Abstract
Psidium friedrichsthalianum (O. Berg) Nied. (Cas) is a guava species from Myrtaceae family whose fruits are very attractive for their organoleptic properties, its economic and nutritional importance and its use as rootstocks. One of the main problems for in vitro propagation of Cas is contamination, and silver nanoparticles (AgNPs), are the most useful in the treatment of plant tissues due to their microbicidal action. Thus, the microbicidal effect of Argovit™ AgNPs (suspension in water, with PVP 18.8 wt% and metallic silver 1.2 wt%, in spheroids form of 35 ± 15 nm, hydrodynamic diameter of 70 nm and zeta potential of − 15 mV) was evaluated either, through the disinfection of Cas shoot tips explants or by dispensing a liquid layer of AgNPs onto the semisolid culture medium during the in vitro establishment phase. The effect of Argovit™ silver nanoparticles in the multiplication rate and the foliar area of Cas was also evaluated. Application of AgNPs 50 mg/L directly to shoot tips during 5 min yielded a contamination rate of 40%, while culture medium sterilization with 5 mg/L of AgNPs as a permanent bilayer reduced shoot contamination rate to 50% compared to 80% for the controls. In addition, Argovit™ silver nanoparticles also enhanced foliar area by 560% (from 0.073 to 0.409 cm2) and multiplication rate by 180% (from 1.286 to 2.286 shoots/explant). The study concluded that the best way for in vitro use of AgNPs on propagation of P. friedrichsthalianum, is as sterilization agent of the culture media because produce additional advantage of enhancing the leaf area and multiplication rate of the shoots.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Psidium friedrichsthalianum (O. Berg) Nied, commonly named Cas or guava Cas, is a species from Myrtaceae family whose fruits are highly prized for their organoleptic properties [1] and its economic and nutritional importance [2]. This specie is also used as a rootstock due to its resistance to Meloidogyne spp. [3]. However, many problems must still be solved.
Plant in vitro culture has greatly contributed to study their growth and development and the influence of the environmental factors on them [4], as well as the large scale propagation of threatened species [5]. However, micropropagation of woody plant species in general has a number of problems, such as a difficult rooting of shootlets [6], shoot tip necrosis [7], phenolization [8, 9], hyperhydricity [10] and a low morphogenetic capacity of the in vitro shoots [11]. In P. friedrichsthalianum, one of the main problems for in vitro propagation of is the presence of contaminants during culture initiation and the subsequent low viability of explants to proceed to the multiplication phase.
Silver nanoparticles (AgNPs) exhibit antimicrobial activity against several plant pathogens [12,13,14] and have also been shown to improve organogenesis, callus induction, somatic embryogenesis, genetic transformation, somaclonal variation and metabolite production [13, 15]. Kim et al. [13] showed that surface disinfection of explants with silver nanoparticles reduces microbial contamination, while their addition to culture media significantly eliminate bacterial contamination and improve the morphology of explants. Both ways produce outstanding results, especially in the cases of addition to tissue culture.
Different AgNPs formulations are available in the market differing in shape, size distribution, the content of metallic silver, synthesis procedure, surface functionalization, and coating agent of the nanoparticles [16,17,18]. All these features help nanoparticles to reach and interact with a wide range of biological targets, playing a significant role in the cytotoxic effects exerted [18,19,20,21].
Based on the reported cytotoxic effects of different formulations of AgNPs, it was decided to use the silver nanoparticles Argovit™, currently approved in Russia and other countries for their use in the in vitro culture of plants, veterinary and human applications [22].
Argovit™ is a silver nanoparticle formulation that was shown to be very useful in the disinfection, shoot multiplication and in the content of micronutrients and macronutrients in sugarcane shoots [23]. In the in vitro culture of vanilla (Vanilla planifolia), Argovit™ nanoparticles reduced in vitro contamination, enhanced shoot multiplication and increased total phenolic content [24]. The use of Argovit™ silver nanoparticles on Vanilla planifolia did not show cytotoxic and genotoxic effects after prolonged exposure (6 weeks) [25]. Silver nanoparticles have also been used to foster plant growth and yield, improve seed germination, enable plant genetic modification, improve bioactive compound production and achieve plant protection [26, 27]. Plant bionanotechnology has been applied as a tool to improve the morphological characteristics of in vitro shoots, to enhance their physiology and to help different biochemical processes in plant cells [28, 29]. AgNPs have been used to strengthen biochemical and physiological phenomena in plants, e.g. photosynthesis [30], growth and development [31], enhanced phenolic and flavonoid production [32] and attenuation of heavy metal toxicity [33], among others. Hence, the use of Argovit™ silver nanoparticles is an excellent option to improve in vitro culture of agronomically interesting woody plants (forest, ornamental, fruit and medicinal trees).
