Abstract
In the present study, phycochemical-loaded silver nanocomplexes (GFAgNPs) were fabricated by using Gracilaria foliifera seaweed extracts as capping agents on silver ions. The growth-stimulating properties of the GFAgNPs were evaluated by using in vitro plant regeneration from axillary nodal explants of Alternanthera sesselis. Explants were sterilized and placed on Murashige and Skoog (MS) medium augmented with various concentrations of GFAgNPs (0.5–3.0 mg L−1), 6-benzyl amino purine (BAP), and kinetin (KIN) (0.5–5.0 mg L−1) for initiation of shoot buds. One hundred percent shoot bud initiation with highest number of shoot buds (112.5 shoots explant−1) was observed on MS medium supplemented with 2.0 mg L−1 GFAgNPs, while shoot bud initiation was 85.3 % with 3.0 mg L−1 BAP. To enhance the percentage of multiple shoot bud proliferation, the mini-shoot buds were subcultured onto MS medium fortified with different doses of GFAgNPs (0.5–3.0 mg L−1), BAP, and KIN (0.5–4.0 mg L−1) in combination with 50 mg L−1 adenine sulfate (AdS). Of the three growth-promoting compounds tested, 100 % of multiple shoot bud regeneration with twofold increased shoots (153.6 shoots culture−1) was obtained on MS medium containing 2.5 mg L−1 GEAgNPs and 50 mg L−1 AdS combination, followed by 3.0 mg L−1 BAP and 50 mg L−1 AdS combination. Elongated shoots were excised from shoot clumps and cultured on half-strength MS medium without auxin but fortified with different concentrations of α-naphthalene acetic acid (NAA) and indole-3-butyric acid (IBA) (0.1–0.5 mg L−1) for rooting. IBA at 0.5 mg L−1 was found to be the best dose for 100 % rooting with 13.5 roots shoot−1. Rooted plantlets were successfully transplanted into plastic cups containing sand and soil mixture, and the acclimatized plantlets were subsequently established in field conditions. The genetic fidelity of in vitro regenerated plants was determined by using random amplified polymorphic DNA (RAPD) analysis. In vitro regenerated plantlets were true to type in nature. The use of phycochemical-coated silver nanocomplex as novel growth-regulating substances was identified as an alternate to commercial cytokinins for large-scale production of genetically uniform plantlets in tissue culture for industrial applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Alternanthera sessilis L. (sessile joyweed), Amarantheceae, is a leafy vegetable found in both wetlands and drylands, and has the ability to grow on a variety of soils. It is distributed in tropical America, Africa, and in temperate Asia. According to WHO, medicinal plants provide a good source to obtain a variety of drugs. In recent years, pharmaceutical companies have invested considerable funds toward developing new medicines by using natural products extracted from herbal plants (Ben Sassi et al. 2007; Coruh et al. 2007). In Alternanthera species, the green leaves are considered as a main source of vitamins, minerals, and fibers for the consumers (Gayathri et al. 2006). Alternanthera sessilis has many medicinal properties, and its leaves and young shoots are consumed in food dishes (Chandrika et al. 2006). This medicinal plant species is used to treat nausea, vomiting, cough, bronchitis, diarrhea, dysentery, diabetes, wounds, and flatulence, anemia, hypertension, and night blindness (Acharya and Pokhrel 2006). Recently, silver nanoparticles were synthesized by using leaf extracts of A. sessilis, and biomolecule-coated nanoparticles showed efficient cytotoxic activity against prostate cancer cells (PC3) and breast cancer cells (MCF-7) (Firdhouse and Lalitha 2013). Therefore, A. sessilis is being exploited for pharmaceutical applications and requires large-scale production to meet the demand.
Plant tissue culture techniques are used for clonal multiplication and in vitro conservation of valuable indigenous germplasm threatened with extinction. High demand for A. sessilis has led to its rapid depletion from its primary habitats. Therefore, in vitro propagation is an alternative crop improvement tool for large-scale multiplication and may increase the number of propagules for cultivation as well as the replacement of natural populations (Johnson et al. 2007; Kumaraswamy and Anuradha 2010). Although there are limited reports on plant regeneration from A. sessilis by using shoot tip explants (Gnanaraj et al. 2011), nodal segments (Gnanaraj et al. 2011), and leaf callus (Singh et al. 2009), the percent of shoot bud multiplication was low. Therefore, the development of a cost-effective plant regeneration protocol for large-scale production of uniform seedlings through micropropagation by using alternate natural plant growth stimulators may reduce the cost substantially. The production of genetically stable in vitro plants similar to the mother plant (explant donor) is also one of the prerequisites for commercial applications (Bhatia et al. 2011).
Natural plant growth stimulators such as cytokinins, auxins, abscisic acid, ethylene, and gibberellins are present in seaweed extracts and modulate cellular metabolism in crop plants, leading to enhanced plant growth (Stirk et al. 2004; Khanet al. 2009; Kumar and Sahoo 2011). It is reported that natural growth-regulating phycomolecules can stimulate seed germination, level of photosynthetic pigment contents, and seedling growth rate and yield in various agricultural crops (Stirk et al. 2004; Khan et al. 2009). Seaweed extracts also may influence in vitro callus induction and plant regeneration via organogenesis and somatic embryogenesis. For example, high-frequency plant regeneration via organogenesis was achieved by using extracts from various cyanobacteria and seaweeds (Nostoc ellipsosporum, Dolichospermum flos-aquae, Oscillatoria acuminata, Gracilaria edulis, Gracilaria salicornia, Sargassum wightii, Padina gymnospora, Padina boergesenii, Gelidiella acerosa) in cotton, tomato, and brinjal (Vinoth et al. 2012; Gurusaravanan et al. 2013; Satish et al. 2015, 2016), and efficient somatic embryogenesis and enhanced percent of plant regeneration were recorded on medium containing different doses of seaweed extracts (Caulerpa scalpelliformis, Gracilaria corticata, G. edulis, and P. boergesenii) in tomato and finger millet (Vinoth et al. 2014; Sathish et al. 2015).
