Abstract
Microbial contamination is a serious problem in temporary immersion systems (TIS) during commercial micropropagation. The use of adequate doses of silver nanoparticles (AgNPs), formulated as Argovit™, is an alternative to reduce the contamination indices and promote development in plants. The aim of this study was to evaluate the antimicrobial and hormetic effects of Argovit on in vitro regeneration of vanilla (Vanilla planifolia) using a TIS. In vitro regenerated shoots were grown in Murashige and Skoog (MS) liquid medium with Argovit at five different concentrations (0, 25, 50, 100 and 200 mg/l) using a temporary immersion bioreactor system (RITA®). At 30 days of culture, contamination percentage was evaluated and shoot regeneration and length were used to determine the hormetic response. Analysis of macro and micronutrient contents was performed. In addition, the effect of Argovit on total phenolic content (TPC), reactive oxygen species (ROS) production, antioxidant capacity (ORAC) and lipid peroxidation (LP-MDA) was determined. Results showed that bacterial contamination was reduced at 50, 100 and 200 mg/l of Argovit. Growth stimulation was observed at 25 and 50 mg/l of Argovit, while significant inhibition was detected at 100 and 200 mg/l of Argovit. Mineral nutrient analysis revealed changes in macro and micronutrient concentrations exerted by Argovit. Moreover, the presence of Argovit induced the production of ROS and increased total phenolic content, antioxidant capacity and lipid peroxidation with a dose-dependent effect. Results suggested that the production of ROS and mineral nutrition are key mechanisms of AgNPs-induced hormesis for vanilla. Therefore, the addition of 50 mg/l of Argovit in the culture media had an antimicrobial and hormetic effect. Use of Argovit could be an efficient strategy for commercial micropropagation of vanilla and other species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vanillin is one of the most highly appreciated fragances in the food, pharmaceutical, and fragrance industries (Bory et al. 2008; Greule et al. 2010; Gallage and Moller 2015) and is extracted from the pods of Vanilla planifolia, an orchid native to the tropical forests of southeastern Mexico (Soto-Arenas and Cribb 2010; Salazar-Rojas et al. 2012). This species is currently listed as threatened due to overexploitation that has decimated the wild populations and reduced genetic diversity (Soto-Arenas 1999; SEMARNAT 2010). Vanilla propagation is limited due to low seed viability and low germination rate (Soto-Arenas 2003; Torres-González et al. 2011). For this reason, it is propagated asexually by cuttings; however, this method does not guarantee the health of the new plantings and is limited to a small number of cuttings per donor plant. In this situation, micropropagation emerges as a fast and efficient alternative for mass propagation of clones of high genetic and phytosanitary quality. Recently, the use of TIS has allowed reducing production costs and increasing multiplication coefficients in this species (Ramos-Castellá et al. 2014; Ramírez-Mosqueda and Iglesias-Andreu 2015a, b). However, contamination is a serious problem in TIS during commercial micropropagation. As an alternative, silver nanoparticles (AgNPs, 1–100 nm in diameter) have good capabilities to eliminate fungal, bacterial and virus contamination without adverse effects on plant growth and development (Tahmasbi et al. 2010; Safavi et al. 2011; Sarmast et al. 2011). Several studies have reported the application of AgNPs for disinfecting explants during in vitro establishment (Sreedhar et al. 2009; Mahna et al. 2013; Arab et al. 2014; Moradpour et al. 2016) and sterilizing the culture medium (Soltanloo et al. 2010; Sharma et al. 2012; Salama 2012; Pokhrel and Dubey 2013; Almutairi and Alharbi 2015).
Argovit™ is a commercial formulation of silver nanoparticles already shown to possess a broad spectrum of antimicrobial activity (Podkopaev et al. 2014; Vazquez-Muñoz et al. 2014). Taking into consideration the reported antimicrobial effects of different formulations of AgNPs, we decided to use the commercial preparation Argovit™ as a source of AgNPs. This product is currently approved in Russia and other countries for use in veterinary and human applications (Borrego et al. 2016). In addition to their antibacterial properties, it has been reported that AgNPs have great influence on plant growth and development such as germination, root-shoot ratio, seedling growth, root growth, root elongation, and senescence inhibition (Shah and Belozerova 2009; Ma et al. 2010). The stimulation response can be seen as “an adaptive compensatory process following an initial disruption in homeostasis,” also defined as direct stimulation hormesis (Calabrese et al. 2016a). Previous investigators have documented evidence that some nanoparticles (silver molybdenum, aluminum nanoparticles and titanium dioxide nanoparticles) can initiate hormesis (Lavicoli et al. 2010; Nascarella and Calabrese 2012; Stovbun et al. 2012; Calabrese 2016b). Hormesis can be defined as “a process in which exposure to a low dose of a chemical agent or environmental factor that is damaging at higher doses induces an adaptive beneficial effect on the cell or organism” (Calabrese and Baldwin 2003; Rattan 2006; Calabrese 2008; Hoffmann 2009; Calabrese et al. 2010).
Plants grown in nutrient medium provided with AgNPs can improve nutrient use efficiency (Lee et al. 2012; Jhanzab et al. 2015). On the other hand, the production of reactive oxygen species (ROS) and the consequent induction of stress-induced antioxidants are key mechanisms in metal ion-induced Hormesis in plants (Poschenrieder et al. 2013). The induction of ROS by mild stress (eustress) leading to the activation of antioxidant defences, stress-signalling hormones, or adaptive growth responses are the most probable pathways for hormetic responses. Growth stimulation in plants due to ROS-induced programmed cell death has not been reported (Poschenrieder et al. 2013). To date, there have been only a few reported studies on the impact of AgNPs on vascular plants, but these have consistently shown that AgNPs have detrimental effects on plant growth (Kumari et al. 2009; Stampoulis et al. 2009; Gubbins et al. 2011; Jiang et al. 2012). Prior to the present study, the effect of a TIS and liquid media on in vitro propagation of vanilla had been reported by Ramos-Castellá et al. (2014) and Ramírez-Mosqueda and Iglesias-Andreu (2016), but no research had been reported using Argovit as antimicrobial agent in a TIS and their hormetic response. The objective of this study was to evaluate the antimicrobial and hormetic effect of Argovit on in vitro regeneration of V. planifolia using a TIS.
Materials and methods
Silver nanoparticles characterization
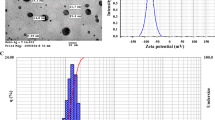
Argovit™ is a formulation of silver nanoparticles, was provided by the Scientific-Production Centre Vector-Vita Ltd, located in Novosibirsk, Russia. Argovit is certified by international institutions for use in veterinary and human health treatments. Argovit solution consists of spherical silver nanoparticles of 35 ± 15 nm, which are clustered silver (12 mg/ml metallic silver) nanoparticles functionalized with 188 mg/ml of polyvinylpyrrolidone (PVP, 10–30 kD) in water with an overall concentration of 200 mg/ml (20%) of AgNPs. Argovit characterization is described by Juarez-Moreno et al. (2016).
