Abstract
Bacterial contamination is a serious problem in plant tissue culture procedures. An experiment was conducted to evaluate the potential of nano silver (NS) to remove bacterial contaminants of valerian nodal explants. This experiment was conducted as a completely randomized design in a factorial arrangement with four replications and each replicate with ten explants. Treatments involved NS at two stages (before and after surface sterilization along with control) with three rates (25, 50 and 100 mg l−1) at three times of soaking (30, 60 and 180 min). Explants were cultured on MS medium supplemented with 5 mg l−1 Kin and 0.1 mg l−1 NAA. Results showed that using 100 mg l−1 of NS solution after surface sterilization resulted in the highest percentage (89%) of disinfected explants. Nano silver solution did not affect the characters measured. On the basis of the data obtained in this experiment, it was concluded that NS had a good potential for removing of the bacterial contaminants in plant tissue culture procedures. As this is the first report on application of NS in in vitro culture techniques, further investigations on other plant species are needed to clarify the effectiveness of NS for the removal of bacterial contaminants in tissue culture of other crops.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Successful tissue culture of all plants depends on the removal of exogenous and endogenous contaminating microorganisms (Buckley et al. 1994; Constantine 1986). Fungi and bacteria are the most common microorganisms to be found on or in plant tissues. To eliminate bacterial contamination during in vitro propagation, different methods have been developed in the last few years (Barrett and Cassells 1994; Hussain et al. 1994; Herman 1996). Antibiotics are commonly used in the medium to eliminate unwanted contaminants from plant systems (Smart et al. 1995). Theoretically, it might seem that all contamination problems could be overcome by the incorporation of one or more antibiotics into the culture medium. However, antibiotics are frequently phytotoxic otherwise may retard or inhibit plant tissue growth. Also, prolonged exposure of cells or tissues to antibiotics can result in the development of genetic change (mutation) in the genes within the organelles (the cytoplasmic genes or cytoplasmic DNA) as well as development of resistance in bacterial cells. Most antibiotics have been shown inhibitory effects in the plants. Streptomycin and chloramphenicol are inhibitors of protein synthesis; rifampicin inhibits nucleic acid synthesis and penicillin inhibits cell-wall membrane synthesis (Pankhurst 1977). Streptomycin alters the sensitivity of chloroplast RNA editing and at phytotoxic levels, white tissue results, owing to the lack of chloroplast differentiation (Karcher and Bock 1998). Inhibition of the cyclic electron flow in chloroplast photosystem I and mitochondrial ATP production inhibition in tobacco by antibiotics such as antimycin A at as low concentration as 1 mM resulted in malformed and bleached leaves (Horvath et al. 2000; Joët et al. 2001). Phytotoxicity of rifampicin, carbenicillin and streptomycin in Clematis, Delphinium, Hosta, Iris and Photinia cultures is also reported (Leifert et al. 1992). Using antibiotics in the media inhibit both multiplication and rooting of Delphinium shoot cultures (Leifert et al. 2000). Teixeira da Silva et al. (2003) reported a decrease in explant survival and biomass reduction, malformation of roots and inhibition of shoot formation in chrysanthemum, and also in tobacco endoreduplication by application of antibiotics in the media. Nano silver (NS) has shown to have antibacterial, antifungal and antiviral effects (Nomiya et al. 2004; Sondi and Salopek-Sondi 2004). Studies have demonstrated that silver ions interact with sulfydryl (–SH) groups of proteins as well as with the bases of DNA leading either to the inhibition of respiratory processes (Bragg and Rannie 1974) or DNA unwinding (Batarseh 2004). Inhibition of cell division and damage to bacterial cell envelopes are also recorded (Richards et al. 1984) and interaction with hydrogen bonding processes has been demonstrated to occur (Russell and Hugo 1994). Interruption of cell wall synthesis resulting in loss of essential nutrients has been shown to occur in yeasts (Wells et al. 1995) and may well occur in other fungi. Antiviral activity of silver ions has been recorded and interaction with –SH groups has been implicated in the mode of action (Thurmann and Gerba 1989). Silver ions clearly do not possess a single mode of action. They interact with a wide range of molecular processes within microorganisms resulting in a range of effects from inhibition of growth, loss of infectivity through cell death. The mechanism depends on both the concentration of silver ions present and the sensitivity of the microbial species to silver. Contact time and temperature can have impact on both the rate and extent of antimicrobial activity (Dibrov et al. 2002). Using antibiotics in medium, as mentioned above, have mutation risks or may show in vitro inhibitory effects. Therefore, using antibiotics without application in the medium may reduce mutation risks and inhibitory effects on them. Nano silver has antimicrobial effects at low concentrations. However, so far there is no report on using NS to eliminate microorganisms in tissue culture procedures. The present study was conducted to evaluate the potential of NS to eliminate fungal and bacterial contaminants in Valeriana officinalis L., which showed high internal contamination in preliminary experiments, explants without application in the medium. The effects of NS solution on growth, proliferation rate and rooting were studied and compared with non-NS treated materials.