Silver nitrate (AgNO3) is a potential ethylene inhibitor which plays a pivotal role on influencing somatic embryogenesis and efficient shoot bud regeneration as well as root formation by inhibiting ethylene biosynthesis and greatly enhances plant regeneration rates in both monocotyledonous and dicotyledonous plants (Giridhar et al. 2003; Kumar et al. 2009). Therefore, establishment of an efficient plant regeneration protocol for mass propagation of genetically uniform plants via tissue culture by using phycomolecule-coated silver nanoparticles as growth-stimulating agents may assist large-scale production of plants at a low cost. Although, the effects of seaweed extracts and silver nitrate has been studied individually on plant tissue culture, the role of phycomolecule-loaded silver nanoparticles on plant regeneration has not been investigated (Sathish et al. 2015). In addition, the possibility of AgNPs causing somoclonal variation needs to be investigated.
The aims of the present study were to determine the potential role of fabricated phycomolecule-loaded silver nanoparticles made using red algal Gracilaria foliifera (Forssk.) Boergs. extracts on plant regeneration from axillary nodal explants of A. sessilis and to detect the genetic fidelity of the regenerated plants.
Materials and methods
Collection and preparation of algal extract
The marine red alga Gracilaria foliifera was collected from the Mandapam coastal area (lat. 09° 17′ N; long. 79° 08′ E), Palk Bay, Tamil Nadu, India, washed several times with running tap water to remove impurities and dried in the shade for 2 weeks. The dried algal sample was ground into fine powder by using a blender and used for nanoparticle synthesis. The algal powder (50 g) was dissolved in 500 mL distilled water and mixed thoroughly at room temperature. Then, it was boiled under microwave irradiation for 10 min, and the extract was filtered through Whatman No. 1 filter paper. Subsequently, the aqueous algal extract was used as the bioreducing and capping agent for synthesis of nanocomplexes.
Synthesis and characterization of phycomolecule-coated silver nanoparticles
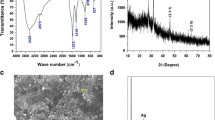
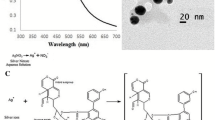
The synthesis of silver nanoparticles was performed by mixing G. foliifera (GF) algal extract with 5 mM aqueous silver nitrate solution (AgNO3) in 1:9 ratio and incubating in the dark overnight at room temperature. The bioreduction of silver ions was monitored in the wavelength range from 400 to 700 nm by using UV-Vis spectrophotometer (Shimadzu, Japan). The reaction mixtures were transferred into sterile tubes and centrifuged at 8000 rpm for 10 min. The nanoparticle pellets were collected and washed several times with sterile distilled water to remove impurities. For characterization, the synthesized nanoparticles were dried at 60 °C for 24 h. The dried samples were compressed into a thin KBr disk and Fourier transform infrared spectroscopy (FTIR PerkinElmer spectrum RXI); spectra were recorded within a range of 4000 to 400 cm−1 in the transmittance mode. The XRD spectrum (Advanced Power X-ray Diffractometer, Brucker, Germany) of 2θ values were recorded ranging from 20 to 60. The structure and shape of the phycochemical-coated silver nanoparticles were determined by using field emission scanning electron microscopy (FESEM, Carl Zeiss ultra 55 model) attached with energy-dispersive X-ray (EDX) spectra analysis.
Plant material and explant preparation
Axillary nodal explants were collected from Alternanthera sessilis plants grown in the Medicinal Plants Garden, Department of Biotechnology, Periyar University, Salem, and used for initiation of in vitro cultures. The selected healthy explants were surface cleaned with Bavistin (carbondazin fungicide) for 10 min to avoid fungal contamination and washed in running tap water for 10 min. Subsequently, they were disinfected with 10 % (v/v) Tween 20 (commercial surfactant) for 20 min, followed by thorough washing under running water for 10 min. Finally, explants were sterilized with 0.1 % (w/v) mercuric chloride solution for 8 min and thoroughly washed with sterile distilled water several times inside the laminar airflow chamber under aseptic conditions.
Media preparation and culture conditions
The culture medium for shoot bud initiation, multiple shoot bud development, and rooting used was Murashige and Skoog (MS) (Murashige and Skoog 1962) basal salts with sucrose 3 % (w/v) as carbon source. After adding suitable concentrations of different growth regulators such as 6-benzyl amino purine (BAP), kinetin (KIN) (0.5–5.0 mg L−1), α-naphthalene acetic acid (NAA), indole-3-butyric acid (IBA) (0.1–0.5 mg L−1), and phycomolecule-coated silver nanoparticles (GFAgNPs) (0.5–3.0 mg L−1), the pH of the medium was adjusted to 5.8 with 0.1 N NaOH or HCl prior to adding 8.0 g L−1 agar. The molten medium (20 mL) was dispensed into culture tubes, plugged tightly with non-absorbent cotton and autoclaved at 121 °C for 20 min.
All the cultures were maintained under 16/8-h light/dark photoperiod with a light intensity of 60 μmol photons m−2 s−1 (cool white fluorescent lights) and maintained in the controlled environment at 25 ± 2 °C.
Shoot bud initiation
Axillary nodal explants (>1.0 cm in length) were cultured on MS medium supplemented with different concentrations of two cytokinins (BAP and KIN, 0.5–5.0 mg L−1) and GFAgNPs (0.5–3.0 mg L−1) individually for shoot bud initiation. The shoot buds emerged directly from the axillary node explants and were further subcultured onto the same fresh medium for further growth and proliferation at 2-week intervals. After two subcultures, they were used for multiple shoot bud proliferation.
Multiple shoot bud proliferation and development
In order to achieve multiple shoot bud development, the initiated shoot buds were excised and cultured onto fresh shoot bud multiplication medium fortified with different concentrations of two cytokinins (BAP and KIN, 0.5–4.0 mg L−1) and GFAgNPs (0.5–3.0 mg L−1) in combination with 50 mg L−1 adenine sulfate (AdS). The shoot buds were subcultured onto the same fresh media combinations for further multiple shoot bud proliferation and development at 2-week intervals. This cycle was repeated for four times (each 14 days) to determine the effect of subculture on the same medium compositions for production of large number of multiple shoots as well as shoot bud elongation.