Plant material and in vitro establishment
For disinfection and in vitro establishment of axillary buds, the method described by Lee-Espinosa et al. (2008) was followed. Axillary buds of vanilla (V. planifolia), between 3 and 5 mm in diameter, were collected from commercial cultivars in Veracruz, Mexico. Isolated buds were excised from the first to the eighth node of the stem and were disinfected with a surfactant solution (1–2 drops of Tween-20; ICI Americas; in 1 l distilled water) and washed under a slow flow of tap water for 45 min, then a higher flow for 10 more min. Subsequently, buds were transferred to a laminar flow hood, immersed in 70% ethanol (v/v) for 30 s and then rinsed three times with sterile distilled water. Finally, the explants were immersed in sodium hypochlorite at 0.6 and 0.3% (v/v) for 10 and 5 min, respectively, and then rinsed three times with sterile distilled water. The in vitro explants were cultivated in MS (Murashige and Skoog 1962) medium supplemented with 2 mg/l benzyladenine, 30 g/l sucrose and 2.2 g/l Phytagel™ (Sigma-Aldrich, St. Louis, MO). The pH of the culture medium was adjusted to 5.8 with 0.1 N sodium hydroxide and then autoclaved at 1 kg/cm2 for 15 min at 120 °C. Culture vessels used were 125-ml baby food jars with 20 ml of medium. All cultures were incubated at 24 ± 2 °C and maintained under fluorescent light (40–50 µmol/m2/s) and a photoperiod of 16 h.
Effect of silver nanoparticles on in vitro contamination, shoot multiplication and chlorophyll contents
After two subcultures of 60 days in solid medium, 2-cm-long vanilla shoots were used as explant. Five explants (two shoots each) were placed in a 1-l Recipient for Automated Temporary Immersion (RITA®) (VITROPIC, Saint-Mathieu-de-Tréviers, France) containing 200 ml of the culture medium described above, without gelling agent. After sterilization of the culture medium, different solutions of Argovit with various concentrations (0, 25, 50, 100 and 200 mg/l) were added to medium. For each treatment, four RITA® were used. The experiment was replicated three times. Incubation conditions were the same as described above. Immersion frequency was according to Ramos-Castellá et al. (2014). It should be noted that gels and/or agars do not allow disseminating AgNPs. Gels act as solid walls for all types of nanoparticles. For this reason it is not recommended to use gels, but rather aqueous solutions. Contamination rate, number of shoots per explant, shoot length, fresh weight, dry weight and chlorophyll (chl) contents were evaluated after 30 days of culture. Moreover, dry matter content was calculated using dry weight/fresh weight × 100. In addition, chl a, chl b, and total chl contents in shoot leaves were determined according to the method of Harborne (1973).
In order to examine the microbicidal capacity of Argovit, microorganisms that appeared during in vitro shoot multiplication were isolated and identified by SENASICA (National Agro-Alimentary Health, Safety and Quality Service, order service 71,988).
Effect of silver nanoparticles on macro and micronutrient contents
After 30 days of in vitro culture treated with different concentrations of Argovit, an analysis of macro and micronutrient contents was performed. The methods used in the present work were described and recommended by Perkin-Elmer (1996). Minerals were analyzed by dry-ashing 1 g of the sample at 550 °C in a furnace. The ash obtained was dissolved in 1.5 N HCl, filtered through an acid-washed paper filter and brought to standard volume (100 ml) with deionized water. Macronutrients: Ca, Mg, K, Fe and micronutrients: Cu, Zn and Mn were determined using atomic absorption spectrophotometry (Perkin-Elmer Analyst 400). Phosphorus (P) content was determined by employing the Vanado-molybdate method and measured with Perkin-Elmer Lambda 25 UV–Vis colorimeter at 630 nm (AOAC 1990). Boron (B) content was determined by spectrophotometric method with curcumin at 540 nm. Total Nitrogen (TN) was determined by digestion with sulphuric acid followed by distillation with NaOH, using from 0.5 to 1 g of leaves according to the micro-Kjeldahl method (Kjeldahl 1883). All measurements were carried out in triplicate.
Effect of silver nanoparticles on total phenolic content, antioxidant capacity, ROS production and lipid perioxidation
For determination of total phenolic content (TPC), vanilla shoots cultured at different concentrations of Argovit were extracted for 3 h at 250 rpm with 50% methanol in water (v/v) using a mass-solvent ratio of 1:10 (w/v) at 30 °C. The supernatant was recovered and filtered with a vacuum pump through a Whatman grade 1 qualitative filter paper. The resulting extract was concentrated in a rotary evaporator to remove methanol. The bath temperature was 60 °C, and the pressure in the vacuum pump 70 to −90 kPa. After the methanol had been removed, the concentrated extract was lyophilized, and the resulting freeze-dried powder was stored at −80 °C. The TPC of the vanilla shoots was examined using the Folin–Ciocalteu method described by Payet et al. (2006) with slight modifications. Briefly, appropriate dilutions of freeze-dried extract in methanol were transferred to a 96-well microplate (Nunc, Roskilde, Denmark) and oxidized with Folin–Ciocalteu reagent at room temperature for 5 min, resulting in a blue color reaction. After that, sodium carbonate (2%) was added to the well. The microplate was immediately placed and agitated in a microplate reader (Synergy HT, Bio-Tek, Winooski, VM) and then allowed to stand for 15 min. The absorbance was measured at 760 nm and TPC was calculated from a calibration curve of gallic acid (10–150 µg/ml) and expressed as milligrams of gallic acid equivalents (GAE) per gram sample. All assays were carried out in triplicate.
The antioxidant capacity (ORAC) was determined according to the method described by Huang et al. (2002). One gram of freeze-dried extract of vanilla shoots was diluted in methanol for quantification. Analyzes were performed at 37 °C using a pH 7.4 phosphate buffer. The peroxide radicals were produced by 2,2′-Azobis(2-amidinopropane) dihydrochloride (AAPH), using fluorescein as substrate and Trolox as standard. Fluorescence was measured every 2 min for one hour and a calibration curve of Trolox at different concentrations (from 10 to 100 μM) was used in each plate read. All determinations were done in triplicate.
The determination of ROS was performed by a direct colorimetric and fluorometric assay that measures Hydogen Peroxide (H2O2) as a reactive oxygen metabolic by-product. Hydrogen Peroxide Assay Kit-ab102500 (CRT scientific, Mexico) was used. The determination was performed as the supplier suggested; briefly, 5 mg of freeze-dried vanilla shoots were homogenized in cold phosphate buffer solution and washed by centrifugation for 2–5 min at 4 °C and 1000×g to remove any insoluble material. The supernatant was collected, transferred to a clean tube and kept on ice for deproteination with Perchloric acid (PCA). 4 M PCA were added to obtain a final concentration of 1 M; the mixture was vortexed and incubated on ice for 5 min. PCA was precipitated by neutralization with 2 M KOH. Then the mixture was centrifuged at 10,000×g for 20 min at 4 °C and the supernatant was collected. The deproteinized samples were used for Hydrogen Peroxide Assay Kit. To calculate the original concentration a dilution factor of final sample was calculated, taking into account initial sample volume + vol PCA + vol KOH. All determinations were done in triplicate.
To evaluate lipid peroxidation, 200 mg of freeze-dried vanilla shoots were homogenized in 4 ml of 0.1% Trichloroacetic Acid (TCA). Then the extract was centrifuged at 10,000×g for 15 min and the supernatant (1 ml) was collected and mixed with 2 ml of 20% TCA and 2 ml of 0.5% Thiobarbituric acid (TBA). The mixture was heated at 95 °C for 30 min in a fume hood and later cooled on ice. The absorbance of supernatant was read at 532 and 600 nm (Synergy HT, Bio-Tek, Winooski, VM). The concentration of the malondialdehyde (MDA), formed by the decomposition of polyunsaturated fatty acids, was calculated using Beer–Lambert’s equation. All determinations were done in triplicate.