This work aimed to evaluate the microbicidal effect of Argovit™ silver nanoparticles during in vitro establishment by surface disinfection of explants in silver nanoparticles solutions as disinfection treatment, and through the addition of a liquid layer of silver nanoparticle suspensions to form a bilayer (semisolid medium-silver nanoparticle suspension) as a culture medium sterilization method. Besides, the plant morphology during in vitro multiplication of P. friedrichsthalianum (O. Berg) Nied established by both procedures was evaluated.
2 Materials and methods
2.1 Silver nanoparticles Argovit™ suspension
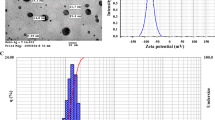
Argovit™ silver nanoparticles were provided by Scientific-Production Centre Vector-Vita Ltd (Novosibirsk, Russia, http://vector-vita.com/) as commercially manufactured product. Argovit™ formulation is a water highly dispersed AgNPs suspension at an overall concentration of 200 mg/mL, with 18.8% polyvinylpyrrolidone (PVP), 1.2% of metallic silver [25]. Characterization of Argovit™ AgNPs is showed in Table 1, as previously reported [34]. Suspensions of Argovit AgNPs with different concentrations (treatments) were prepared by dilution using sterile distilled water.
2.2 Plant material, culture medium and incubation conditions
Apical shoots came from mother plants of Cas (P. friedrichsthalianum (O. Berg) Nied.), cultured in the greenhouse under controlled conditions of temperature, relative humidity, light intensity, and protection from pathogens were used as plant material. Shoot tips (approximately 5 cm of length) were collected and immersed in a 250 mg/L polyvinylpolypyrrolidone (PVPP) solution.
In the laboratory, the apical shoots were reduced in size with a scissor previously disinfected with alcohol (70%) until the shoot tip was 3 cm in length and two pairs of leaves with half-cut blade. Thereinafter all plant material was manipulated in sterile conditions provided by previously sterilized tools (petri box, scalpel and forces) under a laminar flow cabinet.
For in vitro establishment of Cas, shoot tips were cultured on Murashige-Skoog medium [35] supplemented with 30 g/L of sucrose, PVPP 250 mg/L, myoinositol 100 mg/L, Thiamine-HCl 1 mg/L, Benzylaminopurine (BAP) 1 mg/L and Indoleacetic acid (IAA) 0.5 mg/L. For in vitro multiplication of Cas explants, the same medium was used but supplemented with 0.2 mg/L gibberellic acid (GA3).
For all media, pH was adjusted to 5.7–5.8 before addition of 6.65 g/L agar and autoclaved at 121 °C and 1 kg/cm2 for 20 min. All cultures were incubated in a culture room at 25 ± 1 °C with a light intensity of 37.5 µmol m−2 s−1 and a photoperiod of 16 h light from cool-white fluorescent lamps (Philips TLD 32 W/865-NG).
2.3 Use of Argovit AgNPs for disinfection of shoot tips
Shoot tips were disinfected by immersion in AgNPs suspensions (25, 50 and 75 mg/L) during 5 min. For treatment with no AgNPs (used as control) shoot tips were washed with tap water and commercial detergent and then disinfected with a solution of calcium hypochlorite Ca(ClO)2 at 1% for 10 min and washed three times with distilled sterile water.
After disinfection, all explants were reduced in size (1 cm length with leaves eliminated by peduncle) and placed in glass tubes containing 10 ml of in vitro establishment culture medium and incubated in the culture conditions previously described. Fifteen explants were established by treatment and repeated three times (n = 3). Contaminated explants were observed by OPTON stereoscope every 7 days. The percentage of contamination was calculated at 28 days of culture. Fifteen inoculated explants were used as a calculation basis.
2.4 Use of Argovit AgNPs for in vitro sterilization of the culture medium and explant
In a separate experiment, the use of AgNPs for to strengthen sterilization of the culture medium and explant was evaluated. Shoot tips, previously disinfected with a solution of calcium hypochlorite Ca(ClO)2 at 1% for 10 min and washed three times with distilled sterile water, were inoculated in glass tubes with 10 mL of in vitro establishment culture medium. Next, 400 µL of AgNPs suspension at different concentrations (0, 2.5, 5.0, 7.5 and 10 mg/L) were directly dispensed on top of the semisolid medium with the explants, as a permanent bilayer (added in laminar flow cabinet after autoclaving, cooling culture media and explant inoculated). As control (without AgNPs) 400 µL of distilled sterile water was added. Another group of explants (as negative control) was inoculated without addition of any AgNPs suspension or distilled sterile water. After all treatments were set, all cultures were incubated under previously described conditions of culture room. Percentage of explants contamination were recorded after 28 days of culture with the same protocol described above.