In vitro rooting and acclimatization
Elongated shoots (>2.0 cm) were excised from the multiple shoot clumps and cultured onto half-strength MS medium supplemented without and/or with various doses of two auxins NAA and IBA (0.1–0.5 mg L−1) for root initiation. After 4 weeks of culture, shoots with well-developed roots were carefully recovered from the culture tubes, without causing any damage to the root system, and the plantlets were gently washed under running tap water to remove the traces of agar adhering to the roots. Then, the plantlets were transferred to plastic cups containing sand and soil in the ratio 1:2 and covered with a polyethylene bags to maintain high humidity and kept in the culture room under 16/8-h light/dark photoperiod with a light intensity of 60 μmol photons m−2 s−1 (cool white fluorescent lights) and maintained in a controlled environment at 25 ± 2 °C. Plantlets were watered at 2-day interval. After 15 days, the plastic bags were gently removed and the plantlets were allowed to grow in greenhouse conditions for 7 days under 14/10-h light/dark photoperiod with a light intensity of 80 μmol photons m−2 s−1 and maintained at 28 ± 2 °C. The acclimatized plantlets were then transferred to the field conditions for further growth and development.
Isolation of genomic DNA and PCR amplification
In order to confirm the genetic fidelity, a total of 10 regenerated plantlets which were acclimatized in the soil were selected randomly and used for DNA fingerprinting analysis by random amplified polymorphic DNA (RAPD)-PCR technology along with a control mother plant. Total genomic DNA was isolated by cetyltrimethylammonium bromide (CTAB) method (Doyle and Doyle 1990). Fresh leaves (100 mg) were homogenized with 1.0 mL 2× CTAB buffer [2 % (w/v) hexadecyltrimethylammonium ammonium bromide, 1.4 M NaCl, 20 mM EDTA (pH 8.0), 0.1 M Tris-HCl (pH 8.0), 1 % (w/v) polyvinylpolypyrolidone (PVPP), 1.0 % (w/v) β-mercaptoethanol] and incubated in a hot water bath at 65 °C for 20 min. Equal volume of phenol and chloroform mixture was added and centrifuged at 8000 rpm for 10 min. The aqueous phase was transferred into a new sterile microfuge tube and re-extracted with an equal volume of chloroform and centrifuged at 8000 rpm for 10 min. The top aqueous phase was aliquoted into a new microfuge tube, and 0.6 mL 100 % ice-cold isopropyl alcohol was added and mixed thoroughly for DNA precipitation. After centrifugation at 10,000 rpm for 10 min under 4 °C, the DNA pellet was collected and washed with 70 % (v/v) ethanol. Then, the DNA pellet was air dried and dissolved in sterile distilled water (100 μL). The DNA quality was assessed on agarose gel electrophoresis (1 % w/v), and the purity as well as concentration was determined by using UV-Vis spectroscopy before using DNA for PCR amplification reactions.
For initial PCR screening of DNA samples, a total of 100 random decamer primers (Operon Technology Inc., USA) were used. PCR amplification reaction was carried out in a total volume of 20 μL containing 2 μL of 10× PCR buffer (10 mM Tris HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2), 2 μL of 1.5 mM dNTPs (dATP, dGTP, dCTP, and dTTP), 1 μL of 250 nM primers, 0.5 units of Taq DNA polymerase, 2 μL genomic DNA (15 ng), and 13 μL sterile distilled water. DNA amplification reaction was performed in a PCR thermal cycler (Cyber Lab, USA) by using the following amplification profile: initial denaturation at 94 °C for 4 min, followed by 40 cycles consisted of denaturation at 94 °C for 1 min, annealing at 37 °C for 1.5 min, extension at 72 °C for 2 min, and a final extension step at 72 °C for 7 min. After completion of PCR cycles, DNA amplicons were stored at −20 °C until further analysis.
Electrophoretic analysis of PCR-amplified products
The PCR amplicons were mixed with DNA loading buffer and analyzed on 1.5 % (w/v) agarose gel containing 0.5 μg mL−1 ethidium bromide in 1× TAE buffer. Electrophoresis was performed at 50 V for 2 h until the bromophenol blue dye front migrated to the bottom of the gel. The molecular weight marker used was the lambda DNA double digested by EcoRI/HindIII. The amplified DNA bands were visualized by placing the agarose gel under UV transilluminator, and images were captured with gel documentation system (Alpha Imager Gel Documentation System, USA).
Statistical analysis
All experiments were carried out in a completely randomized block (CRB) design, and each experiment had ten replicates and repeated three times. The cultures were visually observed, and the data on percent shoot bud initiation, multiple shoot bud development, and percentage rooting were recorded periodically. The number of shoots and roots was counted visually. Analysis of variance (ANOVA) was performed by using SAS program (SAS Institute 1989). The differences among means were analyzed by Student-Newman-Keuls test at 5 % significance level.
Results
Fabrication and characterization of phycomolecule-loaded silver nanocomplex
During the synthesis of the silver nanoparticles, the reaction mixture color changed from yellow to reddish brown indicating the formation of GFAgNPs. The functional groups involved in the bioreduction of silver ions were also determined by using FTIR analysis. The FTIR spectrum exhibited strong transmittance peaks at 3405 cm−l representing the O–H stretch for strong carboxylic acids, 2908 cm−l for C–H strong stretch assigned alkane group, 2431 cm−l for N–H stretching amines, 1631 cm−l representing C=O strong stretch representing amides, 1411 cm−l for C=C strong stretch assigned to aromatic group, 1004 cm−l for C–H assigned to strong alkanes, and 600 cm−l for C–F stretch representing strong alkyl halides (Fig. 1a). The XRD results show strong intense peaks at 38 and 44 corresponding to Bragg diffraction 111 and 200 lattice plane (Fig. 1b) and confirm the crystalline nature of silver ions. FESEM results showed that the shape of the silver nanoparticles was hexagonal with the range of 2 to 74 nm in size (Fig. 1c). The EDX analysis of GFAgNPs showed a strong peak at 2.4 and 3 keV indicating the presence of a silver element that may be formed due to the surface plasma resonance effect of AgNPs. Some weak peaks of calcium, iron, and magnesium elements were also detected (Fig. 1d).