Statistical analyses
The experimental design was completely randomized. The statistical analysis was performed by one-way ANOVA and means were compared with the Tukey test (p ≤ 0.05) using SPSS v. 22 for Windows. Arcsine transformation was performed for experimental data taken in percentages before subjecting them to statistical analysis.
Results
Effect of silver nanoparticles on in vitro contamination
Contamination showed significant differences for treatments with different Argovit concentrations. In the treatments with 50, 100 and 200 mg/l of Argovit, no contamination was observed. The control treatment and the one with 25 mg/l of Argovit showed 16.66 and 8.33% contamination, respectively. The bacterium Bacillus sp. and the fungal microorganisms Cladosporium cladosporiorides and Penicillium sp. were identified by SENASICA.
Effect of silver nanoparticles on in vitro shoot multiplication and chlorophyll contents
The results showed significant differences (p ≤ 0.05) for shoot multiplication variables and chlorophyll contents among the different Argovit doses evaluated (Table 1). The highest number of shoots per explant was obtained at doses of 25 and 50 mg/l of Argovit, with 14.33 and 14.89, respectively. The lowest number of shoots per explant was obtained at doses of 200 mg/l of Argovit with 4.55 shoots. Regarding shoot length, the greatest length was observed in treatments with 25 and 50 mg/l of Argovit, with 14.33 and 14.89 cm, respectively. The concentrations of 100 and 200 mg/l of Argovit showed the shortest length with 1.14 ± 0.07 and 0.82 ± 0.6 cm, respectively (Fig. 1). The results also showed that fresh weight and dry weight were greatest in shoots under doses of 25 and 50 mg/l of Argovit, with 5076.00 ± 266.25/447.20 ± 37 mg of f/d weight and 5829.00 ± 262.05/438.00 ± 18.42 mg of f/d weight, respectively. The lowest fresh weight was found in the control treatment and at a dose of 200 mg/l of Agrovit, with 26.00 ± 130.29 and 2326.22 ± 156.08 mg, respectively, whereas the lowest dry weight was found at the dose of 200 mg/l of Argovit, with 143.80 ± 12.34 mg. Regarding dry matter percentage, it was significantly decreased as the concentrations of AgNPs increased. The highest dry matter percentage was obtained in the control treatment with 10.24 ± 0.56, while the lowest number was obtained at a dose of 200 mg/l of Argovit with 6.15 ± 0.1. Total chlorophyll and chlorophyll a contents were greater in AgNPs than the control treatment. Chlorophyll b content had no significat effect among AgNP doses (Fig. 2).
Effect of silver nanoparticles on macro and micronutrient contents
Significant differences (p ≤ 0.05) were detected for macro and micronutrient contents in Argovit treatments (Table 2). Macronutrients N and Mg accumulated in higher amounts when plants were exposed to 50, 100 and 200 mg/l of Argovit. K and Ca did not show any difference among the treatments. Depletion in micronutrient use efficiency was detected for Fe and Cu in Argovit treatments. The shoot concentrations of Zn, Mn and B did not show any significant difference among the treatments.
Effect of silver nanoparticles on total phenolic content, antioxidant capacity, ROS production and lipid perioxidation
Differences were found in TPC, ORAC, ROS and LP-MDA contents of vanilla shoots obtained in different treatments with Argovit (Fig. 3). Vanilla shoots grown in medium with 25 and 50 mg/l of Argovit had a significant increase in TPC content. The highest ORAC occurred with the 50 mg/l concentration of Argovit, followed by 25 mg/l, while the lowest occurred with the 200 mg/l concentration. For ROS and LP-MDA generation, results show that both indicators increased significantly as the Argovit concentration in the culture medium was increased. The highest values of these indicators were observed at concentrations of 100 and 200 mg/l of Argovit. These results suggest that even the concentrations of 25 and 50 mg/l of Argovit stimulated the production and accumulation of TPC, obtaining greater ORAC due to the accumulation of such compounds.
Effect of argovit concentration on a total phenolic content (TPC), b antioxidant capacity (ORAC), c reactive oxygen species (ROS) production and d lipid peroxidation (LP-MDA) in V. planifolia after 30 days of in vitro culture in a temporary immersion system (TIS). TPC, expressed as milligrams of gallic acid equivalents per gram of dry weight (mg GAE/g DW). ORAC, quantified by oxygen radical absorbance capacity expressed as trolox equivalents per gram of dry weight (TE/g DW). ROS, expressed as mean fluorescence intensity (MFI). LP-MDA, quantified by malondialdehyde assay (MDA), expressed as nanomol per gram of dry weight (nmol/g DW). Different letters denote statistically significant differences according to Tukey’s test (p ≤ 0.05)
Discussion
Effect of silver nanoparticles on in vitro contamination
Antibiotics have been extensively tested for their ability to inhibit or prevent the growth of bacteria in plant in vitro cultures. However, the use of antibiotics has certain limitations. For example, antibiotics are expensive; their range of efficacy against types of bacteria is often narrow, usually are heat-labile, phytotoxic and only effective against bacteria and not fungi (Arab et al. 2014). The antibacterial effects of Ag salts (e.g., silver nitrate, silver sulfadiazine and silver thiosulphate) have been noticed since antiquity (Silver and Phung 1996). Therefore, it is also important that new types of sterilants are introduced to combat the problems faced during in vitro culture establishment (Moradpour et al. 2016). Recently, nanotechnology has amplified the effectiveness of AgNPs as antimicrobial agents compared to other salts due to their extremely large surface area, which provides better contact with microorganisms (Rai et al. 2009). AgNPs are an effective, fast-acting fungicide against a broad spectrum of fungi including genera such as Aspergillus, Candida, and Saccharomyces. AgNPs show potential antimicrobial effects against infectious organisms including Escherichia coli, Bacillus subtilis, Vibrio cholera, Psuedomonas aeruginosa, Salmonella typhus, and Staphylococcus aureus (Sarsar et al. 2014). It is worth mentioning that, in our study, Bacillus sp., Cladosporium cladosporiorides and Penicillium sp. are microorganisms considered as common external contaminants due to handling and laboratory conditions.
Our results agree with those reported by Mahna et al. (2013), who reported that AgNPs used at low concentrations of 100 mg/l in Arabidopsis seeds had an antimicrobial effect without affecting the germination percentage. Arab et al. (2014) reported that concentrations of 100 and 150 mg/l of AgNPs in nodal segments of G × N15 (hybrid of almond × peach) rootstock in immersion and added directly to the culture medium significantly reduces contamination compared to treatment without AgNPs. Moradpour et al. (2016) found that 10 mg/l of AgNPs in an immersion time of 20 min was effective in reducing microbial contamination without affecting the survival of Hevea brasiliensis explants. Various theories have been put forward as to the possible mechanism for the antimicrobial action of AgNPs. According to Liau et al. (1997), AgNPs interact with sulphur containing proteins on the microbial cell membrane causing disruption. Rai et al. (2009) proposed that AgNPs provide an extremely large surface area for better contact with bacteria. These AgNPs get attached to the cell membrane and easily penetrate inside the bacteria.
