Abstract
Microplastics are a highly concerning pollutant that have gained attention from the scientific community and other regulatory authorities due to their potential risks to organisms and ecosystems. Microplastics are widespread in both terrestrial and aquatic ecosystems and can be found even in Antarctica and deep-sea sediments. The ability to survive for long periods in the environment and their aptitude of inter- and intra-environmental translocation can prompt poor environmental outcomes. The adsorption of heavy metals and other toxic persistent organic pollutants is a further cause for concern. Furthermore, microplastics enable the development of a distinct microbial niche within an ecosystem, which could potentially impair ecosystem function by promoting the growth of selective microbial communities. The acquisition of metal-resistant, antibiotic-resistant genes, and the enrichment of antibiotic-resistant bacteria on microplastic surfaces have recently been reported. Moreover, some studies have also reported the colonization of pathogenic bacterial strains such as Vibrio spp. on microplastic surfaces. This review aims to address the sources of microplastic pollution in the freshwater and marine environments and to discuss their potential functions in the environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plastic has become an indispensable part of human life. It is a synthetic organic polymer produced through the polymerization of monomers procured from fossil fuels, gas, or coal. Certain attributes such as durability, lightweight, resistance to corrosion, and low price have led to the extensive use of plastic-based materials. While plastic was invented a century ago, mass production did not start until the mid-nineteenth century (Ivleva et al. 2017). The annual global production of plastic in 2015 was 320 million tons (PlasticsEurope 2015), and this is increasing annually. Plastics have served human society in many ways, for example, reduced CO2 emission, enhanced consumer health, increased product durability, drinking water storage, and transportation (Andrady and Neal 2009). However, since its extensive use worldwide, it has become recognized as a recalcitrant pollutant in both terrestrial and aquatic ecosystems (Erni-Cassola et al. 2019). Plastics exist in the environment in a broad size range, from macro- to micro-size particles.
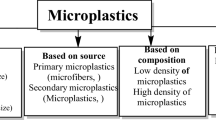
Microplastics, generally, refer to plastic particles that are less than 10 mm in size (Graham and Thompson 2009); however, this classification varies from study to study (Barnes et al. 2009; Claessens et al. 2011; Derraik 2002; Ryan et al. 2009). The existence of microplastic in the environment is becoming a global environmental and health problem. There are two main categories of microplastics: primary microplastics and secondary microplastics. Primary microplastics are plastic particles initially produced at the microscopic size. These are widely used in cosmetics, facial cleansers, and pharmaceutics (drug delivery) (Gregory 1996). Secondary microplastics are plastic particles that are produced from the breakdown of large plastic materials in both terrestrial and aquatic systems (Cole et al. 2011; Ryan et al. 2009). Secondary microplastic is considered the major cause of microplastic pollution in the marine environment (Hidalgo-Ruz et al. 2012).
The distribution and abundance of microplastic in an ecosystem mostly depends on the surrounding anthropogenic activities (Eriksen et al. 2013). In the 1970s, the presence of microplastic was first accentuated in the open ocean (Carpenter and Smith 1972). Since then, a renewed research interest over the last few decades has shown that microplastics are now ubiquitous in the marine environment (Derraik 2002; Moore 2008; Thompson et al. 2004). The distribution, abundance, and ecological consequences of microplastic pollution in the marine environment are hot topics of the current research. Similarly, interest is growing in determining the levels of microplastic pollution in freshwater bodies, including rivers and lakes. Several studies have documented the abundance and distribution of microplastic pollution in freshwater systems, including the water column and sediments (Alam et al. 2019; Eriksen et al. 2013; Nan et al. 2020; Wagner et al. 2014). Riverine and estuaries ecosystems, especially the rivers that flow through populous cities, are considered as dumping sites for microplastic pollution (Eerkes-Medrano et al. 2015). These rivers also serve as the main source of microplastic pollution in the marine environment (Eerkes-Medrano et al. 2015). Domestic waste, littering, and improper waste management are the major routes through which plastic waste enters the riverine systems in populated areas.
Microplastic pollution has been identified as one of the most pervasive and damaging of human stresses in aquatic environments (Wagner et al. 2014). Compared to macroplastic, microplastic pollution can cause serious environmental, ecological, and health issues. The adverse effects and ecological consequences of microplastic pollution have been documented recently in several reports. For example, due to their small size, microplastics can be ingested as food particles by aquatic organisms and hence enter the food chain (Ivleva et al. 2017). Moreover, some studies have reported that microplastics provide a surface for the deposition of persistent organic pollutants such as polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs) (Bakir et al. 2012; Frias et al. 2010). Similarly, heavy metals tend to accumulate on the surface of different microplastic materials (Turner 2016). Furthermore, due to the distinct physiochemical characteristics, microplastics offer a unique habitat for the colonization of microbial communities. These physiognomies could lead to different ecological, microbiological, and evolutionary events. For example, it has been reported that microplastics can act as a reservoir for antibiotic-resistant and metal-resistant genes (Arias-Andres et al. 2018). Similarly, some studies have also found that pathogenic bacteria were able to colonize microplastic surfaces and hence could facilitate the transportation of pathogenic bacteria (Kirstein et al. 2016). This review aims to address the potential sources of microplastic pollution, to review the possible routes by which microplastic enter the environment, and to discuss the environmental consequences of microplastic pollution.
Sources of microplastic
Primary microplastics, those originally synthesized at the microscopic level, are widely used in a variety of manufacturing industries, for example, therapeutics (drug delivery, diagnostics reagent, injectable biomaterial), food science, and exfoliants in personal care products and cosmetics (Kawaguchi 2000). Moreover, microplastic “scrubber,” an important component of facial scrub and hand cleanser, has replaced the traditional use of natural ingredients, such as ground almond, pumice, and oatmeal (Derraik 2002; Fendall and Sewell 2009). A large increase in the use of microplastic “scrubbers” was seen in cosmetic industries after the process was patented in the 1980s (Fendall and Sewell 2009; Gregory 1996). Furthermore, various products such as toothpaste, shampoo, shower gel, liquid makeup, baby lotion, shaving cream, mascara, eye shadow, lotion, hair colors, nail polish, sunscreen, bubble bath have all been reported to be potential sources of microplastics (Conkle et al. 2018; Hintersteiner et al. 2015). According to an investigation of soaps usage conducted by Cosmetics Europe (Europe Cosmetics Industry Association) and Euromonitor International (Consumer database) in Norway and Switzerland, the total annual usage of microplastic beads was 4130 t, resulting in an average discharge of 17.5 ± 10 mg/day per individual (Gouin et al. 2015). In another study conducted by Chang (2015), the annual contribution of microplastics from Berkeley student housing was calculated to be around 5 kg/year. Similarly, the daily discharge from women’s lifestyle products in the UK was around 4594–94,500 microplastic particles (Napper et al. 2015). Furthermore, an investigation on German daily use of care products, including soaps, shower gels, skincare, body cleansers, and sunblock, was around 6.2 g per person per year (Essel et al. 2015). However, the estimated input of microplastic to the environment varies between nations because of different habits and different calculation methods (Galafassi et al. 2019). In addition, a large quantity of microplastics, comprised mostly of melamine, polyester and acrylic, is used in air blasting technology, associated with scrubbers in engine, machines, and boat hulls to remove rust and paint (Browne et al. 2007; Derraik 2002; Gregory 1996).
Secondary plastics are those produced from the breakdown of large plastic material, and the breakdown products enter the environment as a result of environmental weathering of the plastic objects. Over time, the mechanical integrity of the plastic material diminishes due to biological, physical, and chemical action, causing the disintegration of the corresponding material (Browne et al. 2007). Physical factors such as sunlight, including ultraviolet radiation, cause photo-degradation through the oxidation of the polymer matrix (Andrady 2011; Barnes et al. 2009; Moore 2008; Shah et al. 2008). In coastal areas, especially on the beaches, high sunlight and oxygen levels synergize plastic fragmentations (Browne et al. 2007). Furthermore, the continuous effects of turbulence, wave action, and abrasion boost the disintegration of the plastic materials (Barnes et al. 2009). As a result, the plastic material loses its structural integrity and falls apart into small fragments. This process of fragmentation continues until the original macroplastic material turns into microplastic particles. Further disintegration of microplastic particles could result in the formation of nanoplastic particles, with a minimum size of 1.6 µm (Galgani et al. 2010). To overcome the susceptibility to environmental conditions and to enhance the durability of plastic materials, adhesive materials have been added to the polymer matrix; this could result in further environmental and health complications (Talsness et al. 2009).