2.5 In vitro multiplication of apical shoots previously treated with AgNPs
In vitro apical shoots of Cas from the best AgNPs treatments (by disinfection and by sterilization of culture medium) were transferred to the multiplication phase where the AgNPs were not applied. As control treatment, surviving explants from the in vitro establishment experiments were used. These explants were from control treatments (disinfected with hypochlorite and cultivated without AgNPs in the culture medium, as described above). All explants were inoculated in glass test tube containing 10 ml of multiplication culture medium and incubated in conditions of culture room, previously described. Each treatment of five explants were replicated thrice, totaling 15 explants per treatment (n = 15).
After 84 days of cultivation (with three subcultures at intervals of 28 days each in the same type of culture medium), the leaf area and multiplication rate of shoots were evaluated. The number of final shoots per number of initial explants was calculated as multiplication rate for each subculture performed. Leaf area was determined by the gravimetric method according to Chaudhary et al. [36].
2.6 Statistical data processing
Data were evaluated by One-Way ANOVA (p = 0.05) using IBM SPSS Statistics (Version 20, 2016 for Windows) after testing for normality and homogeneity of the variances. For statistical analysis of the contamination percentage, results were transformed according to \(y^{\prime} = 2*arcsin \left( {\sqrt {y/100} } \right)\). The experimental design was totally randomized using Tukey test for Post Hoc multiple comparisons. Experiments were repeated three times, totaling 45 explants per treatment and the experimental unit consisted of a group of fifteen glass tubes containing one explant each (n = 3). For multiplication phase, values were expressed as the means of fifteen replicates per treatment (n = 15).
3 Results and discussion
3.1 Use of Argovit AgNPs for disinfection of shoot tips
The use of Argovit™ silver nanoparticles, for disinfection of Cas explants had significant statistical differences among treatments. Lowest and statistically similar contamination levels under 50% were obtained at 50 and 75 mg/L during 5 min, as shown in Table 2.
Literature about in vitro tissue culture of P. friedrichsthalianum (O. Berg) Nied (Cas) is scanty. In addition, there is no information about Cas explants treatment with AgNPs. Disinfection of Cas was generally performed with NaClO, HgCl2, benomyl (fungicide), rifampicin (bactericide) and AgNO3 [37], but with limited success due to the combined percentage of contamination with bacteria and fungi (above 60%). The results obtained in this work with the use of AgNPs are better having a lower contamination percentage (40%), and all explants that were not contaminated were kept in culture and survived.
3.2 Use of Argovit AgNPs for in vitro sterilization of the culture medium and explant
AgNPs in this experiment were used to reinforce the sterility conditions of the culture medium within test tubes. Table 3 shows the culture medium sterilization results for in vitro establishment of Cas. Contrary to data for other woody species [13], low concentration of this AgNPs formulation (5.0 mg/L) gave better results. The best AgNPs concentration was 5.0 mg/L, where contamination decreased below 50%. No statistical differences were observed between control and 0.0 mg/L treatments. With concentrations higher than 5.0 mg/L, levels of in vitro contamination increased, probably due to the possible hormetic effect of AgNPs [23]. Concentrations used in this experiment were suitable for tissue growing and survival, as no damage was observed to explant foliage.
On the other hand, this is the first time that AgNPs was used for the sterilization of culture medium for in vitro culture of Cas. Argovit™ AgNPs was shown to be an excellent option to reduce in vitro contamination with fungi and bacteria proceeded from both the environment and plant tissues, without cytotoxic or genotoxic effects for specimens exposed for prolonged periods [23, 25]. Here, we showed the effectiveness of the same formulation on woody plant species.
3.3 In vitro multiplication of apical shoots previously treated with AgNPs
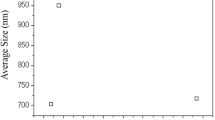
After selecting the best disinfection and sterilization conditions, the explants from the selected treatments were monitored every 28 days for 84 days. Table 4 and Fig. 1 show the differences on leaf area and multiplication rate between different treatments of disinfection and sterilization applied during in vitro establishment of P. friedrichsthalianum (O. Berg) Nied explants with the best concentration of Argovit™ and compared to a control without AgNPs.
