Abstract
With the advancement of nanotechnology, nanomaterials are beginning to be employed in different areas of science, including plant tissue culture. Among nanomaterials, silver nanoparticles (AgNPs) are widely used because of their antibacterial effects. However, knowledge about the effects of AgNPs on in vitro cultivation of plant species is poor. The present study aims to analyse the effects of AgNPs on the in vitro propagation of Campomanesia rufa. Nodal segments were sectioned and cultured in MS medium to induce buds. The MS medium was supplemented with benzylaminopurine, different concentrations of AgNPs or AgNO3. AgNPs were synthesized and characterized as a function of time. The shoots were analysed for number, height and fresh weight. Biochemical and light microscopy and scanning electron microscopy were also performed. Data from the characterization of AgNPs demonstrate that the heating process for sterilization of the culture medium promotes an agglomeration of AgNPs. The lowest concentrations AgNPs (0.385, 0.77 and 1.54 mg L−1) did not affect the in vitro multiplication, since no significant differences were observed in relation to the control, as for the number, height and fresh weight of the shoots formed, however, in the treatments with 15.4 mg L−1 AgNPs and AgNO3, a reduction in the number of shoots was observed. No biochemical, morphological or anatomical changes were observed in the shoots formed. Concludes, AgNPs did not affect the in vitro multiplication at low concentrations but may cause more damage to plant development than the use of AgNO3, depending on the concentration used.
Key message
This manuscript reports that silver nanoparticles not affect the in vitro multiplication of Campomanesia rufa, and the results are important because explain the behavior of AgNPs under high temperatures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanomaterials have unique physicochemical properties that differ distinctly from their macroscopic or even ionic counterparts. These properties usually result from their small size, shape, chemical composition, high surface area and reactivity (Ma et al. 2010). Because they exhibit such unique properties, nanomaterials have been widely used to improve seed germination (Lahiani et al. 2013; Siddiqui and Al-Whaibi 2014), increase plant growth and yield (Kole et al. 2013), to allow genetic modifications in plant cells (Torney et al. 2007) and to improve photosynthetic efficiency (Sharma et al. 2012).
In plant tissue culture, there are promising indications of the utility of nanotechnology. Iron nanoparticles, for example, improved the in vitro development of strawberry seedlings submitted to water stress (Mozafari et al. 2018). CuO nanoparticles stimulated the in vitro production of bioactive compounds in Stevia rebaudiana (Javed et al. 2017) and Brassica rapa spp. pekinensis (Chung et al. 2018).
The effective micropropagation of crop species depends on a range of factors, including the type of culture medium and plant growth regulators (Satish et al. 2015; Venkatachalam et al. 2015; Scalzo et al. 2016; Yu et al. 2017). The application of nanomaterials may be an alternative to optimize protocols of somatic embryogenesis, organogenesis, induction of shoots and roots, superficial disinfection of explants for in vitro culture, and production of secondary metabolites and even to improve or reduce the somaclonal variation of in vitro explants (Kim et al. 2017).
Silver nanoparticles (AgNPs), due to their attractive physiological properties and their recognized antimicrobial action, may have potential use in superficial disinfection processes of explants for in vitro culture (Sarmast and Salehi 2016). AgNPs have been shown to be effective in reducing bacterial contamination in Vanilla planifolia (Spinoso-Castillo et al. 2017) and in the control of contaminants for the in vitro culture of Valeriana officinalis (Abdi et al. 2008), and Olea europaea (Rostami and Shahsavar 2009).
The AgNPs were also efficient in in vitro multiplication of Alternanthera sessilis (Venkatachalam et al. 2017), induction of somatic embryogenesis and plant regeneration of Gloriosa superba (Mahendran et al. 2017), and the promotion of Brassica juncea growth in vitro through the improvement of its antioxidant status (Sharma et al. 2012).
In addition to acting directly on in vitro propagation, silver nanoparticles may also act as inhibitors of the hormone ethylene (Syu et al. 2014; Sarmast et al. 2015). This phytohormone accumulates in culture vessels and may affect the growth and in vitro development of some species (Biddington 1992).
Silver nanoparticle exposure responses in plants have been studied mainly in relation to toxicity (Cvjetko et al. 2017), and less attention has been given to the possibility that silver nanoparticles may support in vitro growth of plant species.