In terrestrial and freshwater ecosystems, secondary microplastics are mostly in a fibrous form, made of polyester, acrylic, and polyamide, originating from washing clothes and usually introduced to the environment at a density of around 100 particles per liter of effluent (Browne et al. 2011; Habib et al. 1998). According to one investigation, the washing of 5–6 kg of garments released around 137,951–6,000,000 particles to the environment (De Falco et al. 2018; Napper and Thompson 2016). Similarly, Pirc et al. (2016) calculated that with each wash, garments lose around 0.00012% of their mass and estimated that every individual release was around 70 g/year of microplastics annually. Moreover, secondary microplastics are also produced from a wide range of materials, including the tyres, car breaks, paints, asphalt, artificial turf, and artificial playgrounds (Galafassi et al. 2019). Secondary microplastic particles can thus be generated from every plastic material that enters the environment, and every plastic material can serve as a potential source of microplastics (Fig. 1). Secondary microplastics are considering the major source of microplastic pollution in the marine environment (Hidalgo-Ruz et al. 2012).
Transportation of microplastic
Specific features of microplastics, such as being lightweight, having different morphologies, and an extremely long shelf life, are making a significant contribution to the inter-/intra-environmental transportation of microplastics. These properties also make microplastics one of the most ubiquitous pollutants on earth. Almost all the plastic and derived plastic materials are produced on land, from where it enters different environments via a variety of routes. In most scenarios, the land act as the first dumping site for microplastics; these then enter the environment through direct littering, improper waste management, accidentally loss during the disposal process, and industrial spillages (Horton and Dixon 2018). The use of sewage sludge as fertilizer in agriculture also introduces a significant amount of plastic (Nizzetto et al. 2016). Compared to urban lands, agricultural and forestry land is more prone to retain the microplastics due to their higher soil permeability and a lower rate of surface water flow (Nizzetto et al. 2016). Moreover, downward drainage and bioturbation can facilitate the transport of microplastic within the soil system, which ultimately causes the deposition of microplastics in deeper layers of soil (Lwanga et al. 2017; Zubris and Richards 2005). Furthermore, soil arthropods (e.g., Folsomia candida and Priosoma minuta) facilitate the transportation of microplastic particles in the soil system (Maaß et al. 2017).
Agricultural runoff, wastewater treatment plants, floods, etc., are the main sources of microplastic input to freshwater systems. Once it enters into an environment, microplastic particles undergo transportation by different mechanisms, which depends on the type of particle. In riverine systems, microplastic transport depends on water current, i.e., rivers with a greater flow have a high capacity to transport large numbers of particles (Horton and Dixon 2018). Alternatively, in slow-moving sections of a river, microplastics are more likely to settle, along with sinking sediment particles, and be buried. However, in lakes and ponds, the rate of sedimentation of microplastic is very high compared to the riverine systems. The physical and chemical properties (shape, buoyancy, chemical composition) of microplastic particles also have a substantial effect on their transport and retention in aquatic systems. For example, microplastics with a density lower than water usually float on the surface, while denser particles sediment out. However, the density of microplastics and other particulate objects does not remain constant because the colonization of microalgae and other microbial communities can increase their density, which then leads to enhanced sinking (Lagarde et al. 2016). At a local scale, sediment deposition and transportation can lead to the translocation of buried microplastic in freshwater systems. Floods (natural disasters) and the progressive change in river channel morphology (long time-scale), however, can also cause erosion of the river banks, which ultimately leads to the re-suspension and mobilization of buried particles (Horton and Dixon 2018).
Riverine systems serve as the biggest source of microplastic input in the oceanic environment. Once they enter the ocean, the microplastic particles can travel great distances and be spread rapidly by water current, winds, turbulence (van Sebille et al. 2012). Microplastics also undergo vertical transportation in the water column. Phenomena such as marine snow, biofouling, and egestion in fecal pellets are considered the major routes involved in the vertical transport of microplastic particles (Cole et al. 2016; Kowalski et al. 2016; Rummel et al. 2017). The size and composition of microplastic particles also affect vertical transport in the water column. Tekman et al. (2020) observed a positive correlation between microplastic size and the abundance of particulate organic matter, which controls the biological processes and leads to the particle settlement. Turbidity currents also play a significant role in the deposition and translocation of microplastic in the seafloor (Pohl et al. 2020).
Microplastic in the fresh and marine environment
Several factors that affect the number and distribution of microplastic particles in an environment have been identified. For example, in addition to physical parameters (pressure, winds, turbulence, wave action, sunlight intensity, etc.), human population, density, anthropogenic activities, distance from the water body, size of water reservoirs, urban waste management practices and the quantity of sewage effluent are important factors (Eerkes-Medrano et al. 2015; Eriksen et al. 2013; Moore et al. 2011). Information on microplastic pollution, accumulation, and their ecological impacts in freshwater systems and terrestrial environments are not well documented compared to the marine environment (House of Commons Science and Technology Committee 2013; Eerkes-Medrano et al. 2015; Thompson et al. 2004). Freshwater ecosystems include rivers, streams, ditches, lakes, and ponds; all have distinct features (Horton and Dixon 2018). Freshwater systems also serve as the dumping site for plastics, act as a source of microplastic pollution to the marine environment, and provide a medium for microplastic production (secondary microplastic). Investigations of microplastic particles in the water column and sediments of freshwater systems across the globe have been conducted by Castañeda et al. (2014), Faure et al. (2012), Imhof et al. (2012), Lechner et al. (2014), Sadri and Thompson 2014, and Wagner et al. (2014), as in Table 1. Microplastic particles have been found in all major rivers and freshwater reservoirs: in China, in the Pearl River and Pearl River estuary (Yan et al. 2019), in the Yellow River (Duan et al. 2019) in the Three Gorges Reservoirs (Zhang et al. 2019a, b), in the Yangtze River (Xiong et al. 2019), America: in the Los Angeles Basin (Moore et al. 2011), in the Lawrence River (Castañeda et al. 2014) and the Great Lakes (Eriksen et al. 2013), in the rivers and lakes of Europe; Geneva Lake (Faure et al. 2012), Italian lake Gerda (Imhof et al. 2012), Austrian Danube River (Lechner et al. 2014), the German, Elbe, Necker, Mosel, and Rhine rivers (Wagner et al. 2014). Furthermore, Table 1 summarizes the distribution and abundance of microplastic in these freshwater systems.
The uncontrolled disposal of waste produced from the onshore activities ultimately enters the ocean. Plastic materials are ubiquitous in marine habitats, i.e., they can be found in beaches, polar regions, and even in the deep-sea sediments (Browne et al. 2011; Goldberg 1997; Law et al. 2010). It has been reported that around 80% of microplastic items in the marine environment originates from terrestrial sources (Andrady 2011). Approximately half of the global population lives near coastal regions, and the microplastic debris resulting from anthropogenic activities most likely enters the ocean via rivers and domestic and industrial drainage systems (Derraik 2002; Moore 2008; Thompson et al. 2005). Microplastics are varyingly distributed across the different habitats of the oceanic environment (Table 2). For example, in two independent studies, van Sebille et al. (2015) reported 90–235 thousand tons, and Eriksen et al. (2013) reported 66 thousand tons of plastic debris floating on surface seawater. Similarly, at the shoreline the highest concentration of microplastics, 50,000 particles/kg, was detected on East Frisian Island (Liebezeit and Dubaish 2012) and 285.673 particles/m3 on a coastline in South Korea (Kim et al. 2015). Browne et al. (2011) observed a correlation between microplastic abundance and anthropogenic activities by identifying the sources and sinks of microplastic pollution along shorelines worldwide. Table 2 shows the distribution and abundance of microplastic in marine ecosystems at different locations.
Many previous studies have documented that freshwater systems, including rivers, are the major source of microplastic input into the marine environment. According to Moore et al. (2011), in which they quantified the microplastic particles in the water sample of two Los Angeles rivers, extrapolating the data showed that these rivers alone could introduce around 2 billion microplastic particles into the ocean within 3 days. Similarly, it has been estimated that on an annual basis, around 13.6 thousand tons of plastic debris entered the South China Sea via the Pearl River (Lebreton et al. 2017). Natural disasters and extremes weather, including floods or hurricanes, enhance the transportation of terrestrial waste into the ocean (Browne et al. 2011; Thompson et al. 2005). Moore et al. (2002) found that following a storm the transfer of neustonic plastic particles (< 4.55 mm in diameter) into Californian waters near the entrance of the Los Angeles stormwater conveyance system increased from 10 plastic particles/m3 to 60 particles/m3. Coastal tourism, shipping (commercial and recreational), oil rigs, and aquaculture practices are all causes of microplastic pollution in the marine environment. While secondary microplastic is the major source of microplastic pollution in the marine environment, physical parameters such as winds, sunlight, ultraviolet radiation, wave action, and turbulence are all essential factors contributing to the creation of these particles and their transportation to other ecosystems.