Characterization of synthesized phycochemical-loaded silver nanocomplex (GFAgNPs) using Gracilaria foliifera seaweed extracts. a FTIR spectral analysis of the synthesized GFAgNPs. b XRD pattern of the phycosynthesized GFAgNPs. c FESEM image of the fabricated GFAgNPs. d EDX spectrum of the synthesized GFAgNPs exhibited the strong signal of silver ion
Initiation of shoot buds
In the present study, an efficient protocol for high-frequency plant regeneration from axillary nodal explants obtained from field grown A. sessilis plants was established. Shoot bud initiation occurred in the presence of growth regulators after 2 weeks of culture (Fig. 2a). Subculturing at 2-week intervals enhanced the percent of shoot bud initiation as well as the number of shoot buds. The percent of response varied depending on the type and concentration of growth regulator (nanoparticles and cytokinins) used in the medium. The frequency of shoot bud initiation ranged from 29.2 to 100 % [shoot bud development was observed in all the cultures at specific doses (100 %)], and the number of shoots was in the range of 17.5 to 112.5 shoots explant−1 after four subcultures (Table 1). Among the BAP concentrations tested, the highest shoot bud regeneration (85.3 %) with 47.0 shoots explant−1 was obtained on MS medium containing 3.0 mg L−1 BAP, while the lowest shoot bud initiation (29.26 %) was obtained on MS medium fortified with 0.5 mg L−1 BAP (Table 1). Both the frequencies of shoot bud induction as well as the number of shoot buds increased with increasing the concentrations of BAP up to 3.0 mg L−1; however, the percent of shoot bud regeneration declined when the BAP concentration was increased beyond 3.0 mg L−1. In the case of KIN, the highest frequency of shoot bud induction was 76.7 % on MS medium supplemented with 3.0 mg L−1 followed by 60.1 %, whereas KIN at 0.5 mg L−1 was found to be the less effective for shoot bud initiation (23.5 %) (Table 1). The shoot bud initiation as well as number shoots was decreased when the KIN dose was increased beyond the optimal dose (3.0 mg L−1) in the medium. Among the different doses of GFAgNPs tested in the medium, 100 % shoot bud initiation with maximum number of shoot buds (112 shoots explant−1) was obtained on MS medium augmented with 2.0 mg L−1 and GFAgNP at 0.5 mg L−1 produced 40.5 % shoot bud regeneration (Table 1). Of the three growth-regulating compounds tested, the frequency of shoot bud initiation was found to be highest with GFAgNPs, followed by BAP and KIN, and it was statistically significant at P < 0.05 level.
In vitro plant regeneration via direct multiple shoot bud proliferation from axillary nodal explants of Alternanthera sessilis. a Shoot bud induction. b, c Multiple shoot bud development and proliferation. d Elongation of shoots. e Root initiation from the regenerated shoot. d Plantlet growing in plastic cups containing soil mixture
Multiple shoot bud proliferation and elongation
To enhance the multiple shoot bud development rate, in vitro raised shoot clumps were subsequently dissected into mini-shoots (four to five shoots) and transferred onto MS medium supplemented with various concentrations of BAP or KIN (0.5–4.0 mg L−1) and GFAgNPs (0.5–3.0 mg L−1) in combination with 50 mg L−1 AdS for multiple shoot bud proliferation (Table 2). The cultures were frequently subcultured into fresh medium with the same hormonal combinations at 2-week intervals and repeated for 4 cycles to obtain maximum number of shoots (Fig. 2b, c). The percentage of multiple shoot bud proliferation ranged between 38.5 to 100 % (Table 2). Of the BAP and AdS combinations used, maximum shoot bud regeneration (96.8 %) with the highest number of multiple shoots (78.2 shoots culture−1) was obtained on MS medium containing 3.0 mg L−1 BAP + 50 mg L−1 AdS combination followed by 2.0 mg L−1 BAP (Table 2). Among the KIN and AdS combinations, the KIN (2.0 mg L−1) and AdS (50 mg L−1) combination was found to be the optimal for maximum shoot bud regeneration (82.8 %) with 64.7 shoots culture−1, while the lowest mean percent of multiple shoots (38.5 %) was produced on MS medium containing 0.5 mg L−1 KIN +50 mg L−1 AdS combination with 28.6 shoots culture−1 (Table 2). Medium supplemented with GFAgNPs + AdS combinations exhibited maximum percent of multiple shoot proliferation as well as mean number of shoots per culture. The highest percent of multiple shoot development (100 %) with maximum mean number of shoots (153.6 shoots culture−1) was observed on MS medium containing 2.5 mg L−1 GFAgNPs + 50 mg L−1 AdS combination followed by 3.0 mg L−1 GFAgNPs + 50 mg L−1 AdS combination, whereas the 0.5 mg L−1 GFAgNPs + 50 mg L−1 AdS combination gave 51.4 % of multiple shoot bud proliferation with 38.5 shoots culture−1 (Table 2). The addition of AdS with three growth regulators not only favored multiple shoot bud proliferation but also promoted shoot bud elongation (Fig. 2d). The rate of multiple shoot bud induction and mean number of shoots was significantly enhanced with increasing concentrations of growth regulators in the medium but, beyond the optimum level, lead to a decrease. Out of three growth regulator combinations tested, the GFAgNPs + AdS combination significantly increased multiple shoot bud development as well as number of shoots compared to the BAP + AdS and KIN + AdS combinations.
Rooting and acclimatization
Elongated shoots were excised from the shoot cluster and transferred onto half-strength MS medium with or without different concentrations of two auxins. The roots emerged directly from the cut end of the shoots without intervening callus phase after 2-week culture. Root initiation frequency varied depending on the type and dose of auxin used in the medium (Table 3). Although the in vitro shoots produced roots on hormone-free medium, they were thin and fewer compared to the auxin-supplemented medium. The rooting percentage ranged from 54.2 to 100 %. Among the NAA doses tested, the highest rooting (96.7 %) with 11.53 roots shoot−1 was obtained on half-strength MS medium supplemented with 0.5 mg L−1 NAA, while 56.6 % of rooting with 4.8 roots shoot−1 was obtained at 0.1 mg L−1 NAA (Table 3). In the case of IBA, maximum rooting recorded was 100 % on half-strength MS medium fortified with 0.5 mg L−1 which produced highest number of roots also (Table 3). In general, rooting was found to increase with increasing concentrations of both the auxins in the medium. However, IBA showed maximum frequency of root initiation as well as number of roots/shoot compared to NAA in the present study (Fig. 2e), and the data were statistically significant at P < 0.05 level.
