Abstract
Rare earth elements, a type of abiotic elicitor, were used to investigate the shift in the mechanism of flavonoid accumulation from primary metabolites in Ginkgo suspension cell culture. The changes in flavonoids, mineral ions, photosynthetic pigments, chlorophyll fluorescence and antioxidant enzymes with varying Ce3+ (Ce(NO3)3) doses (0.01–5.0 mM) were studied. Low doses of Ce3+ (0.01–0.1 mM) improved cell growth and the highest increased dry weight was 2.67 g L−1 medium at 0.1 mM Ce3+ dose. Moderate doses (0.5–1.0 mM) limited cell growth and initiated a self-protective mechanism through modulation of the pigments, ions and flavonoid content, chlorophyll fluorescence and antioxidant enzymes, while high doses (2.0–5.0 mM) inhibited the cell growth and even caused cell mortality. The chlorophyll content, K+, Zn2+ and growth mass peaked at 0.1 mM Ce3+. The Fv/Fm, and Fv′/Fm′ values, and the amounts of P, Ca2+, Fe2+ and Zn2+ reached a maximum at 0.5 mM Ce3+ while the flavonoids content was highest at 1.0 mM Ce3+ (11.1 mg L−1 medium). The antioxidant enzyme activities were high from 0.05 to 0.5 mM Ce3+. Moreover the changes of chlorophyll fluorescence images of the suspension cells were consistent with the cell growth, relative electrical conductivity (REC) and malondialdehyde (MDA) content with increasing Ce3+ doses. Our findings suggest doses of 0.1–0.5 mM Ce3+ in Ginkgo cells shifted the metabolism from primary to secondary processes, diverting the metabolism from growth to defense related pathways as a result of the flavonoids accumulation. The high ratio of Car/Chl t, high Car content and 0.5–1.0 mM Ce3+ were beneficial for the accumulation of flavonol glycosides in Ginkgo suspension cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Flavonoid biosynthesis is closely linked to environmental induction and stress, and is up-regulated in response to a wide range of stimulators including biotic and abiotic stresses (Agati et al. 2012). Abiotic elicitors include high salinity, drought stress, heavy metals, rare earth elements (REEs), ozone, and ultraviolet irradiation, etc. Methyl jasmonate (MJ), Salicylic acid (SA), KCl, NaCl, fungal endophytes and UV-B irradiation have been tested as inducers in Ginkgo cell culture for production of bilobalide, ginkgolides and flavonoids (Kang et al. 2009; Hao et al. 2009; Chen et al. 2014).
Rare earth elements (REEs) are generic terms representing 17 chemical elements which include 15 lanthanide series elements such as lanthanum (La), cerium (Ce), plus scandium (Sc) and yttrium (Y) (Wang et al. 2012). REEs have been applied as micro-fertilizers to improve plant growth and yield in China (Fitriyanto et al. 2011). Cerium and lanthanum are two major kinds of rare earth elements which have been used to induce the secondary metabolites production in plant cell culture as an elicitor (Table 1).
A few researchers have reported the positive or negative effects of REEs on plant physiological metabolism depending on the dosage, time, plant species, growth conditions, and specific REEs used (Xu and Chen 2011; Wu et al. 2001; Chu et al. 2014). The toxicity and damage of surplus REEs on plant growth may be as a result of REEs ions from the cell wall moving into the intracellular space through Ca channels, leading to a blockage of Ca2+ uptake (Liu et al. 2009). Therefore, REEs were recognized as Ca channel antagonists and are known to inhibit Ca2+-dependent enzymes or other proteins (Shtangeeva and Ayrault 2007). Additionally, the phospholipase A2 activation involved in Ce4+-induced taxol production was speculated to be associated with a JA-dependent signaling pathway. Furthermore, Ce4+ induced programmed cell death (PCD) in Taxus cuspidate cells was observed (Yang et al. 2008). In the Ce4+ induced cellular apoptosis signaling pathway and taxol biosynthesis, phospholipase D (PLD) and the rapid accumulation of PA may be required for the initial MAPK activity in Taxus cuspidata suspension cultures (Yang et al. 2009).
Despite the available literature focused on the yield and the mechanism of action of REEs on taxol biosynthesis in Taxus cell cultures, the underlying mechanisms of REEs on Ginkgo flavonoid metabolism has not been explored. Only the accumulation of flavonoids and growth in Ginkgo cell culture were studied, there is no data on the correlation of flavonoids and primary metabolism in Ginkgo cell culture under cerium induction. Therefore, an in-depth study into the mechanism of the shift between primary and flavonoid metabolism is needed in Ginkgo cell culture in the presence of cerium.
The aim of this work was to investigate the relationship of flavonol glycoside production with photosynthetic pigment, chlorophyll fluorescence, mineral content, and antioxidant systems in the presence of varying cerium nitrate doses in suspension Ginkgo cells. The results should help to improve our understanding of how the mechanism of REEs increases the accumulation of flavonol glycosides, and be beneficial for in-depth analysis of the biosynthetic pathways of flavonol glycosides in suspension cell culture.