Microplastics as vectors
Microplastic as a reservoir of heavy metals
Due to their unique physicochemical characteristics, microplastics offer a distinct surface for chemical acquisition, pollutant accumulation, and microbial communities. Because of their low degradation rates, microplastics can persist in the environment for decades or even centuries. The long-lasting presence of microplastics in the aquatic environment is considered a threat to many aquatic animals. In addition to aesthetic concerns, plastic debris poses several threats to marine organisms, such as entrapment, choking, entanglement, and suffocation (Boren et al. 2006; Browne et al. 2008). Plastic materials can also act as a cause of organic pollution to biotic organisms because organic pollutants, such as polycyclic aromatic hydrocarbons and polychlorinated hydrocarbons, tend to accumulate on the microplastic surfaces. Plastic surfaces have usually been considered to be inert for the acquisitions of heavy metals; however, metals accumulated during storage in a plastic container and during the experimental processes are generally stated problems (Cobelo-Garcia et al. 2007; Fischer et al. 2007; Weijuan et al. 2000). Table 3 summarizes some important environmental functions of microplastic particles.
Recently, many studies reported that heavy metals accumulate on microplastic surfaces in the marine environment. Brennecke et al. (2016) studied the adsorption of Cu and Zn metals on the surfaces of aged polyvinyl chloride (PVC) and virgin polystyrene (PS) microplastics in marine waters. They concluded that heavy metals leached from antifouling paint tended to absorb on the surface of the studied microplastics; moreover, PVC absorbed a relatively high concentration of metals compared to PS. Similarly, Turner (2016) detected the presence of heavy metals, metalloids, and other toxic elements on the surface of marine plastic debris. Microbeads that are extensively used in cosmetic products could adsorb lead (Pb) onto their surface from the surrounding sediments (Boucher et al. 2016). In another study, a higher concentration of different heavy metals was detected on the surface of microplastic compared to the surrounding seawater, which further demonstrates the sorption of heavy metals onto microplastic surfaces (Marsic-Lucic et al. 2018). The previous investigation also showed that aged microplastic particles have a higher capacity of metals sorption compared to virgin particles. Wang et al. (2020) detected a higher concentration of Zn2+ and Cu2+ sorption onto the surface of aged polyethylene terephthalate (PET) particles compared to their virgin counterpart in aqueous solution. Guo et al. (2020) also identified several factors such as the types of microplastic, pH, ionic strength, and humic acid that effected the adsorption of Cd2+ onto the microplastic surfaces. Similarly, Tang et al. (2020) extensively investigated the Pb(II) uptake mechanism onto nylon particle surfaces in a batch culture experiment. They observed that Pb(II) adsorption was significantly dependent on solution initial pH, NaCl concentration, and fulvic acid concentration. Moreover, they also detected that hydroxyl ions on the surface of aged nylon particles play a fundamental role in controlling Pb(II) adsorption. Sodium dodecylbenzene sulfonate (SDBS), an anionic surfactant, has a broad range of applications, such as personal care products, shampoo, hand-washer, household, and laundry detergents. Zhang et al. (2020a, b) reported that SDBS significantly increases the adsorption capacity of polyethylene microplastic. However, the exact mechanism by which metal ions interact and are adsorbed onto microplastic items in the natural environment is not well understood. Along with the intrinsic properties of the participants (microplastics and heavy metals), the chemical nature of the surrounding environment can also affect this interaction. In addition, the attachment of metals containing small particles with microplastic surfaces possibly facilitates microplastic/metals interaction (Holmes et al. 2012). The accumulation of heavy metals on microplastic surfaces could cause additional complications if ingested by an aquatic organism and could enter the food chain. More research is required to explain the mechanism of how heavy metals accumulate on microplastic surfaces and to address the subsequent ecological, environmental, and health implications.
Microplastic as a carrier of organic pollutants
The accumulation of persistent and toxic organic pollutants on microplastic particles is a highly concerning issue. The sorption of persistent organic pollutants could trigger many environmental problems as it can promote the translocation of these pollutants, can increase their recalcitrancy, and can enter the food cycle via ingestion by animals. Several studies have been reported that show the adsorption of environmentally concerned toxic contaminants, such as polychlorinated biphenyls (PCBs), polycyclic aromatic hydrocarbon (PAHs), and organochlorine pesticides (OCPs), onto microplastics. Carpenter and Smith (1972) reported for the first time the presence of PCBs at a concentration of 5000 ng/g on the surfaces of plastic debris in seawater. Similarly, Gregory (1978) detected the presence of PCBs in high concentrations on the surface of virgin polyethylene granules recovered from coastal sediments of New Zealand beaches. In the last two decades, several studies had been conducted around the globe and reported the presence of PCBs on microplastic surfaces at a variety of concentrations (Endo et al. 2005; Frias et al. 2010; Heskett et al. 2012; Rios et al. 2007). Similarly, PAHs, an important class of persistent organic pollutants, had been discovered on the surface of microplastic particles in several studies. For example, Rios et al. (2007) detected PAHs at a concentration of 39–1200 ng/g on plastic debris collected from the North Pacific Gyre, Hawaii, Guadalupe Island, and Mexico beaches. Teuten et al. (2007) observed a 106 higher phenanthrene (PAH) concentration on microplastics compared to the surrounding water concentration. Moreover, a survey carried out by International Pellet Watch (IPW), by collecting microplastic samples from 75 locations in 26 countries, found higher PAHs concentrations on Sao Torpes Beach, Portugal (24,400 ng/g), and the Forth Estuary in the UK (162,900 ng/g) (Yeo et al. 2017). Similarly, OCPs are an important group of synthetic chlorinated hydrocarbons mostly used in agriculture and chemical industries. Dichlorodiphenyltrichloroethane (DDTs) and related compounds were widely used chemicals in the agriculture sectors in the past (currently banned in many countries). In four coastal sites of Japan, DDTs were detected on microplastic at varied concentrations, ranging from 0.61 to 3.1 ng/g (Mato et al. 2001). In another study conducted on OCPs, DDT was found at concentrations ranging from 64.4 to 87.7 ng/g on microplastic particles ingested by seabirds (Colabuono et al. 2010).
The adsorption mechanism of toxic organic pollutants on microplastic surfaces is complex, varied for different chemicals and relatively unexplored process (Verla et al. 2019). Contaminants can adsorb onto microplastic surfaces via three possible mechanisms: (1) adsorption as hydrophobic adsorbents, (2) biofilm-mediated adherence, and (3) additive materials in plastic resins (Verla et al. 2019). Due to their hydrophobicity, organic pollutants are reluctant to attach to floating particles (microplastics). On the other hand, hydrophobic microplastics have a large surface area-to-volume ratio, which makes them an ideal surface for chemical adsorption. Also, environmental weathering of plastic material enhances the capacity of the sorption of different organic pollutants. It has been reported that aged microplastic items exhibit a higher capacity for pollutant accumulation than virgin particles (Fotopoulou and Karapanagioti 2012). This might be due to the fact that environmental weathering removes their surface topology, i.e., makes the surface porous, rough, and irregular, which ultimately increases the surface area. In addition to the physical disruption, environmental weathering also alters the chemical properties of the particle surface. For example, Fotopoulou and Karapanagioti (2012) reported that environmental erosion of polyethylene particles produced a negative charge on the particle surface in seawater. This phenomenon could facilitate the adsorption of specific organic pollutants and other positively charged contaminants. In addition, because microplastics are produced from different plastic materials, they will have a distinct chemical composition, which could also affect the adsorption of organic pollutants. For example, Rochman et al. (2013) found that low-density polyethylene, high-density polyethylene, and propylene-derived microplastics adsorbed higher concentrations of PAHs and PCBs than polyvinyl chloride and polyethylene terephthalate-derived particles (Fig. 1). This attribution of plastic particles has been used to quantify the amount of persistent organic pollutants (POPs) in the aquatic environment (Huckins et al. 1993; Lohmann 2012). Particle size also affects the pattern of pollutant adsorption. For example, Ma et al. (2019) found that small polyvinyl chloride particles had a stronger adsorption capacity and greater distribution coefficient kd of triclosan than large particles.
Microplastic provide a distinct microbial niche
Understanding the interactions between microbial communities and microplastic particles of different origins is gaining attention. Naturally, in the environments (terrestrial, aquatic, marine), microorganisms tend to attach and colonize surfaces, including both natural and synthetic. The attachment and colonization of microorganisms, including bacteria, fungi, viruses, archaea, algae, and protozoans, on surfaces, is generally known as “biofilm formation.”