In this context, the present study has the objective of analysing the effect of silver nanoparticles on the micropropagation of Campomanesia rufa (O. Berg) Nied. C. rufa is a fruit-bearing species of the Cerrado with potential for food commercialization, but it presents difficulties for propagation in the natural environment.
Materials and methods
Synthesis of silver nanoparticles
Silver nanoparticles were synthesized according to the methodology described by Turkevich et al. (1951) with adaptations. A solution was prepared with silver nitrate (0.18 g L−1) and sodium carboxymethylcellulose (0.6 g L−1), which was maintained under constant heating and stirring. At 95 °C, an aqueous solution of sodium citrate (1%) was added thereto. The concentration of the silver nanoparticle solution was quantified by UV–Vis absorption spectroscopy (model UV-1800, Shimadzu).
Characterization of silver nanoparticles
The culture media containing the silver nanoparticle solution at the corresponding concentrations, after autoclaving, were characterized by means of dynamic light scattering (DLS) and zeta potential, performed with the Malvern 3000 Zetasizer equipment.
Three replicates were used for each treatment. For analysis of the solutions, 0.5 mL of each sample suspension was combined with 100 mL of deionized water and sonicated in an ultrasonic tip (Brason) for 1 min at a power of 450 W.
Bud induction
Buds (4 cm) induced from established in vitro plantlets of Campomanesia rufa were transferred to MS medium (Murashige and Skoog 1962) plus 5.62 µM BAP (6-benzylaminopurine) and 30 g L−1 sucrose (Sant’Ana et al. 2018). The medium was supplemented with different concentrations of silver nanoparticles (0.0, 0.385, 0.77, 1.54 and 15.4 mg L−1) or silver nitrate at a concentration of 0.18 g L−1, which corresponds to the same concentration used for the synthesis of stock solution of AgNPs. This concentration is equivalent to 0.114 g L−1 of Ag+ ions.
The medium was solidified with 7.0 g L−1 agar, and the pH was adjusted to 5.7 ± 0.1 before autoclaving at 121 °C and 1 atm pressure for 20 min. The culture was maintained in a growth room for 90 days at a temperature of 25 °C on a 16-h photoperiod (fluorescent lamps radiating 36 µmol of photons m−2 s−1) for shoot multiplication and subsequent growth evaluations, biochemistry, light microscopy and scanning electron microscopy.
After 90 days of culture, the number and size of the shoots were analysed, and the total fresh mass of the shoots. The size of the shoots was measured using a ruler.
Biochemical analysis of antioxidant metabolism
At 90 days of culture, samples of the shoots formed were removed and stored in a freezer at − 80 °C until biochemical analysis.
The enzymatic extract for biochemical analysis was obtained by the maceration in liquid nitrogen of 200 mg of fresh material from the aerial part of the shoots, to which was added 1.5 mL of an extraction buffer composed of potassium phosphate buffer (400 mM) in pH 7.8, 15 µL EDTA (10 mM), 75 µL ascorbic acid (200 mM) and 1035 µL water (Biemelt et al. 1998). The extract was centrifuged at 13,000×g for 10 min at 4 °C, and the supernatant collected for analysis of superoxide dismutase (SOD) was evaluated according to Giannopolitis and Ries (1977).
SOD activity was assessed by the ability of the enzyme to inhibit nitrotetrazolium blue photoreduction, NBT (Giannopolitis and Ries 1977). We added 5 mL aliquots of the supernatants from treatments with different concentrations of AgNPs and silver nitrate to incubation medium containing 100 mM potassium phosphate buffer (pH 7.8), 70 mM methionine, 10 µM EDTA, 1 mM NBT, 0.2 mM riboflavin and water. Samples and a control composed of the same reaction medium without the sample were illuminated with a 20 W fluorescent lamp for 7 min. Readings were performed at 560 nm where a unit of SOD corresponds to the amount of enzyme required to inhibit the NBT photoreduction at 50%.
Light microscopy
For the anatomical studies, leaf and stem samples from 90 days of culture were collected and stored in alcohol 70% (v/v) until the anatomical sections were taken. To prepare the slides, leaf and stem samples were sectioned at approximately 0.5 cm2, and these sections were dehydrated in increasing ethylic series and included in methacrylate (Historesin, Leica Instruments, Heidelberg, Germany).
Transverse sections of the leaves and stems were made with an 8 µM thick microtome, stained with toluidine blue (O’Brien et al. 1964) and sealed using stained glass (Acrilex).