Materials and methods
Suspension cell culture and cerium nitrate treatment
The cell lines were attained from young leaves of Ginkgo biloba L. cv., ‘Da Fozhi’, and subcultured by diluting 50 mL into 100 mL fresh medium in 250 ml flasks every 12 days in liquid MS medium (no agar) containing 30 g L−1 sucrose, 1.0 mg L−1 NAA (Naphthaleneacetic acid) and 0.5 mg L−1 KT (Kinetin) on a rotary shaker at 100 rpm (Chen et al. 2014). Eight cerium nitrate (Ce (NO3)3, Sinopharm Chem. Reag. Co., Ltd, Shanghai, China) filter sterilized doses including 0 (control), 0.01, 0.05, 0.1, 0.5, 1.0, 2.0 and 5.0 mM Ce3+ (all final concentrations) were added into the medium on the 6th day after inoculation, and the cells were harvested 7 days after the addition of cerium nitrate. All plant growth regulators were filter sterilized via a syringe filter (0.22 micrometer) then added into the autoclaved medium and the pH of the medium was adjusted to 5.8. Cultures were incubated at 25 ± 1 °C with a 14 h photoperiod using 55 µmol m−2 s−1 fluorescent illumination.
Cell growth analysis
Cell growth was primarily measured by cell fresh weight (FW) and dry weight (DW). Half of the fresh cells were weighed and stored at −70 °C for the active compound assay, and the other half was dried at 60 °C for 12 h until a constant weight (DW) was obtained for determination of flavonoids and mineral elements. The FW and DW were calculated as shown below:
here, FW1 (DW1) is final fresh (dry) weight of cells; FW2 (DW2) is initial fresh (dry) weight of cells i.e. the weight before addition of cerium nitrate into culture medium.
Flavonoids contents and phenylalanine ammonia-lyase (PAL) activity
The flavonoid extraction was performed using the method of Hao et al. (2009). HPLC was performed according to Chen et al. (2014) on an Agilent 1200 HPLC (Agilent Inc., USA). The analytical column was a C18 column (ODS, 4.6 × 250 mm, 5.0 μm), while the injection volume was 10 μL. The mobile phase consisted of methanol and 0.4 % phosphoric acid in H2O (55:45, v/v) with a flow rate of 1.0 mL/min. The column temperature was 30 °C and the wavelength was monitored at 360 nm.
The three flavonol glycosides (quercetin, kaempferol, isorhamnetin) (μg g−1 DW) contents and total flavonoids content (μg g−1 DW) or flavonoids yield (mg DW L−1 medium) were calculated as shown of Chen et al. (2014).
PAL (EC 4.3.1.5) activity was determined by the method of Koukol and Conn (1961). PAL activity was measured at 290 nm based on the production of cinnamic acid.
Photosynthetic pigment contents and imaging chlorophyll fluorescence
The chlorophyll (a and b) absorbance was measured at 645 and 663 nm and carotenoids were determined at 470 nm according to Lichtenthaler (1987). Chlorophyll fluorescence measurements were performed according to the method of Osorio et al. (2013) by using a chlorophyll fluorescence imager (CF Imager, Technologica, UK). The maximal quantum yield of PSII photochemistry (Fv/Fm), the photochemical efficiency of PSII in the light (Fv′/Fm′), the photochemical quenching coefficient qP (Fq′/Fv′), and the non-photochemical quenching coefficient NPQ (Fm/Fm′−1) were calculated using the software supplied by the imager manufacturer.
Ion contents
According to the method of Maria et al. (2012), the dry sample (200 mg) was digested with 0.1 M HNO3 in a closed microwave system. The concentration of ions was analyzed using an ICS-900 (Dionex, Sunnyvale, CA, USA).
Antioxidant enzymes, membrane permeability and lipid peroxidation
The enzyme extract method was performed by the method of (Chen et al. 2014). The Superoxide dismutase (SOD, EC 1.15.1.1), Catalase (CAT, EC 1.11.1.6) and POD (guaiacol peroxidase, EC1.11.1.7) activities were assayed according to the methods of Dong et al. (2010), respectively. One unit (U) of SOD was defined as inhibiting 50 % of NBT reduction at 560 nm. One unit (U) of CAT or POD was defined as 1 μmol or 1 nmol H2O2 decomposed in 1 min, respectively.
The relative electrical conductivity (REC) and the malondialdehyde (MDA) were measured according to Chen et al. (2014). The absorbance of the supernatant was monitored at 532 and 600 nm. An extinction coefficient of 155 mM−1 cm−1 was used to calculate the MDA concentration.
Statistical analysis
Each treatment had three replications (flasks) within each experiment and the independent experiment repeated two times. The analytical assays data were collected from six replicates (n = n = 3). The data were expressed as mean values ± SD (standard deviation). The data were analyzed using one-way ANOVA and the data generated used SPSS version 17.0 (SPSS Inc., IL, USA) analysis and Duncan’s multiple-range test (DMRT). Associations were also examined by simple correlation analysis using SPSS version 17.0.
Results
Cell growth response to varying Ce3+ doses
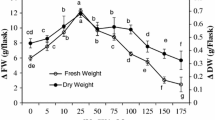
Figure 1 shows the effects of Ce3+ doses on cell growth after adding cerium nitrate into the medium for the 7th day. We select this day to perform all experiments, since it is well-known that a high total flavonoids production happens at approximately 1 week after elicitors’ supplementation (Peng and He 2013; Wang et al. 2015; Hao et al. 2009). The same tendency were found for the cell ΔFW and ΔDW after adding Ce3+, while cell growth (ΔFW and ΔDW) was significantly enhanced from 0.01 to 0.1 mM Ce3+ and gradually decreased from 0.5–5.0 mM Ce3+ treatments compared with the control. The ΔFW at Ce3+ doses <2.0 mM and ΔDW at Ce3+ doses ≤2.0 mM were lower than the control values and showed the suspension cell growth was limited. The most significant increases in cell ΔFW and ΔDW were observed at 0.1 mM, at 87.5 and 100 % higher than those of the control cells, respectively.