Sterilization of the culture media with 5 mg/L of AgNPs show the best results, statistically different from the other treatments in terms of leaf area and multiplication rate. A leaf area of 0.409 cm2 and a multiplication rate of 2.286 (high considering the recalcitrance of this species) represent an increase of 5.6 times for the former and almost double for the latter, respectively, compared with control. Previously it was shown that AgNPs Argovit low concentrations stimulate the growth of in vitro regeneration of vanilla (Vanilla planifolia Jacks.) [24], Sugarcane (Saccharum spp. Cv. Mex 69-290) [23] and Allium cepa [38], three monocotyledonous species, but this is the first time in a dicotyledonous woody plant.
Contrary to the sterilization of culture medium, the explant surface disinfection reduced explant contamination in the same rate as sterilization of the culture media but without any significant changes in leaf area and multiplication rate. Silver nanoparticles application has been bit analyzed in the in vitro culture of different woody plants [39,40,41,42,43,44]. The results above show that Argovit™ is a satisfying solution not only for explant disinfection but also as a growth promoter. This growth promoting function is not exclusive to this nanoparticle formulation. Several studies indicated the phytomodulatory effect of AgNPs biologically synthesized from extracts of cocoa pods [12, 32, 33] and others work explained a very effective effect on the superficial sterilization of Ocimum tissues with a stimulating effect on callus formation, as a result of the absorption of AgNPs by the explants [15].
It may be concluded that prolonged exposure time to low concentrations of this AgNPs formulation is a key factor for observation of beneficial effects, such as growth promotion in Cas explants. Application of this AgNPs formulation in other crops has produced good results. In vanilla and sugar cane, besides the disinfection activity, a hormetic effect modulated by ROS overproduction induced by AgNPs was observed [23,24,25]. As far as we know, this effect has not been reported for AgNPs on woody plants.
Other AgNPs formulations were employed to disinfect woody plant species [39,40,41,42,43,44]; unfortunately, no data regarding size, coating agent and silver content of these formulations were provided. Table 5 shows the exposure time, AgNPs concentration ranges used as well as the disinfection effectiveness ranges found on other woody species, ranging from 3 to 180 min and from 50 to 1000 mg/L, respectively, and coupled with a lack of disinfection capacity up to significant results nearing 50% disinfection.
Disinfection effectiveness strongly depends on the plant species and contamination grade, among other factors, whereby a direct comparison among treatments from the different works is not feasible. However, from Table 5 it is possible to suggest that the AgNPs formulation used here could be a valuable disinfection alternative as a contamination rate of around 40% was achieved with 50 mg/L AgNPs and a short immersion of 5 min.
Although there are no previous results on the use of AgNPs as microbicide in Cas in vitro culture, low to good results were reported for other woody plant species. Table 4 shows a comparison among the different obtained results among some woody plants and P. friedrichsthalianum (O. Berg) Nied. with the use of silver nanoparticles in the in vitro propagation. Unfortunately, scarce characetrization data do not allow to identify a general trend between the physicochemical characteristics of AgNPs and their antimicrobial and growth promotion effects on woody explants. Application of 100-1000 mg/L AgNPs in the surface of leaf explants of Vitis vinifera ‘Farkhi’, ‘Khoshnave’ and ‘Rashe’ decreased the contamination rate by 10–80% [42], and controlled internal contaminants in olive explants without negative effects on the morphology and growth of explants [39]. Good results have also been obtained in the reduction of internal and external contamination in both immersion and MS medium, in G × N15 (hybrid of almond × peach) rootstocks [43]. Sarmast et al. [40] reported that surface sterilization of explants of Araucaria excelsa and subsequent AgNPs immersion reduced contamination, while adding 400 mg/L AgNPs in the culture medium decreased contamination from 81.25 to 18.75%. In Tecomella undulata, 60 mg/L of AgNPs improved shoot number, shoot length and percentage of produced shoots [44], whereas 10 mg/L AgNPs improved callus formation [41]. Table 4 shows a comparison among the different obtained results among some woody plants and P. friedrichsthalianum (O. Berg) Nied. with the use of silver nanoparticles in the in vitro propagation.