The prepared slides were observed and photographed under a Zeiss Scope AX10® microscope coupled to the digital camera and photomicrographed with Axio Vision R.L. 4.8® software. The presence or absence of cellular damage was determined.
Scanning electron microscopy
Leaf and stem segments of the shoots were fixed in Karnovski aqueous solution for 24 h, after which the material remained in glycerol for 30 min. After this period, the stem and leaf segments were segmented into liquid nitrogen (LN). Thereafter, dehydration was accomplished in increasing series of acetone (25, 50, 75, and 100%) for 10 min.
In the next step, the material was dried in the critical point equipment, and the cuts were adhered to the stubs and covered by a layer of metallic gold. The segments were observed with a JEOL T200 scanning electron microscope.
Results
Characterization of silver nanoparticles
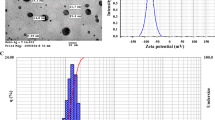
In DLS analysis, the presence of silver nanoparticles in the culture medium was observed in both the lowest (0.385 mg L−1) and the highest concentration (15.4 mg L−1), which indicates the presence of silver ions in the medium used to induce new buds in C. rufa.
The AgNPs present in medium were larger than the AgNPs of the stock solution (155.2 nm). This increase in size is related to autoclaving of the culture medium. The high heating (121 °C) for sterilization promoted the agglomeration of nanoparticles, which resulted in the increase in size compared to the solution of non-autoclaved AgNPs (Fig. 1).
The Zeta Potential, close to zero, and the Polydispersity Index (PDI) show that the synthesized solution is unstable and tends to aggregate. The instability of the solution, associated with the high temperature to which the AgNPs were subjected during sterilization of the medium, contributed to the increase in size of AgNPs (Fig. 2a, b).
Growth analysis
Exposure of C. rufa nodal segments to different concentrations of AgNPs did not affect the induction of buds at the concentrations of 0.385, 0.77, and 1.54 mg L−1 compared to the conditions with no AgNPs. However, at the concentration of 15.4 mg L−1 and in the silver nitrate treatment, there was a reduction of approximately 90% in the number of new shoots (Figs. 3, 4).
Number of shoots obtained from Campomanesia rufa at 90 days of culture, induced by BAP (5.6 µM) and submitted to silver nitrate (180 mg L−1) or different concentrations of silver nanoparticles (0.385, 0.77, 1.54 and 15.4 mg L−1). The averages followed by the same letter do not differ significantly from one another by the Scott-Knott test at the 5% probability level
Consequently, with the reduction in the number of shoots, the total fresh mass was also reduced by approximately 80% in the silver nitrate and 15.4 mg L−1 AgNPs conditions, relative to the control (Fig. 5). However, the length of shoots obtained (averaging 1.1 cm) was not influenced by exposure to silver nanoparticles.
Total fresh mass of the shoots obtained from Campomanesia rufa at 90 days of culture, induced by BAP (5.6 µM) and submitted to silver nitrate (180 mg L−1) or different concentrations of silver nanoparticles (0.385, 0.77, 1.54 and 15.4 mg L−1). The averages followed by the same letter do not differ significantly from one another by the Scott-Knott test at the 5% probability level
Biochemical analysis of antioxidant metabolism
No significant differences were observed among treatments in the superoxide dismutase activity (SOD) by the Scott-Knott test at the 5% probability level, which showed a mean activity of 0.51 U mg− 1 FM (Table 1).
Light microscopy
The photomicrographs of the leaf blade of C. rufa showed uniseriate epidermis with stomata present only on the abaxial face and dorsiventral heterogeneous mesophyll composed of a layer of palisade parenchyma and two layers of lacunae (Fig. 6).
Transverse cuts of Campomanesia rufa leaf and stem segments at 90 days of culture, resulting from the induction of BAP shoots (5.6 µM), submitted to silver nitrate (0.18 g L−1) or silver nanoparticles (AgNPs). a and b control, c and d silver nitrate, e and f 0.77 mg L−1 AgNPs. PP palisade parenchyma, SP spongy parenchyma. In b highlight for stomata and in f prominence for secretory gland. Bar: 10 µM
Light microscopy showed secretory glands abundant in the leaves and stem. In Campomanesia xanthocarpa, it was reported that these glands have lipid content (Gogosz et al. 2010). No cell damage was observed in the leaves and stems, regardless of the concentration of AgNPs or silver nitrate, in comparison to the control condition.