Effects of Ce3+doses on Ginkgo suspension cell of ΔFW and ΔDW (in 1L media) subjected to cerium nitrate elicitation for 7 days. Values are mean ± SD (standard deviation, n = 3). Means followed by different letters above the bars indicate significant differences (p < 0.05) among treatments according to the Duncan’s multiple range test
Flavonoid content and PAL activity responses to varying Ce3+ doses
The effects on Ginkgo suspension cells subjected to elevated levels of Ce3+ on the total amounts of flavonoids, three flavonol glycosides (quercetin, kaempferol and isorhamnetin) and PAL were determined and are shown in Fig. 2. The concentration of these components increased as the Ce3+ dose significantly increased from 0.01 to 1.0 mM, then decreased from 2.0 to 5.0 mM, but all Ce3+ treatments induced flavonoid accumulation more than that of the control (Fig. 2a–d). The maximum values observed at 1.0 mM Ce3+ were 1.04, 2.76, 1.97, and 2.06 folds (1.49 mg g−1 DW) higher for quercetin, kaempferol, isorhamnetin and total flavonoids content compared with the control (p < 0.05), respectively. The next highest amounts of these were found at 0.5 mM Ce3+ treatment (Fig. 2a–d). By comparison, flavonoids yield were only increased by 0.39 fold or 0.09 fold at 5.0 mM Ce3+ treatment, respectively (Fig. 2e). The PAL activity showed a similar trend as the flavonoids yields, with the highest activity observed at 0.5 mM Ce3+ treatment (Fig. 2f).
Quercetin, kaemferol, isorhamnetin (a–c) and total flavonoids content (d) and flavonoids yield (e) and PAL activity (f) in Ginkgo suspension cells subjected to cerium nitrate elicitation for 7 days. Values are mean ± SD (standard deviation, n = 3). Means followed by different letters above the bars indicate significant differences (p < 0.01) among treatments according to the Duncan’s multiple range test
Photosynthetic pigment content response to varying Ce3+doses
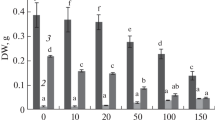
Suspension cell chlorophyll concentrations (Chl a, Chl b, Chl t (Chl t = Chl a + Chl b), Chl a/b, carotenoids (Car) and Car/Chl t are shown in Fig. 3. Exposure to 0.01–0.1 mM Ce3+ caused an increase in the contents of chlorophyll a (Chl a) and chlorophyll b (Chl b), and total chlorophyll (Chl t) while a gradual decrease was observed for treatments at 0.5–5.0 mM Ce3+. The Chl b content was particularly reduced at these Ce3+ levels compared with the control. The maximum values of Chl t was observed at 0.1 and 5.0 mM Ce3+ at 34.1 and 147.6 % higher than that of the control, respectively (p < 0.05). There was no significant difference in the Chl t, Chl a, and Chl b content between 0.05 and 0.1 mM Ce3+ treatments (p > 0.05) (Fig. 3a–c). In contrast, little change in the carotenoid (Car) content was observed at 0.01 and 0.1 mM, while a significant enhancement was observed at 0.5–2.0 mM Ce3+ treatment compared with the control, and the peak value was examined at 0.5 mM Ce3+. No significant difference in Car content was observed between 0.01 and 5.0 mM Ce3+ treatment (p > 0.05) (Fig. 3e).
The chlorophyll a and b, total chlorophyll content Chl t (Chl t = Chl a + Chl b) (a–c), ratio Chl a/b (d), carotenoid content (Car) (e) and ratio of carotenoid/total chlorophyll (Car/Chl t) (f) in Ginkgo suspension cells subjected to cerium nitrate elicitation for 7 days. Values are mean ± SD (n = 3). Means followed by different letters above the bars indicate significant differences (p < 0.05) among treatments according to the Duncan’s multiple range test
The Chl a/b and Car/Chl t ratios both decreased in the presence of 0.01–0.1 mM Ce3+ and were lower than the control, while they significantly increased from 0.5 to 2.0 mM Ce3+, especially at 0.5 mM Ce3+. The maximum ratios of Chl a/b occurred at 0.5 mM Ce3+ where it was 183.1 % of the control, and at 5.0 mM Ce3+ treatment where it was 107.4 % of the control (Fig. 3d), while the Car/Chl t ratio at 0.5 mM was 145.4 % of the control (p < 0.05) (Fig. 3f). There were no significant differences among the 0.01–0.1 mM Ce3+ treatments and between the 0.5–2.0 mM Ce3+ treatments for Car/Chl t ratios.
