A., & Yadav, K. K. (2020). Lead toxicity: Health hazards, infuence on food chain, and sustainable remediation approaches. International Journal of Environmental Research and Public Health, 17(7), 2179.
Li, W., Zu, B., Yang, Q., Huang, Y., & Li, J. (2022). Adsorption of lead and cadmium by microplastics and their desorption behavior as vectors in the gastrointestinal environment. Journal of Environmental Chemical Engineering, 10(3), 107379.
Li, Y., Wang, X., Wang, Y., Sun, Y., Xia, S., & Zhao, J. (2022). Effect of biofilm colonization on Pb (II) adsorption onto poly (butylene succinate) microplastic during its biodegradation. Science of the Total Environment, 833, 155251.
Lin, Q., Liu, E., Zhang, E., Li, K., & Shen, J. (2016). Spatial distribution, contamination and ecological risk assessment of heavy metals in surface sediments of Erhai Lake, a large eutrophic plateau lake in southwest China. CATENA, 145, 193–203.
Lin, Z., Hu, Y., Yuan, Y., Hu, B., & Wang, B. (2021). Comparative analysis of kinetics and mechanisms for Pb (II) sorption onto three kinds of microplastics. Ecotoxicology and Environmental Safety, 208, 111451.
Liu, G., Dave, P. H., Kwong, R. W., Wu, M., & Zhong, H. (2021a). Influence of microplastics on the mobility, bioavailability, and toxicity of heavy metals: A review. Bulletin of Environmental Contamination and Toxicology, 107, 710–721.
Liu, S. Y., Leung, M.M.-L., Fang, J.K.-H., & Chua, S. L. (2021). Engineering a microbial ‘trap and release’mechanism for microplastics removal. Chemical Engineering Journal, 404, 127079.
Liu, S., Huang, J., Zhang, W., Shi, L., Yi, K., Yu, H., Zhang, C., Li, S., & Li, J. (2022a). Microplastics as a vehicle of heavy metals in aquatic environments: A review of adsorption factors, mechanisms, and biological effects. Journal of Environmental Management, 302, 113995.
Liu, S., Huang, J., Zhang, W., Shi, L., Yi, K., Zhang, C., Pang, H., Li, J., & Li, S. (2022). Investigation of the adsorption behavior of Pb (II) onto natural-aged microplastics as affected by salt ions. Journal of Hazardous Materials, 431, 128643.
Liu, Y., Zhang, J., Cao, W., Hu, Y., & Shen, W. (2022). The influence of Pb (II) adsorption on (Non) biodegradable microplastics by UV/O3 oxidation treatment. Journal of Environmental Chemical Engineering, 10(6), 108615.
Mariana, M., Abdul Khalil, H. P. S, Mistar, E. M., Yahya, E. B., Alfatah, T., Danish, M., & Amayreh, M. (2021). Recent advances in activated carbon modification techniques for enhanced heavy metal adsorption. Journal of Water Process Engineering, 43, 102221.
Maršić-Lučić, J., Lušić, J., Tutman, P., Varezić, D. B., Šiljić, J., & Pribudić, J. (2018). Levels of trace metals on microplastic particles in beach sediments of the island of Vis, Adriatic Sea, Croatia. Marine Pollution Bulletin, 137, 231–236.
Massányi, P., Massányi, M., Madeddu, R., Stawarz, R., & Lukáč, N. (2020). Effects of cadmium, lead, and mercury on the structure and function of reproductive organs. Toxics, 8(4), 94.
Massos, A., & Turner, A. (2017). Cadmium, lead and bromine in beached microplastics. Environmental Pollution, 227, 139–145.
Mishra, S., et al. (2019). Heavy metal contamination: An alarming threat to environment and human health. In R. Sobti, N. Arora, & R. Kothari (Eds.), Environmental biotechnology: For sustainable future. Springer.
Moumakwa, N. L. (2023). Application of agricultural waste and residues derived fibers in the building construction industry: A review. AIP Conference Proceedings, 2581(1), 020005.
Moyal, J., Dave, P. H., Wu, M., Karimpour, S., Brar, S. K., Zhong, H., & Kwong, R. W. (2023). Impacts of Biofilm Formation on the Physicochemical Properties and Toxicity of Microplastics: A Concise Review. Reviews of Environmental Contamination and Toxicology, 261(1), 1–17.