Regenerated plantlets were successfully established in the field conditions with 85 % survival rate. The field-established plants did not show any phenotypic variations and flowered normally similar to mother plants.
Genetic fidelity analysis by RAPD-PCR
In order to confirm the genetic uniformity, RAPD-PCR-based DNA fingerprinting analysis was carried out by using the genomic DNA samples from in vitro regenerated field grown plants and control mother plant. Of the 100 random oligonucleotide primers used for screening with DNA samples, 17 primers produced clear and reproducible DNA banding patterns. The RAPD banding patterns recorded after amplification of genomic DNA from 11 randomly selected in vitro regenerated plantlets as well as from the mother plant were scored for presence as 1 (band appeared) and absence as 0 (band disappeared) for each primer individually. About 111 PCR amplicons were generated with an average of 6.5 DNA bands per primer (Table 4). The number of scorable amplified bands per random primer varied from 4 (OPB-04) to 10 (OPB-11). Each primer produced a unique set of DNA bands ranging from 300 to 2000 bp (Fig. 3). Out of the 111 DNA amplicons observed, 109 bands were monomorphic RAPD products and only 2 bands showed polymorphism. The frequency of monomorphism was 98.7 % among the regenerants and mother plants tested (Table 4). Results clearly showed the homogeneity of the regenerated plants and its genetic uniformity with that of the mother A. sesselis plant. The present study indicates that the RAPD-based DNA fingerprinting approach is an effective molecular tool to detect the genetic variability among in vitro regenerated plants in A. sesselis.
Genetic fidelity analysis of in vitro regenerated plantlets of Alternanthera sessilis L. using RAPD markers. RAPD banding pattern generated by amplification of DNA of mother plant (C) along with 11 in vitro raised field grown plants with a OPB-11 primer, b OPC-09 primer, and c OPC-19 primer. Molecular size marker (lane M), 11 randomly selected in vitro regenerated field grown plantlets (lanes T1–T11), and control mother plant (lane C)
Discussion
Phycofabrication of silver nanocomplex using seaweed extract
The fabrication of silver nanocomplexes by using a seaweed extract was successful in the present study as indicated by the reaction mixture color changing from yellow to reddish brown due to the surface plasmon resonance (SPR). Similar results were reported by Kalaiarasi et al. (2015). FTIR spectrum of GFAgNPs showed strong peaks at 3405, 2908, 2431, 1631, 1411, 1004, and 600 cm−1 indicating the presence of different functional groups such as amides, alkanes, carboxylic acids, and aromatic compounds. FTIR results suggest that different phycomolecules from seaweed extracts might be involved in the reduction, stabilization, and capping of silver nanoparticles. It has been reported that the observed carboxylic acid groups might be palmitic acid, palmatoyl acid, or lauric acid (Batista et al. 2011). The crystalline nature of the nanoparticles produced in the present study denotes the presence of cubic structures in the GFAgNP (Khan et al. 2011). The presence of silver ions in GFAgNPs was confirmed, and the absorption peak at 2.4 and 3.0 keV is a typical characteristic of silver nanocomplexes due to SPR, indicating the successful formation of AgNPs by using seaweed extract. These results are in agreement with the recent reports (Jeeva et al. 2014; Kalaiarasi et al. 2015). Similarly, Krishnaraj et al. (2012) also produced AgNPs in the range of 2–50 nm, by using Azardiracta indica leaf extracts.
Effect of phycomolecule-coated silver nanocomplex and cytokinin on plant regeneration
The major focus of this research was to investigate the role of the phycomolecule-loaded silver nanoparticles synthesized from G. foliifera (GFAgNPs) seaweed extracts as novel plant growth-stimulating complex on in vitro regeneration of A. sesselis. Increased rate of shoot bud development was recorded with different doses of growth regulators, and it was positively correlated with concentrations of GFAgNPs, BAP, and KIN up to the optimal level and declined at higher doses. It is presumed that the higher concentrations might be toxic and affect the cell growth and development. GFAgNP complex showed maximum frequency of shoot bud initiation (100 %) over synthetic cytokinins (BAP and KIN). The positive role of BAP on in vitro shoot bud regeneration is well documented in various plant species (Ganesan and Huyop 2010; Thiyagarajan and Venkatachalam 2012a, 2013; Kumar et al. 2015; Skala et al. 2015). Both the percentage of shoot bud initiation and number of shoots decreased significantly on MS medium fortified with KIN when compared with GFAgNP complex and BAP. However, the positive effect of KIN on shoot bud regeneration was previously reported in Stevia rebaudiana (Thiyagarajan and Venkatachalam 2012a) and Gymnema sylvestre (Thiyagarajan and Venkatachalam 2013). Of the two cytokinins tested in the present study, BAP was found to be the best cytokinin for initiation of multiple shoot buds; however, lower dose of GFAgNPs showed superior in vitro plant regeneration effect in A. sesselis.
Silver nitrate plays a pivotal role for enhanced rate of in vitro shoot bud induction in the culture medium (Kumar et al. 2009). The fabricated GFAgNPs showed maximum percent of shoot bud induction in A. sesselis, and this may be due to the presence of natural growth-promoting molecules coated with silver nanocomplex which act as superior plant growth-stimulating compounds compared to the synthetic cytokinins used in the present experiment (Gurusaravanan et al. 2013; Vinoth et al. 2012, 2014). The number of multiple shoot formation increased with increasing the doses of cytokinins and silver nanoparticles in the medium; however, there was twofold increase in multiple shoot bud development recorded on medium with 2.5 mg L−1 phycomolecule-loaded silver nanoparticles (153.6 shoots culture−1). Multiple shoot bud regeneration and number of shoots slightly declined at higher doses of cytokinins as well as silver nanoparticles. Park et al. (2012) also reported the growth-promoting effect of silver nitrate under in vitro in Sinningia speciosa. These results suggested that the addition of silver nanoparticles in the medium greatly promoted the rate of multiple shoot bud development as well as the number of shoots in A. sesselis.