Scanning electron microscopy
For all treatments, the electron photomicrographs reveal many simple and uniserial trichomes on both the adaxial and abaxial faces of the C. rufa leaf blades, which were grown in vitro. The trichomes are located both leaf veins and interveins (Fig. 7).
Scanning electron microscopy photomicrography of the abaxial surface of foliar segments of Campomanesia rufa at 90 days of culture, resulting from the induction of shoots from BAP (5.6 µM), submitted to silver nitrate (180 mg L−1) or different concentrations of silver nanoparticles (AgNPs). a control, b silver nitrate, c 0.385 mg L−1 AgNPs, d 0.77 mg L−1 AgNPs, e 1.54 mg L−1 AgNPs and f 15.4 mg L−1 AgNP. Arrowhead: in b stomata and in c trichome
The stomata were observed only on the abaxial sides of the leaves and were classified as paracytic, with reniform guard cells, being more prominent than the fundamental epidermal cells (Fig. 7).
The stomata of the leaves treated with silver nitrate showed larger stomatal openings than seen in the control and in the treatments with AgNPs. No leaf surface damage was observed, regardless of the concentration of AgNPs or the presence of silver nitrate, in comparison to the control.
Discussion
No significant changes were observed among conditions without addition of AgNPs and treatments with low concentrations of AgNPs (0.385, 0.77 and 1.54 mg L−1), regarding the number, height and fresh weight of the shoots.
This low toxicity of the AgNPs may be related to the size of the AgNPs, as observed by the characterization of the culture medium the high temperature experienced by the medium during autoclaving may be promotes agglomeration of the nanoparticles, thus producing particles that exceed the nano scale (1 at 100 nm). As has been demonstrated, effects of AgNPs on plant cells vary with size of the nanoparticles (Geisler-Lee et al. 2012).
In a study conducted with Arabidopsis, it was found that AgNPs of 45 ± 5 nm promoted a higher growth rate with less induction of oxidative stress compared to AgNPs of 8 ± 2 nm that caused the highest rate of inhibition of growth and higher levels of oxidative stress (Syu et al. 2014). In Spirodela polyrhiza, it was shown that silver microparticles did not cause oxidative stress to the detriment of silver nanoparticles; consequently, no changes in antioxidant metabolism were observed compared to the control (Jiang et al. 2014).
These results may be related to the size of pores in plant cell walls, which are generally in the range of a few nanometres (Carpita et al. 1979; Adani et al. 2011), much smaller than the size of the silver particles. The relatively large size of the silver particles may prevent them from easily penetrating plant cell walls and thus cause less damage than the smaller AgNPs that can pass through the pores.
In treatments with 15.4 mg L−1 of AgNPs and with silver nitrate (180 mg L−1), similar results were observed. In both treatments, a reduction in the number and the fresh mass of the shoots was observed. However, the concentration of AgNO3 is higher than the concentration of AgNPs, suggesting that AgNPs may be more toxic than AgNO3 for plant species.
In similar concentrations AgNPs have been observed to be more toxic to Lolium multiflorum plants than AgNO3, and AgNPs toxicity caused significant reductions in root growth rate and changes in the morphology of root systems (Yin et al. 2011). Likewise, AgNO3 was shown to be more effective in improving growth rates (root and shoot length and leaf area) than the AgNPs for the in vitro culture of Solanum tuberosum (Bagherzadeh Homaee and Ehsanpour 2015).
In Allium cepa, AgNO3 was more toxic than AgNPs coated with citrate, polyvinylpyrrolidone (PVP) or cetyltrimethylammonium bromide (CTAB) (Cvjetko et al. 2017). AgNO3 was also shown to be more toxic than AgNPs in Brassica sp., due to the lower accumulation of AgNPs and better APX and CAT activities, which resulted in lower oxidative stress (Vishwakarma et al. 2017). In Brassica juncea, inhibition of root length and chlorophyll content in plants exposed to AgNO3 at the same concentrations of AgNPs was observed (Pandey et al. 2014).
In Cucumis sativus cortical cell degeneration and disintegration of the endoderm in AgNO3 treatments were more prominent than in treatments with AgNPs, although both were toxic to the growth and development of this species (Tripathi et al. 2017).
In Capsicum annuum, both AgNPs and Ag+ ions similarly affected the development of the plant, decreasing its height and biomass as a result of the increase of total Ag+ content in the plant tissues (Vinković et al. 2017).
