Abstract
The effects of iron nanoparticles and salicylic acid (SA) on strawberry (Fragaria × ananassa Duch.) plants in conditions of drought stress were surveyed under in vitro conditions to find the optimum combination for strawberry tissue culture. Cuttings of the Queen Elisa cultivar were surveyed in a three-way factorial experiment with three replications in 2015. The results showed that drought stress significantly affected all measured parameters of strawberry plantlets under in vitro condition in a negative way. SA compensated for the negative effects of drought stress on strawberry plantlets and improved their growth parameters under in vitro culture. Strawberry plantlets treated with iron nanoparticles were able to cope with stressful conditions better than untreated ones. This study found that iron, a micronutrient in plant growth and in vitro development, greatly influenced the plantlets’ growth parameters and other measured traits. These results indicate that the efficiency of tissue culture and in vitro culture of strawberries could be improved by increased application of iron in the form of nanoparticles. The results might also indicate that the application of iron nanoparticles along with SA can be a useful method for providing higher quantity and quantity in the in vitro culture of strawberries, and could be used for adapting strawberry plants to drought before transplanting them in the field.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Strawberries (Fragaria × ananassa Duch.) are important fruits and excellent dietary sources of nutrients due to their unique taste, flavor, ascorbic acid, potassium, fiber, other secondary metabolites, and simple sugars (Diengngan et al. 2016). Berry yield is a polygenic trait and greatly influenced by environmental conditions. Strawberries are a high-value agricultural product worldwide, and their cultivation has great economic impacts. Stolons or runners are the most common methods for strawberry propagation, although they pose difficulties in achieving healthy and vigorous daughter plants (clones or stocks). Many researchers have thus suggested in vitro techniques as essential tools for agricultural propagation (among other purposes such as germplasm preservation). Horticultural strawberry micropropagation under in vitro conditions has now been used for more than 26 years (Diengngan et al. 2016). A number of studies have also shown that in vitro propagation of this plant, like other horticultural plants, is effective in achieving disease-free clones (Diengngan et al. 2016; Ghaderi et al. 2015; Sowik et al. 2016). It has been asserted that by using tissue culture and in vitro condition, several million clone plants can be generated from a mother plant each year (Diengngan et al. 2016).
Strawberries are relatively sensitive to environmental stresses such as drought and salinity (Sowik et al. 2016). Drought stress is one of the most significant environmental stresses limiting the agricultural production of strawberries worldwide, affecting the plants’ anatomical, physiological, and enzymatic features. Drought stunts the plants’ branches and dramatically decreases their yield (Natsheh et al. 2015). In arid and semi-arid areas, problems such as low precipitation, high evaporation and temperature and poor management of water resources further limit the cultivation of the strawberries (de Ribou et al. 2013). Drought influences the plant growth and triggers a reduction in the leaves’ expansion as well as stomatal closure; moreover, photosynthesis decreases as the result of osmotic stress from the soil’s low water potential (Seemann and Critchley 1985). The drought-related osmotic stress, in turn, usually affects the absorption of some nutrients and increases intra-cellular ionic concentrations, which leads to slowed plant growth and development (Todaka et al. 2015).
In addition to stunted growth and decreased plant expansion, drought and other environmental stresses have negative influences on the plants’ nutritional content. Nano-fertilizers, which plants can be quickly and completely absorb, can compensate for iron shortages (Tarafdar et al. 2015). The use of nano-fertilizers can be considered as an effective step to attain sustainable agriculture. Micronutrients can increase plants’ resistance to the negative effects of toxic ions. Higher concentrations of iron in nutritional solutions can compensate for the impacts of salinity (Uauy et al. 2006). Uauy et al. (2006) reported that spraying iron, zinc, and magnesium over wheat shoots led to improved growth characteristics. Maciel et al. (2004) stated that the negative impacts of saline environs could be relieved by the application of iron sulfate. The application of iron nano-fertilizer has been shown to increase the selective plasma membrane permeability properties of the root, and to reduce the absorption and accumulation of sodium; this improves the potassium per sodium ratio in shoot, thus increasing the plant’s resistance to salinity stress (Taiz et al. 2015).
Salicylic acid (SA), an endogenous plant-growth regulator, as well as influencing different physiological and biochemical functions in plants, including growth, development, and productivity (Hayat et al. 2010), is a naturally occurring signaling molecule that has an important role in establishing and signaling a defense response against various biotic and abiotic stresses (Ghaderi et al. 2015). Antioxidant enzyme activity, which plays an important role in protecting plants against oxidative damage by detoxifying super oxide radicals (Ray et al. 2012), is altered in the presence of SA. High antioxidant-enzyme activity improves plant resistance to oxidative damage caused by reactive oxygen species (Saed-Moucheshi et al. 2014b). Hayat et al. (2010) have reported that SA can effectively alleviate toxic effects in plants resulting from exposure to various abiotic stresses. Based on different reports SA enhanced the leaf area and dry matter production in corn and soybean (Khan et al. 2003), Brassica juncea (Fariduddin et al. 2003), wheat (Hayat et al. 2005) and strawberry (Ghaderi et al. 2015) under drought or salinity stress. Increases in root and shoot fresh weight and yield of strawberry by SA application under drought stress has been also reported (Jamali et al. 2011).
Tissue culture using the in vitro method can potentially produce uniform strawberry stocks and clone plants with high efficiency, as well as offer the means to limit the influence of drought stress with plant treatments such as the application of micronutrient elements and plant regulators. The aim of the current study was to investigate the effects of iron nano-particles and SA on different biochemical, physiological, and morphological traits of strawberry under water deficit and in vitro propagation, and to determine their interactions. It is worth mentioning that this research is among the first studies to consider the effect of iron nanoparticles along with a SA hormonal mixture on strawberry cultivation under in vitro conditions under drought stress.