Materials and methods
Nano silver preparation
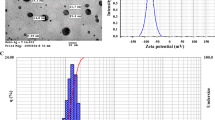
The nano-particles used in this experiment were silver particles 35 nm (average) in size. Figure 1 show the transmission electron microscopy (TEM) micrograph of silver (Ag) nano-particles. The base working fluid was pure water. Ag nano-fluids were prepared using a two-step method. Ag nano-particles were prepared first. They were produced using a catalytic chemical vapour deposition method (Nanocid Company Method). The Ag nano-particles were then added to pure water. No surfactant was used in the Ag nano-fluid suspensions. The mixture was prepared using an ultrasonic homogenizer. Nano-fluid concentrations at 25, 50 and 100 mg l−1 were used in this study. Some atomic and physical properties of NS used in this study are presented in Table 1.
Plant materials and culture conditions
Greenhouse grown valerian (Valeriana officinalis L.) mother plants were used in this study. These plants were tested by culturing their stem explants in potato dextrose agar (PDA) medium for internal contamination assay. The explants were first surface sterilized with 70% ethanol for 1 min and 10% Clorox (containing 5.25% sodium hypochlorite) for 1 min and then rinsed four times with sterilized distilled water. The cause of internal contamination was identified with special laboratory methods, in the Department of Plant Pathology, Shiraz University, as Xanthomonas genus. After testing, the mother plants were divided into two groups: with internal contamination (group 1) and without internal contamination (group 2).
For group 1, a number of 20–25 cm stems were cut and transferred to the laboratory immediately. They were cut to the length of about 0.5–1 cm and prewashed in water supplemented with 10 drops of a weak household detergent solution for 10 min and then placed under running tap water for at least 30 min. Nano silver solution at different concentrations (25, 50 and 100 mg l−1) and exposure times (30, 60, 180, 300, 600 and 1,200 min) was used at two stages; before and after surface sterilization along with the control. Initial experiment showed that in high exposure times (300, 600 and 1,200 min) explants turned to bleach. Therefore, high exposure time’s results were omitted.
For the treatment without surface sterilization, after prewashing in water and keeping under running tap water, nodal segments were dipped at appropriate times and concentrations of NS solution. After this treatment, the explants were rinsed four times with sterilized distilled water. For the treatment before surface sterilization, after dipping explants in NS solution, the explants were surface sterilized (as mentioned above). For the treatment after surface sterilization, the explants were rinsed with sterilized distilled water and dipped in NS solution with appropriate concentrations at different times. After recut, the sterilized explants were dipped in NS solution before being transferred to the culture vessels. After sterilization, about 1 cm single node explants were cultured on a modified MS (Murashige and Skoog 1962) medium containing salts, organic constituents, 30 g l−1 sucrose, 8 g l−1 agar and 5 mg l−1 Kin and 0.1 mg l−1 NAA (Abdi 2006). The pH of media was adjusted to 5.8 by 0.1 N HCl before autoclaving for 15 min at 121°C and 1.5 kg cm−2 pressure. Cultures were kept under a 16 h photoperiod of 30 mm m−2 s−1 light intensity emitted by two cool white fluorescent lamps at 25 ± 3°C.
Explants in group 2 were just prewashed, surface sterilized and cultured without NS treatment. All the cultural conditions in this group were similar to those in group 1.
Data collecting
The percentages of infected explants were recorded 3 days after culture for without surface sterilization treatment. For estimation of size and growth of bacterial and fungal colonies the grades of 1 (lowest) to 5 (highest) contamination were given. For other treatments, the percentages of infected explants were recorded 3 weeks after cultures. The experiment was conducted as a completely randomized design in a factorial arrangement with four replications and each replicate with ten explants. Means were compared using Duncan’s new multiple range test (DNMRT) at 5% probability level. Impact of the NS on subsequent shoot formation and rooting was assessed in four subcultures with 4 week intervals.