From the point of production to final sinking, microplastics undergo inter-/intra-environmental transportation. During this process and in final settlement, microorganisms are able to colonize the surface of microplastic particles (Schluter et al. 2015). Biofilm formation is a complex process, and understanding the mechanism on different microplastic surfaces is challenging (Rummel et al. 2017), particularly in the aquatic environment (fresh and marine) where the chemical and biological heterogeneity changes with time and place. Once microplastics are released into the environment, they attract the attachments of organic and inorganic substances. It has been reported that within seconds of primary exposure to the ambient environment, a thin coating layer of organic and inorganic substances forms on virgin microplastic surfaces. This thin coating of organic and inorganic substances is generally known as the “conditioning film” (Loeb and Neihof 1975) and is considered a major factor in the establishment of a biofilm. The chemical constituency of these conditioning films can direct the type of colonizing microbial communities (Jones et al. 2007; Taylor et al. 1997). The distinct physiochemical characteristics of different microplastic particles can also influence the composition of the conditioning film, which in turn could direct the assembly of microbial communities. Different chemicals trigger different stimuli, i.e., they might be chemoattracting, which would attract microbial communities, or it might be a chemorepellent, which would repel microbial communities. However, different microbial communities may respond differently to different chemical substances.
The attachment of various chemicals, including nutrients on microplastic surfaces, provides an additional advantage to the colonizing microbial communities. For example, it can provide physical support, can provide a relatively stable nutrient supply, and provide a stable habitat that could help microorganisms to resist environmental stresses (Oberbeckmann et al. 2015; Shen et al. 2019). These properties of microplastics might facilitate the attachment of biofilm-forming microbial communities, which could cause ecosystem compartmentalization. The distinct composition of microbial communities between microplastic particles surfaces and the surrounding environment (water, sediment, soil) has been documented in many reports (Fig. 1). For example, Ogonowski et al. (2018) demonstrated the impact of plastic and non-plastic microparticles on the composition of microbial communities. They also observed that substrate hydrophobicity was the major factor of variation in community structure on different surfaces.
Microplastic microbial communities have a lower diversity and richness compared to natural surfaces (Miao et al. 2019). Microplastics not only affect microbial community differentiation but also influence the functionality of microbial communities (Miao et al. 2019). Early reports also suggested that different microbial communities occurred on different plastic debris, including low-density polyethylene, polyethylene terephthalate (PET), and polypropylene (Oberbeckmann et al. 2015). Similarly, the work of Frere et al. (2018) showed that variation in microbial communities depended on the type of microplastic particle rather than the size. Therefore, it has been suggested that microplastics develop a distinct microbial niche known as the “plastosphere”. This acquisition of distinct microbial phylotypes on different types of plastic materials could have environmental and ecological implications. For example, microplastics could promote the growth and succession of some microbial phylotypes while hindering the development of others, which might affect the ecological functions of the microbial communities. As discussed earlier, microplastics provide a substrate for organic pollutant deposition, which might favor the colonization of organic pollutants degrading microbial phylotype (Curren and Leong 2019).
Microplastic as a vector of antibiotic-resistant and bacterial pathogens
Owing to the low weight and high buoyancy of microplastics, they undergo both inter- and intra-environmental transportation from the point of production to the ultimate settling sites. This transportation of microplastic particles prompts some ecological, environmental, and public health implications. It has been reported that plastic material might act as a reservoir for antibiotic and metal resistance genes in the marine environment (Yang et al. 2019). Zhang et al. (2020a, b) demonstrated that microplastics not only act as a reservoir for antibiotic-resistant genes, but also provide a substrate for the enrichment of multi-antibiotic resistance bacteria (MRAB) in mariculture systems. A range of antibiotic-resistant genes, including tetracycline, penicillin, sulfafurazole, and erythromycin-resistant, was detected in the genome of bacterial strains recovered from some microplastic particles (Zhang et al. 2020a, b). Moreover, microplastics facilitate the exchange of genetic materials via horizontal gene transfer among the microbial communities (Arias-Andres et al. 2018). This phenomenon could aid the spread of antibiotic-resistant, metal-resistant, and virulence genes among microbial communities. In addition to the antibiotic-resistant bacteria, microplastic is also starting to harbor various human, aquatic animals, and plant pathogenic bacterial strains (Virsek et al. 2017; Wingender and Flemming 2011; Wu et al. 2019; Zhou et al. 2019). Wu et al. (2019) observed that Pseudomonas monteilii and Pseudomonas mendocina, which are opportunistic human pathogens, were selectively enriched on microplastic surfaces rather than on natural surfaces. Similarly, the plant pathogen, Pseudomonas syringae, also tends to accumulate and enrich on the microplastic surfaces. Moreover, Vibrio, which is a ubiquitous, ecologically and metabolically active marine animal and plankton-associated bacterial group, has been detected on a variety of microplastic surfaces (Foulon et al. 2016; Schmidt et al. 2014; Zettler et al. 2013). Vibrio, being a diverse bacterial group, encompasses several human and animal pathogens, including Vibrio cholerae, Vibrio coralliilyticus, Vibrio harveyi, Vibrio splendidus, Vibrio parahaemolyticus, Vibrio alginolyticus, and Vibrio fluvialis. Most of these pathogenic Vibrio species have been detected on microplastic particles, indicating that microplastics can provide a habitat for the colonization and enrichment of Vibrio species (Foulon et al. 2016; Kirstein et al. 2016; Zettler et al. 2013). In addition, fish pathogens, such as Aeromonas salmonicida, were found on microplastics collected from north Adriatic seawater (Virsek et al. 2017).
Microplastics possess many of the properties (enriching antibiotic resistance, colonizing pathogens, and enabling their transportation) that might cause severe public health, ecological, and commercial problems. For example, the ingestion of pathogen-loaded microplastic particles could lead to infections in fresh and marine water organisms. By consuming raw, ready to eat, and uncooked food, it could also cause infections in the human population. Vibrio species, which preferentially colonize microplastics, were the causative agents of several seafood-borne outbreaks (Elmahdi et al. 2016; Tran et al. 2013). Microplastic particles could also be a vector of infection spread in aquaculture, such as shrimp aquaculture, which could cause severe economic losses.
Microplastic particles are a proven hot spot for the acquisition of antibiotics, enriching antibiotic-resistant bacterial strains, and for the colonization of pathogens. These properties of microplastics can accelerate infection in aquaculture and mariculture farms by promoting the diffusion of pathogens. Due to their buoyancy and mobility, microplastics might promote the translocation of pathogens from one environment to another. For example, Goldstein et al. (2014) observed several coral pathogens on plastic debris recovered from the eastern and western Pacific. Furthermore, a folliculinid ciliate (Halofolliculina spp.), which is a coral pathogen causing skeletal eroding band (SED) (Rodríguez et al. 2008), was originally discovered and thought to be limited to Indian and South Pacific Ocean, but was later found in Caribbean (Cróquer et al. 2006) and Hawaiian corals (Palmer and Gates 2010). The actual mechanism of spread of SED is unknown; however, from the frequent recovery of the pathogens on the plastic debris, it has been speculated that plastic materials could facilitate the spread (Dameron et al. 2007; Pham et al. 2012). Hence, microplastics can facilitate the invasion of a new habitat by a pathogen, where they can proliferate and can harm local community structure, impair water quality, and also threaten human health (Kirkpatrick et al. 2004; Kirstein et al. 2016; Shen et al. 2019; Zettler et al. 2013).
Conclusion and future perspectives
Interest has been growing in understanding and assessing the environmental, ecological, and health (human and other animals) consequences of microplastic pollution. Microplastic comes from diverse sources and enters the environment, including terrestrial, freshwater, and marine water, via different routes. Due to their extremely long environmental persistence, microplastic can survive in the environment for decades, even for centuries. The ease of transportation and the attachment of environmental and public health concerning pollutants, including PAHs and PCBs, could have serious implications. Moreover, the enrichment of antibiotic-resistant bacteria and wildlife pathogenic bacteria is the most critical aspect of microplastic pollution. Keeping in view the adverse impacts, more research and understanding is required to comprehensively address all the possible hazardous threats prompted by microplastic pollution. The following studies should be conducted in the future:
-
1.
Currently, different names, such as microplastic, mesoplastic, nanoplastic, and microparticles, have been used for plastic particles. It is important to devise a standard classification system for the nomenclature of microplastic particles.
-
2.