Materials and methods
Plant materials
The current experiment was conducted to compare different levels of the interaction effects of iron nanoparticles, SA, and drought stress on strawberry (Fragaria × ananassa Duch.) in vitro. Strawberry runner tips (~1 cm in length) of the Queen Elisa cultivar, which is commonly cultivated, were used in this experiment during 2014–2015. Runners were separated from the greenhouses growing maternal plant and immediately transported to the tissue culture laboratory of the University of Kurdistan. To remove soil and contaminants, the runners were washed for about 30 min with tap water along with a washing liquid. After that, the samples were placed in a laminar hood as a sterile environment and kept in ethanol 70% for about 30 s. In the next stage, the samples were placed in 1.2% solution of sodium hypochloride for 15 min. The samples were then washed for 5, 10, and 15 min with distilled water. Runner tips of Queen Elisa cultivar were placed on Murashige and Skoog (1962) (MS) medium containing 3% sucrose, 0.7% (w/v) agar (Merck KGaA Company) and benzyl adenine (BA) (Sigma Aldridge Company Ltd.) 2 ppm + indole-3-butyric acid (IBA) (Sigma Aldridge Company Ltd.) 0.01 ppm. Then, the pH of the media was adjusted to 5.8 using NaOH or HCl. Afterward 40 mL of each prepared medium mixture was added to 250 mL glass jars. The media were sterilized by autoclaving for 15 min at 121 °C and 1.2 bar. After the media were cooled and firmed, the lids of the glass jars were sealed. To prevent subsequent contamination, the jars were kept in sterilized plastic bags. The glass jars were placed in a growth chamber at 25 ± 1 °C temperature under a photo period of 16 h light and 8 h dark with fluorescent lights (38 µE m−2 s−1) at 60% humidity conditions. After 56 days regenerated plants were used as explant experiment. Regenerated shoots (~3 cm) were isolated from the proliferating cultures and transferred to sterile glass jar containing 40 ml of medium (four shoots per jar), as experiment materials. The treatments consisted of drought stress at three levels (0, 5, and 10%) of polyethylene glycol (PEG 6000, Sigma Aldridge Company Ltd.), iron nanoparticles (Fe3O4, Nanozino Copanmy, 40–53 nanometer size) was coated by L-Sistein (Sigma Aldridge Company Ltd.) at three levels (0.0, 0.08, and 0.8 ppm) and SA (Sigma Aldridge Company Ltd.) at three levels of 0.0, 0.01, 0.05 mM. FeEDTA (Na2EDTA = 37.3 ppm + FeSO4·7H2O = 27.8 ppm) in 27 ppm concentration was considered as 0.0 ppm iron nanoparticles and defined as control. In total, three experiments were conducted. Experiment (1) Effect of PEG (0, 5, 10%) on branch number. Experiment (2) Combined effect of PEG (0, 5%) and iron (0.0, 0.08 and 0.8 ppm iron nanoparticle) on branch number. Experiment (3) Combined effect of PEG (0, 5%), iron (0.0, 0.8 ppm iron nanoparticle) and SA (0.0, 0.01, 0.05 mM) on branch number and other phenotypical traits. The applied treatments were mixed with other elements to produce the medium for each in vitro unit. The media were prepared based on MS, supplemented with same as plants’ regulatory hormones that explained in the regeneration section. The specific treatments for each unit were added separately, and all media were placed in the same conditions as described above.
Growth characteristics
Branch number, branch length (cm), root fresh weight (g), root length (cm), shoot fresh weight (g), Shoot dry weight (g) and total plant fresh weight (g) were measured at the end of the experiment. Regenerated plants were weighed individually for their fresh weight and kept in black paper bags. Bags were then kept in an oven at 70 °C for 48 h, after which dry weight was determined by weighing the dried shoots. Samples for physiological and biochemical traits were taken from in vitro conditions during the experiment. Precise rulers and scales were used to measure the plantlets’ lengths and weights. The sum of root and shoot fresh weight was used as the total weight for each plantlet.
Physiological characteristics
Fully expanded fresh leaves from each plantlet sample were precisely weighed using a digital scale (precision of 0.001 g) at fresh weight, saturated weight, and dry weight to determine relative water content (RWC). These weights were then used to calculate percent RWC for each sample (Karimi et al. 2012). The samples’ membrane-stability index (MSI) was measured based on the method of Sairam (1994).
Biochemical characteristics
The levels of chlorophyll a and b and carotenoid were calculated using the method of Lichtenthaler and Buschmann (2001) using a spectrophotometer tool to read the absorbance at 470, 663, and 645 nm. To measure total carbohydrate content, 0.1 g fresh tissue from the samples was weighed. It was then ground in a mortar and mixed with 5 mL ethanol 95%, and its extract was transferred to falcons that were then centrifuged for 10 min at 3500 rpm. The final supernatants were separated, then transferred to other falcons and placed in a bain marie bath for about 20 min at 100 °C, after which they were removed to cool to room temperature. Their light absorption was then measured at 625 nm. The free proline content in each sample was measured based on the method described by Bates et al. (1973), and total protein content was measured using the method of Bradford (1976). Ahmad et al.’s (2008) method was used to measure malondialdehyde (MDA) content. Hydrogen peroxide (H2O2) content was calculated at a wavelength of 390 nanometers spectrophotometrically as described by Pick and Mizel (1981). Using the methods of Flohé and Günzler (1984) and Beauchamp and Fridovich (1971), peroxidase (POD) and superoxide dismutase (SOD) activity were measured using spectrophotometry.
All spectrophotometry traits were measured using a spectrophotometer (UV-2100 model suv, New Jersey) and centrifuging was conducted using a centrifuge (HETTCH model MICRO, Germany).
Iron content
Iron content in plantlets as a mineral element was measured using the method described by Gupta et al. (2007), in which the samples’ ash was mixed with HCI and flame atomic absorption (Perkin Elmer Company, model 200) was applied.
Statistical analysis
This study was carried out based on a three-way factorial experiment arranged in a completely randomized design (CRD) with three glass jars as replications. Each glass jar contained four explants so for each treatment combination, totally 3 × 4 (e.g. 12) explants were analyzed. Mean of explants in each gloss jar was considered as the trait’s value for each replication. Means of shoot number were compared only for PEG treatment levels alone. Since, mean comparison showed a significant difference between control and PEG levels, while no significant difference was observed between 5 and 10% PEG application, data for 10% PEG application were excluded and the experiment was continued with data of only control (0% PEG) and 5% PEG application. At next, two-way ANOVA for mean comparison for PEG application and iron nanoparticles application was carried out. The results showed that there is no significant difference between control (0.0 ppm) and 0.08 ppm iron nano application under both 0 and 5% PEG, while their difference with 0.8 iron nano application were significant. Therefore, data related to 0.08 ppm iron nano application were excluded again and the experiment was continued with 0.0 and 0.8 ppm iron nanoparticles application. Finally, for the branch number which was measured in all treatments (at three levels of PEG application, three levels of iron nano applications, and three levels of SA application), all other parameters were measured on two levels of PEG application (0 and 5%), two levels of iron nano application (0.0 and 0.8 ppm), and three levels of SA (0.0, 0.01, and 0.05 mM). Analysis of variances was carried out in SAS v.9.4 after normality test of residuals and homogeneity test of variances. Means of treatments were compared using least significant difference (LSD) method. Bar graphs were drowning in Excel 2007. Residuals for branch number were not normal. Therefore, root square transformation for this trait was used. ANOVA and mean comparisons were based on transformed data while the values for branch number were reported according to the original data.