Synergistic effect of AdS with GEAgNPs and cytokinins on multiple shoot bud development
AdS has been used for enhanced rate of in vitro shoot bud multiplication in the presence of cytokinins. The benefits of adenine sulfate are often noticed only if it is used along with cytokinins such as BAP or KIN (van Staden et al. 2008). Adenine sulfate exhibits a synergistic effect with other cytokinins on enhanced shoot bud proliferation, and AdS was used effectively as an adjuvant for shoot bud development in Pterocarpus marsupium (Husain et al. 2008). Hasan et al. (2010) obtained maximum number of shoot bud induction in the presence of adenine sulfate. BAP and KIN along with AdS enhanced the rate of multiple shoot bud induction in Psoralea corylifolia (Jeyakumar and Jayabalan 2002), S. rebaudiana (Thiyagarajan and Venkatachalam 2012a), and G. sylvestre (Thiyagarajan and Venkatachalam 2013). The superiority of BAP and AdS combinations on multiple shoot bud formation in Swertia chirata (Chaudhuri et al. 2007), Phyllanthus fraternus (Upadhyay et al. 2013), S. rebaudiana (Thiyagarajan and Venkatachalam 2012a), and G. sylvestre (Thiyagarajan and Venkatachalam 2013). In the present study, the impact of AdS in combination with silver nanocomplex, BAP, and KIN was evaluated and GFAgNPs showed an significantly enhanced percentage of multiple shoot proliferation as well as number of shoots per culture over the synthetic cytokinin combinations. This may be due to the possible presence of different kinds of natural plant growth-promoting compounds in the seaweed extracts (Khan et al. 2011; Gurusaravanan et al. 2013; Vinoth et al. 2012, 2014; Satish et al. 2015, 2016). It is presumed that the increased percentage as well as number of shoot buds was due to the action of silver nanocomplex along with AdS.
Influence of auxins on root initiation
In this study, although root formation occurred from elongated shoots on half-strength medium, the roots were thin and weak. In contrast, thick and strong roots were produced from shoots cultured on medium fortified with auxin-supplemented medium. Of the two auxins examined, IBA was the better auxin for rooting compared to NAA. Both the percentage of rooting and the number of roots were positively correlated with auxin concentrations. Similar rooting response was reported in S. rebaudiana (Thiyagarajan and Venkatachalam 2012a) and G. sylvestre (Thiyagarajan and Venkatachalam 2013). IBA produces the best rooting response in a wide range of plants such as Benincasa hispida (Thomas and Sreejesh 2004), Melothria maderaspatana (Baskaran et al. 2009), and Rhaponticum carthamoides (Skala et al. 2015). Regenerated plantlets were established in the field, and they did not show any morphological variability. The main reason for the absence of genetic variability among regenerated plants could be multiple shoot bud development via direct organogenesis in which no callus phase was involved.
Determination of genetic fidelity of regenerants by RAPD-PCR
In the present study, the genetic fidelity of the in vitro-developed plantlets of A. sessilis was determined by RAPD-DNA fingerprinting analysis. RAPD molecular markers are being effectively used to assess the genetic variability found within the genomic sequence of the in vitro-regenerated plants (Kumar et al. 2015; Skala et al. 2015). Results indicated that about 100 random oligonucleotide primers were tested and only 1.3 % DNA polymorphism was recorded. The occurrence of DNA polymorphism in tissue culture and mother plants is due to the disruption of normal cell developmental responses and includes point mutations such as mismatch repair, DNA methylation, chromosomal aberrations at DNA base level (addition, insertion, deletion), and gene repression or activation (Guo et al. 2006; Pathak and Dhawan 2012). Similarly, a low percent of DNA polymorphism (2 %) has been reported earlier in Brassica oleracea (Qin et al. 2006). Most recently, Kumar et al. (2015) reported about 10–12 % polymorphism among in vitro-regenerated plantlets from B. oleracea by RAPD analysis. In contrast, Skala et al. (2015) reported that RAPD data exhibited nearly 35.3 % polymorphism in tissue culture plants mainly because of the intervening with callus phase during indirect regeneration method in R. carthamoides. The low percent of genetic variability noticed may be due to the successive medium changes (Werner et al. 2015). In this study, about 98.7 % monomorphic DNA bands were recorded with all the primers tested in the regenerated plants describing genetic stability in the Alternanthera. There are many reports on molecular characterization of micropropagated plants by the RAPD technique especially to confirm the clonal fidelity and genetic stability among tissue culture grown plants and donor in different plant species including Chlorophytum borivilianum (Samantaray and Maiti 2010), Jatropha curcas (Sharma et al. 2011) Cuphea procumbens (Fatima et al. 2012), Eclipta alba (Singh et al. 2012) and S. rebaudiana (Thiyagarajan and Venkatachalam 2012b). Results clearly showed that the in vitro shoot regeneration from axillary nodal explants via direct organogenesis without any intervening callus phase is a highly reliable method for large-scale production of genetically uniform plants (true to type).
Conclusion
In conclusion, this report describes the successful bioengineering and characterization of phycomolecule-coated silver nanocomplex and its impact on enhanced rate of multiple shoot regeneration in A. sessilis. Plant regeneration via direct organogenesis was achieved, and phycomolecule-loaded silver nanocomplex with AdS combination exhibited significantly highest percent of multiple shoot regeneration with maximum number of shoots than BAP and KIN. Auxin was essential to produce efficient root system from regenerated shoots. Molecular analysis confirmed the absence of genetic variability among regenerated populations. Results of this study showed that the phycomolecule-coated silver nanocomplex could be used in the medium as alternate to readily available synthetic plant growth hormones to produce commercial-scale tissue culture plants for industry applications in the future. The cost for phycomolecule-coated silver nanocomplex production is seven times lower than the cost of plant growth regulators used for plant tissue culture. To the best of our knowledge, this is the first report on high-frequency plant regeneration by using phycomolecule-coated silver nanocomplex and evaluation of genetic fidelity of in vitro-propagated A. sesselis plants.