Microbicidal effects are possible because silver has a wide antibacterial spectrum, and it can kill many of pathogenic bacteria and virus [45,46,47,48] and by the antimicrobial and viricidal potential of silver nanoparticles through different mechanisms such as AgNPs adhesion to microbial cells, penetration inside the cells, generation of ROS and free radical, modulation of microbial signal transduction pathways [49,50,51,52]. Likewise, due to the inhibition of virus penetration into the cell, interaction with virus genome, inhibition of genome replication, inhibition of protein synthesis and inhibition of assembly and release of virions [53, 54]. Meanwhile the growth promoting effect may be possible due to an induction of genes associated with growth regulators. Syu et al. [55] verified the effect of size and shape of AgNPs on Arabidopsis plant growth and gene expression and verified that AgNPs activate Arabidopsis gene expression of indoleacetic acid protein 8 (IAA8) and reduced the expression of ACC synthase 7 (ACS7) and ACC oxidase 2 (ACO2), among others. All this suggesting that AgNPs acted as inhibitors of ethylene and growth promoter [55]. For recalcitrant woody plant species in early stage in vitro culture, such as some Myrtaceae, it is important to have an agent that promotes the growth of the explants and thus reduce rejection losses and increase multiplication rates, especially for commercial micropropagation.
For practical application of AgNPs effects revealed in this work for P. friedrichsthalianum (O. Berg) Nied are necessary future studies. The most important experiments will be related to the following stages of plant growth (in vitro rooting, adaptation, ex vitro growth rate, etc.)
4 Conclusions
The use of silver nanoparticles has different advantages in the in vitro culture of plants. Argovit™ silver nanoparticles reduce the contaminant levels during the establishment of woody plant cultures and improve the morphology in the multiplication phase of the treated plants. A 50 mg/L Argovit™ silver nanoparticles application for 5 min reduced explant contamination in P. friedrichsthalianum (O. Berg) Nied. (Cas) to 50%. Upon sterilization of the culture medium with 5 mg/L, the percentage of contamination in Cas shoots was reduced to 40%. Argovit™ silver nanoparticles also enhanced leaf area growth by 560% (from 0.073 to 0.409 cm2) and multiplication rate by 180% (from 1.286 to 2.286 shoots/explant) in the multiplication phase when explants had been established with these AgNPs and these feature was maintained until the fifth subculture. The best way for in vitro use of AgNPs on propagation of Cas, is the sterilization of the culture medium, as it produces the same decontamination effect as surface disinfection of explants but coupled with a 10 times lower concentration and the additional advantage of enhancing the leaf area and multiplication rate of the shoots.
References
Cuadrado-Silva CT, Pozo-Bayón MÁ, Osorio C (2017) Identification of aroma compounds and precursors of sour guava (Psidium friedrichsthalianum Nied.) following a sensomics approach. Eur Food Res Technol 243(1):1–10
Granados-Chinchilla F, Villegas E, Molina A, Arias C (2016) Composition, chemical fingerprinting and antimicrobial assessment of Costa Rican cultivated guavas (Psidium friedrichsthalianum (O. Berg) Nied. and Psidium guajava L.) essential oils from leaves and fruits. Nat Prod Chem Res 4:236
de Oliveira DF, do Nascimento SA, Rebouças ER (2018) Germination of Psidium friedrichsthalianum (O. Berg) Nied. seeds under different temperature and storage conditions. J Seed Sci 40(3):246–252
Espinosa CA, Puente CA, García S (2018) In vitro plant tissue culture: means for production of biological active compounds. Planta 348:1–18
Ibrahim FH, Alhenzab MT, Elbadawi AO (2018) Establishment of an efficient in vitro regeneration protocol for mass propagation via organogenesis of endangered plant Rhanterium epapposum in Qatar. In: Qatar foundation annual research conference proceedings, vol 1. HBKU Press Qatar, pp EEPD85