Results
Morphological and growth parameter
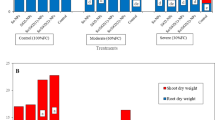
Figure 1 is presenting the mean comparison for branch number regarding different levels of PEG application. The highest number of branches achieved in no application of PEG (0% PEG as control of drought) which showed significant statistical difference from two other levels of PEG applications (5 and 10%). The difference between 5 and 10% application of PEG was not significant. Since the difference between 5 and 10% of PEG application was not significant and they showed similar effects on branch numbers of the explants, in the next stage, the plants were attributed to 10% PEG were not included in the analyses. Consequently, in order for considering the impacts of iron nanoparticles application, mean comparison according to LSD at 5% level (LSD 5%) was implemented for each level of PEG applications (0 and 5%), separately. No significant difference was observed between 0.0 and 0.08 ppm iron nano application under both PEG levels (Fig. 2), while the application of 0.8 ppm iron nano resulted in significant difference from the two other levels. Subsequently, the data attributed to the application of 0.08 ppm iron nano were set aside and the rest of the data were used for the next stage. Finally, three factors factorial ANOVA was carried out for all other measured parameters including morphological and growth related traits, physiological traits, and also biochemical traits with two level of PEG application (0 and 5%), two levels of iron nano application (0.0 and 0.8 ppm) and three levels of SA application (0.0, 0.01 and 0.05 mM). Table S1 (see Supplementary Material) presents the results of analysis of variance for growth-related traits and morphological traits. Except for root length regarding the PEG application and dry weight regarding iron nanoparticles application, the effect PEG and iron nano applications showed significant effect for all other parameters in Table S1 (see Supplementary Material). Also, the main effect of SA applications were significant for all growth-related and morphological traits. Two-way interaction effect of PEG and iron nano was significant only for root weight and total plant weight. Except shoot fresh weight and total plant weight, the interaction between PEG and SA applications was significant for all other growth-related and morphological parameters. Branch length and root weigh are the only parameters that were affected by interaction iron nano and SA application. The final three-way interaction effect of PEG, iron nao, and SA was significant only for root weight.
Three-way mean comparison for branch number in Fig. 3 showed that drought stress caused significant reduction in branch number of all samples. The application of both SA and iron nanoparticles improved the branch numbers of all samples even under drought stress condition. Higher concentration of SA improved number of branches. The highest number of branches was obtained in 0.8 ppm iron nano application and highest level of SA application (0.05 mM) under no drought stress condition. The lowest number of branches obtained under no application of SA and no application of iron nano under drought stress condition. Figure 4 shows the results of mean comparison for total plant weight. The difference between application of SA and iron nano under no stress condition and stressful one were significant. Higher total plant weight obtained under no stress condition and the higher concentration of both SA and iron nano. The highest total plant weight achieved in application of 0.8 ppm iron nano and 0.05 mM SA under no stress condition while the lowest total plant weight was observed in no application of SA and iron nano under drought stress condition. For all other morphological and growth-related parameters that are presented in the Table 1, drought stress caused significant reductions in the mean values for all traits: branch length, root weight, root length, shoot fresh and dry weight compared to the control samples. The highest mean values for branch length was observed in the control level of drought (PEG 0%), the highest level of iron nanoparticles application (0.8 ppm), and the medium-level application of SA (0.01 mM); their lowest mean values were observed in highest level of drought (PEG 10%) with no application of either iron nanoparticles or SA. The strawberry plantlets that had been treated with no drought stress, 0.8 ppm iron nanoparticle, and 0.8 mM salicylic acid showed the highest fresh weight of root, shoot, and those with no application of iron and SA under drought stress showed the lowest weights.
Physiological traits
Tables S2 (see Supplementary Material) and 2 are presenting the analysis of variance and mean comparison for all physiological and biochemical parameters measured in this study. Drought stress reduced the values for both physiological traits, RWC and MSI, under all treatments; the higher the stress, the lower the values (Table 2). Iron-nanoparticles and salicylic-acid applications as treatments for strawberry plantlets generally increased the values for both traits, with higher concentrations resulting in higher RWC and MSI under all drought-stress levels. The highest mean percent values for both RWC and MSI were obtained with the highest application of SA and iron under no drought stress; in contrast, the lowest mean percent values were recorded with no application of SA and iron under drought stress.
Biochemical traits
Pigment content
Chlorophyll a, chlorophyll b, total chlorophyll, and carotenoid contents of the strawberry plantlets for each treatment were measured as pigments’ content. Drought conditions had a reverse correlation with pigment content and significantly decreased pigments’ content. Application of both SA and iron nanoparticles resulted in higher content of these pigments than under control conditions. Higher concentrations of both SA and iron nanoparticles resulted in higher pigment content: the highest levels of these four pigments were recorded with the highest SA (0.05 mM) and iron nanoparticles (0.8 ppm) application and no drought stress; the lowest levels were recorded with application of either SA or iron nanoparticles and under highest drought-stress conditions (PEG 10%).
Carbohydrates, protein, and proline content
Table 2 also shows the mean comparison for free total soluble carbohydrates, free proline, and total protein in strawberry plantlets. Generally, all of these three traits increased in drought conditions. Under the control conditions and also drought-stress condition, application of SA and iron nanoparticles resulted in higher levels of total soluble carbohydrates, total protein, and free proline. In contrast, the simultaneous application of SA and iron nanoparticles, and thus their interaction, resulted in the highest levels of these substances under drought stress condition.