The effect of the chemical composition of the conditioning film on the attachment of subsequent microbial communities and their functions are also important aspects to be investigated.
-
3.
More research is needed to explore the phenomenon of gene exchange at the genomic and transcriptomic levels to identify the key microbial phylotypes involved in this exchange.
-
4.
Research is needed to explore the mechanisms of enhanced antibiotic-resistance development on microplastics. It will be important to include the intrinsic properties of the microplastic particles, or to determine whether the accumulation is due to the various substances and other organic and inorganic contaminants on the microplastics. This is because many reports have documented that heavy metals, PAHs, and PCBs play a significant role in antibiotic-resistance development.
-
5.
The colonization and transport of pathogens (humans, other animals, and plants) by microplastic should be further explored. More research is also needed to determine the potential role of microplastic in pathogen transportation and disease outbreaks.
References
Abidli S, Antunes JC, Ferreira JL, Lahbib Y, Sobral P, Menif NTE (2018) Microplastics in sediments from the littoral zone of the north Tunisian coast (Mediterranean Sea). Estuar Coast Shelf Sci 205:1–9
Alam FC, Sembiring E, Muntalif BS, Suendo V (2019) Microplastic distribution in surface water and sediment river around slum and industrial area (case study: Ciwalengke River, Majalaya district, Indonesia). Chemosphere 224:637–645
Andrady AL (2011) Microplastics in the marine environment. Mar Pollut Bull 62:1596–1605
Andrady AL, Neal MA (2009) Applications and societal benefits of plastics. Philos Trans R Soc B 364:1977–1984
Arias-Andres M, Klumper U, Rojas-Jimenez K, Grossart HP (2018) Microplastic pollution increases gene exchange in aquatic ecosystems. Environ Pollut 237:253–261
Bagaev A, Khatmullina L, Chubarenko I (2018) Anthropogenic microlitter in the Baltic Sea water column. Mar Pollut Bull 129:918–923
Bakir A, Rowland SJ, Thompson RC (2012) Competitive sorption of persistent organic pollutants onto microplastics in the marine environment. Mar Pollut Bull 64:2782–2789
Barnes DK, Galgani F, Thompson RC, Barlaz M (2009) Accumulation and fragmentation of plastic debris in global environments. Philos Trans R Soc B 364:1985–1998
Blumenroder J, Sechet P, Kakkonen JE, Hartl MGJ (2017) Microplastic contamination of intertidal sediments of Scapa Flow, Orkney: a first assessment. Mar Pollut Bull 124:112–120
Bordos G, Urbanyi B, Micsinai A, Kriszt B, Palotai Z, Szabo I, Hantosi Z, Szoboszlay S (2019) Identification of microplastics in fish ponds and natural freshwater environments of the Carpathian basin, Europe. Chemosphere 216:110–116
Boren LJ, Morrissey M, Muller CG, Gemmell NJ (2006) Entanglement of New Zealand fur seals in man-made debris at Kaikoura, New Zealand. Mar Pollut Bull 52:442–446
Boucher C, Morin M, Bendell LI (2016) The influence of cosmetic microbeads on the sorptive behavior of cadmium and lead within intertidal sediments: a laboratory study. Reg Stud Mar Sci 3:1–7
Brennecke D, Duarte B, Paiva F, Caçador I, Canning-Clode J (2016) Microplastics as vector for heavy metal contamination from the marine environment. Estuar Coast Shelf Sci 178:189–195
Browne MA, Galloway T, Thompson R (2007) Microplastic—an emerging contaminant of potential concern? Integr Environ Asses 3:559–566
Browne MA, Dissanayake A, Galloway TS, Lowe DD, Thompson RC (2008) Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.). Environ Sci Technol 42:5026–5031
Browne MA, Crump P, Niven SJ, Teuten E, Tonkin A, Galloway T, Thompson R (2011) Accumulation of microplastic on shorelines worldwide: sources and sinks. Environ Sci Technol 45:9175–9179
Caldwell J, Petri-Fink A, Rothen-Rutishauser B, Lehner R (2019) Assessing meso- and microplastic pollution in the Ligurian and Tyrrhenian Seas. Mar Pollut Bull 149:110572
Carpenter EJ, Smith KL Jr (1972) Plastics on the Sargasso Sea surface. Science 175:1240–1241
Castañeda RA, Avlijas S, Simard MA, Ricciardi A, Smith R (2014) Microplastic pollution in St. Lawrence River sediments. Can J Fish Aquat Sci 71:1767–1771
Chang M (2015) Reducing microplastics from facial exfoliating cleansers in wastewater through treatment versus consumer product decisions. Mar Pollut Bull 101:330–333
Claessens M, De Meester S, Van Landuyt L, De Clerck K, Janssen CR (2011) Occurrence and distribution of microplastics in marine sediments along the Belgian coast. Mar Pollut Bull 62:2199–2204
Cobelo-Garcia A, Turner A, Millward GE, Couceiro F (2007) Behaviour of palladium(II), platinum(IV), and rhodium(III) in artificial and natural waters: influence of reactor surface and geochemistry on metal recovery. Anal Chim Acta 585:202–210
Colabuono FI, Taniguchi S, Montone RC (2010) Polychlorinated biphenyls and organochlorine pesticides in plastics ingested by seabirds. Mar Pollut Bull 60:630–634
Cole M, Lindeque P, Halsband C, Galloway TS (2011) Microplastics as contaminants in the marine environment: a review. Mar Pollut Bull 62:2588–2597
Cole M, Lindeque PK, Fileman E, Clark J, Lewis C, Halsband C, Galloway TS (2016) Microplastics alter the properties and sinking rates of zooplankton faecal pellets. Environ Sci Technol 50:3239–3246
Conkle JL, Baez Del Valle CD, Turner JW (2018) Are we underestimating microplastic contamination in aquatic environments? Environ Manag 61:1–8
Cróquer A, Bastidas C, Lipscomb D (2006) Folliculinid ciliates: a new threat to Caribbean corals? Dis Aquat Organ 69:75–78
Curren E, Leong SCY (2019) Profiles of bacterial assemblages from microplastics of tropical coastal environments. Sci Total Environ 655:313–320
Dameron OJ, Parke M, Albins MA, Brainard R (2007) Marine debris accumulation in the Northwestern Hawaiian Islands: an examination of rates and processes. Mar Pollut Bull 54:423–433
De Falco F, Gullo MP, Gentile G, Pace ED, Cocca M, Gelabert L, Brouta-Agnesa M, Rovira A, Escudero R, Villalba R, Mossotti R, Montarsolo A, Gavignano S, Tonin C, Avella M (2018) Evaluation of microplastic release caused by textile washing processes of synthetic fabrics. Environ Pollut 236:916–925
Derraik JGB (2002) The pollution of the marine environment by plastic debris: a review. Mar Pollut Bull 44:842–852
Dikareva N, Simon KS (2019) Microplastic pollution in streams spanning an urbanisation gradient. Environ Pollut 250:292–299
Ding L, Mao RF, Guo X, Yang X, Zhang Q, Yang C (2019a) Microplastics in surface waters and sediments of the Wei River, in the northwest of China. Sci Total Environ 667:427–434
Ding J, Jiang F, Li J, Wang Z, Sun C, Wang Z, Fu L, Xiangyu Ding NX, He C (2019b) Microplastics in the coral reef systems from Xisha islands of South China Sea. Environ Sci Technol 53:8036–8046
Duan Z, Zhao S, Zhao L, Duan X, Xie S, Hai Zhang H, Liu Y, Peng Y, Liu C, Wang L (2019) Microplastics in Yellow River Delta wetland: occurrence, characteristics, human influences, and marker. Environ Pollut 258:11323
Eerkes-Medrano D, Thompson RC, Aldridge DC (2015) Microplastics in freshwater systems: a review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res 75:63–82
Elmahdi S, DaSilva LV, Parveen S (2016) Antibiotic resistance of Vibrio parahaemolyticus and Vibrio vulnificus in various countries: a review. Food Microbiol 57:128–134
Endo S, Satoshi Endo Takizawa R, Okuda K, Takada H, Chiba K, Kanehiro H, Ogi H, Yamashita R, Date T (2005) Concentration of polychlorinated biphenyls (PCBs) in beached resin pellets: variability among individual particles and regional differences. Mar Pollut Bull 50:1103–1114