Effect of silver nanoparticles on in vitro shoot multiplication and chlorophyll contents
AgNPs concentrations applied in this study showed significant differences in shoot multiplication and elongation. The 25 and 50 mg/l AgNPs treatments increased shoot number and length, while the highest concentrations (100 and 200 mg/l of Argovit) inhibited both processes. The fact that low doses of AgNPs have a favorable effect on plant development has been previously observed. Lu et al. (2002) found a benefical effect of AgNPs for Glycine max seed germination and growth. According to Sharon et al. (2010), AgNPs act as growth simulators. In the other hand, the inhibitory effect of using high concentrations of AgNPs has been previously reported by other authors. Kumari et al. (2009) reported that AgNPs at 100 mg/l with particle size <100 nm, type of stabilizer citrate, (provided by Sigma-Aldrich, St. Louis, MO, USA) decrease the mitotic index (27.62%) and cause multiple chromosomal breaks and cell disintegration in Allium cepa. Stampoulis et al. (2009) have demonstrated that AgNPs of 100 mg/l with particle size <100 nm, type of stabilizer citrate, (provided by Sigma-Aldrich, St. Louis, MO, USA) inhibit seed germination, growth and ‘transpiration volume’ of Cucurbita pepo plants. In Arabidopsis thaliana, Geisler-Lee et al. (2013) demonstrated that 534.72 mg/l of AgNPs with particles of three sizes, 20, 40 and 80 nm, stabilizer citrate and phosphate, (from Ted Pella Inc., Redding, CA, USA) inhibited seedling and root elongation. Nair and Chung (2014a, b) showed an increase in lipid peroxidation and ROS production in plant tissues as well as the activation of genes related to oxidative stress at 400 and 500 mg/l of CuONPs. Lee et al. 2012 observed that AgNPs with particle size ranged from 5 to 25 nm, type of stabilizer citrate, (provided by ABC Nanotech Daejeon, KOR) reduced growth in Phaseolus radiatus and Sorghum bicolor cultivated in soil or agar-based medium. Amooaghaie et al. (2015) found that AgNPs at 100 mg/l with silver particle size approximately 40 nm, type of stabilizer not mentioned, (from Shanghai Huzheng Nanotechnology Co. LTD AGS-WMB1000C, Shanghai, CHN), reduced germination in Brassica nigra. Similarly, Gubbins et al. (2011) reported that AgNPs at 160 mg/l could inhibit the growth of Lemna minor at 100, 125 and 150 mg/l with particle size of 10–20 nm and citrate as a stabilizer.
The present results demonstrated that the total chlorophyll and chlorophyll a contents appeared greater in V. planifolia shoots growing under AgNPs, while the lowest chlorophyll contents were found in the control treatment. These results are consistent in that Argovit-AgNPs have an important role in the synthesis of photosynthetic pigments. Similar results were found by Najafi and Jamei et al. (2014 ) who reported that applying 50 mg/l of AgNPs in Vigna radiata plants dramatically increased total c|hl, chl-a, chl-b and root fresh weight but did not affect shoot fresh weight. AgNPs are beneficial for Glycine max seed germination and growth (Lu et al. 2002), and act as growth simulators (Sharon et al. 2010). Homaee and Ehsanpour (2015) in Solanum tuberosum reported that 2 mg/l of AgNPs with particle size of 20 nm, Polyvinylpyrrolidone (PVP) as a stabilizer, (from US Research Nanomaterials Inc., Houston, TX, USA) improved some growth parameters such as dry weight, root length and leaf area. Furthermore, AgNPs exhibited toxicity at 10 and 20 mg/l concentrations.
Different hypotheses could explain the increase in shoot number and length, the first being related to the increase in the accumulation of N, Mg and Fe. Together, these elements are associated with biosynthesis of chlorophyll, which is a molecule vital for photosynthesis during development. The second hypothesis, put forward by Yin et al. (2012), suggests that AgNP-induced damage may cause the loss of gravitropism in roots through disruption of auxin transport. In our opinion, one of the effects of auxins is related to apical dominance, in that losing apical dominance favors the development of new shoots. However, high concentrations of AgNPs not only affect auxin transport, but also induce phytotoxicity. Probably, phytotoxicity caused by the silver ion reduced shoot number and length.
Effect of silver nanoparticles on macro and micronutrient contents
Vanilla shoots grown in culture media with silver nanoparticles accumulated a larger amount of N and Mg, compared to the control treatment. Nitrogen is a constituent of many important molecules, including proteins, nucleic acids, certain hormones (e.g., indole-3-acetic acid; cytokinin, etc.), and chlorophyll (Hopkins and Huner 2004). By far the largest proportion of Mg is found in the porphyrin moiety of the chlorophyll molecule, but it is also required to stabilize ribosome structure and is involved as an activator for numerous critical enzymes. Magnesium is critical to reactions involving ATP, where it serves to link the ATP molecule to the active site of the enzyme. Magnesium is also an activator for both ribulosebisphosphate carboxylase and phosphoenolpyruvate carboxylase, two critical enzymes in photosynthetic carbon fixation (Hopkins and Huner 2004). Iron and Copper were the only micronutrients with reduced levels in the shoots caused by the treatment with AgNPs. Fe is related to two important functions in the plant, it is part of the catalytic group for many redox enzymes and it is required for the synthesis of chlorophyll and a constituent of several oxidase enzymes, such as catalase and peroxidase (Hopkins and Huner 2004). Copper (Cu) is an essential micronutrient which is required for normal growth and development of plants (Li et al. 2015). It is a vital component involved in various processes, including the electron transfer reactions of respiration and photosynthesis or the removal of superoxide radicals (Adrees et al. 2015). At the cellular level, Cu plays an important role in signalling of transcription, protein trafficking machinery, oxidative phosphorylation, and iron mobilization (Da Costa and Sharma 2016). This explains why plants had lower growth at high concentrations of AgNPs, as they could block nutrient transportation by ionic channel competition.
To date, there is not enough information to form a clear idea of how AgNPs could affect the uptake of macro and microelements. Zuverza-Mena et al. (2016) suggest that it is possible that AgNPs physically block the diffusion pathway or the channels for active absorption. Shoots obtained in this experiment showed no significant differences in P, K, Ca, Cu, Zn and Mn contents. This can be achieved by immobilization in culture media or, alternatively, these micronutrients are probably less required during stress metabolism. Martínez-Fernández et al. (2016), in Helianthus annuus in a hydroponic culture, found that the application of iron oxide nanoparticles (100 mg/l) reduced the concentrations of the macronutrients Ca, K, Mg and S in the shoot relative to the control plants. Jhanzab et al. (2015), in Triticum aestivum, soaked the pot soil with different AgNPs concentrations, finding that 25 mg/l of AgNPs significantly enhanced the growth and yield attributes, N–P–K uptake and nutrient use efficiency, while a 75 mg/l concentration resulted in a decrease in grain yield.
Effect of silver nanoparticles on total phenolic content, antioxidant capacity, ROS production and lipid perioxidation
In Eichhornia crassipes (Mart) Solms, Rani et al. (2016) observed a quantitative increase in the amounts of total phenol contents at all concentrations with a peak at 10 mg/l of AgNPs with particle size <100 nm, Polyvinylpyrrolidone (PVP) as a stabilizer, (provided by Sigma-Aldrich, St. Louis, MO, USA) and increased SOD activity was found in the plants treated with higher concentrations of AgNPs. According to Yasur and Rani (2013), AgNPs can generate ROS and the plant tries to reverse this effect by increasing production of phenolic compounds with high antioxidant activity. Results obtained in this work suggest that vanilla shoots increase their production of phenolic compounds to try to counter the production of ROS produced due to Argovit. However, it was observed that at high concentrations (100 and 200 mg/l of Argovit), phenolic compound content and antioxidant capacity decrease significantly, while ROS production increases. These results suggest that Argovit at high concentrations inhibit the production of phenolic compounds and antioxidant capacity, thereby favoring increased ROS production. According to López-Moreno et al. (2016), an increase in ROS inside cellular membranes is due to environmental factors that induce stress to the plant. Heavy metals increase ROS inside cellular membranes, causing considerable damage and disrupting normal cellular activity in plants. This trend can be attributed to the accumulation of AgNPs in plant tissues which should have induced an oxidative stress response. This fact probably causes a decrease in shoot number and length, since having a higher concentration of reactive oxygen species enhances apoptosis. This effect also has an impact on the macroscopic appearance of leaf tissue, as shown in Fig. 1.