Hydrogen-peroxide and MDA content
H2O2 and MDA as an indication of the rate of damage caused by ROS to plant were measured; the results are shown in Table 2. Under all situations, drought conditions caused higher hydrogen-peroxide levels, which indicate ROS production, and higher MDA levels, which indicate lipid peroxidation. The highest level of drought stress resulted in the highest production of both of these substances. Application of SA and iron nanoparticles somewhat ameliorated the negative effect of drought and decreased the content of H2O2 MDA; the application of greater concentrations of both SA and iron nanoparticles led to lower the levels of both substances. The lowest levels were observed in drought stress (PEG 5%) with the application of neither SA nor iron nanoparticle, and for the control level of drought with the highest concentrations of both SA and iron nano. Generally, the lowest levels of H2O2 and MDA were achieved in two situations: drought and no SA or iron application.
Antioxidant-enzyme activity
Two kind of enzymatic antioxidant consist of SOD and POD were measured for considering the effects of treatment on antioxidant state of the strawberry plantlets which are presented Table 2. The drought stress was in a direct correlation with the activities of these antioxidants in which higher drought leaded to higher activity content of all measured pigments (Table 2). Application of both SA and iron nanoparticles resulted in higher activities of these traits in compare to control. On the other hand, higher concentration of both SA and iron nanoparticles caused antioxidants to achieved higher activities; so that the highest content of these traits were achieved for highest SA (0.05 mM) and iron nanoparticles (0.8 ppm) application under highest drought stress level. On the contrary, the lowest contents of SOD and POD were resulted in no application of SA and iron nanoparticles with no stress condition.
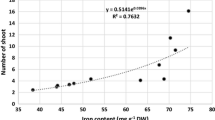
Iron content
Drought stress reduced the iron content, while the application of iron nanoparticles and SA increased the iron content (Table 2). The highest mean content of iron was obtained with the highest application of salicylic acid and iron nanoparticles under no drought stress; in contrast, the lowest mean content was recorded for drought stress without the application of either SA or iron nanoparticles.
Discussion
As strawberries are a major horticultural plant worldwide, many efforts have been made to provide higher yields and quality. Nonetheless, its genetic base and its polyploidy nature and other phenotypic characteristics have placed constraints on the ability of breeding to manage its regeneration and production in the field and in alternative natural growing environments (Palei et al. 2015). In vitro culture and micropropagation of this plant can effectively substitute for natural strawberry propagation (Bhat 2014). However, before in vitro propagation can be applied as a commercially viable method, different components and nutritional and hormonal elements must be extensively evaluated. Higher branching and more vigorous growth have often been observed where in vitro culture is used. Different researchers have provided evidence in support of the positive effects of in vitro tissue culture on strawberry growth, production of crowns, and generation of runners, length of petiole, overall yield, and number of inflorescences per crown compared to strawberry plants propagated conventionally (Diengngan et al. 2016; Palei et al. 2015). Plants’ growth and development in in vitro conditions mainly depends on the nutrition elements and hormonal content of the medium, along with controls to environmental temperature and humidity. George et al. (2008b) reported that mineral nutrients in the medium can effect cellular differentiation in combination with growth hormones and influence the phenotypic characteristics of the plants. Thus, the contents of the nutritional medium and the concentration of each hormone should be surveyed to reach optimum production and regeneration of highly vigorous strawberry plants in in vitro conditions. SA as an influential hormone and iron nanoparticles as a micro element were studied the current research to consider their effects on plants’ characteristics under conditions of drought stress in in vitro culture.
Drought stress is the most common abiotic stress among all plant species in arid and semi-arid areas. It prompts a substantial reduction in photosynthesis, although the precise degree to which it does so depends on the specific plant’s photosynthetic pigments and tissue (Saed-Moucheshi et al. 2015). During drought stress, the accumulation of solutes such as proteins, soluble carbohydrates, and free amino acids like proline are considered to be an influential stress-tolerance mechanism (Ghaderi et al. 2015). Drought stress reduces the osmotic potential of the plant tissue, usually represented by RWC and other physiological traits; these osmotic solutes accumulate to sustain the cells’ water potential at a level that permits their survival (Pirasteh-Anosheh et al. 2016). Plants’ flexibility and adaptability to this stress varies substantially among plant species, and even genotypes within a specific species, due to differences at the molecular and genetic levels as well as the circumstances of gene expression (Hossain et al. 2015). Drought stress also stimulates the generation of reactive oxygen species (ROS) containing H2O2, singlet oxygen (O1 −), superoxide radicals (O2 −), and hydroxyl radicals (OH−) (Hossain et al. 2016). Disproportionate ROS production damages most of the macromolecules inside plant tissues, such as lipids, nucleic acids, and proteins (Saed-Moucheshi et al. 2014a). Under these conditions, plants are obliged to develop and employ different types of defense mechanisms; among these, enzymatic antioxidants as ROS scavengers play a substantial role in maintaining of photosynthesis (Malik et al. 2014). The main enzymatic antioxidant shields in plant cells contain catalase (CAT), SOD, and POD (Chen et al. 2016). Induced drought stress in this study using PEG significantly reduced all the measured traits related to plants’ growth. Aside from root length, all other growing and morphological traits in this study (branch number, shoot fresh weight, branch length, root fresh weight, and total plant fresh weight) decreased in the strawberry plantlets as the drought-stress level, as induced by the application of increasing concentrations of polyethylene glycol, increased. The root length of the strawberry plantlets under almost all treatments increased under drought stress. When water potential in each culture medium is reduced through increasing the PEG concentration, the plantlets’ roots cannot take in enough water for growth of the plant; in this situation, either the plant can consume more energy to enhancing the length of the root, enabling it to travel through the medium seeking higher water potential, or it can bring higher concentrations of solutes toward root tissues and cells by consuming more energy to lower the potential of the root compared to the culture medium (Pirasteh-Anosheh et al. 2016). Normally most plants employ both strategies. Accumulating the solutes is a normal strategy for all tissue in plants to obtain water that is required for survival under drought stress (Naik and Al-Khayri 2016). RWC significantly decreased under drought stress in the current study. Furthermore, soluble solutes, including total soluble carbohydrates, total protein, and free proline significantly increased under drought stress in this study. Reduced water potential in plants also compelled plants to close their stomata to avoid wasting water by evapotranspiration, leading to dramatically decreased photosynthesis and reduced energy production, which in turn stunted growth. The pigment contents of strawberry plantlets in the current study decreased under drought stress, which may be mainly due to the generation of high levels of reactive oxygen species, resulting in damage to the cells’ photosynthetic components. MDA and H2O2 as indices representing the severity of drought stress (Parvin et al. 2015) significantly increased under drought stress compared to the control conditions; in contrast, the membrane stability index declined under all drought-stress levels in all treatments, which produced high levels of damage to cell membranes. SOD and POD activities increased in response to higher concentrations of polyethylene glycol, which simulated corresponding levels of drought stress. Under drought stress the generation of ROS increased beyond its normal steady state to the point at which it triggered higher enzymatic antioxidant activities as a means to cope with the drought stress (Tang et al. 2014).