Results
Group 1
Using NS solution without surface sterilization did not affect the contamination. In control, visible fungal contaminations were observed only 3–5 days after culture, while, the bacterial contaminations were observed 7–10 days after culture, in the other treatments, colony appearance was delayed by at least 6–8 and 12–18 days for fungal and bacterial contaminations, respectively. The size and growth of the colonies varied significantly among treatments. In the control treatment, growth of the colonies was quick; whereas, in the other treatments depending on exposure time and concentration of NS solution the influence on growth was negligible (Table 2).
Cultures subjected to NS solution treatment before surface sterilization showed low percentage of disinfected valerian explants (Table 3). In all the treatments, the percentage of fungal contamination was zero. Among the treatments, highest percentages of disinfection (32%) were observed when the explants were dipped in 100 mg l−1 NS solution for 180 min.
Using NS solution after surface sterilization was successful. Treatment with 100 mg l−1 of NS for 180 min after rinsing the explants in sterilized distilled water was the most successful disinfection treatment. This treatment had significant differences with other treatments. The 11% contamination left after this treatment was bacterial contamination (data not shown). Also, this treatment did not have any negative impact on measured characters in micropropagation of valerian in four subsequent subcultures.
Group 2
As mentioned above, explants in this group did not show any contamination during culture period. Comparing this group with explants obtained from NS solution treatment after surface sterilization did not show any significant differences in measured characters (proliferation rate, leaf number, percentage of fresh weight, number of rooted explants, number of roots and root length) in micropropagation of valerian in four subsequent subcultures (data not shown).
Discussion
Novel approaches for controlling the contamination in plant tissue culture, screening the contaminants, disinfestation of explant methods using activated charcoal or diethylpyrocarbonate, elimination of microbial contaminants using density gradient centrifugation, flexible container system, repeated subculture, low free-water medium, acidification, egg white lysozyme and antibiotics are reviewed in the book edited by Herman (1996). Silver and its compounds have long been used as antimicrobial agents (Brown and Anderson 1968; Russell and Hugo 1994; Herman 1996). The most important silver compound currently in use is silver sulfadiazine (AgSD), although silver metal, silver acetate, silver nitrate and silver protein have antimicrobial effect too. Using AgNO3 as silver compound against infection in tissue culture is common (Herman 1996). Our results showed that silver in nano size can similarly control the bacterial infection in tissue culture conditions. Also, subcultures indicated that bacterial contaminations were removed because the late appearance of contamination was not observed in subsequent subcultures. In general, using NS solution after surface sterilization had acceptable influence on the bacterial contaminants control without any adverse effects on growth characters in micropropagation of valerian. However, it was not effective in controlling the fungi in this experiment. The differences in the effects of NS treatment before and after surface sterilization may be due to the presence of NS in NS solution treatment after surface sterilization at the cut end of the explants inside the medium. After recut, the sterilized explants were dipped in NS solution before being transferred to the culture vessels. However, in NS solution treatment, before surface sterilization explants were washed with distilled water and then transferred to the medium. A method has been suggested by Salehi and Khosh-Khui (1997) for controlling bacterial contamination in miniature roses. They used gentamicin solution after surface sterilization. Using NS may be more convenient and less toxic than using antibiotics in the medium. Furthermore, using other methods for controlling the infection like first acidification of the medium and later regulation of pH to normal condition (Leifert et al. 2000) and microbial culture filtrate (Hussain et al. 1994) may be time consuming methods in tissue culture techniques. Showing acceptable antibacterial activity in this investigation is in agreement with the results obtained by other investigators (Nomiya et al. 2004; Sondi and Salopek-Sondi 2004).
Since, this is the first report of NS application in the tissue culture methods, further studies are needed on using this chemical in in vitro culture of other species.