References
Acharya E, Pokhrel B (2006) Ethno-medicinal plants used by Bantar of Bhaudaha, Morang, Nepal. Our Nature 4:96–103
Baskaran P, Velayutham P, Jayabalan N (2009) In vitro regeneration of Melothria maderaspatana via indirect organogenesis. In Vitro Cell Dev Biol Plant 45:407–413
Batista LM, Almeida CLF, Falcao HS, Lima GRM, Montenegro CA, Lira NS, Athayde-Filho PF, Rodrigues LC, Souza MFV, Barbosa-Filho JM (2011) Bioactivities from marine algae of the genus Gracilaria. Int J Mol Sci 12:4550–4573
Ben Sassi AF, Skhiri B, Aouni M (2007) Investigation of some medicinal plants from Tunisia for antimicrobial activities. Pharm Biol 15:421–428
Bhatia R, Singh KP, Sharma TR, Jhang T (2011) Evaluation of the genetic fidelity of in vitro-propagated gerbera (Gerbera jamesonii Bolus) using DNA-based markers. Plant Cell Tissue Organ Cult 104:131–135
Chandrika UG, Svanberg U, Jansz ER (2006) In vitro accessibility of ß-carotene from cooked Sri Lankan green leafy vegetables and their estimated contribution to vitamin a requirement. J Sci Food Agricult 86:54–61
Chaudhuri RK, Pal A, Jha TB (2007) Production of genetically uniform plants from nodal explants of Swertis chirata Buch.-ham. Ex wall.—an endangered medicinal herb. In Vitro Cell Dev Biol Plant 43:467–472
Coruh I, Gornez AA, Ercisli S (2007) Total phenolics, mineral elements, antioxidant and antibacterial activities of some edible wild plants in Turkey. Asian J Chem 19:5755–5762
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Fatima N, Ahmad N, Anis M (2012) In vitro propagation of Cuphea procumbens Orteg. and evaluation of genetic fidelity in plantlets using RAPD markers. J Plant Biochem Biotechnol 21:51–57
Firdhouse JM, Lalitha P (2013) Biosynthesis of silver nanoparticles using the extract of Alternanthera sessilis-antiproliferative effect against prostate cancer cells. Cancer Nanotechnol 4:137–143
Ganesan K, Huyop F (2010) In vitro regeneration of Citrullus lanatus cv. Round Dragon. J Biol Sci 10:131–137
Gayathri BM, Balasuriya K, Gunawardena GSPS, Rajapakse RPVJ, Dharmaratne HRW (2006) Toxicological studies of the water extract of green leafy vegetable sessile joy weed (Alternanthera sessilis). Res Comm Curr Sci 91:1517–1520
Giridhar P, Indu EP, Vijaya Ramu D, Ravishankar GA (2003) Effect of silver nitrate on in vitro shoot growth of coffee. Trop Sci 43:144–146
Gnanaraj WE, Antonisamy JM, Subramanian KM, Nallyan S (2011) Micropropagation of Alternanthera sessilis (L.) using shoot tip and nodal segments. Iranian J Biotech 9:3–10
Guo WL, Gong L, Ding ZF, Li YD, Li FX, Zhao SP, Liu B (2006) Genomic instability in phenotypically normal regenerants of medicinal plant Codonopsis lanceolata Benth. Et Hook. F., as revealed by ISSR and RAPD markers. Plant Cell Rep 25:896–906
Gurusaravanan P, Vinoth S, Kumar S, Thajuddin N, Jayabalan N (2013) Effect of cyanobacterial extracellular products on high-frequency in vitro induction and elongation of Gossypium hirsutum L organs through shoot apex explants. J Genet Eng Biotechnol 11:9–16
Hasan MN, Nigar S, Rabbi MAK, MizanS B, Rahman MS (2010) Micropropagation of strawberry (Fragaria x ananassa Duch.). Int J Sustain Crop Prod 5:36–41
Husain MK, Anis M, Shahzad A (2008) In vitro propagation of a multipurpose leguminous tree (Pterocarpus marsupium Roxb.) using nodal explants. Acta Physiol Plant 30:353–359
Jeeva K, Thiyagarajan M, Elangovan V, Geetha N, Venkatachalam P (2014) Caesalpinia coriaria leaf extracts mediated biosynthesis of metallic silver nanoparticles and their antibacterial activity against clinically isolated pathogens. Ind Crop Prod 52:714–720
Jeyakumar M, Jayabalan N (2002) In vitro plant regeneration from cotyledonary node of Psoralea corylifolia L. Plant Tissue Cult 12:125–129
Johnson M, Yasmin N, Sonali D, Rajasekarapandian M (2007) The role of cytokinin and auxin in organogenesis of Passiflora mollissima and evaluation of biochemical changes using isozyme. Eth J Sci Technol 4:27–36
Kalaiarasi K, Prasannaraj G, Sahi SV, Venkatachalam P (2015) Phytofabrication of biomolecule-coated metallic silver nanoparticles using leaf extracts of in vitro-raised bamboo species and its anticancer activity against human PC3 cell lines. Turk J Biol 39:223–232
Khan W, Rayirath UP, Subramanian S, Jithesh MN, Rayorath P, Hodges DM, CritchleyAT CJS, Norrie J, Prithivraj B (2009) Seaweed extracts as biostimulants of plant growth and development. J Plant Growth Regul 28:386–399
Khan W, Hiltz D, Critchley AT, Prithiviraj B (2011) Bioassay to detect Ascophyllum nodosum extract-induced cytokinin-like activity in Arabidopsis thaliana. J Appl Phycol 23:409–414
Krishnaraj C, Jagan EG, Ramachandran R, Abirami SM, Mohan N, Kalaichelvan PT (2012) Effect of biologically synthesized silver nanoparticles on Bacopa monnieri (Linn.) Wettst. plant growth metabolism. Process Biochem 47:651–658
Kumar G, Sahoo D (2011) Effect of seaweed liquid extract on growth and yield of Triticum aestivum var. Pusa Gold J Appl Phycol 23:251–255
Kumar V, Giridhar P, Ravishankar GA (2009) AgNO3—a potential regulator of ethylene activity and plant growth modulator. Electr J Biotech 12:1–15