SA as a regulatory element in plants is acknowledged to influence numerous biochemical and physiological activities (Khan et al. 2003; Shakirova et al. 2016). The growth parameters of the strawberry plantlets in this study showed improvement with the application of SA in the growth medium under all drought levels. RWC and MSI in the current study showed that regardless of the drought level, these parameters increased in strawberry plantlets treated with SA, and that higher SA concentration caused higher values for both RWC and MSI. Further, in most of the experimental situations, application of the SA under all drought levels increased pigment levels. Similarly, the levels of proline, total protein, and carbohydrates increased when SA was applied. In contrast, the application of SA reduced the concentrations of H2O2 and MDA. SOD and POD activity rose in all levels of drought stress when SA was applied. These results clearly indicating that, SA substantially improves strawberry plantlets’ characteristics and compensates for the negative effects of drought. Similar results for maize and soybean have been reported by Khan et al. (2003), who found that the application of SA and its components enhanced dry shoot weigh and leaf. Sun et al. (2013) observed increases in substances adjusting plants’ osmotic content, such as proline and total soluble sugar, as well as higher antioxidant activity, were observed as a result of the application of SA in strawberry plants, and Ghaderi et al. (2015) observed similar results for strawberry cultivars related to antioxidant activities and osmoregulators. Exogenous application of SA in red bayberry (Myric rubra) seedlings of three different genotypes showed that the treated plants had higher proline content, chlorophyll content, RWC, rate of photosynthesis, SOD activity, and stomatal conductance, although they showed lower enzyme activity for catalase and electrolyte conductivity compared to untreated plants (Ying et al. 2013). In Nimir et al.’s (2016) study of Iranian melons (Cucumis melo L.), the application of SA increased chlorophyll content (SPAD), fruit ripening duration and TSS compared to the control. Different water treatments and salicylic-acid levels had no significant effects on number of fruit per plant in the current study. Waheed and Madi (2016) investigated the effects of ascorbic acid and SA under salinity stress on the biochemical content, enzyme activities, and growth parameters of date palm under in vitro conditions. In their study shoots were micro-propagated and the tissues were excised and sampled at 2.5–3 cm length from the proliferation medium, then independently cultured on MS medium. The results indicated that maximum growth and chlorophyll content of shoots was observed after 75 days of culturing in the medium supplemented with 50 mg L−1 SA and 100 mg L−1 ascorbic acid in both stress and non-stress conditions. The in vitro culture led to increased antioxidant-enzyme activities (SOD and APX) in the medium that contained SA (75 mg L−1) and ascorbic acid (100 mg L−1) when salt-stress severity was increased. The shoot-protein patterns revealed some remarkable changes in protein expression. The results of SDS–PAGE in this experiment showed that the stress brought about the synthesis of higher protein bands. The application of SA and ascorbic acid under salt-stress conditions increased the additional bands of protein synthesis of different types. Naik and Al-Khayri (2016) have stated that the application of SA as a supplementary element in the medium of in vitro cultures in an appropriate concentration can increase resistance of the plants to water deficits and other abiotic stresses under in vitro conditions. Shakirova (2007) carried out a work on seed priming by SA and found that this treatment clearly enhanced seed germination in line with seedling growth in wheat seedlings. In contrast, Fariduddin et al.’s (2003) study on B. juncea found a significantly lower accumulation of dry matter with the application by spraying of higher concentrations of SA. In another study, He et al. (2005) stated that the shoots’ dry and fresh weight and leaf number and weight of wheat seedlings that had been treated with SA as a priming strategy had significantly higher values than the control. In Khodary’s (2004) exploration of the enhancement of the carbohydrate content in maize with exogenous application of SA, substantial increases in pigment levels, rate of photosynthesis, and growth characteristics of maize were reported with the application of SA. Hussein et al. (2007) reported higher values for all growth characteristics of wheat plants under salinity stress for which SA had been applied to the foliage; moreover, the plants treated with SA showed much higher proline levels under salinity stress. Eraslan et al. (2007) surveyed the effect of exogenous application of SA on carrots under the combined stress of boron toxicity and salinity, finding that SA significantly increased the activity of antioxidants in the roots and improved anthocyanin and carotenoid contents in line with higher root dry matter and overall growth of carrots compared plants not treated with SA. They also found that the application of SA enhanced proline accumulation both in root and shoot. The application of higher concentrations of SA have been shown to inhibit bud formation in Funariahy gromatica, indicating the plants’ sensitivity to high concentrations of SA (Christianson and Duffy 2002). Hayat et al. (2008) surveyed tomato plants (L. esculentum) were surveyed under drought-stress conditions and the application of SA, finding that high concentrations of SA led to significant declines in the leaf water potential, membrane stability index, photosynthesis-related traits, and RWC, significant increases in enzymatic antioxidant activities (SOD, POX, and CAT). However, this study showed that application of particular concentrations of SA increased other parameters in addition to enzymatic antioxidant activity. Since drought stress induces the production of ROS in plants, leading to oxidative stress, managing the stress through the application of related components can increase the plants’ ability to cope with the stress, improving their stability. In the current study the generation of H2O2 and MDA as harmful components under drought stress was significantly reduced with the application of SA, and enzymatic antioxidant activity increased. These results indicate that SA is an effect substance to apply to strawberry plants that are under drought stress. Our results are supported by Knörzer et al. (1999), who explained that the application of suitable concentrations of SA can improve the antioxidant system efficiency in plants.