Eo S, Hong SH, Song YK, Lee J, Lee J, Shim WJ (2018) Abundance, composition, and distribution of microplastics larger than 20 mm in sand beaches of South Korea. Environ Pollut 238:894–902
Eriksen M, Mason S, Wilson S, Box C, Zellers A, Edwards W, Farley H, Amato S (2013) Microplastic pollution in the surface waters of the Laurentian Great Lakes. Mar Pollut Bull 77:177–182
Erni-Cassola G, Zadjelovic V, Gibson MI, Christie-Oleza JA (2019) Distribution of plastic polymer types in the marine environment; a meta-analysis. J Hazard Mater 369:691–698
Essel R, Engel L, Carus M (2015) Sources of microplastics relevant to marine protection in Germany. Umwelt Bundesamt Publisher, TEXTE 64/2015, pp 1–45. https://www.umweltbundesamt.de/publikationen/sources-of-microplastics-relevant-to-marine
Faure F, Corbaz M, Baecher H, De Alencastro LF (2012) Pollution due to plastics and microplastics in lake Geneva and in the Mediterranean sea. Arch Des Sci 65:157–164
Fendall LS, Sewell MA (2009) Contributing to marine pollution by washing your face: microplastics in facial cleansers. Mar Pollut Bull 58:1225–1228
Fischer AC, Kroon JJ, Verburg TG, Teunissen T, Wolterbeek HT (2007) On the relevance of iron adsorption to container materials in small-volume experiments on iron marine chemistry: 55Fe-aided assessment of capacity, affinity and kinetics. Mar Chem 107:533–546
Fotopoulou KN, Karapanagioti HK (2012) Surface properties of beached plastic pellets. Mar Environ Res 81:70–77
Foulon V, Le Roux F, Lambert C, Huvet A, Soudant P, Paul-Pont I (2016) Colonization of polystyrene microparticles by Vibrio crassostreae: light and electron microscopic investigation. Environ Sci Technol 50:10988–10996
Frere L, Maignien L, Chalopin M, Huvet A, Rinnert E, Morrison H, Kerninon S, Cassone AL, Lambert C, Reveillaud J, Paul-Pont I (2018) Microplastic bacterial communities in the Bay of Brest: influence of polymer type and size. Environ Pollut 242:614–625
Frias JP, Sobral P, Ferreira AM (2010) Organic pollutants in microplastics from two beaches of the Portuguese coast. Mar Pollut Bull 60:1988–1992
Galafassi S, Nizzetto L, Volta P (2019) Plastic sources: a survey across scientific and grey literature for their inventory and relative contribution to microplastics pollution in natural environments, with an emphasis on surface water. Sci Total Environ 693:133499
Galgani F, Fleet D, Van Franeker J, Katsanevakis S, Maes T, Mouat J, Oosterbaan L, Poitou I, Hanke G, Thompson R, Amato E, Birkun A, Janssen C (2010) Marine strategy framework directive: task group 10 report marine litter. JRC Sci Tech Rep. https://doi.org/10.2788/86941
Goldberg ED (1997) Plasticizing the seafloor: an overview. Environ Technol 18:195–201
Goldstein MC, Carson HS, Eriksen M (2014) Relationship of diversity and habitat area in North Pacific plastic-associated rafting communities. Mar Biol 161:1441–1453
Gouin T, Avalos J, Brunning I, Brzuska K, de Graaf J, Kaumanns J, Koning T, Meyberg M, Rettinger K, Schlatter H, Thomas J, Welie R, Wolf TV (2015) Use of microplastic beads in cosmetic products in Europe and their estimated emissions to the North Sea environment. SOFW J 141:40–46
Graham ER, Thompson JT (2009) Deposit- and suspension-feeding sea cucumbers (Echinodermata) ingest plastic fragments. J Exp Mar Biol Ecol 368:22–29
House of Commons Science and Technology Committee (2013) Water quality: priority substances. First report of session 2013–14, vol 1, pp 1–25
Gregory MR (1978) Accumulation and distribution of virgin plastic granules on New Zealand beaches. N Z J Mar Freshw Res 12:399–414
Gregory MR (1996) Plastic ‘scrubbers’ in hand cleansers: a further (and minor) source for marine pollution identified. Mar Pollut Bull 32:867–871
Guo X, Hu G, Fan X, Jia H (2020) Sorption properties of cadmium on microplastics: the common practice experiment and a two-dimensional correlation spectroscopic study. Ecotoxicol Environ Saf 190:110118
Habib D, Locke DC, Cannone LJ (1998) Synthetic fibers as indicators of municipal sewage sludge, sludge products, and sewage treatment plant effluents. Water Air Soil Pollut 103:1–8
He B, Goonetilleke A, Ayoko GA, Rintoul L (2020) Abundance, distribution patterns, and identification of microplastics in Brisbane River sediments, Australia. Sci Total Environ 700:134467
Heskett M, Takada H, Yamashita R, Yuyama M, Ito M, Geok YB, Ogata Y, Kwan C, Heckhausen A, Taylor H, Powell T, Morishige C, Young D, Patterson H, Robertson B, Bailey E, Mermoz J (2012) Measurement of persistent organic pollutants (POPs) in plastic resin pellets from remote islands: toward establishment of background concentrations for International Pellet Watch. Mar Pollut Bull 64:445–448
Hidalgo-Ruz V, Gutow L, Thompson RC, Thiel M (2012) Microplastics in the marine environment: a review of the methods used for identification and quantification. Environ Sci Technol 46:3060–3075
Hintersteiner I, Himmelsbach M, Buchberger WW (2015) Characterization and quantitation of polyolefin microplastics in personal-care products using high-temperature gel-permeation chromatography. Anal Bioanal Chem 407:1253–1259
Holmes LA, Turner A, Thompson RC (2012) Adsorption of trace metals to plastic resin pellets in the marine environment. Environ Pollut 160:42–48
Horton AA, Dixon SJ (2018) Microplastics: an introduction to environmental transport processes. Wiley Interdiscip Rev WIREs Water 5:e1268
Huang Y, Yan M, Xu K, Nie H, Gong H, Wang J (2019) Distribution characteristics of microplastics in Zhubi Reef from South China Sea. Environ Pollut 255:113133
Huckins JN, Manuweera GK, Petty JD, Mackay D, Lebo JA (1993) Lipid-containing semipermeable membrane devices for monitoring organic contaminants in water. Environ Sci Technol 27:2489–2496
Imhof HK, Schmid J, Niessner R, Ivleva NP, Laforsch C (2012) A novel, highly efficient method for the separation and quantification of plastic particles in sediments of aquatic environments. Limnol Oceanogr Methods 10:524–537
Ivleva NP, Wiesheu AC, Niessner R (2017) Microplastic in aquatic ecosystems. Angew Chem Int Ed 56:1720–1739
Jiang Y, Yang F, Zhao Y, Wang J (2020) Greenland Sea Gyre increases microplastic pollution in the surface waters of the Nordic Seas. Sci Total Environ 712:136484
Jones PR, Cottrell MT, Kirchman DL, Dexter SC (2007) Bacterial community structure of biofilms on artificial surfaces in an estuary. Microb Ecol 53:153–162
Kawaguchi H (2000) Functional polymer microspheres. Prog Polym Sci 25:1171–1210
Kim IS, Chae DH, Kim SK, Choi S, Woo SB (2015) Factors influencing the spatial variation of microplastics on high-tidal coastal beaches in Korea. Arch Environ Contam Toxicol 69:299–309
Kirkpatrick B, Fleming LE, Squicciarini D, Backer LC, Clark R, Abraham W, Benson J, Cheng YS, Johnson D, Pierce R, Zaias J, Bossart GD, Baden DG (2004) Literature review of Florida red tide: implications for human health effects. Harmful Algae 3:99–115
Kirstein IV, Kirmizi S, Wichels A, Garin-Fernandez A, Erler R, Loder M, Gerdts G (2016) Dangerous hitchhikers? Evidence for potentially pathogenic Vibrio spp. on microplastic particles. Mar Environ Res 120:1–8
Kor K, Mehdinia A (2020) Neustonic microplastic pollution in the Persian Gulf. Mar Pollut Bull 150:110665
Kowalski N, Reichardt AM, Waniek JJ (2016) Sinking rates of microplastics and potential implications of their alteration by physical, biological, and chemical factors. Mar Pollut Bull 109:310–319
Lagarde F, Olivier O, Zanella M, Daniel P, Hiard S, Caruso A (2016) Microplastic interactions with freshwater microalgae: hetero-aggregation and changes in plastic density appear strongly dependent on polymer type. Environ Pollut 215:331–339