With these data, it is clear that the effect of AgNPs and silver nitrate is still controversial, with results that vary widely from species to species. These results make evident the need for further studies to understand the effects of AgNPs on plant cells and to better explore the intrinsic properties of nanoparticles.
No significant differences in antioxidant metabolism were observed among the treatments, regardless of the concentrations of AgNPs or silver nitrate. However, it has been reported that the exposure of plants to nanomaterials can increase the production of reactive oxygen species (ROS), such as singlet oxygen (1O2), superoxide (O2•−), hydrogen peroxide (H2O2) and hydroxyl radical (OH•) (Hossain et al. 2015).
ROS influence the growth and development of a plant, so they need to be rapidly metabolized by the antioxidant system to avoid oxidative stress conditions. Enzyme metabolism for ROS detoxification in plants is essential for the protection of plant cells and their organelles against the toxic effects of ROS (Mittler 2017). Thus, when a plant is subjected to some type of abiotic stress, the increase in the activity of antioxidant enzymes is remarkable (Gill et al. 2015). It is important to evaluate the physiological conditions of plant cells in this context, as these conditions effect their growth and development.
SOD is the first line of defence of the antioxidant system, catalysing the decomposition of O2•− in O2 and H2O2, and CAT and APX catalyse the dismutation of H2O2 in H2O and O2 (Das and Roychoudhury 2014).
It is reported that Ag+ ions released from AgNPs induce oxidative stress through the generation of ROS. In vitro multiplication of sugarcane by temporary immersion, an increase of ROS and lipid peroxidation have been reported when different concentrations of AgNPs were added to MS medium (Bello–Bello et al. 2017). In Solanum tuberosum, an increase in H2O2 and lipid peroxidation was also observed in explants cultured in vitro in the presence of AgNPs or silver nitrate (Bagherzadeh Homaee and Ehsanpour 2015).
However, in the present study, it is possible to verify that AgNPs were probably not provoking the production of ROS in C. rufa seedlings grown in vitro, since SOD activity was not significantly changed by the addition of AgNPs or silver nitrate. The analysis of the images obtained by light and scanning microscopy showed no cellular damage in the vegetal cells. However would have been expected if high concentrations of H2O2, (which can potentially cause plant cell death) had been present.
The greater opening of the stomata in the silver nitrate treatment is related to the positive effect of AgNO3 in the reduction of hyperhydricity. It has been reported that the presence of AgNO3 in the culture medium reduces the hyperhydricity of explants cultivated in vitro by promoting greater stomatal opening, resulting in greater water outflow from the plantlets and reducing the accumulation of water in the tissues (Gao et al. 2017).
In Bacopa monnieri, no morphological changes or toxic effects were observed in plants treated with AgNPs or silver nitrate by scanning electron microscopy. However, in light microscopy, small changes were observed in xylem elements within stems and roots of plants treated with AgNPs and AgNO3 (Krishnaraj et al. 2012). In Oryza sativa, the presence of AgNPs of 150 nm at 10 and 100 mg L−1 caused alterations in the anatomy of plantlets’ leaves (Thuesombat et al. 2014). These results demonstrate that both the size and concentration of AgNPs may affect the development of plant species. There is need for further study to better understand the effects of AgNPs, since in the literature, the results remain controversial and inconclusive.
Conclusions
AgNPs did not affect the in vitro multiplication of C. rufa at low concentrations, and the results for silver nitrate (180 mg L−1) were similar to the results for the 15.4 mg L−1 concentration of AgNPs. These results indicate that AgNPs may cause more damage to plant development than AgNO3, depending on the concentration employed.
The characterization of the culture media containing AgNPs shows that the heat treatment drastically increases the size of the AgNPs and that this can significantly alter the unique properties present only in nanoparticles. These results are important because they help explain the behaviour of AgNPs under high temperatures and suggest opportunities for future research on the effects of heat-treated AgNPs on plant cells.