Benmahioul B, Dorion N, Kaid-Harche M, Daguin F (2012) Micropropagation and ex vitro rooting of pistachio (Pistacia vera L.). Plant Cell Tissue Org 108(2):353–358
Surakshitha NC, Soorianathasundaram K, Ganga M, Raveendran M (2019) Alleviating shoot tip necrosis during in vitro propagation of grape cv. Red Globe Sci Hortic 248:118–125
Restrepo C, Gómez FA, Gil A, Torres JM, Urrea AI (2018) In vitro propagation of avocado (Persea americana Mill.) cv. Hass through morphogenesis. Acta Agron 67(1):160–167
Kozgar MI, Shahzad A (2012) An improved protocol for micropropagation of teak tree (Tectona grandis L.). Rend Lincei Sci Fis 23:195–202. https://doi.org/10.1007/s12210-012-0176-2
Cuenca B, Sánchez C, Aldrey A, Bogo B, Blanco B, Correa B, Vidal N (2017) Micropropagation of axillary shoots of hybrid chestnut (Castanea sativa × C. crenata) in liquid medium in a continuous immersion system. Plant Cell Tissue Org 131(2):307–320
Bello-Bello JJ, Iglesias L, Sánchez L, Casas J, Santana N (2012) In vitro regeneration of Pinus brutia Ten. var. eldarica (Medw.) through organogenesis. Afr J Biotechnol 11(93):15982–15987
Azeez L, Lateef A, Wahab A, Rufai M, Salau A, Ajayi E, Ajayi M, Adegbite M, Adebisi B (2019) Phytomodulatory effects of silver nanoparticles on Corchorus olitorius: its antiphytopathogenic and hepatoprotective potentials. Plant Physiol Biochem 136:109–117
Kim DH, Gopal J, Sivanesan I (2017) Nanomaterials in plant tissue culture: the disclosed and undisclosed. RSC Adv 7(58):36492–36505
Lateef A, Folarin B, Oladejo S, Akinola P, Beukes L, Gueguim-Kana E (2018) Characterization, antimicrobial, antioxidant, and anticoagulant activities of silver nanoparticles synthesized from Petiveria alliacea L. leaf extract. Prep Biochem Biotechol 48(7):646–652
Adebomojo A, AbdulRahaman A (2020) Surface sterilization of Ocimum seeds and tissues with biosynthesized nanosilver and its effects on callus induction. In: IOP conference series: materials science and engineering, vol 1. IOP Publishing, pp 012024
Adelere I, Lateef A (2016) A novel approach to the green synthesis of metallic nanoparticles: the use of agro-wastes, enzymes, and pigments. Nanotechnol Rev 5:567–587
Lateef A, Oladejo S, Akinola P, Aina D, Beukes L, Folarin B, Gueguim-Kana E (2020) Facile synthesis of silver nanoparticles using leaf extract of Hyptis suaveolens (L.) Poit for environmental and biomedical applications. In: IOP conference series: materials science and engineering, vol 1. IOP Publishing, pp 012042
Pulit-Prociak J, Banach M (2016) Silver nanoparticles—a material of the future…? Open Chem 14:76–91. https://doi.org/10.1515/chem-2016-0005
Akintayo G, Lateef A, Azeez M, Asafa T, Oladipo I, Badmus J, Ojo S, Elegbede J, Gueguim-Kana E, Beukes L (2020) Synthesis, bioactivities and cytogenotoxicity of animal fur-mediated silver nanoparticles. In: IOP conference series: materials science and engineering, vol 1. IOP Publishing, pp 012041
Sayed R, Saad H, Hagagy N (2017) Silver nanoparticles: characterization and antibacterial properties. Rend Lincei Sci Fis. https://doi.org/10.1007/s12210-017-0663-6
Yekeen T, Azeez M, Lateef A, Asafa T, Oladipo I, Badmus J, Adejumo S, Ajibola A (2017) Cytogenotoxicity potentials of cocoa pod and bean-mediated green synthesized silver nanoparticles on Allium cepa cells. Caryologia 70(4):366–377
Juarez-Moreno K, Gonzalez EB, Giron-Vazquez N, Chávez-Santoscoy RA, Mota-Morales JD, Perez-Mozqueda LL, Garcia-Garcia MR, Pestryakov A, Bogdanchikova N (2017) Comparison of cytotoxicity and genotoxicity effects of silver nanoparticles on human cervix and breast cancer cell lines. Hum Exp Toxicol 36(9):931–948
Bello-Bello JJ, Chavez-Santoscoy RA, Lecona-Guzman CA, Bogdanchikova N, Salinas-Ruíz J, Gómez-Merino FC, Pestryakov A (2017) Hormetic response by silver nanoparticles on in vitro multiplication of sugarcane (Saccharum spp. Cv. Mex 69-290) using a temporary immersion system. Dose-Response 15(4):1–9