Law KL, Morét-Ferguson S, Maximenko NA, Proskurowski G, Peacock EE, Hafner J, Reddy CM (2010) Plastic accumulation in the North Atlantic subtropical gyre. Science 329:1185–1188
Lebreton LC, Zwet VD, Damsteeg JW, Slat B, Andrady A, Reisser J (2017) River plastic emissions to the world’s ocean. Nat Commun. https://doi.org/10.1038/ncomms15611
Lechner A, Keckeis H, Lumesberger-Loisl F, Zens B, Krusch R, Tritthart M, Glas M, Schludermann E (2014) The Danube so colourful: a potpourri of plastic litter outnumbers fish larvae in Europe’s second largest river. Environ Pollut 188:177–181
Liebezeit G, Dubaish F (2012) Microplastics in beaches of the East Frisian islands Spiekeroog and Kachelotplate. Bull Environ Contam Toxicol 89:213–217
Lin L, Zuo LZ, Peng JP, Cai LQ, Fok L, Yan Y, Li HX, Xu XR (2018) Occurrence and distribution of microplastics in an urban river: a case study in the Pearl River along Guangzhou City, China. Sci Total Environ 644:375–381
Liu F, Olesen KB, Borregaard AR, Vollertsen J (2019) Microplastics in urban and highway stormwater retention ponds. Sci Total Environ 671:992–1000
Loeb GI, Neihof RA (1975) Marine conditioning films. Adv Chem 145:319–335
Lohmann R (2012) Critical review of low-density polyethylene’s partitioning and diffusion coefficients for trace organic contaminants and implications for its use as a passive sampler. Sci Total Environ 46:606–618
Lwanga EH, Gertsen H, Gooren H, Peters P, Salanki T, van der Ploeg M, Besseling E, Koelmans AA, Geissen V (2017) Incorporation of microplastics from litter into burrows of Lumbricus terrestris. Environ Pollut 220:523–531
Ma J, Zhao J, Zhu Z, Li L, Yu F (2019) Effect of microplastic size on the adsorption behavior and mechanism of triclosan on polyvinyl chloride. Environ Pollut 254:113104
Maaß S, Daphi D, Lehmann A, Rillig MC (2017) Transport of microplastics by two collembolan species. Environ Pollut 225:456–459
Marsic-Lucic J, Lusic J, Tutman P, Bojanic Varezic D, Siljic J, Pribudic J (2018) Levels of trace metals on microplastic particles in beach sediments of the island of Vis, Adriatic Sea, Croatia. Mar Pollut Bull 137:231–236
Mato Y, Isobe T, Takada H, Kanehiro H, Ohtake C, Kaminuma T (2001) Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environ Sci Technol 35:318–324
Miao L, Wang P, Hou J, Yao Y, Liu Z, Liu S, Li T (2019) Distinct community structure and microbial functions of biofilms colonizing microplastics. Sci Total Environ 650:2395–2402
Moore CJ (2008) Synthetic polymers in the marine environment: a rapidly increasing, long-term threat. Environ Res 108:131–139
Moore CJ, Moore SL, Weisberg SB, Lattin GL, Zellers AF (2002) A comparison of neustonic plastic and zooplankton abundance in Southern California’s coastal waters. Mar Pollut Bull 44:1035–1038
Moore CJ, Lattin GL, Zellers AF (2011) Quantity and type of plastic debris flowing from two urban rivers to coastal waters and beaches of Southern California. JICZM 11:65–73
Naidoo T, Glassom D (2019) Sea-surface microplastic concentrations along the coastal shelf of KwaZulu-Natal, South Africa. Mar Pollut Bull 149:110514
Naji A, Esmaili Z, Khan FR (2017) Plastic debris and microplastics along the beaches of the Strait of Hormuz, Persian Gulf. Mar Pollut Bull 114:1057–1062
Nan B, Su L, Kellar C, Craig NJ, Keough MJ, Pettigrove V (2020) Identification of microplastics in surface water and Australian freshwater shrimp Paratya australiensis in Victoria, Australia. Environ Pollut 259:113865
Napper IE, Thompson RC (2016) Release of synthetic microplastic plastic fibres from domestic washing machines: effects of fabric type and washing conditions. Mar Pollut Bull 112:39–45
Napper IE, Bakir A, Rowland SJ, Thompson RC (2015) Characterisation, quantity and sorptive properties of microplastics extracted from cosmetics. Mar Pollut Bull 99:178–185
Nizzetto L, Futter M, Langaas S (2016) Are agricultural soils dumps for microplastics of urban origin? Environ Sci Technol 50:10777–10779
Oberbeckmann S, Löder MGJ, Labrenz M (2015) Marine microplastic-associated biofilms—a review. Environ Chem 12:551–562
Ogonowski M, Motiei A, Ininbergs K, Hell E, Gerdes Z, Udekwu KI, Bacsik Z, Gorokhova E (2018) Evidence for selective bacterial community structuring on microplastics. Environ Microbiol 20:2796–2808
Palmer CV, Gates RD (2010) Skeletal eroding band in Hawaiian corals. Coral Reefs 29:469–469
Park TJ, Lee SH, Lee MS, Lee JK, Lee SH, Zoh KD (2020) Occurrence of microplastics in the Han River and riverine fish in South Korea. Sci Total Environ 708:134535
Peng G, Bellerby R, Zhang F, Sun X, Li D (2020) The ocean’s ultimate trashcan: hadal trenches as major depositories for plastic pollution. Water Res 168:115121
Pham PH, Jung J, Lumsden JS, Dixon B, Bols NC (2012) The potential of waste items in aquatic environments to act as fomites for viral haemorrhagic septicaemia virus. J Fish Dis 35:73–77
Phuong NN, Poirier L, Lagarde F, Kamari A, Zalouk-Vergnoux A (2018) Microplastic abundance and characteristics in French Atlantic coastal sediments using a new extraction method. Environ Pollut 243:228–237
Pico Y, Alvarez-Ruiz R, Alfarhan AH, El-Sheikh MA, Alshahrani HO, Barcelo D (2020) Pharmaceuticals, pesticides, personal care products and microplastics contamination assessment of Al-Hassa irrigation network (Saudi Arabia) and its shallow lakes. Sci Total Environ 701:135021
Piehl S, Mitterwallner V, Atwood EC, Bochow M, Laforsch C (2019) Abundance and distribution of large microplastics (1–5 mm) within beach sediments at the Po River Delta, northeast Italy. Mar Pollut Bull 149:110515
Pirc U, Vidmar M, Mozer A, Krzan A (2016) Emissions of microplastic fibers from microfiber fleece during domestic washing. Environ Sci Pollut Res 23:22206–22211
PlasticsEurope (2015) Plastics - The Facts 2015. details are available at http://www.plasticseurope.org/Document/plastics-the-facts-2015.aspx?FolID=2, 2015
Pohl F, Eggenhuisen JT, Kane IA, Clare MA (2020) Transport and burial of microplastics in deep-marine sediments by turbidity currents. Environ Sci Technol. 54:4180–4189
Rios LM, Moore C, Jones PR (2007) Persistent organic pollutants carried by synthetic polymers in the ocean environment. Mar Pollut Bull 54:1230–1237
Rochman CM, Manzano C, Hentschel BT, Simonich SL, Hoh E (2013) Polystyrene plastic: a source and sink for polycyclic aromatic hydrocarbons in the marine environment. Environ Sci Technol 47:13976–13984
Rodríguez S, Cróquer A, Guzmán HM, Bastidas C (2008) A mechanism of transmission and factors affecting coral susceptibility to Halofolliculina sp. infection. Coral Reefs 28:67–77
Rose D, Webber M (2019) Characterization of microplastics in the surface waters of Kingston Harbour. Sci Total Environ 664:753–760
Rummel CD, Jahnke A, Gorokhova E, Kühnel D, Schmitt-Jansen M (2017) Impacts of biofilm formation on the fate and potential effects of microplastic in the aquatic environment. Environ Sci Technol Lett 4:258–267
Ryan PG, Moore CJ, van Franeker JA, Moloney CL (2009) Monitoring the abundance of plastic debris in the marine environment. Philos Trans R Soc B 364:1999–2012
Sadri SS, Thompson RC (2014) On the quantity and composition of floating plastic debris entering and leaving the Tamar Estuary, Southwest England. Mar Pollut Bull 81:55–60
Schluter J, Nadell CD, Bassler BL, Foster KR (2015) Adhesion as a weapon in microbial competition. ISME J 9:139–149
Schmidt VT, Reveillaud J, Zettler E, Mincer TJ, Murphy L, Amaral-Zettler LA (2014) Oligotyping reveals community level habitat selection within the genus Vibrio. Front Microbiol 5:563
Shah AA, Hasan F, Hameed A, Ahmed S (2008) Biological degradation of plastics: a comprehensive review. Biotechnol Adv 26:246–265