Munoz, M., Ortiz, D., Nieto-Sandoval, J., de Pedro, Z. M., & Casas, J. A. (2021). Adsorption of micropollutants onto realistic microplastics: Role of microplastic nature, size, age, and NOM fouling. Chemosphere, 283, 131085.
Nagoya, S., Nakamichi, S., & Kawase, Y. (2019). Mechanisms of phosphate removal from aqueous solution by zero-valent iron: A novel kinetic model for electrostatic adsorption, surface complexation and precipitation of phosphate under oxic conditions. Separation and Purification Technology, 218, 120–129.
Negahban, S., & Mokarram, M. (2021). Potential ecological risk assessment of Ni, Cu, Zn, Cd, and Pb in roadside soils. Earth and Space Science, 8(4), e2020EA001120.
Nguyen, M.-K., Rakib, M. R. J., Lin, C., Hung, N. T. Q., Le, V.-G., Nguyen, H.-L., Malafaia, G., & Idris, A. M. (2023). A comprehensive review on ecological effects of microplastic pollution: An interaction with pollutants in the ecosystems and future perspectives. TrAC Trends in Analytical Chemistry, 168, 117294.
Nowicka, B. (2022). Heavy metal–induced stress in eukaryotic algae—mechanisms of heavy metal toxicity and tolerance with particular emphasis on oxidative stress in exposed cells and the role of antioxidant response. Environmental Science and Pollution Research, 29(12), 16860–16911.
Obasi, P. N., & Akudinobi, B. B. (2020). Potential health risk and levels of heavy metals in water resources of lead–zinc mining communities of Abakaliki, southeast Nigeria. Applied Water Science, 10(7), 1–23.
Obeng-Gyasi, E. (2019). Sources of lead exposure in various countries. Reviews on Environmental Health, 34(1), 25–34.
Özgenç, E., Keleş, E., & Yıldız Töre, G. (2023). Assessment of biomarker-based ecotoxic effects in combating microplastic pollution - A review. Global NEST Journal, 26(1), 05398.
Padha, S., Kumar, R., Dhar, A., & Sharma, P. (2022). Microplastic pollution in mountain terrains and foothills: A review on source, extraction, and distribution of microplastics in remote areas. Environmental Research, 207, 112232.
Pandey, G., & Madhuri, S. (2014). Heavy metals causing toxicity in animals and fishes. Research Journal of Animal, Veterinary and Fishery Sciences, 2(2), 17–23.
Paz-Sabillón, M., Torres-Sánchez, L., Piña-Pozas, M., Del Razo, L. M., & Quintanilla-Vega, B. (2023). Prenatal Exposure to Potentially Toxic Metals and Their Effects on Genetic Material in Offspring: A Systematic Review. Biological Trace Element Research, 201(5), 2125–2150.
Prajapati, A., Narayan Vaidya, A., & Kumar, A. R. (2022). Microplastic properties and their interaction with hydrophobic organic contaminants: A review. Environmental Science and Pollution Research, 29(33), 49490–49512.
Prokić, M. D., Radovanović, T. B., Gavrić, J. P., & Faggio, C. (2019). Ecotoxicological effects of microplastics: Examination of biomarkers, current state and future perspectives. TrAC Trends in Analytical Chemistry, 111, 37–46.
Purwiyanto, A. I. S., Suteja, Y., Ningrum, P. S., Putri, W. A. E., Agustriani, F., Cordova, M. R., & Koropitan, A. F. (2020). Concentration and adsorption of Pb and Cu in microplastics: Case study in aquatic environment. Marine Pollution Bulletin, 158, 111380.
Qiang, L., Cheng, J., Mirzoyan, S., Kerkhof, L. J., & Häggblom, M. M. (2021). Characterization of microplastic-associated biofilm development along a freshwater-estuarine gradient. Environmental Science & Technology, 55(24), 16402–16412.
Qiongjie, W., Yong, Z., Yangyang, Z., Zhouqi, L., Jinxiaoxue, W., & Huijuan, C. (2022). Effects of biofilm on metal adsorption behavior and microbial community of microplastics. Journal of Hazardous Materials, 424, 127340.
Qiu, B., Tao, X., Wang, H., Li, W., Ding, X., & Chu, H. (2021). Biochar as a low-cost adsorbent for aqueous heavy metal removal: A review. Journal of Analytical and Applied Pyrolysis, 155, 105081.