References
Abdi Gh (2006) Factors affecting micropropagation of valerian (Valeriana officinalis L.) MSc Thesis, Shiraz University, Iran
Barrett C, Cassells AC (1994) An evaluation of antibiotics for the elimination of Xanthomonas campestris pv pelargonii (brown) from Pelargonium x domesticum cv. ‘Grand Slam’ explants in vitro. Plant Cell Tissue Organ Cult 36:169–175
Batarseh KI (2004) Anomaly and correlation of killing in the therapeutic properties of silver (I) chelating with glutamic and tartaric acids. J Antimicrob Chemother 54:546–548
Bragg PD, Rannie DJ (1974) The effect of silver ions on the respiratory chain of E coli. Can J Microbiol 20:883–889
Brown MRW, Anderson RA (1968) The bactericidal effect of silver ions on Pseudomonas aeruginosa. J Pharm Pharmacol 20(Suppl):1S–3S
Buckley PM, Reed BM (1994) Antibiotic susceptibility of plant-associated bacteria. HortScience 29:434 (Abst)
Constantine DR (1986) Micropropagation in the commercial environment. In: Withers L, Alderson PG (eds) Plant tissue culture and its agricultural applications. Butterworth, London, pp 175–186
Dibrov P, Dzioba J, Khoosheh K, Gosink K, Claudia C (2002) Chemiosmotic mechanism of antimicrobial activity of Ag+ in Vibrio cholerae. Antimicrob Agents Chemother 46:2668–2670
Herman EB (ed) (1996) Microbial contamination of plant tissue cultures. Agritech Consultants Inc, Shrub Oak, USA, 84 p
Horvath EM, Peter SO, Joët T (2000) Targeted inactivation of the plastid ndhB gene in tobacco results in an enhanced sensitivity of photosynthesis to moderate stomatal closure. Plant Physiol 123:1337–1350
Hussain S, Lane SD, Price DN (1994) A preliminary evaluation of the use of microbial culture filtrates for the control of contaminants in plant tissue culture systems. Plant Cell Tissue Organ Cult 36:45–51
Joët T, Couranc L, Horvath EM, Medgyesy P, Peltier G (2001) Increased sensitivity of photosynthesis to antimycin A induced by inactivation of the chloroplast ndhB gene. Evidence for a participation of the NADH-dehydrogenase complex to cyclic electron flow around photosystem I. Plant Physiol 125:1919–1929
Karcher D, Bock R (1998) Site-selective inhibition of plastid RNA editing by heat shock and antibiotics: a role for plastid translocation in RNA editing. Nucleic Acids Res 26:1185–1190
Leifert C, Cammota H, Waites WM (1992) Effect of combinations of antibiotics on micropropagated Clematis, Delphinium, Hosta, Iris and Photinia. Plant Cell Tissue Organ Cult 29:153–160
Leifert C, Waites B, Keetley JW, Wright SM, Nicholas JR, Waites WM (2000) Effect of medium acidification on filamentous fungi, yeasts and bacterial contaminants in Delphinium tissue cultures. Plant Cell Tissue Organ Cult 42:149–155
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nomiya K, Yoshizawa A, Tsukagoshi K, Kasuga NC, Hirakava S, Watanabe J (2004) Synthesis and structural characterization of silver (I), aluminium (III) and cobalt (II) complexes with 4-isopropyltropolone (hinokitiol) showing noteworthy biological activities. Action of silver (I)-oxygen bonding complexes on the antimicrobial activities. J Inorg Biochem 98:46–60
Pankhurst CE (1977) Symbiotic effectiveness of antibiotic-resistant mutants of fast and slow-growing strains of Rhizobium nodulating Lotus species. Can J Microbiol 23:1026–1033
Salehi H, Khosh-Khui M (1997) A simple procedure of disinfection of ‘Baby Masquerade’ miniature rose explants. Sci Hortic 68:145–148
Richards RME, Taylor RB, Xing DKL (1984) Effect of silver on whole cells and spheroplasts of a silver resistant Pseudomonas aeruginosa. Microbios 39:151–158
Russell AD, Hugo WB (1994) Antimicrobial activity and action of silver. Prog Med Chem 31:351–371
Smart DR, Ferro A, Ritchie K, Bugbee BG (1995) On the use of antibiotics to reduce rhizoplane microbial populations in root physiology and ecology investigations. Physiol Plant 95:533–540
Sondi I, Salopek-Sondi B (2004) Silver nano particles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci 275:177–182
Teixeira da Silva JA, Duong NT, Michi T, Seiichi F (2003) The effect of antibiotic on the in vitro growth response of chrysanthemum and tobacco stem transverse thin cell layers (tTcLs). Sci Hortic 97:397–410
Thurmann RB, Gerba CP (1989) The molecular mechanisms of copper and silver ion disinfection of bacteria and viruses. Critic Rev Environ Cont 18:295–315
Wells TN, Scully P, Paravicini G, Proudfoot AE, Payton MA (1995) Mechanism of irreversible inactivation of phosphomannose isomerases by silver ions and flamazine. Biochemistry 24:896–903
Acknowledgments
The authors are grateful to Nanocid Company (Tehran, Iran) for advice and for generous gift of silver samples.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Kononowicz.
Rights and permissions
About this article
Cite this article
Abdi, G., Salehi, H. & Khosh-Khui, M. Nano silver: a novel nanomaterial for removal of bacterial contaminants in valerian (Valeriana officinalis L.) tissue culture. Acta Physiol Plant 30, 709–714 (2008). https://doi.org/10.1007/s11738-008-0169-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-008-0169-z