Chlorophyll fluorescence response to varying Ce3+doses
The maximum quantum yield of photosystem-II (Fv/Fm), the photochemical efficiency of PSII in the light (Fv′/Fm′), the non-photochemical quenching coefficient (qp) and the non-photochemical quenching (NPQ) were monitored by using a chlorophyll fluorescence imager (Fig. 4). Similar trends in the Fv/Fm and Fv′/Fm′ values were observed (Fig. 4a, b). At doses of Ce3+ ≤ 0.1 mM, both Fv/Fm and Fv′/Fm′ were higher than the control but were not significantly different between them (p > 0.05), however at doses of over 0.1 mM Ce3+, these values began to decrease and became lower than the control. For both parameters, the highest values were obtained at 0.1 mM Ce3+ treatment. In contrast, the lowest values were obtained at 5.0 mM Ce3+ treatment, where the Fv/Fm value decreased by 32.9 % and the Fv′/Fm′ value decreased by 41.1 % compared with the control, respectively. Conversely, the qP value was not significantly different between 0.01 and 0.1 mM Ce3+ compared with the control, while it was significantly increased from 0.5 to 5.0 mM Ce3+. The highest qp value was obtained at 5.0 mM Ce3+ where it was increased by 34.2 % compared with the control (Fig. 4c). The same trend was observed for the NPQ value from 0.01 to 0.1 mM Ce3+ although no significant difference was found compared with the control (p > 0.05). However, the NPQ value was higher (54.8 %) than the control at 0.1–0.5 mM Ce3+ (Fig. 4d).
Chlorophyll fluorescence images of maximum PSII photochemical efficiency (Fv/Fm) (a), photochemical efficiency of PSII in the light (Fv′/Fm′) (b), photochemical quenching (qp) (c), and non-photochemical quenching (NPQ) (d) in Ginkgo suspension cells subjected to cerium nitrate elicitation for 7 days. Values are mean ± SD (n = 3). Means followed by different letters above the bars indicate significant differences (p < 0.01) among treatments according to the Duncan’s multiple range test
No significant difference were observed in the color of the cell Fv/Fm and Fv′/Fm′ images at 0.01 M Ce(NO3)3 treatment compared with the control, however the color of these two images from 0.5 to 1.0 mM Ce(NO3)3 were deeper in red than the control (Fig. 5). The color of the Fv′/Fm′ images at 5.0 mM Ce(NO3)3 was observed to contain only a small amount of red area and a large amount of blue area (Fig. 5). These changes were in accordance with the cell color changes (Fig. 6).
Ion content response to varying Ce3+ doses
The cerium dose effects on the absorption of mineral elements in the suspension cell medium after treatment are shown in Fig. 7. Potassium (K) absorption was substantially higher in the suspension cells exposed to between 0.01 and 0.5 mM Ce3+ comparison with the control. No differences were observed between the control and 5.0 Ce3+ dose (p > 0.05). The highest absorption of K was found at 0.1 mM Ce3+ (Fig. 7a). Phosphorus (P) absorption was also higher than the control for all Ce3+ treatments. At 0.5 mM Ce3+, the absorption of P was 134.8 % more than the control. There was no significant difference with the control between the Ce3+ treatments except at 0.5 mM (Fig. 7b). The highest level of Mg ion was observed at 0.1 mM Ce3+ treatment and the lowest level was at 5.0 mM treatment (p < 0.05), while no significant differences were found at other Ce3+ treatments, compared with the control (p > 0.05) (Fig. 7c). The calcium (Ca) content were significantly reduced and lower than that of the control under the Ce3+ treatments except 0.1 and 0.5 mM. The 0.1 and 0.5 mM Ce3+ treatments could maintained the Ca level in Ginkgo cells and no significant differences were found between 0.1 and 0.5 mM and control treatments (p > 0.05) (Fig. 7d).
Mineral elements (K, P, Ca, Mg, Zn, Fe, Cu) (a–g) in Ginkgo suspension cells subjected to cerium nitrate elicitation for 7 days. Values are mean ± SD (n = 3). Means followed by different letters above the bars indicate significant differences (p < 0.05) among treatments according to the Duncan’s multiple range test
As shown in Fig. 7e–g, the Ce3+ doses differentially affected the accumulation of microelements (Fe, Zn, Cu) in Ginkgo suspension cells. In this study, Ce3+ treatment increased Zinc (Zn) accumulation from 0.05 to 1.0 mM while no significant difference was noted for the other treatments compared with the control. Interestingly, at a low Ce3+ dose (0.01 mM), the Zn content in the cells was similar to the control and as the high Ce3+ doses (2.0–5.0 mM). There was a significant increase of Zn at 0.1 mM (104.6 %) and 0.5 mM (102.4 %) Ce3+ in the suspension cells compared with the control (Fig. 7e). Fe accumulation was significantly reduced in the cells (p < 0.05) at all Ce3+ dose treatments. The lowest reduction of Fe content was found at 0.5 mM Ce3+ (only 21.1 % of Fe was reduced), whereas reduction of Fe was 70.8 % at 5.0 mM Ce3+ compared with the control (Fig. 7f). Similar results were observed for copper (Cu). The Ce3+ treatments reduced Cu accumulation in this study. However, there was no significant difference between 0.1, 0.5, 1.0 mM Ce3+ treatments and the control. Cu accumulation only decreased by 17.6 % at 0.1 mM Ce3+ treatment whereas it was reduced by 74.5 % at 5.0 mM Ce3+ treatment (Fig. 7g). The percent of increased or decreased of mineral element contents compared to control was appeared in Table 2.
Response of antioxidant enzyme activities to varying Ce3+ doses
The activities of the major ROS (reactive oxygen species) scavenging enzymes, the antioxidant enzymes SOD, POD and CAT, after Ce3+ treatment are shown in Fig. 8. An increase in SOD activity was observed from 0.05 to 0.5 mM Ce3+ doses in Ginkgo cells while no significant changes were observed for other Ce3+ treatments (p > 0.05) (Fig. 8a). However, the CAT activities of the suspension cells for 0.1,1.0 and 2.0 mM Ce3+ dose treatments were significantly higher than those of the control. The cells exposed to 0.5 mM Ce3+ treatment showed an enhancement of over 94 % in CAT activity (Fig. 8b). In contrast, the POD activity significantly increased from 0.05 to 5.0 mM Ce3+ in Ginkgo suspension cells where the highest increase over the control was 139 % at 0.05 mM Ce3+. No change in the POD activity was observed at a low level (0.01 mM) of Ce3+ (Fig. 8c).