Spinoso-Castillo JL, Chavez-Santoscoy RA, Bogdanchikova N, Pérez-Sato JA, Morales-Ramos V, Bello-Bello JJ (2017) Antimicrobial and hormetic effects of silver nanoparticles on in vitro regeneration of vanilla (Vanilla planifolia Jacks. ex Andrews) using a temporary immersion system. Plant Cell Tissue Org 129(2):195–207
Bello-Bello JJ, Spinoso-Castillo JL, Arano-Avalos S, Martínez-Estrada E, Arellano-García M, Pestryakov A, Toledano-Magaña Y, García-Ramos J, Bogdanchikova N (2018) Cytotoxic, genotoxic, and polymorphism effects on Vanilla planifolia jacks ex andrews after long-term exposure to argovit® silver nanoparticles. Nanomaterials 8(10):754
Wang P, Lombi E, Zhao F-J, Kopittke PM (2016) Nanotechnology: a new opportunity in plant sciences. Trends Plant Sci 21(8):699–712
Ruttkay-Nedecky B, Krystofova O, Nejdl L, Adam V (2017) Nanoparticles based on essential metals and their phytotoxicity. J Nanobiotechnol 15(1):33
Siddiqui MH, Al-Whaibi MH, Firoz M, Al-Khaishany MY (2015) Role of nanoparticles in plants. In: Nanotechnology and plant sciences. Springer, Cham, pp 19–35
Ramírez-Mosqueda MA, Iglesias-Andreu LG, Favián-Vega E, Teixeira JA, Leyva-Ovalle OR, Murguía-González J (2019) Morphogenetic stability of variegated Vanilla planifolia Jacks. Plants micropropagated in a temporary immersion system (TIB®). Rend Lincei Sci Fis. https://doi.org/10.1007/s12210-019-00813-9
Rico CM, Peralta-Videa JR, Gardea-Torresdey JL (2015) Chemistry, biochemistry of nanoparticles, and their role in antioxidant defense system in plants. In: Siddiqui M, Al-Whaibi M, Mohammad F (eds) Nanotechnology and plant sciences. Springer, Cham, pp 1–17
Siddiqui MH, Al-Whaibi MH, Firoz M, Al-Khaishany MY (2015) Role of nanoparticles in plants. In: Siddiqui M, Al-Whaibi M, Mohammad F (eds) Nanotechnology and plant sciences. Springer, Cham, pp 19–35
Azeez L, Lateef A, Adebisi S (2017) Silver nanoparticles (AgNPs) biosynthesized using pod extract of Cola nitida enhances antioxidant activity and phytochemical composition of Amaranthus caudatus Linn. Appl Nanosci 7(1–2):59–66
Azeez L, Adejumo A, Lateef A, Adebisi S, Adetoro R, Adewuyi S, Tijani K, Olaoye S (2019) Zero-valent silver nanoparticles attenuate Cd and Pb toxicities on Moringa oleifera via immobilization and induction of phytochemicals. Plant Physiol Biochem 139:283–292
Valenzuela-Salas L, Girón-Vázquez N, García-Ramos J, Torres-Bugarín O, Gómez C, Pestryakov A, Villarreal-Gómez L, Toledano-Magaña Y, Bogdanchikova N (2019) Antiproliferative and antitumour effect of nongenotoxic silver nanoparticles on melanoma models. Oxid Med Cell Longev 2019:1–12. https://doi.org/10.1155/2019/4528241
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15(3):473–497
Chaudhary P, Godara S, Cheeran A, Chaudhari A (2012) Fast and accurate method for leaf area measurement. Int J Comput Appl 49(9):22–25
Ramírez M, Sierralta S, Urdaneta A (1999) Evaluation of surface desinfectants on the in vitro establishment of Psidium guajava L. and Psidium friedrichsthalianum (Berg) Nierdz. Plant Sci 156(2):125–135
Casillas-Figueroa F, Arellano-García M, Leyva-Aguilera C, Ruíz-Ruíz B, Vázquez-Gómez R, Radilla-Chávez P, Chávez-Santoscoy R, Pestryakov A, Toledano-Magaña Y, García-Ramos J, Bogdanchikova N (2020) Argovit™ silver nanoparticles effects on allium cepa: plant growth promotion without cyto genotoxic damage. Nanomaterials 10(1386):1–20. https://doi.org/10.3390/nano10071386
Rostami AA, Shahsavar A (2009) Olive “Mission” explants. Asian J Plant Sci 8(7):505–509
Sarmast M, Salehi H, Khosh-Khui M (2011) Nano silver treatment is effective in reducing bacterial contaminations of Araucaria excelsa R. Br. var. glauca explants. Acta Biol Hung 62(4):477–484
Aghdaei M, Salehi H, Sarmast MK (2012) Effects of silver nanoparticles on Tecomella undulata (Roxb) seem, micropropagation. Adv Hortic Sci 26:21–24
Gouran A, Jirani M, Mozafari AA, Koshesh M, Ghaderi N, Zaheri S (2014) Effect of silver nanoparticles on grapevine leaf explants sterilization at in vitro conditions. In: 2nd National conference of nanotechnology from theory to application, Jami Institute Isfahan, Iran, pp 1–6