Rummel, C. D., Jahnke, A., Gorokhova, E., Kühnel, D., & Schmitt-Jansen, M. (2017). Impacts of biofilm formation on the fate and potential effects of microplastic in the aquatic environment. Environmental Science & Technology Letters, 4(7), 258–267.
Sajid, M., Basheer, C., Narasimhan, K., Buhmeida, A., Al Qahtani, M., & Al-Ahwal, M. S. (2016). Persistent and endocrine disrupting organic pollutants: Advancements and challenges in analysis, health concerns and clinical correlates. Nature Environment and Pollution Technology, 15(2), 733.
Sassykova, L., Aubakirov, Y., Sendilvelan, S., Tashmukhambetova, Z. K., Faizullaeva, M., Bhaskar, K., Batyrbayeva, A., Ryskaliyeva, R., Tyussyupova, B., & Zhakupova, A. (2019). The main components of vehicle exhaust gases and their effective catalytic neutralization. Oriental Journal of Chemistry, 35(1), 110.
Sharifinia, M., Bahmanbeigloo, Z. A., Keshavarzifard, M., Khanjani, M. H., & Lyons, B. P. (2020). Microplastic pollution as a grand challenge in marine research: A closer look at their adverse impacts on the immune and reproductive systems. Ecotoxicology and Environmental Safety, 204, 111109.
Sharma, S., Basu, S., Shetti, N. P., Nadagouda, M. N., & Aminabhavi, T. M. (2021). Microplastics in the environment: Occurrence, perils, and eradication. Chemical Engineering Journal, 408, 127317.
Shen, M., Song, B., Zeng, G., Zhang, Y., Teng, F., & Zhou, C. (2021). Surfactant changes lead adsorption behaviors and mechanisms on microplastics. Chemical Engineering Journal, 405, 126989.
Singh, N., Kumar, A., Gupta, V. K., & Sharma, B. (2018). Biochemical and molecular bases of lead-induced toxicity in mammalian systems and possible mitigations. Chemical Research in Toxicology, 31(10), 1009–1021.
Sooriyakumar, P., Bolan, N., Kumar, M., Singh, L., Yu, Y., Li, Y., Weralupitiya, C., Vithanage, M., Ramanayaka, S., & Sarkar, B. (2022). Biofilm formation and its implications on the properties and fate of microplastics in aquatic environments: A review. Journal of Hazardous Materials Advances, 6, 100077.
Souri, Z., Cardoso, A. A., da-Silva, C. J., de Oliveira, L. M., Dari, B., Sihi, D., & Karimi, N. (2019). Heavy metals and photosynthesis: Recent developments. In Photosynthesis, productivity and environmental stress (pp. 107–134).
Stabnikova, O., Stabnikov, V., Marinin, A., Klavins, M., & Vaseashta, A. (2022). The role of microplastics biofilm in accumulation of trace metals in aquatic environments. World Journal of Microbiology and Biotechnology, 38(7), 117.
Ta, A. T., & Babel, S. (2020). Microplastic contamination on the lower Chao Phraya: Abundance, characteristic and interaction with heavy metals. Chemosphere, 257, 127234.
Tang, S., Lin, L., Wang, X., Yu, A., & Sun, X. (2021). Interfacial interactions between collected nylon microplastics and three divalent metal ions (Cu (II), Ni (II), Zn (II)) in aqueous solutions. Journal of Hazardous Materials, 403, 123548.
Tong, S., Schirnding, Y. E. V., & Prapamontol, T. (2000). Environmental lead exposure: a public health problem of global dimensions. Bulletin of the world health organization, 78(9), 1068–1077.
Tourinho, P. S., Kočí, V., Loureiro, S., & van Gestel, C. A. (2019). Partitioning of chemical contaminants to microplastics: Sorption mechanisms, environmental distribution and effects on toxicity and bioaccumulation. Environmental Pollution, 252, 1246–1256.
Tu, C., Chen, T., Zhou, Q., Liu, Y., Wei, J., Waniek, J. J., & Luo, Y. (2020a). Biofilm formation and its influences on the properties of microplastics as affected by exposure time and depth in the seawater. Science of the Total Environment, 734, 139237.
Tu, C., Zhou, Q., Zhang, C., Liu, Y., & Luo, Y. (2020b). Biofilms of microplastics. In D. He & Y. Luo (Eds.), Microplastics in terrestrial environments: Emerging contaminants and major challenges (pp. 299–317). Springer International Publishing.