In Solanum tuberosum, Homaee and Ehsanpour (2015) found that applying 2 mg/l of AgNPs with particle size of 20 nm, Polyvinylpyrrolidone (PVP) as a stabilizer, (from US Research Nanomaterials Inc., Houston, TX, USA) decreases shoot length. The most prominent symptoms of oxidative stress generation in biological membranes can be observed due to the peroxidation of lipids (Rajeshwari et al. 2016). Thus, the fatty acid can get converted into toxic lipid peroxides by the hydroperoxyl radical, and thus destroy the biological membranes (Kumari et al. 2011). In this study lipid peroxidation increased significantly when raising the Argovit concentration, indicating that ROS production caused by AgNPs is capable of enhancing lipid peroxidation. The plant tries to counter this effect by inducing the production of phenolic compounds; however, at high concentrations of Argovit (100 and 200 mg/l), ROS production is so high that it probably destabilizes the cell membrane and causes cell death. In a study conducted by Nair and Chung (2015) on germination of Vigna radiata L. in the presence of 20 and 50 mg/l of AgNPs with particle size of 20 nm, type of stabilizer citrate and phosphate, (from Ted Pella Inc., Redding, CA, USA), it was shown that the higher the AgNPs concentration in the culture medium, the greater the ROS production in the plant tissues.
Effect of silver nanoparticles as an elicitor of hormesis
This study demonstrates a hormetic effect of studied Argovit-AgNPs on shoot number and length in vanilla. It has been claimed that low concentrations of toxic metals induce hormetic effects through activating plant stress defense mechanisms (Poschenrieder et al. 2013). The beneficial effects of hormesis may arise from endogenous over-compensatory changes that the cell and organism use to repair or prepare for damage from larger magnitude, adaptively similar external threats from the environment (Wiegant et al. 2011; Stark 2012). Hormesis is understood as a dynamic adaptive response or biological plasticity of a complex living system at the level of the whole organism to intermittent mild stressors of various categories (Calabrese 2013). Calabrese and Mattson (2011) have used hormesis as a quantitative estimate of biological plasticity due to adaption through the activation of defenses by cross signaling. Other researchers have proposed that organisms exposed to intermittently-timed hormetic stimuli could shift and shape epigenetic expression toward increased resilience against stressors of higher intensity, disease, and their aging (Vaiserman 2011; Stark 2012).
Hormetic stress response is characterized by activation of cellular defenses, in particular increasing antioxidant systems and proteins related to cellular survival (Luna-Lopez et al. 2014). Plant cell walls function as natural sieves. According to Thuesombat et al. (2014), the smaller sizes of AgNPs had the higher ability for penetrating into plant roots, as AgNPs with the smaller sizes could be found at higher concentration in root tissues. The pore size of plant cell walls is usually in the range of a few nanometers (Carpita et al. 1979). This may partially explain why our AgNPs with hydrodynamic size, which is 70 nm, have effects on the evaluated variables. They are gradually absorbed while their ionic forms are immediately included into the biochemical reactions (Taran et al. 2016). Nanoparticles take part in the electron transfer in plants and thus increase the activity of plant enzymes, intensify photosynthesis processes, and have a direct influence on plant mineral nutrition (Hong et al. 2005; Kole et al. 2013; Du et al. 2015; Razzaq et al. 2016). Therefore, the size and other important characteristics could also modify the biological properties of nanoparticles. In this study, Argovit had effect on bacterial contamination, development, nutrient accumulation, metabolism of antioxidants and ROS generation. Results obtained in the present work show a relationship between shoot number and length with antioxidant activity promoted by the application of different concentrations of Argovit. Therefore, the effect observed in the hormetic shoot multiplication phase may be explained by the following mechanism: the increase in shoot production and length in the case of the 50 mg/l concentration of Argovit is probably due to the accumulation of N and Mg, a response observed at all Argovit concentrations evaluated. Additionally, at the 25 and 50 mg/l concentrations of Argovit, oxidative stress increased but was offset by the high antioxidant capacity, resulting in intensive shoot production. Finally, at the 100 and 200 mg/l concentration of Argovit, oxidative stress continued to increase while antioxidant capacity decreased, leading to the reduction in shoot number and length.
The addition of silver compounds to culture medium is being used in tissue culture activities. Silver nitrate (AgNO3) and silver thiosulphate (Ag(S2O3)2)3 have been used as an inhibitor of the physiological action of ethylene to enhance shoot production (Hyde and Phillips 1996; Santana-Buzzy et al. 2006; San et al. 2015). The inhibiting effect of AgNO3 and (Ag(S2O3)2)3 on ethylene synthesis in plant tissues is believed to act through the attachment of Ag+ to ethylene binding sites. In this study devoted to the application of Argovit in plant tissue culture, the concentration of Argovit (3.0 mg/l metallic silver concentration) with polivinilpirollidine (PVP) as a stabilizer is indicated. This will allow future comparisons of biological activities per 1 mg of active component (metallic silver). Before this paper, the concentration of metallic silver as an active component was not mentioned in similar publications. Metallic silver concentration can change drastically depending on AgNPs type. For example, in our case the metallic silver concentration represents only 5% wt., while PVP content is 95% wt. of total AgNPs concentration. Works on the application of nanomaterials recommend presenting detailed information on their characteristics. This research study indicates the interaction of the explant source with the environment and the effective application of the silver nanoparticles in reducing bacterial contamination. The application of 50 mg/l of Argovit was the most appropriate concentration to reduce bacterial contamination and produce a hormetic response on growth and differentiation. Argovit had an effect on the synthesis of photosynthetic pigments, nutrient accumulation, metabolism of antioxidants and ROS generation. The results demonstrated that using Argovit in culture medium significantly affects in vitro parameters of development, nutrient contents, metabolism of antioxidants and ROS generation of vanilla. Moreover, N and Mg contents in the shoots increased significantly (by 300 and 118% respectively) when Argovit was applied compared to the control plants, while Fe and Cu contents changed considerably. Oxidative stress caused by Argovit increased ROS production and lipid peroxidation. As a response mechanism, antioxidant capacity also increased, up to the 50 mg/l concentration. From this Argovit concentration upwards, antioxidant capacity decreased and this probably caused the decrease in shoot number and length. Results obtained in this promising work open up the possibility of wide applications of this type of AgNPs in agriculture. We suggest more studies to evaluate the potential of using Argovit in plant tissue culture.