Shen M, Zhu Y, Zhang Y, Zeng G, Wen X, Yi H, Ye S, Ren X, Song B (2019) Micro(nano)plastics: unignorable vectors for organisms. Mar Pollut Bull 139:328–331
Shruti VC, Jonathan MP, Rodriguez-Espinosa PF, Rodríguez-González F (2019) Microplastics in freshwater sediments of Atoyac River basin, Puebla City, Mexico. Sci Total Environ 654:154–163
Su L, Sharp SM, Pettigrove VJ, Craig NJ, Nan B, Du F, Shi H (2020) Superimposed microplastic pollution in a coastal metropolis. Water Res 168:115140
Suaria G, Perold V, Lee JR, Lebouard F, Aliani S, Ryan PG (2020) Floating macro- and microplastics around the Southern Ocean: results from the Antarctic circumnavigation expedition. Environ Int 136:105494
Talsness CE, Andrade AJ, Kuriyama SN, Taylor JA, vom Saal FS (2009) Components of plastic: experimental studies in animals and relevance for human health. Philos Trans R Soc B 364:2079–2096
Tang S, Lin L, Wang X, Feng A, Yu A (2020) Pb(II) uptake onto nylon microplastics: interaction mechanism and adsorption performance. J Hazard Mater 386:121960
Taylor GT, Zheng D, Lee M, Troy PJ, Gyananath G, Sharma SK (1997) Influence of surface properties on accumulation of conditioning films and marine bacteria on substrata exposed to oligotrophic waters. Biofouling 11:31–57
Tekman MB, Wekerle C, Lorenz C, Primpke S, Hasemann C, Gerdts G, Bergmann M (2020) Tying up loose ends of microplastic pollution in the Arctic: distribution from the sea surface through the water column to deep-sea sediments at the HAUSGARTEN observatory. Environ Sci Technol. https://doi.org/10.1021/acs.est.9b06981
Teuten EL, Rowland SJ, Galloway TS, Thompson RC (2007) Potential for plastics to transport hydrophobic contaminants. Environ Sci Technol 41:7759–7764
Thompson RC, Thompson Richard C, Olsen Y, Mitchell RP, Davis A, Rowland SJ, John AWG, McGonigle D, Russell AE (2004) Lost at sea: where is all the plastic? Science 304:838
Thompson R, Moore C, Andrady A, Gregory M, Takada H, Weisberg S (2005) New directions in plastic debris. Science 310:1117
Tran L, Nunan L, Redman RM, Mohney LL, Pantoja CR, Fitzsimmons K, Lightner DV (2013) Determination of the infectious nature of the agent of acute hepatopancreatic necrosis syndrome affecting penaeid shrimp. Dis Aquat Organ 105:45–55
Turner A (2016) Heavy metals, metalloids and other hazardous elements in marine plastic litter. Mar Pollut Bull 111:136–142
van Sebille E, England MH, Froyland G (2012) Origin, dynamics and evolution of ocean garbage patches from observed surface drifters. Environ Res Lett 7:044040
van Sebille E, Wilcox C, Lebreton L, Maximenko N, Hardesty BD, Franeker JA, Eriksen M, Siegel D, Galgani F, Law KL (2015) A global inventory of small floating plastic debris. Environ Sci Technol Lett 10:124006
Verla AW, Enyoh CE, Verla EN, Nwarnorh KO (2019) Microplastic–toxic chemical interaction: a review study on quantified levels, mechanism and implication. SN Appl Sci. 1:1400
Virsek MK, Lovsin MN, Koren S, Krzan A, Peterlin M (2017) Microplastics as a vector for the transport of the bacterial fish pathogen species Aeromonas salmonicida. Mar Pollut Bull 125:301–309
Wagner M, Scherer C, Alvarez-Muñoz D, Brennholt N, Bourrain X, Buchinger S, Fries E, Grosbois C, Klasmeier J, Marti T, Rodriguez-Mozaz S, Urbatzka R, Vethaak AD, Winther-Nielsen M, Reifferscheid G (2014) Microplastics in freshwater ecosystems: what we know and what we need to know. Environ Sci Eur 26:2–9
Wang Q, Zhang Y, Wangjin X, Wang Y, Meng G, Chen Y (2020) The adsorption behavior of metals in aqueous solution by microplastics effected by UV radiation. J Environ Sci 87:272–280
Weijuan L, Youqian D, Zuyi T (2000) Americium(III) adsorption on polyethylene from very dilute aqueous solutions. J Radioanal Nucl Chem 250:497–500
Wingender J, Flemming HC (2011) Biofilms in drinking water and their role as reservoir for pathogens. Int J Hyg Environ Health 214:417–423
Wu X, Pan J, Li M, Li Y, Bartlam M, Wang Y (2019) Selective enrichment of bacterial pathogens by microplastic biofilm. Water Res 165:114979
Xiong X, Wu C, Elser JJ, Mei Z, Hao Y (2019) Occurrence and fate of microplastic debris in middle and lower reaches of the Yangtze River—from inland to the sea. Sci Total Environ 659:66–73
Yan M, Nie H, Xu K, He Y, Hu Y, Huang Y, Wang J (2019) Microplastic abundance, distribution and composition in the Pearl River along Guangzhou city and Pearl River estuary, China. Chemosphere 217:879–886
Yang Y, Liu G, Song W, Ye C, Lin H, Li Z, Liu W (2019) Plastics in the marine environment are reservoirs for antibiotic and metal resistance genes. Environ Int 123:79–86
Yeo BG, Takada H, Hosoda J, Kondo A, Yamashita R, Saha M, Maes T (2017) Polycyclic aromatic hydrocarbons (PAHs) and hopanes in plastic resin pellets as markers of oil pollution via international pellet watch monitoring. Arch Environ Contam Toxicol 73:196–206
Zettler ER, Mincer TJ, Amaral-Zettler LA (2013) Life in the “plastisphere”: microbial communities on plastic marine debris. Environ Sci Technol 47:7137–7146
Zhang K, Su J, Xiong X, Wu X, Wu C, Liu J (2016) Microplastic pollution of lakeshore sediments from remote lakes in Tibet plateau, China. Environ Pollut 219:450–455
Zhang K, Chen X, Xiong X, Ruan Y, Zhou H, Wu C, Lam PKS (2019a) The hydro-fluctuation belt of the Three Gorges Reservoir: source or sink of microplastics in the water? Environ Pollut 248:279–285
Zhang B, Wu D, Yang X, Teng J, Liu Y, Zhang C, Zhao J, Yin X, You L, Liu Y, Wang Q (2019b) Microplastic pollution in the surface sediments collected from Sishili Bay, North Yellow Sea, China. Mar Pollut Bull 141:9–15
Zhang W, Zhang L, Hua T, Li Y, Zhou X, Wang W, You Z, Wang H, Li M (2020a) The mechanism for adsorption of Cr(VI) ions by PE microplastics in ternary system of natural water environment. Environ Pollut 257:113440
Zhang Y, Lu J, Wu J, Wang J, Luo Y (2020b) Potential risks of microplastics combined with superbugs: enrichment of antibiotic resistant bacteria on the surface of microplastics in mariculture system. Ecotoxicol Environ Saf 187:109852
Zhao S, Zhu L, Wang T, Li D (2014) Suspended microplastics in the surface water of the Yangtze Estuary System, China: first observations on occurrence, distribution. Mar Pollut Bull 86:562–568
Zhou R, Zeng S, Hou D, Liu J, Weng S, He J, Huang Z (2019) Occurrence of human pathogenic bacteria carrying antibiotic resistance genes revealed by metagenomic approach: a case study from an aquatic environment. J Environ Sci China 80:248–256
Zubris KA, Richards BK (2005) Synthetic fibers as an indicator of land application of sludge. Environ Pollut 138:201–211
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (nos. 91951205, 31850410475), the China Postdoctoral Science Foundation (no. 2018M643294), and Guangdong Basic and Applied Basic Research Foundation, China (no. 2020A1515011139). This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-track Research Funding Program.
Author information
Authors and Affiliations
Contributions
MA and W-JL designed and wrote the manuscript. J-LL helped in data collection and making figure and tables. P-DW and WH helped in writing and critical review of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All the authors contributed equally and declared no conflict of interest.
Animal and human rights statement
No human or animal subjects were used during the course of this research.
Additional information
Edited by Chengchao Chen.
Rights and permissions
About this article
Cite this article
Ahmad, M., Li, JL., Wang, PD. et al. Environmental perspectives of microplastic pollution in the aquatic environment: a review. Mar Life Sci Technol 2, 414–430 (2020). https://doi.org/10.1007/s42995-020-00056-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42995-020-00056-w