Kumar P, Gambhir G, Gaur A, Srivastava DK (2015) Molecular analysis of genetic stability in in vitro regenerated plants of broccoli (Brassica oleracea L. var. italica). Current Sci 109:1470–1475
Kumaraswamy M, Anuradha M (2010) Micropropagation of Pogostemon cablin Benth. through direct regeneration for production of true to type plants. Plant Tissue Cult Biotechnol 20:81–89
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol Plant 15:473–497
Park EH, Bae H, Park WT, Kim YB, Chae SC, Park SU (2012) Improved shoot organogenesis of gloxinia (Sinningia speciosa) using silver nitrate and putrescine treatment. Plant Omics J 5:6–9
Pathak H, Dhawan V (2012) ISSR assay for ascertaining genetic fidelity of micropropagated plants of apple rootstock Merton 793. In Vitro Cell Dev Biol Plant 48:137–143
Qin Y, Li HL, Guo YD (2006) High frequency embryogenesis, regeneration of broccoli (Brassica oleracea var. italica) and analysis of genetic stability by RAPD. Sci Hortic 111:203–208
Samantaray S, Maiti S (2010) An assessment of genetic fidelity of micropropagated plants of Chlorophytum borivilianum using RAPD markers. Biol Plant 54:334–338
Satish L, Rameshkumar R, Rathinapriya P, Pandian S, Rency AS, Sunitha T, Ramesh M (2015) Effect of seaweed liquid extracts and plant growth regulators on in vitro mass propagation of brinjal (Solanum melongena L.) through hypocotyl and leaf disc explants. J Appl Phycol 27:993–1002
Satish L, Rathinapriya P, Rency AS, Ceasar SA, Pandian S, Rameshkumar R, Ramesh M (2016) Somatic embryogenesis and regeneration using Gracilaria edulis and Padina boergesenii seaweed liquid extracts and genetic fidelity in finger millet (Eleusine coracana). J Appl Phycol 28:2083–2098
Sharma S, Pamidimarri DVNS, Ananda KGV, Reddy MP (2011) Assessment of genetic stability in micropropagules of Jatropha curcas genotypes by RAPD and AFLP analysis. Ind Crop Prod 34:1003–1009
Singh A, Kandasamy T, Odhav B (2009) In vitro propagation of Alternanthera sessilis (sessile joyweed), a famine food plant. Afr J Biotech 8:5691–5695
Singh SK, Rai MK, Sahoo L (2012) An improved and efficient micropropagation of Eclipta alba through transverse thin cell layer culture and assessment of clonal fidelity using RAPD analysis. Ind Crop Prod 37:328–333
Skala E, Bkowska RG, Sitarek P, Kuz’ma L, Błau A, Wysokin’ska H (2015) Rhaponticum carthamoides regeneration through direct and indirect organogenesis, molecular profiles and secondary metabolite production. Plant Cell Tiss Organ Cult 123:83–98
Stirk WA, Arthur GD, Lourens AF, Novak O, Strnad M, Van Staden J (2004) Changes in cytokinin and auxin concentrations in seaweed concentrates when stored at an elevated temperature. J Appl Phycol 16:31–39
Thiyagarajan M, Venkatachalam P (2012a) Large scale in vitro propagation of Stevia rebaudiana (Bert) for commercial application: pharmaceutically important and antidiabetic medicinal herb. Ind Crop Prod 37:111–117
Thiyagarajan M, Venkatachalam P (2012b) Evaluation of the genetic fidelity of in vitro propagated natural sweetener plant (Stevia rebaudiana Bert.) using DNA-based markers. Plant Cell Biotech Mol Biol 13:99–104
Thiyagarajan M, Venkatachalam P (2013) A reproducible and high frequency plant regeneration from mature axillary node explants of Gymnema sylvestre (Gurmur)—an important antidiabetic endangered medicinal plant. Ind Crop Prod 50:517–524
Thomas TD, Sreejesh KR (2004) Callus induction and plant regeneration from cotyledonary explants of ash gourd (Benincasa hispida L.). Sci Hort 100:359–367
Upadhyay R, Tiwari KN, Singh K (2013) High frequency shoots regeneration for mass multiplication of Phyllanthus fraternus Webster—an important antiviral and hepatoprotective plant. Appl Biochem Biotechnol 169:2303–2314
van Staden J, Zazimalova E, George EF (2008) Plant growth regulators II: cytokinins, their analogues and antagonist. In: George EF, Hall M, DE Kleck GJ (eds) Plant propagation by tissue culture. Springer, Dordrecht pp, pp. 205–226
Vinoth S, Gurusaravanan P, Jayabalan N (2012) Effect of seaweed extracts and plant growth regulators on high-frequency in vitro mass propagation of Lycopersicon esculentum L (tomato) through double cotyledonary nodal explants. J Appl Phycol 24:1329–1337
Vinoth S, Gurusaravanan P, Jayabalan N (2014) Optimization of somatic embryogenesis protocol in Lycopersicon esculentum L. using plant growth regulators and seaweed extracts. J Appl Phycol 26:1527–1537
Werner ET, Soares TCB, Gontijo ABPL, Souza Neto JD, do Amara 1JAT (2015) Genetic stability of micropropagated plants of Crambe abyssinica Hochst using ISSR markers. Genet Mol Res 14:16450–16460
Acknowledgments
Authors gratefully acknowledge the Department of Biotechnology, Periyar University, Salem, for providing necessary facilities to carry out this experiment. Authors wish to thank the anonymous reviewers as well as editor for making excellent comments on this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Venkatachalam, P., Malar, S., Thiyagarajan, M. et al. Effect of phycochemical coated silver nanocomplexes as novel growth-stimulating compounds for plant regeneration of Alternanthera sessilis L.. J Appl Phycol 29, 1095–1106 (2017). https://doi.org/10.1007/s10811-016-0977-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-016-0977-2