Arab MM, Yadollahi A, Hosseini-Mazinani M, Bagheri S (2014) Effects of antimicrobial activity of silver nanoparticles on in vitro establishment of G × N15 (hybrid of almond × peach) rootstock. J Genet Eng Biotechnol 12(2):103–110
Sarmast MK, Niazi A, Salehi H, Abolimoghadam A (2015) Silver nanoparticles affect ACS expression in Tecomella undulata in vitro culture. Plant Cell Tissue Org 121(1):227–236
Kawashita M, Tsuneyama S, Miyaji F, Kokubo T, Kozuka H, Yamamoto K (2000) Antibacterial silver-containing silica glass prepared by sol–gel method. Biomaterials 21(4):393–398
Zhao Q, Liu Y, Wang C (2005) Development and evaluation of electroless Ag-PTFE composite coatings with anti-microbial and anti-corrosion properties. Appl Surf Sci 252(5):1620–1627
Betts AJ, Dowling DP, McConnell ML, Pope C (2005) The influence of platinum on the performance of silver–platinum anti-bacterial coatings. Mater Des 26(3):217–222
Zhao L, Ashraf MA (2015) Influence of silver-hydroxyapatite nanocomposite coating on biofilm formation of joint prosthesis and its mechanism. West Indian Med J 64(5):506
Dakal TC, Kumar A, Majumdar RS, Yadav V (2016) Mechanistic basis of antimicrobial actions of silver nanoparticles. Front Microbiol 7:1831. https://doi.org/10.3389/fmicb.2016.01831
Mishra S, Singh BR, Singh A, Keswani C, Naqvi AH, Singh HB (2014) Biofabricated silver nanoparticles act as a strong fungicide against Bipolaris sorokiniana causing spot blotch disease in wheat. PLoS ONE 9(5):e97881
Wang L, Hu C, Shao L (2017) The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int J Nanomedicine 12:1227. https://doi.org/10.2147/IJN.S121956
Zhang Y, Chen Y-Y, Huang L, Chai Z-G, Shen L-J, Xiao Y-H (2017) The antifungal effects and mechanical properties of silver bromide/cationic polymer nano-composite-modified Poly-methyl methacrylate-based dental resin. Sci Rep UK 7(1):1547
Gaikwad S, Ingle A, Gade A, Rai M, Falanga A, Incoronato N, Russo L, Galdiero S, Galdiero M (2013) Antiviral activity of mycosynthesized silver nanoparticles against herpes simplex virus and human parainfluenza virus type 3. Int J Nanomedicine 8:4303–4314. https://doi.org/10.2147/IJN.S50070
Akbarzadeh A, Kafshdooz L, Razban Z, Dastranj Tbrizi A, Rasoulpour S, Khalilov R, Kavetskyy T, Saghfi S, Nasibova AN, Kaamyabi S (2018) An overview application of silver nanoparticles in inhibition of herpes simplex virus. Artif Cell Nanomed B 46(2):263–267
Syu Y, Hung J, Chen J, Chuang H (2014) Impacts of size and shape of silver nanoparticles on Arabidopsis plant growth and gene expression. Plant Physiol Biochem 83:57–64
Acknowledgements
This research was supported by the Bioplant Center (University of Ciego de Ávila, Cuba). The authors would like grateful to Mrs. Yarianne Lezcano and Mr. Osbel Mosqueda for their collaborations and skilled technical assistances. The authors also would like give a special grateful to Tomsk Polytechnic University Competitiveness Enhancement Program, project VIU-ISHBMT-197/2020; RFBR project 18-29-24037 and the BIOALI-CYTED network (P117RT0522) for creating the enabling framework for collaboration.
Author information
Authors and Affiliations
Contributions
IA, JCG, NB, AP, ME and OC designed the research and wrote the paper; IA and NG conducted the experiments and analyzed the data; IA and OC had primary responsibility for the final content. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors do not have any conflict of interests.
Human and animal rights
This research did not involve experiment with humans or animals.
Informed consent
It was obtained from all of participants included in these studies. Additional informed consent was obtained from all individual participants for whom identifying information is included in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Andújar, I., González, N., García-Ramos, J.C. et al. Argovit™ silver nanoparticles reduce contamination levels and improve morphological growth in the in vitro culture of Psidium friedrichsthalianum (O. Berg) Nied.. SN Appl. Sci. 2, 2110 (2020). https://doi.org/10.1007/s42452-020-03948-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-03948-9