The antioxidant enzymes of SOD (a), CAT (b) and POD (c) activities in Ginkgo suspension cells subjected to cerium nitrate elicitation for 7 days. Values are mean ± SD (n = 3). Means followed by different letters above the bars indicate significant differences (p < 0.05) among treatments according to the Duncan’s multiple range test
Membrane potential, lipid peroxidation response to varying Ce3+doses
Application of varying Ce3+ doses to Ginkgo suspension cells significantly enhanced the membrane permeability and the membrane deterioration as reflected by the increased relative conductivity (REC) and MDA content. As shown in Fig. 9a, the REC of the Ginkgo cells did not significantly increase in the presence of 0.01–0.5 mM Ce3+ compared with the control. However the REC sharply increased in the presence of 1.0–5.0 mM Ce3+ where it was 291 % higher than the control at 5.0 mM Ce3+. Ce3+ treatment also caused an increase in the MDA content in suspension cells, however, the magnitude of the increase was slightly higher at doses from 0.01 to 0.5 mM while it sharply increased at doses from 1.0 to 5.0 mM. The MDA content at 5.0 mM Ce3+ treatment was 51.7 % larger than the control (Fig. 9b).
The relative conductivity (REC) (a) and MDA (b) content in Ginkgo suspension cells subjected to cerium nitrate elicitation for 7 days. Values are mean ± SD (n = 3). Means followed by different letters above the bars indicate significant differences (p < 0.01) among treatments according to the Duncan’s multiple range test
Discussion
Our present research showed varying Ce3+ dose treatments significantly changed suspension cell growth, chlorophyll content, chlorophyll fluorescence, ion content and flavonol glycosides in Ginkgo biloba L. Low doses (0.01–0.1 mM) had a positive effect and improved cell growth. The maximum increase of dry and fresh weight was found at 0.1 mM Ce treatment. The moderate dose (0.5–1.0 mM) treatments limited the cell growth, while the high dose (2.0–5.0) mM treatments inhibited the cell growth or even caused cell mortality especially at 5.0 mM (Fig. 1). These findings were verified by the photos of the suspension cells (Fig. 6), and images of the chlorophyll fluorescence (Fig. 5) to Ce3+ treatment. Similar results have also been obtained by others (Xu and Chen 2011; Huang et al. 2010; dosSantos et al. 2014). These results indicated the positive or negative effects of REEs on suspension cell physiological metabolism mainly depended on the dosage of Ce3+. In this work, treatment with less than 0.5 mM Ce3+ had positive effects on Ginkgo suspension cell growth.
REEs such as La3+, Ce3+, Nd3+, and Y3+ have been used to stimulate taxol, flavonoid, crocin, and catharanthine production in Taxus, Tetrastigma hemsleyanum, Saussurea medusa, Crocus sativus cells and callus (Table 1). In the present work, moderate doses of Ce3+ (0.5–1.0 mM) significantly increased the accumulation of total flavonoids, and three flavonol glycosides in the Ginkgo suspension cells, whereas the low or high doses (such as 0.01–0.05 and 5.0 mM) resulted in no obvious increases in the above mentioned compounds compared with the control. PAL is a key branch point enzyme which regulates phenylalanine biosynthesis into phenolic compounds (Dong et al. 2010). The maximum PAL activity was also found at moderate doses of Ce3+ (0.5–1.0 mM) and was consistent with the accumulation of flavonoids in Ginkgo suspension cells. These results indicated that stimulation with Ce3+ switched primary metabolism to secondary metabolism and doses in the range of 0.1–0.5 mM Ce3+ may initiate a shift from primary to secondary metabolism in Ginkgo cells.
Appropriate doses of Ce increases the chloroplast pigment content, and maintain the structure of the chloroplast, while a high dose of Ce reduces these parameters (Wang et al. 2012; Maksimović et al. 2014). A similar result was found in the present work where 0.01–0.1 mM Ce3+ treatments gradually increased the chlorophyll a and b, while doses higher than 0.1 mM Ce3+ (0.5–5.0 mM) gradually decreased the content of these pigments. Unlike the changes observed for chlorophyll, significant increases in carotenoids were found at 0.5–2.0 mM Ce3+ treatments. Moreover, the Chl a/b ratio increased about one fold in the presence of 0.5–5.0 mM Ce3+ and the Car/Chl t ratio showed the same trend, where at higher doses (0.5–5.0 mM Ce3+), the ratio increased about two folds compared to that at lower (0.01–0.1 mM Ce3+). These results suggest that higher doses of Ce (0.5–5.0 mM) primarily disturbed Chl b biosynthesis while it stimulated the biosynthesis of Car. Furthermore, the high ratio of Car/Chl t and high Car content were beneficial to the accumulation of flavonol glycosides (Fig. 3). These results were strongly supported by the chlorophyll fluorescence results and similar to the results of Osorio et al. (2013).