References
Adrees M, Ali S, Rizwan M, Ibrahim M, Abbas F, Farid M, Zia-urRehman M, Irshad MK, Bharwana SA (2015) The effect of excess copper on growth and physiology of important food crops: a review. Environ Sci Pollut Res 22:8148–8162
Almutairi ZM, Alharbi A (2015) Effect of silver nanoparticles on seed germination of crop plants. Int Sch Sci Res Innov 9(6):551–555
Amooaghaie R, Tabatabaei F, Ahadi AM (2015) Role of hematin and sodium nitroprusside in regulating Brassica nigra seed germination under nanosilver and silver nitrate stresses. Ecotoxicol Environ Saf 113:259–270. doi:10.1016/j.ecoenv.2014.12.017
AOAC (1990) Official methods of analysis of the AOA, 16th edn. Association of Official Analytical Chemists, Washington
Arab M, Yadollahi M, Hosseini-Mazinani A, Bagheri S (2014) Effects of antimicrobial activity of silver nanoparticles on in vitro establishment of G × N15 (hybrid of almond × peach) rootstock. J Genet Eng Biotechnol 12:103–110. doi:10.1016/j.jgeb.2014.10.002
Borrego B, Lorenzo G, Mota-Morales JD, Almanza-Reyes H, Mateos F, Lopez-Gil E, De la Losa N, Burmistrov VA, Pestryakov AN, Brun A, Bogdanchikova N (2016) Potential application of silver nanoparticles to control the infectivity of Rift Valley fever virus in vitro and in vivo. Nanomedicine 12:1185–1192. doi:10.1016/j.nano.2016.01.021
Bory S, Lubinsky P, Risterucci AM, Noyer JL, Grisoni M, Duval MF, Besse P (2008) Patterns of introduction and diversification of Vanilla planifolia (Orchidaceae) in Reunion Island (Indian Ocean). Am J Bot 95:805–815. doi:10.3732/ajb.2007332
Calabrese EJ (2008) Converging concepts: adaptive response, preconditioning, and the Yerkes–Dodson law are manifestations of hormesis. J Ageing Res Rev 7:8–20
Calabrese EJ (2013) Biphasic dose responses in biology, toxicology and medicine: accounting for their generalizability and quantitative features. Environ Pollut 182:452–460. doi:10.1016/j.envpol.2013.07.046
Calabrese EJ (2016a) Preconditioning is hormesis part I: documentation, dose-response features and mechanistic foundations. Pharmacol Res. doi:10.1016/j.phrs.2015.12.021
Calabrese EJ (2016b) Preconditioning is hormesis part II: How the conditioning dose mediates protection: dose optimization within temporal and mechanistic frameworks. Pharmacol Res. doi:10.1016/j.phrs.2015.12.020
Calabrese EJ, Baldwin LA (2003) Hormesis at the National Toxicology Program (NTP): evidence of hormetic dose responses in NTP doserange studies. Nonlinear Biol Toxicol Med 1:455–467
Calabrese EJ, Mattson MP (2011) Hormesis provides a generalized quantitative estimate of biological plasticity. J Cell Commun Signal 5:25–38. doi:10.1007/s12079-011-0119-1
Calabrese V, Cornelius C, Mancuso C, Lentile R, Stella AM, Butterfield DA (2010) Redox homeostasis and cellular stress response in aging and neurodegeneration. Methods Mol Biol 610:285–308. doi:10.1007/978-1-60327-029-8_17
Carpita N, Sabularse D, Montezinos D, Delmer DP (1979) Determination of the pore size of cell walls of living plant cells. Science 205:1144–1147. doi:10.1126/science.205.4411.1144
Da Costa MVJ, Sharma PK (2016) Effect of copper oxide nanparticles on growth, morphology, photosynthesis, and antioxidant response in Oriza sativa. Photosynthetica 54(1):110–119. doi:10.1007/s11099-015-0167-5
Du W, Gardea-Torresdey JL, Ji R, Yin Y, Zhu J (2015) Physiological and biochemical changes imposed by CeO2 nanoparticles on wheat: a life cycle field study. Environ Sci Technol 49:11884–11893. doi:10.1021/acs.est.5b03055
Gallage NJ, Moller BL (2015) Vanillin-bioconversion and bioengineering of the most popular plant flavor and its de novo byosynthesis in the vanilla orchid. Mol Plant 8:40–57. doi:10.1016/j.molp.2014.11.008
Geisler-Lee J, Wang Q, Yao Y, Zhang W, Geisler M, Li K, Huang Y, Chen Y, Kolmakov A, Ma X (2013) Phytotoxicity, accumulation and transport of silver nanoparticles by Arabidopsis thaliana. Nanotoxicology 7:323–337. doi:10.3109/17435390.2012.658094
Greule M, Tumino L, Kronewald T, Hener U, Schleucher J, Mosandl A, Keppler F (2010) Improved rapid authentication of vanillin using δ13 C and δ2 H values. Eur Food Res Technol 231:933–941. doi:10.1007/s00217-010-1346-z
Gubbins EJ, Batty LC, Lead JR (2011) Phytotoxicity of silver nanoparticles to Lemna minor L. Environ Pollut 159:1551–1559. doi:10.1371/journal.pone.0047674
Harborne JB (1973) Nitrogen compounds. In: Harborne JB (ed) Phytochemical methods. Springer, The Netherlands, pp 166–211
Hoffmann GR (2009) A perspective on the scientific, philosophical, and policy dimensions of hormesis. Dose Response 7:1–51. doi:10.2203/dose-response.08-023.Hoffmann
Homaee MB, Ehsanpour AA (2015) Physiological and biochemical responses of potato (Solanum tuberosum) to silver nanoparticles and silver nitrate treatments under in vitro conditions. Indian J Plant Physiol 20:353–359. doi:10.1007/s40502-015-0188-x
Hong F, Zhou J, Liu C, Yang F, Wu C, Zheng L, Yang P (2005) Effect of nano-TiO2 on photochemical reaction of chloroplasts of spinach. Biol Trace Elem Res 105:269–279. doi:10.1385/BTER:105:1-3:269
Hopkins WG, Huner NPA (2004) Introduction to plant physiology. Wiely, New York
Huang D, Ou B, Hampsch-Woodill M, Flanagan JA, Prior RL (2002) High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J Agric Food Chem 50:4437–4444. doi:10.1021/jf0201529
Hyde CL, Phillips GC (1996) Silver nitrate promotes shoot development and plant regeneration of chili pepper (Capsicum annuum L.) via organogenesis. In Vitro Cell Dev Biol Plant 32:72–80. doi:10.1007/BF02823134
Jhanzab HM, Razzaq A, Jilani G, Rehman A, Hafeez A, Yasmeen F (2015) Silver nano-particles enhance the growth, yield and nutrient use efficiency of wheat. Int J Agron Agri Res 7:15–22
Jiang HS, Li M, Chang FY, Li W, Yin LY (2012) Physiological analysis of silver nanoparticles and AgNO3 toxicity to Spirodela polyrhiza. Environ Toxicol Chem 31:1880–1886. doi:10.1002/etc.1899
Juarez-Moreno KO, Gonzalez EB, Giron-Vazquez N, Chavez A, Mota-Morales JD, Perez-Mozqueda LL, García-García MR, Pestryakov A, Bogdanchikova N (2016) Comparison of cytotoxicity and genotoxicity effects of silver nanoparticles on human cervix and breast cancer cell lines. Human Exper Toxicol 1–18. doi:10.1177/0960327116675206.