Plants require nutrients for their growth, development, and reproduction (including the production of fruit and seeds). These elements can be divided into macronutrients and micronutrients. Even though micronutrients are required in slight quantities for plant production and growth, their deficiency can trigger large disruptions in plants’ morpho-physiological and biochemical traits and metabolic processes. Mn, Fe, Cu, Zn, B and Mo The micronutrients manganese, iron, copper, zinc, boron, and molybdenum have been shown to be necessary for plants’ health (Mortvedt 1991). Iron deficiency is a widespread agricultural problem in many crops, especially in calcareous soils. In these soils, total iron is high but occurs in chemical forms not available to plant roots (Lindsay and Schwab 1982). In this situation the application of iron in the form of nanoparticles might be a way to compensate for this problem. Environmental stresses, particularly drought and salinity, as affect the uptake of some micronutrients such as iron, and disturb their absorption by the roots, leading to severe problem in plant health. The response of plants to iron inadequacy, in particular, prompts a series of morpho-physiological changes in the roots to enable the use of soluble iron compounds in the rhizosphere. Iron nanoparticles are a newly produced compound that can easily be absorbed by plant roots, readily compensating for drought- and salinity-induced iron deficiency. The results of the current study on the effects of iron nanoparticles on strawberry plantlets under drought-stress conditions indicate that all growth and morphological parameters measured in this study were higher in plantlets treated with iron nanoparticles regardless of the presence or degree of drought stress. However, the influence of SA was clearly higher under drought stress, which suggests that it can be used to compensate for stressful conditions. The root length of the strawberry plantlets under drought stress with the application of iron nanoparticles was greater than the control. These morphological adjustments in the roots might be the result of iron nanoparticles’ higher capability to interact with root hairs, thus enabling the root to seek more water as it grows longer. Higher root growth leads to higher intake of water; correspondingly, in the current study the plantlets treated with iron nanoparticles had a higher RWC than the control plantlets. The application of iron nanoparticles also enhanced the plantlets’ levels of pigment, carbohydrates, proline, and protein. The application of iron nanoparticles improved the plantlets’ membrane stability index, which might be the result of higher activity for the enzymatic antioxidants SOD and POD, and a correspondingly lower concentration of H2O2. Sharma et al. (1996) found that iron has an essential role in plant metabolism, such as activating catalase enzymes associated with SOD, as well as in photorespiration, glycolate metabolism, and chlorophyll synthesis. When iron is applied in the form of nanoparticles, it can be very useful in enabling strawberries to readily absorb required quantities of iron and increase their stability under conditions of drought stress. Said-Al Ahl and Mahmoud (2010) have stated that plant yield on many soils is limited by poor iron availability, rather than a low iron content in the soil. Therefore, the application of iron nanoparticles could be an appropriate strategy to enhance the absorption of iron through the plants’ roots. Ghasemi et al. (2010) reported that, in most of the cases, the application of iron nanoparticles has substantial positive effects on the biochemical composition and growth parameters of soybean. Abountiolas (2016) highlighted the importance of adjusting the concentration of micronutrients to cope with stresses under both in vitro and in vivo conditions. This study stressed that micronutrients, particularly iron, are major components of in vitro culture, and controlling the level of micronutrients is much more important in in vitro culture than in vivo. Organs and tissues of the plant grown in vitro with an artificial medium should be supported by the nutrients essential for their growth. The use of tissue culture as a tool for plant propagation is greatly influenced by the content of the medium, including its micronutrient levels. Potassium, nitrogen, phosphorus, calcium, sulphur, and magnesium are are required in large amounts in tissue culture, and are thus called major plant nutrients. Some other elements, termed minor plant nutrients, include iron, copper, nickel, chlorine, zinc, molybdenum, boron, and manganese (Mn) (George et al. 2008b). Although iron is considered to be a micronutrient for plant growth, in the current study it showed a great influence on growth parameters and other measured traits compared to control plants. These results indicate that the efficiency of tissue culture and in vitro cultivation of strawberries could be improved, even under drought-stress conditions, by increased application of iron in the form of nanoparticles.
Conclusion
Drought stress significantly affected all measured parameters of strawberry plantlets under in vitro cultivation. The negative impacts of drought resulted in stunted growth and production of fruit and decreased pigment levels, RWC, and MSI, but increased enzymatic antioxidant activity, proline content, protein content, and carbohydrate content. SA as a plant-growth regulator could effectively compensate for the negative effects of drought stress on strawberries and improve the growth parameters of the plantlets. This study’s results indicate that SA is an appropriate component to be used in in vitro conditions to compensate for the negative effects of drought stress on strawberries. Furthermore, since optimizing plant nutritional content is very important in the production of high yield with high quality in micro-propagation culture and media, iron in form of nanoparticles could be a suitable strategy. Strawberry plantlets treated with iron nanoparticles added to MS culture could cope with stressful conditions better than untreated plantlets. Iron in the form of nanoparticles can be a very useful method to allow strawberry plants to readily absorb required quantities of iron and to increase the plants’ stability under drought stress. The application of both SA and iron nanoparticles improved the quantity and quality of the strawberry plantlets’ morphological and growth-related characteristics under in vitro culture and compensated for the negative effects of drought stress. It is recommended that they be used together under this situation in in vitro strawberry cultivation.