References
Abdi G, Salehi H, Khosh-Khui M (2008) Nano silver: A novel nanomaterial for removal of bacterial contaminants in valerian (Valeriana officinalis L.) tissue culture. Acta Physiol Plant 30:709–714. https://doi.org/10.1007/s11738-008-0169-z
Adani F, Papa G, Schievano A et al (2011) Nanoscale structure of the cell wall protecting cellulose from enzyme attack. Environ Sci Technol 45:1107–1113. https://doi.org/10.1021/es1020263
Bagherzadeh Homaee M, Ehsanpour AA (2015) Physiological and biochemical responses of potato (Solanum tuberosum) to silver nanoparticles and silver nitrate treatments under in vitro conditions. Indian J Plant Physiol 20:353–359. https://doi.org/10.1007/s40502-015-0188-x
Bello-Bello JJ, Chavez-Santoscoy RA, Lecona-Guzmán CA et al (2017) Hormetic response by silver nanoparticles on in vitro multiplication of sugarcane (Saccharum spp. Cv. Mex 69–290) using a temporary immersion system. Dose Response 15:1559325817744945. https://doi.org/10.1177/1559325817744945
Biddington NL (1992) The influence of ethylene in plant tissue culture. Plant Growth Regul 11:173–187. https://doi.org/10.1007/BF00024072
Biemelt S, Keetman U, Albrecht G (1998) Re-aeration following hypoxia or anoxia leads to activation of the antioxidative defense system in roots of wheat seedlings. Plant Physiol 116:651–658. https://doi.org/10.1104/pp.116.2.651
Carpita N, Sabularse D, Montezinos D, Delmer DP (1979) Determination of the pore size of cell walls of living plant cells. Science 205:1144–1147. https://doi.org/10.1126/science.205.4411.1144
Chung I-M, Rekha K, Rajakumar G, Thiruvengadam M (2018) Production of bioactive compounds and gene expression alterations in hairy root cultures of chinese cabbage elicited by copper oxide nanoparticles. Plant Cell Tissue Organ Cult. https://doi.org/10.1007/s11240-018-1402-0
Cvjetko P, Milošić A, Domijan A-M et al (2017) Toxicity of silver ions and differently coated silver nanoparticles in Allium cepa roots. Ecotoxicol Environ Saf 137:18–28. https://doi.org/10.1016/J.ECOENV.2016.11.009
Das K, Roychoudhury A (2014) Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front Environ Sci 2:53. https://doi.org/10.3389/fenvs.2014.00053
Gao H, Xu P, Li J et al (2017) AgNO3 prevents the occurrence of hyperhydricity in Dianthus chinensis L. by enhancing water loss and antioxidant capacity. Vitr Cell Dev Biol 53:561–570. https://doi.org/10.1007/s11627-017-9871-0
Geisler-Lee J, Wang Q, Yao Y et al (2012) Phytotoxicity, accumulation and transport of silver nanoparticles by Arabidopsis thaliana. Nanotoxicology 7(3):323–337. https://doi.org/10.3109/17435390.2012.658094
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol 59:309–314. https://doi.org/10.1104/PP.59.2.309
Gill SS, Anjum NA, Gill R et al (2015) Superoxide dismutase—mentor of abiotic stress tolerance in crop plants. Environ Sci Pollut Res 22:10375–10394. https://doi.org/10.1007/s11356-015-4532-5
Gogosz AM, Cosmo NL, Bona C, Souza LA de (2010) Morfoanatomia da plântula de Campomanesia xanthocarpa O. Berg. (Myrtaceae). Acta Bot Brasilica 24:613–623. https://doi.org/10.1590/S0102-33062010000300003
Hossain Z, Mustafa G, Komatsu S (2015) Plant responses to nanoparticle stress. Int J Mol Sci 16:26644–26653. https://doi.org/10.3390/ijms161125980
Javed R, Mohamed A, Yücesan B et al (2017) CuO nanoparticles significantly influence in vitro culture, steviol glycosides, and antioxidant activities of Stevia rebaudiana Bertoni. Plant Cell Tissue Organ Cult 131:611–620. https://doi.org/10.1007/s11240-017-1312-6
Jiang H-S, Qiu X-N, Li G-B et al (2014) Silver nanoparticles induced accumulation of reactive oxygen species and alteration of antioxidant systems in the aquatic plant Spirodela polyrhiza. Environ Toxicol Chem 33:1398–1405. https://doi.org/10.1002/etc.2577
Kim DH, Gopal J, Sivanesan I (2017) Nanomaterials in plant tissue culture: the disclosed and undisclosed. RSC Adv 7:36492–36505. https://doi.org/10.1039/C7RA07025J