Chlorophyll fluorescence has been used as a sensitive tool to diagnose the damage caused by environmental stresses (Zhang et al. 2014). Imaging of chlorophyll fluorescence provides an ideal screening platform which allows multiple plants and sample heterogeneity to be monitored under identical conditions at the same time (Murchie and Lawson 2013). In present work, the effect of Ce on chlorophyll fluorescence parameters in Ginkgo cells was dependent on the Ce dose. When the Ce3+ doses were greater than 0.1 mM (0.5–5.0), the Fv/Fm and Fv′/Fm′ ratios decreased while the qp and NPQ (0.5–1.0 mM) values increased in Ginkgo suspension cells (Fig. 4). The Fv/Fm ratio is thought to represent the electron capacity captured in the PSII reaction center. The decrease in the Fv/Fm and Fv′/Fm′ ratios may work to protect the PSII and the integrity of the thylakoid membrane in the presence of high doses of Ce. The increases of the qp and NPQ values may be caused by carotenoid accumulation from moderate doses of Ce treatments, while xanthophylls play a critical part in the NPQ value (Adams III et al. 1999). These results indicated Ginkgo suspension cells contained a self photo-protective regulatory mechanism at moderate doses of Ce (0.5–1.0 mM). These results were also in agreement with the findings of chlorophyll fluorescence in rice (Zhang et al. 2014; Chen et al. 2014).
Low dose of REEs (Ce and La) could increase the uptake of mineral elements, and be beneficial to photosynthesis in plants, while high doses of REEs could limit their uptake (Peralta-Videa et al. 2014). Interestingly, we found the accumulation of K and P was significant increased at 0.1 and 0.5 mM Ce3+ treatments, respectively, while the other Ce dose treatments did not significantly promote the accumulation of these elements compared with the control (Fig. 7a, b). These findings were similar to the work of Corral-Diaz et al. (2014). The increase of K uptake was believed to have been brought about because REEs stimulated the synthesis of Abscisic acid (ABA) which then increases the uptake of K (Zhao et al. 2014). These results suggested the effect of Ce on these mineral nutrients required a dose threshold, and lower or higher doses of Ce treatment had no significant effects on the accumulation of these elements.
Mg, Ca, Zn, Fe and Cu are fundamental in the photosynthesis process and the biosynthesis of pigments. Hong et al. (2005) found REEs were mainly bound to chlorophyll and located in the chloroplast membrane as well as in the thylakoid. REEs (Ce3+ or La3+) can enter the chlorophyll and replace the Mg2+ ion, then form Ce-chlorophyll or La-chlorophyll in the spinach chlorophyll molecule under conditions deficient in Mg (Ze et al. 2009). However, the accumulation of Mg was not significantly dependent on the dose of Ce in Ginkgo cells compared with the control in this work. It seems probable that Ce3+ may act as a substitute for Mg2+ only under conditions of Mg deficiency in cells (Fig. 7d).
Calcium is a secondary messenger that relates to biotic and abiotic environmental stressors, hormone biosynthesis, and development in plants (Peralta-Videa et al. 2014). It was known that Ce could fully displace Ca because the ionic radius of Ce was similar to that of Ca in biological systems (Paoli et al. 2014). Calcium accumulation was significantly reduced in Ginkgo cells in the presence of varying Ce3+ doses except from 0.1 to 0.5 mM (p < 0.05) (Fig. 7c). Thus, improper doses of Ce(NO3)3 might interfere with the function of Ca channels leading to a blockage of Ca2+ uptake in Ginkgo cells.
Fe and Cu accumulations were significantly reduced in Ginkgo cells at all Ce doses (Fig. 7f, g), while Zn accumulation was significantly increased in the presence of 0.01–2.0 mM Ce3+ (Fig. 7e). Iron is utilized in electron transport or stored complexed with ferritin and copper ion is an element of plastocyanin in chloroplasts (Grusak et al. 1999). However, there is an ion antagonism between zinc ion and the Fe or Cu ion, and an increase of Zn2+ might displace Fe2+ or Cu 2+ from their binding sites (Corral-Diaz et al. 2014), therefore causing decreases in the accumulation of Fe and Cu. Additionally, low concentrations of Fe and Cu compared with the control could also indicate that these elements are bound to Ce and stuck on the cell wall (Zhao et al. 2014). However, the chlorophyll and carotenoid amounts did not decrease with a reduction in the Fe2+ or Cu2+ concentration in Ginkgo cells from 0.01 to 0.5 mM Ce(NO3)3 treatment, likely as a result of an increasing Zn2+ concentration.
High dose of REEs may cause the generation of ROS and lead to oxidative stress (Rico et al. 2013) in plant cells. Antioxidant enzymes (SOD, CAT, POD) play an important role in the defense mechanisms of plants. SOD dismutates \({\text{{O}}_{2^\cdot}}^-\) to H2O2 while CAT and POD catalyze the conversion of H2O2 to H2O (Tian et al. 2015; Chen et al. 2014). In the present work, the 0.05–0.1, 0.1–2.0 and 0.05–5.0 mM Ce3+ doses were all observed to increase the activities of SOD, CAT, and POD in Ginkgo cells relative to the control, respectively. This indicated the doses of 0.05–0.5 mM Ce3+ were the sensitivity and key levels, in these levels Ginkgo cells maybe divert their metabolism from growth to defense by higher antioxidant enzymes activities. These results were also in agreement with the work of others (Corral-Diaz et al. 2014; Rico et al. 2013).