Kjeldahl J (1883) A new method for the determination of nitrogen in organic matter. Z Anal Chem 22:366–382
Kole C, Kole P, Randunu KM, Choudhary P, Podila R, Ke PC (2013) Nanobiotechnology can boost crop production and quality: first evidence from increased plant biomass, fruit yield and phytomedicine content in bitter melon (Momordica charantia). BMC Biotechnol 9:37. doi:10.1186/1472-6750-13-37
Kumari MS, Mukherjee A, Chandrasekaran N (2009) Genotoxicity of silver nanoparticles in Allium cepa. Sci Total Environ 407:5243–5246. doi:10.1016/j.scitotenv.2009.06.024
Kumari MS, Khan S, Pakrashi S, Mukherjee A, Chandrasekaran N, Hazard J (2011) Cytogenetic and genotoxic effects of zinc oxide nanoparticles on root cells of Allium cepa. J Hazard Mater 190:613–621. doi:10.1016/j.jhazmat.2011.03.095
Lavicoli I, Calabrese EJ, Nascarella MA (2010) Exposure to nanoparticles and hormesis. Dose Response 8 4):501–517. doi:10.2203/dose-response
Lee WM, Kwak JI, An YJ (2012) Effect of silver nanoparticles in crop plants Phaseolus radiatus and Sorghum bicolor: media effect on phytotoxicity. Chemosphere 86:491–499. doi:10.1016/j.chemosphere.2011.10.013
Lee-Espinosa HE, Murguía-González J, García-Rosas B, Córdova-Contreras AL, Laguna-Cerda A, Mijangos-Cortés JO, Barahona-Pérez LF, Iglesias-Andréu LG, Santana-Buzzy N (2008) In vitro clonal propagation of vanilla (Vanilla planifolia ‘Andrews’). Hortic Sci 43:454–458
Li S, Zhang G, Gao W, Zhao X, Deng C, Lu L (2015) Plant growth, development and change in GSH level in safflower (Carthamus tinctorius L.) exposed to copper and lead. Arch Biol Sci Belgrade 67(2):385–396. doi:10.2298/ABS140910006L
Liau SY, Read DC, Pugh WJ, Furr JR, Russell AD (1997) Interaction of silver nitrate with readily identifiable groups: relationship to the antibacterial action of silver ions. Lett Appl Microbiol 25:279–283
López-Moreno ML, Avilés LL, Pérez NG, Irizarry BÁ, Perales O, Cedeno, Mattei Y (2016) Effect of cobalt ferrite (CoFe2O4) nanoparticles on the growth and development of Lycopersicon lycopersicum (tomato plants). Sci Total Environ 550:45–52. doi:10.1016/j.scitotenv.2016.01.063
Lu C, Zhang C, Wen J, Wu G, Tao M (2002) Research of the effect of nanometer materials on germination and growth enhancement of Glycine max and its mechanism. Soybean Sci 21:168–171
Luna–López A, González-Puertos VY, López-Diazguerrero NE, Königsberg M (2014) New considerations on hormetic response against oxidative stress. J Cell Commun Signal 8:323–331. doi:10.1007/s12079-014-0248-4
Ma Y, Kuang L, He X, Bai W, Ding Y, Zhang Z, Zhao Y, Chai Z (2010) Effect of rare earth oxide nanoparticles on root elongation of plants. Chemosphere 78:273–279. doi:10.1016/j.chemosphere.2009.10.050
Mahna N, Vahed SZ, Khani S (2013) Plant in vitro culture goes nano: nanosilver-mediated decontamination of ex vitro explants. J Nanomed Nanotechol 4:161. doi:10.3389/fpls.2016.01330
Martínez-Fernández D, Barroso D, Komárek M (2016) Root water transport of Helianthus annuus L. under iron oxide nanoparticle exposure. Environ Sci Pollut Res 23(2):1732–1741. doi:10.1007/s11356-015-5423-5
Moradpour M, Aziz MA, Abdullah SNA (2016) Establishment of in vitro culture of rubber (Hevea brasiliensis) from field-derived explants: effective role of silver nanoparticles in reducing contamination and browning. J Nanomed Nanotechnol 7:375. doi:10.4172/2157-7439.1000375
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Nair PG, Chung I (2014a) A mechanistic study on the toxic effect of copper oxide nanoparticles in soybean (Glycine max L.) root development and lignification of root cells. Biol Trace Element Res 162:342–352
Nair PG, Chung I (2014b) Impact of copper oxide nanoparticles exposure on Arabidopsis thaliana growth, root system development, root lignificaion, and molecular level changes. Environ Sci Pollut Res 21:12709–12722
Nair PG, Chung I (2015) Physiological and molecular level studies on the toxicity of silver nanoparticles in germinating seedlings of mung bean (Vigna radiata L.). Acta Physiol Plant 37:1–11. doi:10.1007/s11738-014-1719-1
Najafi S, Jamei R (2014) Effect of silver nanoparticles and Pb (NO3)2 on the yield and chemical composition of mung bean (Vigna radiata). J Stress Physiol Biochem 10:317–325
Nascarella MA Calabrese EJ (2012) A method to evaluate hormesis in nanoparticle dose-responses. Dose Response 10:344–354. doi:10.2203/dose-response.10-025
Payet B, Shum CSA, Smadja J (2006) Comparison of the concentrations of phenolic constituents in cane sugar manufacturing products with their antioxidant activities. J Agric Food Chem 54:7270–7276
Perkin-Elmer B (1996) Analytical methods for atomic absorption spectroscopy. The Perkin Elmer Corporation, Norwalk, CT, pp 132–145
Podkopaev DO, Shaburova LN, Balandin GV, Kraineva OV, Labutina NV, Suvorov OA (2014) Comparative evaluation of antimicrobial activity of silver nanoparticles. Nanotechnol Rus 9:93–97
Pokhrel LR, Dubey B (2013) Evaluation of developmental responses of two crop plants exposed to silver and zinc oxide nanoparticles. Sci Total Environ 45:321–332. doi:10.1016/j.scitotenv.2013.02.059
Poschenrieder C, Cabot C, Martos S, Gallego B, Barceló J (2013) Do toxic ions induce hormesis in plants? Plant Sci 212:15–25. doi:10.1016/j.plantsci.2013.07.012
Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27:76–83. doi:10.1016/j.biotechadv.2008.09.002
Rajeshwari A, Suresh S, Chandrasekaran N, Mukherjee A (2016) Toxicity evaluation of gold nanoparticles using an Allium cepa bioassay. RSC Adv 6:24000–24009. doi:10.1039/c6ra04712b
Ramírez-Mosqueda MA, Iglesias-Andreu LG (2015a) Indirect organogenesis and assessment of somaclonal variation in plantlets of Vanilla planifolia Jacks. Plant Cell Tissue Organ Cult 123:657–664
Ramírez-Mosqueda MA, Iglesias-Andreu LG (2016) Evaluation of different temporary immersion systems (BIT®, BIG, and RITA®) in the micropropagation of Vanilla planifolia Jacks. In Vitro Cell Dev Biol Plant 52:154–160. doi:10.1007/s11627-015-9735-4
Ramírez-Mosqueda MA, Iglesias-Andreu LG, Luna-Rodríguez M, Castro-Luna AA (2015b) In vitro phytotoxicity of culture filtrates of Fusarium oxysporum f. sp.vanillae in Vanilla planifolia Jacks. Sci Hortic 197:573–578
Ramos-Castellá A, Iglesias-Andreu LG, Bello-Bello J, Lee-Espinosa H (2014) Improved propagation of vanilla (Vanilla planifolia Jacks. ex Andrews) using a temporary immersion system. In Vitro Cell Dev Biol Plant 50:576–581
Rani PU, Yasur J, Loke KS, Dutta D (2016) Effect of synthetic and biosynthesized silver nanoparticles on growth, physiology and oxidative stress of water hyacinth: Eichhornia crassipes (Mart) Solms. Acta Physiol Plant 38:58. doi:10.1007/s11738-016-2074-1