Kole C, Kole P, Randunu KM et al (2013) Nanobiotechnology can boost crop production and quality: first evidence from increased plant biomass, fruit yield and phytomedicine content in bitter melon (Momordica charantia). BMC Biotechnol 13:37. https://doi.org/10.1186/1472-6750-13-37
Krishnaraj C, Jagan EG, Ramachandran R et al (2012) Effect of biologically synthesized silver nanoparticles on Bacopa monnieri (Linn.) Wettst. plant growth metabolism. Process Biochem 47:651–658. https://doi.org/10.1016/j.procbio.2012.01.006
Lahiani MH, Dervishi E, Chen J et al (2013) Impact of carbon nanotube exposure to seeds of valuable crops. ACS Appl Mater Interfaces 5:7965–7973. https://doi.org/10.1021/am402052x
Ma X, Geiser-Lee J, Deng Y, Kolmakov A (2010) Interactions between engineered nanoparticles (ENPs) and plants: Phytotoxicity, uptake and accumulation. Sci Total Environ 408:3053–3061. https://doi.org/10.1016/J.SCITOTENV.2010.03.031
Mahendran D, Kishor PBK, Geetha N, Venkatachalam P (2017) Phycomolecule-coated silver nanoparticles and seaweed extracts induced high-frequency somatic embryogenesis and plant regeneration from Gloriosa superba L. J Appl Phycol. https://doi.org/10.1007/s10811-017-1293-1
Mittler R (2017) ROS are good. Trends Plant Sci 22:11–19. https://doi.org/10.1016/J.TPLANTS.2016.08.002
Mozafari A, Havas F, Ghaderi N (2018) Application of iron nanoparticles and salicylic acid in in vitro culture of strawberries (Fragaria × ananassa Duch.) to cope with drought stress. Plant Cell Tissue Organ Cult 132:511–523. https://doi.org/10.1007/s11240-017-1347-8
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
O’Brien TP, Feder N, McCully ME (1964) Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 59:368–373. https://doi.org/10.1007/BF01248568
Pandey C, Khan E, Mishra A et al (2014) Silver nanoparticles and its effect on seed germination and physiology in Brassica juncea L. (Indian Mustard) Plant. Adv Sci Lett 20:1673–1676. https://doi.org/10.1166/asl.2014.5518
Rostami AA, Shahsavar A (2009) Nano-Silver particles eliminate the in vitro contaminations of olive “Mission” explants. Asian J Plant Sci. https://doi.org/10.3923/ajps.2009.505.509
Sant’Ana CRO, Paiva R, Reis MV et al (2018) In vitro propagation of Campomanesia rufa: an endangered fruit species. Ciência e Agrotecnologia 42(4):372–380. https://doi.org/10.1590/1413-70542018424011018
Sarmast MK, Salehi H (2016) Silver nanoparticles: an influential element in plant nanobiotechnology. Mol Biotechnol 58:441–449. https://doi.org/10.1007/s12033-016-9943-0
Sarmast MK, Niazi A, Salehi H, Abolimoghadam A (2015) Silver nanoparticles affect ACS expression in Tecomella undulata in vitro culture. Plant Cell Tissue Organ Cult 121:227–236. https://doi.org/10.1007/s11240-014-0697-8
Satish L, Rameshkumar R, Rathinapriya P et al (2015) Effect of seaweed liquid extracts and plant growth regulators on in vitro mass propagation of brinjal (Solanum melongena L.) through hypocotyl and leaf disc explants. J Appl Phycol 27:993–1002. https://doi.org/10.1007/s10811-014-0375-6
Scalzo J, Donno D, Miller S et al (2016) Effect of genotype, medium and light on in vitro plant proliferation of Vaccinium spp. New Zeal J Crop Hortic Sci 44:231–246. https://doi.org/10.1080/01140671.2016.1206946
Sharma P, Bhatt D, Zaidi MGH et al (2012) Silver nanoparticle-mediated enhancement in growth and antioxidant status of Brassica juncea. Appl Biochem Biotechnol 167:2225–2233. https://doi.org/10.1007/s12010-012-9759-8
Siddiqui MH, Al-Whaibi MH (2014) Role of nano-SiO2 in germination of tomato (Lycopersicum esculentum seeds Mill.). Saudi J Biol Sci 21:13–17. https://doi.org/10.1016/J.SJBS.2013.04.005
Spinoso-Castillo JL, Chavez-Santoscoy RA, Bogdanchikova N et al (2017) Antimicrobial and hormetic effects of silver nanoparticles on in vitro regeneration of vanilla (Vanilla planifolia Jacks. ex Andrews) using a temporary immersion system. Plant Cell Tissue Organ Cult 129:195–207. https://doi.org/10.1007/s11240-017-1169-8