The lipid peroxidation (MDA) and electrolyte leakage (REC) were greatly enhanced at 1.0–5.0 mM Ce3+ doses in Ginkgo cells. The results suggest that the decreased activities of SOD, and CAT at higher Ce doses (>1.0 mM) were inefficient to remove \({\text{{O}}_{2^\cdot}}^-\) and H2O2 leading to enhanced membrane damage. Furthermore, the higher the concentration of Ce treatment, the less effective the enzymes were at removing ROS and the greater the damage to the cells.
Additionally, the associations between the total flavonoid content and the P, Ca2+, Zn2+, and Car amounts, and the Car/Chl t ratio, the SOD, CAT, POD, PAL activities, ∆GW were significantly positive (p < 0.01 or 0.05), but were negatively correlated with Chl b, Fv/Fm, Fv′/Fm′ respectively. However, the correlation of Car/Chl t with flavonoid, Car, and REC, MDA, qp was significantly positive (p < 0.01 or 0.05) (Table 3) while its correlation with Cu, Chl a, Chl b, total Chl t content, Fv/Fm, and Fv′/Fm′ is negative, respectively. Importantly, PAL activity was positively correlation with six mineral elements content (not including Fe), Car, total flavonoids, SOD, CAT, ∆GW, and ∆FW. While ∆GW was positively correlation with K, P, Ca, Zn, Chl b, total flavonoids, SOD, POD, qp, NPQ (Table 3). These correlations suggest that flavonoid biosynthesis is closely related to photosynthetic pigments, mineral ions and antioxidant enzymes. The increase of the Car/Chl t ratio, P, Ca2+ and Zn2+ content, and the decrease of Chl content, Fv/Fm, and Fv′/Fm′ by REEs could lead to an increase in the accumulation of flavonoids. The changes in the Car/Chl t ratio, Ca2+, Zn2+ and Car concentrations, and chlorophyll fluorescence in Ginkgo suspension cells were likely indicators that the metabolism was shifting from primary to secondary processes (e.g. as a result of the flavonoids accumulation) (Table 3).
In summary, 0.1 mM Ce(NO3)3 promoted the Ginkgo cell growth by increasing the Chl and Fv/Fm value, ions content, while 0.5–1.0 mM Ce(NO3)3 limited the cell growth and promoted flavonoid accumulation and antioxidant enzyme activities. These observed changes in flavonoids and antioxidant enzyme activities might be a self–protection mechanism to alleviate the environment stress in Ginkgo cells. Induction of 1.0 mM Ce(NO3)3 may be beneficial for in vitro production of flavonol glycosides in Ginkgo suspension cell culture.
Abbreviations
- Chl:
-
Chlorophyll
- Chl a/b :
-
Chlorophyll a/b ratio
- Car:
-
Carotenoid
- Chl t/Car:
-
Rate of total chlorophyll to Carotenoid
- PSII:
-
Photosystem II
- F v /F m :
-
Maximum PSII photochemical efficiency
- Fv′/Fm′:
-
Photochemical efficiency of PSII in the light
- qp :
-
Photochemical quenching
- NPQ:
-
Non-photochemical quenching
- NAA:
-
Naphthaleneacetic acid
- KT:
-
Kinetin
- REC:
-
Relative electrical conductivity
- MDA:
-
Malondialdehyde
- SOD:
-
Superoxide dismutase
- CAT:
-
Catalase
- POD:
-
Guaiacol peroxidase
- PAL:
-
Phenylalanine ammonia-lyase
- ROS:
-
Reactive oxygen species
References
Adams WW III, Demmig-Adams B, Logan BA, Barker DH, Osmond CB (1999) Rapid changes in xanthophyll cycle-dependent energy dissipation and photosystem II efficiency in two vines, Stephania japonica and Smilax australis, growing in the understory of an open Eucalyptus forest. Plant Cell Environ 22:125–136
Agati G, Azzarello E, Pollastri S, Tattini M (2012) Flavonoids as antioxidants in plants: location and functional significance. Plant Sci 196:67–76
Chen Y (2005) The research of cell and tissue cultures, and flavonoids metabolic regulation of Ginkgo biloba L. in vitro [D]. Doctoral dissertation of Nanjing Forestry University (in Chinese)
Chen SA, Zhao B, Wang XD, Yuan XF, Wang YC (2004) Promotion of the growth of Crocus sativus cells and the production of crocin by rare earth elements. Biotechnol Lett 26:27–30
Chen Y, Lin FZ, Yang H, Yue L, Hu F, Wang JL, Luo YY, Cao FL (2014) Effect of varying NaCl doses on flavonoid production in suspension cells of Ginkgo biloba L.: relationship to chlorophyll fluorescence, ion homeostasis, antioxidant system and ultrastructure. Acta Physiol Plant 36:3173–3187
Chu WY, Cai SJ, Fu YY, Li FF, Xu T, Qiu H, Xu QS (2014) The toxicity of cerium nitrate to Elodea canadensis: subcellular distribution, chemical forms and physiological effects. Acta Physiol Plant 36:2491–2499
Corral-Diaz B, Peralta-Videa JR, Alvarez-Parrilla E, Rodrigo-García J, Osuna-Avila P, Niu GH, Hernandez-Viezcas JA, Gardea-Torresdey JL (2014) Cerium oxide nanoparticles alter the antioxidant capacity but do not impact tuber ionome in Raphanus sativus (L). Plant Physiol Biochem 84:277–285