References
Abountiolas M (2016) In vitro and in vivo antioxidant capacity of synthetic and natural polyphenolic compounds identified from strawberry and fruit juices. Dissertation, University of South Florida. http://scholarcommons.usf.edu/etd/6057 Accessed 9 Mar 2016
Ahmad R, Tripathi AK, Tripathi P, Singh S, Singh R, Singh RK (2008) Malondialdehyde and protein carbonyl as biomarkers for oxidative stress and disease progression in patients with chronic myeloid leukemia. In Vivo 22(4):525–528
Bates L, Waldren R, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39(1):205–207
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44(1):276–287
Bhat S (2014) Studies on in vitro induced mutations in strawberry (Fragaria X ananassa Duch.). Dissertation, CCSHAU. KrishiKosh. Chaudhary Charan Singh Haryana Agricultural University. http://krishikosh.egranth.ac.in/bitstream/1/77012/1/CCSHAU-293729-Bhat%2C%20Sandhya.pdf
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. J Anal Biochem 72(1–2):248–254
Chen Z, Chen M, Jiang M (2016) Hydrogen sulfide alleviates mercury toxicity by sequestering it in roots or regulating reactive oxygen species productions in rice seedlings. Plant Physiol Biochem 111:179–192
Christianson ML, Duffy SH (2002) Dose-dependent effect of salicylates in a moss, Funaria hygrometrica. J Plant Growth Regul 21(3):200–208
de Ribou SB, Douama F, Hamanta O, Frohlich MW, Negrutiua I (2013) Plant science and agricultural productivity: why are we hitting the yield ceiling? Plant Sci 210:159–176
Diengngan S, Mahadevamma M, Murthy B (2016) Efficacy of in vitro propagation and crown sizes on the performance of strawberry (Fragaria × ananassa Duch) cv. festival under field condition. J Agric Sci Technol 18(1):255–264
Eraslan F, Inal A, Gunes A, Alpaslan M (2007) Impact of exogenous salicylic acid on the growth, antioxidant activity and physiology of carrot plants subjected to combined salinity and boron toxicity. Sci Hortic 113(2):120–128. https://doi.org/10.1016/j.scienta.2007.03.012
Fariduddin Q, Hayat S, Ahmad A (2003) Salicylic acid influences net photosynthetic rate, carboxylation efficiency, nitrate reductase activity, and seed yield in Brassica juncea. Photosynthetica 41(2):281–284. https://doi.org/10.1023/B:PHOT.0000011962.05991.6c
Flohé L, Günzler WA (1984) [12] Assays of glutathione peroxidase. Methods Enzymol 105:114–120. https://doi.org/10.1016/S0076-6879(84)05015-1
George EF, Hall MA, De Klerk G-J (2008b) The components of plant tissue culture media I: macro-and micro-nutrients. In Plant propagation by tissue culture. 3rd edn. Springer, Dordrecht, pp 65–113
Ghaderi N, Normohammadi S, Javadi T (2015) Morpho-physiological responses of strawberry (Fragaria × ananassa) to exogenous salicylic acid application under drought stress. J Agric Sci Technol 17(1):167–178
Ghasemi R, Zarei M, Torki M (2010) Adding medicinal herbs including garlic (Allium sativum) and thyme (Thymus vulgaris) to diet of laying hens and evaluating productive performance and egg quality characteristics. Am J Anim Vet Sci 5(2):151–154
Gupta R, Wall T, Baxter L (2007) Impact of mineral impurities in solid fuel combustion. Springer, New York
Hayat S, Fariduddin Q, Ali B, Ahmad A (2005) Effect of salicylic acid on growth and enzyme activities of wheat seedlings. Acta Agron Hung 53(4):433–437. https://doi.org/10.1556/AAgr.53.2005.4.9
Hayat S, Hasan SA, Fariduddin Q, Ahmad A (2008) Growth of tomato (Lycopersicon esculentum) in response to salicylic acid under water stress. J Plant Interact 3(4):297–304
Hayat Q, Hayat S, Irfan M, Ahmad A (2010) Effect of exogenous salicylic acid under changing environment: a review. Environ Exp Bot 68(1):14–25. https://doi.org/10.1016/j.envexpbot.2009.08.005
He Y, Liu Y, Cao W, Huai M, Xu B, Huang B (2005) Effects of salicylic acid on heat tolerance associated with antioxidant metabolism in Kentucky bluegrass. Crop Sci 45(3):988–995. https://doi.org/10.2135/cropsci2003.0678
Hossain MA, Bhattacharjee S, Armin S-M, Qian P, Xin W, Li H-Y, Burritt DJ, Fujita M, Tran L-SP (2015) Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: insights from ROS detoxification and scavenging. Front Plant Sci 6:420. https://doi.org/10.3389/fpls.2015.00420
Hossain MA, Burritt DJ, Fujita M (2016) Cross-stress tolerance in plants: molecular mechanisms and possible involvement of reactive oxygen species and methylglyoxal detoxification systems. In Abiotic stress response in plantsm, Wiley, Weinheim, pp 323–375
Hussein M, Balbaa L, Gaballah M (2007) Salicylic acid and salinity effects on growth of maize plants. Res J Agric Biol Sci 3(4):321–328
Jamali B, Eshghi S, Tafazoli E (2011) Vegetative and reproductive growth of strawberry plants cv.‘Pajaro’affected by salicylic acid and nickel. J Agric Sci Technol 13:895–904
Karimi S, Yadollahi A, Nazari-Moghadam R, Imani A, Arzani K (2012) In vitro screening of almond (Prunus dulcis (Mill.)) genotypes for drought tolerance. J Biol Environ Sci 6(18):263–270
Khan W, Prithiviraj B, Smith DL (2003) Photosynthetic responses of corn and soybean to foliar application of salicylates. J Plant Physiol 160(5):485–492. https://doi.org/10.1078/0176-1617-00865
Khodary S (2004) Effect of salicylic acid on the growth, photosynthesis and carbohydrate metabolism in salt-stressed maize plants. Int J Agric Biol 6(1):5–8
Knörzer OC, Lederer B, Durner J, Böger P (1999) Antioxidative defense activation in soybean cells. Physiol Plant 107(3):294–302. https://doi.org/10.1034/j.1399-3054.1999.100306.x
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: measurement and characterization by UV–VIS spectroscopy. Curr Protoc Food Anal Chem. https://doi.org/10.1002/0471142913.faf0403s01
Lindsay W, Schwab A (1982) The chemistry of iron in soils and its availability to plants. J Plant Nutr 5(4–7):821–840. https://doi.org/10.1080/01904168209363012
Maciel R, Sant’Anna G, Dezotti M (2004) Phenol removal from high salinity effluents using Fenton’s reagent and photo-Fenton reactions. Chemosphere 57(7):711–719. https://doi.org/10.1016/j.chemosphere.2004.07.032
Malik B, Pirzadah TB, Tahir I, Rehman RU, Hakeem KR, Abdin M (2014) Plant signaling: response to reactive oxygen species. In Plant signaling: understanding the molecular crosstalk. Springer, New Delhi, pp 1–38