Syu Y, Hung J-H, Chen J-C, Chuang H (2014) Impacts of size and shape of silver nanoparticles on Arabidopsis plant growth and gene expression. Plant Physiol Biochem 83:57–64. https://doi.org/10.1016/J.PLAPHY.2014.07.010
Thuesombat P, Hannongbua S, Akasit S, Chadchawan S (2014) Effect of silver nanoparticles on rice (Oryza sativa L. cv. KDML 105) seed germination and seedling growth. Ecotoxicol Environ Saf 104:302–309. https://doi.org/10.1016/j.ecoenv.2014.03.022
Torney F, Trewyn BG, Lin VS-Y, Wang K (2007) Mesoporous silica nanoparticles deliver DNA and chemicals into plants. Nat Nanotechnol 2:295–300. https://doi.org/10.1038/nnano.2007.108
Tripathi A, Liu S, Singh PK et al (2017) Differential phytotoxic responses of silver nitrate (AgNO3) and silver nanoparticle (AgNPs) in Cucumis sativus L. Plant Gene 11:255–264. https://doi.org/10.1016/J.PLGENE.2017.07.005
Turkevich J, Stevenson PC, Hillier J (1951) A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss Faraday Soc 11:55. https://doi.org/10.1039/df9511100055
Venkatachalam P, Kalaiarasi K, Sreeramanan S (2015) Influence of plant growth regulators (PGRs) and various additives on in vitro plant propagation of Bambusa arundinacea (Retz.) Wild. A recalcitrant bamboo species. J Genet Eng Biotechnol 13:193–200. https://doi.org/10.1016/J.JGEB.2015.09.006
Venkatachalam P, Malar S, Thiyagarajan M et al (2017) Effect of phycochemical coated silver nanocomplexes as novel growth-stimulating compounds for plant regeneration of Alternanthera sessilis L. J Appl Phycol 29:1095–1106. https://doi.org/10.1007/s10811-016-0977-2
Vinković T, Novák O, Strnad M et al (2017) Cytokinin response in pepper plants (Capsicum annuum L.) exposed to silver nanoparticles. Environ Res 156:10–18. https://doi.org/10.1016/j.envres.2017.03.015
Vishwakarma K, Shweta G, Upadhyay N et al (2017) Differential phytotoxic impact of plant mediated silver nanoparticles (AgNPs) and silver nitrate (AgNO3) on Brassica sp. Front Plant Sci 8:1501. https://doi.org/10.3389/fpls.2017.01501
Yin L, Cheng Y, Espinasse B et al (2011) More than the ions: the effects of silver nanoparticles on Lolium multiflorum. Environ Sci Technol 45:2360–2367. https://doi.org/10.1021/es103995x
Yu R, Baniaga AE, Jorgensen SA, Barker MS (2017) A successful in vitro propagation technique for resurrection plants of the Selaginellaceae. Am Fern J 107:96–104. https://doi.org/10.1640/0002-8444-107.2.96
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001. Sincere thanks to the Foundation for Research Support of the State of Minas Gerais (FAPEMIG) and the National Council for Scientific and Technological Development (CNPq), for financial support. We are also grateful to the National Laboratory of Nanotechnology for Agribusiness - Embrapa Instrumentation - São Carlos-SP, where the silver nanoparticles were characterized.
Author information
Authors and Affiliations
Contributions
CT, MR planned the study, performed the experiments, analyzed the results and wrote the manuscript. RP research supervisor, contributed with scientific advice, corrected and revised the final version of the manuscript. JO, JM, PC and DS assisted in the planning and supervision of the study, performed the experiments, analyzed the results and corrected the manuscript. PC assisted performed experiments and analyzed the results. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors state that the present study was conducted in the absence of commercial or financial relationships that could result in a potential conflict of interest.
Additional information
Communicated by M. Angeles Revilla.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Timoteo, C.d., Paiva, R., dos Reis, M.V. et al. Silver nanoparticles in the micropropagation of Campomanesia rufa (O. Berg) Nied. Plant Cell Tiss Organ Cult 137, 359–368 (2019). https://doi.org/10.1007/s11240-019-01576-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-019-01576-9