Rattan SI (2006) Hormetic modulation of aging and longevity by mild heat stress. Dose Response 3:533–546
Razzaq A, Ammara R, Jhanzab HM, Mahmood T, Hafeez A, Hussain S (2016) A novel nanomaterial to enhance growth and yield of wheat. J Nanosci Technol 2:55–58
Safavi K, Esfahanizadeh M, Mortazaeinezahad DH, Dastjerd H (2011) The study of nano silver (NS) antimicrobial activity and evaluation of using ns in tissue culture media. Int Conf Life Sci Technol IPCBEE 3:159–161
Salama HMH (2012) Effects of silver nanoparticles in some crop plants, common bean (Phaseolus vulgaris L.) and corn (Zea mays L.). Int Res J Biotechnol 3:190–197
Salazar-Rojas VM, Herrera-Cabrera BE, Delgado-Alvarado A, SotoHernández M, Castillo-González F, Cobos-Peralta M (2012) Chemotypical variation in Vanilla planifolia Jack. (Orchidaceae) from the Puebla-Veracruz Totonacapan region. Genet Res Crop Evol 59:875–887
San B, Li, Hu Q, Reighard GL, Luo H (2015) Adventitious shoot regeneration from in vitro cultured leaf explants of peach rootstock Guardian® is significantly enhanced by silver thiosulfate. Plant Cell Tissue Organ Cult 120:757. doi:10.1007/s11240-014-0645-7
Santana-Buzzy N, Canto-Flick A, Iglesias-Andreu LG, Montalvo-Peniche MC, López-Puc G, Barahona-Pérez F (2006) Improvement of in vitro culturing of Habanero pepper by inhibition of ethylene effects. HortScience 41:405–409
Sarmast M, Salehi H, Khosh-Khui M (2011) Nano silver treatment is effective in reducing bacterial contaminations of Araucaria excelsa R. Br. var. glauca explants. Acta Biol Hung 62:477–484. doi:10.1556/ABiol.62.2011.4.12
Sarsar V, Selwal KK, Selwal MK (2014) Nanosilver: potent antimicrobial agent and its biosynthesis. Afr J Biotechnol 13:546–554
SEMARNAT (Secretaría de Medio Ambiente y Recursos Naturales) (2010) Norma Oficial Mexicana (NOM-059-ECOL-2001) de Protección especial de especies nativas de México de Flora y Fauna silvestres. Diario Oficial de la Federación, Marzo 6, pp 2–56 (in Spanish)
Shah V, Belozerova I (2009) Influence of metal nanoparticles on the soil microbial community and germination of lettuce seeds. Water Air Soil Pollut 197:143–148
Sharma P, Bhatt D, Zaidi MGH, Saradhi PP, Khanna PK, Arora S (2012) Silver nanoparticle-mediated enhancement in growth and antioxidant status of Brassica juncea. Appl Biochem Biotechnol 167:2225–2233
Sharon M, Choudhary AK, Kumar R (2010) Nanotechnology in agricultural diseases and food safety. J Phytol 2:83–92
Silver S, Phung LT (1996) Bacterial heavy metal resistance: new surprises. Annu Rev Microbiol 50:753–789
Soltanloo H, Alimohammadi M, Ramezanpour SS, Bagher M, Najar B (2010) Nansilver colloid: a novel antimicrobial candidate applicable in plant tissue culture medium. Austr J Basic Appl Sci 4(10):5338–5345
Soto-Arenas MA (1999) Filogeografía y recursos genéticos de las vainillas de México. Instituto Chinoin AC. SNIB-CONABIO proyecto No. J101, México (in Spanish)
Soto-Arenas MA (2003) Vanilla. In: Pridgeon AM, Cribb PJ, Chase MW, Rasmussen FN (eds) Genera Orchidacearum, vol 3, Orchidoideae (Part 2) Vanilloideae. Oxford University Press, Oxford, pp 321–334
Soto-Arenas MA, Cribb P (2010) A new infrageneric classification and synopsis of the genus vanilla plum. ex Mill. (Orchidaceae: Vanillinae). Lakesteriana 9:355–398
Sreedhar RV, Venkatachalam L, Neelwarne B (2009) Hyperhydricityrelated morphologic and biochemical changes in vanilla (Vanilla planifolia). J Plant Growth Regul 28:46–57
Stampoulis D, Sinha SK, White JC (2009) Assay-dependent phytotoxicity of nanoparticles to plants. Environ Sci Technol 43:9473–9479. doi:10.1021/es901695c
Stark M (2012) The sandpile model: optimal stress and hormesis. Dose Response 10:66–74. doi:10.2203/dose-response.11-010.Stark
Stovbun SV, Kiselev AV, Zanin AM, Kalinina TS, Voronina TA, Mikhailov AI (2012) Effects of physicochemical forms of phenazepam and Panavir on their action at ultra-low doses. Bull Exp Biol Med 153:455–458
Tahmasbi D, Zharghami R, Vatanpour AA Chaeich M (2010) Effects of nanosilver and nitrogen biofertilizer on yield and yield components of potato microtubes. Inter J Agric Biol 13:986–990
Taran N, Batsmanova L, Kovalenko M, Okanenko A (2016) Impact of metal nanoform colloidal solution on the adaptive potential of plants. Nanoscale Res Lett 11:89. doi:10.1186/s11671-016-1294-z
Thuesombat P, Hannongbua S, Akasit S, Chadchawan S (2014) Effect of silver nanoparticles on rice (Oryza sativa L. cv. KDML 105) seed germination and seedling growth. Ecotoxicol Environ Saf 104:302–309
Torres-González MJ, Aguirre-Medina JF, Iracheta-Donjuan L (2011) Germinación de semillas y obtención de plántulas de Vanilla planifolia Andrews en condiciones in vitro. Agroproductividad 4:3–8 (in Spanish)
Vaiserman AM (2011) Hormesis and epigenetics: is there a link?. Ageing Res Rev 10:413–421. doi:10.1016/j.arr.2011.01.004
Vazquez-Muñoz R, Avalos-Borja M, Castro-Longoria E (2014). Ultrastructural analysis of Candida albicans when exposed to silver nanoparticles. PloS One 9:e108876. doi:10.1371/journal.pone.0108876
Wiegant FA, Prins HA, Van Wijk R (2011) Postconditioning hormesis put in perspective: an overview of experimental and clinical studies. Dose Response 9:209–224. doi:10.2203/dose-response.10-004
Yasur J, Rani PU (2013) Environmental effects of nanosilver: impact on castor seed germination, seedling growth, and plant physiology. Environ Sci Pollut Res 20:8636–8648. doi:10.1007/s11356-013-1798-3
Yin L, Colman BP, McGill BM, Wright JP, Bernhardt ES (2012) Effects of silver nanoparticle exposure on germination and early growth of eleven wetland plants. PLoS One 7:e47674. doi:10.1371/journal.pone.0047674
Zuverza-Mena N, Armendariz R, Peralta-Videa JR, Gardea-Torresdey JL (2016) Effects of silver nanoparticles on radish sprouts: root growth reduction and modifications in the nutritional value. Front Plant Sci 7:9. doi:10.3389/fpls.2016.00090
Acknowledgements
This work was supported by Mexican PAPIIT-UNAM No IT200114 and CONACyT No 270242 projects.
Author contributions
All authors listed have made substantial, direct and intellectual contribution to the work, and approved it for publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Spinoso-Castillo, J.L., Chavez-Santoscoy, R.A., Bogdanchikova, N. et al. Antimicrobial and hormetic effects of silver nanoparticles on in vitro regeneration of vanilla (Vanilla planifolia Jacks. ex Andrews) using a temporary immersion system. Plant Cell Tiss Organ Cult 129, 195–207 (2017). https://doi.org/10.1007/s11240-017-1169-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-017-1169-8