Mortvedt JJ (1991) Micronutrient fertilizer technology. In Micronutrients in agriculture, 2rd edn. SSSA Book Series, Madison, WI, pp 523–548
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol Plant 15:473–497
Naik P, Al-Khayri J (2016) Impact of abiotic elicitors on in vitro production of plant secondary metabolites: a review. J Adv Res Biotech 1(2):7
Natsheh B, Abu-Khalaf N, Mousa S (2015) Strawberry (Fragaria ananassa Duch.) plant productivity quality in relation to soil depth and water requirements. Int J Plant Res 5(1):1–6. https://doi.org/10.5923/j.plant.20150501.01
Nimir NE, Guisheng Z, Guo W-S, Ma B, Shiyuan L, Yonghui W (2016) Effect of foliar application of ga3, kinetin, and salicylic acid on ions content, membrane permeability and photosynthesis under salt stress of sweet sorghum. Can J Plant Sci. https://doi.org/10.1139/CJPS-2016-0110
Palei S, Das AK, Rout GR (2015) In vitro studies of strawberry-an important fruit crop: a review. J Plant Sci Res 31(2):115–131
Parvin S, Javadi T, Ghaderi N (2015) Proline, protein, rwc and msi contents affected by paclobutrazol and water deficit treatments in strawberry cv. Paros Cercetări Agronomice în Moldova 1(161):107–114. https://doi.org/10.1515/cerce-2015-0022
Pick E, Mizel D (1981) Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J Immunol Methods 46(2):211–226. https://doi.org/10.1016/0022-1759(81)90138-1
Pirasteh-Anosheh H, Saed-Moucheshi A, Pakniyat H, Pessarakli M (2016) Stomatal responses to drought stress. In Water stress and crop plants: a sustainable approach. Vol. 2, Wiley, Chichester, pp 24–40
Ray PD, Huang B-W, Tsuji Y (2012) Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 24(5):981–990. https://doi.org/10.1016/j.cellsig.2012.01.008
Saed-Moucheshi A, Pakniyat H, Pirasteh-Anosheh H, Azooz M (2014a) Role of ROS as signaling molecules in plants. In Ahmad P. (ed) Reactive oxygen species, antioxidant network and signaling in plants. Springer, New York, pp 585–626
Saed-Moucheshi A, Shekoofa A, Pessarakli M (2014b) Reactive oxygen species (ROS) generation and detoxifying in plants. J Plant Nutr 37(10):1573–1585. https://doi.org/10.1080/01904167.2013.868483
Saed-Moucheshi A, Hasheminasab H, Khaledian Z, Pessarakli M (2015) Exploring morpho-physiological relationships among drought resistance related traits in wheat genotypes using multivariate techniques. J Plant Nutr 38(13):2077–2095. https://doi.org/10.1080/01904167.2015.1009099
Said-Al Ahl H, Mahmoud AA (2010) Effect of zinc and/or iron foliar application on growth and essential oil of sweet basil (Ocimum basilicum L.) under salt stress. Ozean J Appl Sci 3(1):97–111
Sairam R (1994) Effects of homobrassinolide application on plant metabolism and grain yield under irrigated and moisture-stress conditions of two wheat varieties. Plant Growth Regul 4(2):173–181. https://doi.org/10.1007/BF00025220
Seemann JR, Critchley C (1985) Effects of salt stress on the growth, ion content, stomatal behaviour and photosynthetic capacity of a salt-sensitive species, Phaseolus vulgaris L. Planta 164(2):151–162. https://doi.org/10.1007/BF00396077
Shakirova F (2007) Role of hormonal system in the manifestation of growth promoting and antistress action of salicylic acid Salicylic acid a plant hormone. In Hayat S & Ahmad A (eds) Salicyclic acid—a plant hormaone. Springer, Dordrecht, pp 69–89
Shakirova F, Allagulova CR, Maslennikova D, Klyuchnikova E, Avalbaev A, Bezrukova M (2016) Salicylic acid-induced protection against cadmium toxicity in wheat plants. Environ Exp Bot 122:19–28. https://doi.org/10.1016/j.envexpbot.2015.08.002
Sharma YK, León J, Raskin I, Davis KR (1996) Ozone-induced responses in Arabidopsis thaliana: the role of salicylic acid in the accumulation of defense-related transcripts and induced resistance. Proc Natl Acad Sci 93(10):5099–5104
Sowik I, Borkowska B, Markiewicz M (2016) The activity of mycorrhizal symbiosis in suppressing Verticillium wilt in susceptible and tolerant strawberry (Fragaria x ananassa Duch.) genotypes. Appl Soil Ecol 101:152–164. https://doi.org/10.1016/j.apsoil.2016.01.021
Sun C, Wang D, Hu Y, Li X, Zhang W, Sun J, Gao X (2013) Effects of salicylic acid on physiological characteristics of strawberry leaves under drought stress. Eur J Hortic Sci 78(3):106–111
Taiz L, Zeiger E, Møller IM, Murphy A (2015) Plant physiology and development. Sinauer Associates, Incorporated, Sinauer Associates Incorporated, Sunderland
Tang X, Zhao C, Wen G, Wang W, Wang C, Sun Y, Bai X (2014) Physiological mechanism for anthocyanins to strengthen the drought tolerance of plants. Agric Sci Technol 15(11):1935–1941
Tarafdar J, Rathore I, Thomas E (2015) Enhancing nutrient use efficiency through nano technological interventions. Indian J Fertil 11(12):46–51
Todaka D, Shinozaki K, Yamaguchi-Shinozaki K (2015) Recent advances in the dissection of drought-stress regulatory networks and strategies for development of drought-tolerant transgenic rice plants. Front Plant Sci 6:84. https://doi.org/10.3389/fpls.2015.00084
Uauy C, Distelfeld A, Fahima T, Blechl A, Dubcovsky J (2006) A NAC gene regulating senescence improves grain protein, zinc, and iron content in wheat. Science 314(5803):1298–1301. https://doi.org/10.1126/science.1133649
Waheed A-M, Madi A (2016) Influence of salicylic acid (SA) and ascorbic acid (ASA) on’in vitro’propagation and salt tolerance of date palm (‘Phoenix dactylifera’ L.) cv.’Nersy’. Aust J Crop Sci 10(7):969–976
Ying Y, Yue Y, Huang X, Wang H, Mei L, Yu W, Zheng B, Wu J (2013) Salicylic acid induces physiological and biochemical changes in three Red bayberry (Myric rubra) genotypes under water stress. Plant Growth Regul 71(2):181–189. https://doi.org/10.1007/s10725-013-9818-3
Funding
This study was funded by University of Kurdistan (Grant Number 4.14363).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Henryk Flachowsky.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mozafari, A., Havas, F. & Ghaderi, N. Application of iron nanoparticles and salicylic acid in in vitro culture of strawberries (Fragaria × ananassa Duch.) to cope with drought stress. Plant Cell Tiss Organ Cult 132, 511–523 (2018). https://doi.org/10.1007/s11240-017-1347-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-017-1347-8