Vardhan, K. H., Kumar, P. S., & Panda, R. C. (2019). A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. Journal of Molecular Liquids, 290, 111197.
Vareda, J. P., Valente, A. J., & Durães, L. (2019). Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: A review. Journal of Environmental Management, 246, 101–118.
Viana, G. M., Priestman, D. A., Platt, F. M., Khan, S., Tomatsu, S., & Pshezhetsky, A. V. (2020). Brain pathology in mucopolysaccharidoses (MPS) patients with neurological forms. Journal of Clinical Medicine, 9(2), 396.
Wang, Q., Zhao, H., Chen, J., Gu, K., Zhang, Y., Zhu, Y., Zhou, Y., & Ye, L. (2009). Adverse health effects of lead exposure on children and exploration to internal lead indicator. Science of the Total Environment, 407(23), 5986–5992.
Wang, F., Zhang, M., Sha, W., Wang, Y., Hao, H., Dou, Y., & Li, Y. (2020). Sorption behavior and mechanisms of organic contaminants to nano and microplastics. Molecules, 25(8), 1827.
Wang, B., Wang, C., & Hu, Y. (2022). Sorption behavior of Pb (II) onto polyvinyl chloride microplastics affects the formation and ecological functions of microbial biofilms. Science of the Total Environment, 832, 155026.
Wang, M., Xiao, Y., Li, Y., & Liu, J. (2023). Optimistic effects of galaxolide and polystyrene microplastic stress on the physio-biochemical characteristics and metabolic profiles of an ornamental plant. Plant Physiology and Biochemistry, 196, 350–360.
Wen, B., Jin, S.-R., Chen, Z.-Z., Gao, J.-Z., Liu, Y.-N., Liu, J.-H., & Feng, X.-S. (2018). Single and combined effects of microplastics and cadmium on the cadmium accumulation, antioxidant defence and innate immunity of the discus fish (Symphysodon aequifasciatus). Environmental Pollution, 243, 462–471.
Witkowska, D., Słowik, J., & Chilicka, K. (2021). Heavy metals and human health: Possible exposure pathways and the competition for protein binding sites. Molecules, 26(19), 6060.
Wu, B., Wu, X., Liu, S., Wang, Z., & Chen, L. (2019). Size-dependent effects of polystyrene microplastics on cytotoxicity and efflux pump inhibition in human Caco-2 cells. Chemosphere, 221, 333–341.
Zhang, Q., He, Y., Cheng, R., Li, Q., Qian, Z., & Lin, X. (2022). Recent advances in toxicological research and potential health impact of microplastics and nanoplastics in vivo. Environmental Science and Pollution Research, 29(27), 40415–40448.
Zhou, Y., Yang, Y., Liu, G., He, G., & Liu, W. (2020). Adsorption mechanism of cadmium on microplastics and their desorption behavior in sediment and gut environments: The roles of water pH, lead ions, natural organic matter and phenanthrene. Water Research, 184, 116209.
Zhu, D., Chen, Q.-L., An, X.-L., Yang, X.-R., Christie, P., Ke, X., Wu, L.-H., & Zhu, Y.-G. (2018). Exposure of soil collembolans to microplastics perturbs their gut microbiota and alters their isotopic composition. Soil Biology and Biochemistry, 116, 302–310.
Zou, J., Liu, X., Zhang, D., & Yuan, X. (2020). Adsorption of three bivalent metals by four chemical distinct microplastics. Chemosphere, 248, 126064.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). The author does not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
Enes Özgenç planned and designed all stages of this study, collected and compiled the data, and wrote and edited the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
The author has read, understood, and has complied as applicable with the statement on “Ethical Responsibilities of Authors” as found in the Instructions for Authors.
Consent to Participate
Not applicable.
Consent to Publication
The author confirms that this paper has not been published before, that it is not under consideration for publication elsewhere, and that its publication has been approved by Enes Özgenç.
Conflict of Interest
The author declares no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Özgenç, E. Evaluation of the Spreading Dynamics and Interactions of Lead-Carrier Microplastics Affected by Biofilm: A Mini-Review. Water Air Soil Pollut 235, 281 (2024). https://doi.org/10.1007/s11270-024-07090-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-024-07090-9