Cui TB, Zhang CY, Zheng HP, Guo Y, Jiang LL (2002) The influence of rare earth elements on growth and accumulation of secondary metabolites in Ginkgo suspension culture cells. J Guangdong Agr Sci 5:29–31 (in Chinese)
Dong JN, Wan GW, Liang ZS (2010) Accumulation of salicylic acidinduced phenolic compounds and raised activities of secondary metabolic and antioxidative enzymes in Salvia miltiorrhiza cell culture. J Biotechnol 148:99–104
dosSantos RW, Schmidt Éder C, deLFelix MR, Polo LK, Kreusch M, Pereira DT, Costa GB, Simioni C, Chowd F, Ramlov F, Maraschin M, Bouzon ZL (2014) Bioabsorption of cadmium, copper and lead by the red macroalga Gelidium floridanum: physiological responses and ultrastructure features. Ecotox Environ Safe 105:80–89
Fitriyanto NA, Fushimi M, Matsunaga M, Pertiwiningrum A, Iwama T, Kawai K (2011) Molecular structure and gene analysis of Ce3+ induced methanol dehydrogenase of Bradyrhizobium sp. MAFF211645. J Biosci Bioeng 111(6):613–617
Grusak MA, Pearson JN, Marentes E (1999) The physiology of micronutrient homeostasis in field crops. Field Crops Res 60:41–56
Hao GP, Du XH, Zhao FX, Shi RJ, Wang JM (2009) Role of nitric oxide in UV-B-induced activation of PAL and stimulation of flavonoid biosynthesis in Ginkgo biloba callus. Plant Cell Tiss Organ Cult 97:175–185
Hong FS, Wang L, Tao Y (2005) Mechanism of LaCl3 on increasing photosystem II activity of spinach. Chin J Chem 23(5):617–621
Huang SF, Li ZY, Wang XQ, Wang QX, Hu FF (2010) Cerium caused life span shortening and oxidative stress resistance in Drosophila melanogaster. Ecotox Environ Safe 73:89–93
Kang SM, Min JY, Kim YD, Karigar CS, Kim SW, Goo GH, Choi MS (2009) Effect of biotic elicitorson the accumulation of bilobalide and ginkgolides in Ginkgo biloba cell cultures. J Biotechnol 139:84–88
Koukol J, Conn EE (1961) The metabolism of aromatic compounds in higher plants. Purification and properties of the l-phenylalanine de aminase of Hordeum vulagare. J Biol Chem 236:2692–2698
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic membranes. Method Enzymol 148:350–382
Liu C, Cao WQ, Lu Y, Huang H, Chen L, Liu XQ, Hong FS (2009) Cerium under calcium deficiency influence on the antioxidative defense system in spinach plants. Plant Soil 323:285–294
Maksimović I, Kastori R, Putnik-Delić M, Borišev M (2014) Effect of yttrium on photosynthesis and water relations in young maize plants. J Rare Earth 32(4):372–378
Maria F, Stanisław W, Halina M, El Zbieta RS, Apolonia S, Jolanta BK (2012) Membrane permeability and micro-and macroelement accumulation in spring wheat cultivars during the short-term effect of salinity and PEG induced water stresses. Acta Physiol Plant 34:985–995
Murchie EH, Lawson T (2013) Chlorophyll fluorescence analysis: a guide to good practice and understanding some new applications. J Exp Bot 64:3983–3998
Osorio ML, Goncalves S, Coelho N, Osorio J, Romano A (2013) Morphological, physiological and oxidative stress markers during acclimatization and field transfer of micropropagated Tuberaria major plants. Plant Cell Tiss Organ Cult 115:85–97
Ouyang J, Wang X, Zhao B, Yuan X, Wang Y (2003) Effects of rare earth elements on the growth of Cistanche deserticola cells and the production of phenylethanoid glycosides. J Biotechnol 102:129–134
Paoli E, Fiorini S, Munzi S, Sorbo A, Basile S, Loppi S (2014) Uptake and acute toxicity of cerium in the lichen Xanthoria parietina L. Ecotox Environ Safe 104:379–385
Peng X, He JY (2013) The inhibitory effect of Ca2+ on the flavonoid production of Tetrastigma hemsleyanum suspension cells induced by metal elicitors. In Vitro Cell Dev Biol plant 49:550–559
Peng X, Zhou SL, He JY, Ding L (2013) Influence of rare earth elements on metabolism and related enzyme activity and isozyme expression in Tetrastigma hemsleyanum cell suspension cultures. Biol Trace Elem Res 152:82–90
Peralta-Videa JR, Hernandez-Viezcas JA, Zhao LJ, Corral Diaz B, Ge Y, Priester JH, Holden PA, Gardea-Torresdey JL (2014) Cerium dioxide and zinc oxide nanoparticles alter the nutritional value of soil cultivated soybean plants. Plant Physiol Biochem 80:128–135
Rico CM, Morales MI, McCreary R, Castillo-Michel H, Barrios AC, Hong J, Tafoya A, Lee WY, Varela-Ramirez A, Peralta-Videa JR, Gardea-Torresdey JL (2013) Cerium oxide nanoparticles modify the antioxidative stress enzyme activities and macromolecule composition in rice seedlings. Environ Sci Technol 47(24):14110–14118
Shtangeeva I, Ayrault S (2007) Effects of Eu and Ca on yield and mineral nutrition of wheat (Triticum aestivum) seedlings. Env Exp Bot 59:49–58
Tian J, Jiang FL, Wu Z (2015) The apoplastic oxidative burst as a key factor of hyperhydricity in garlic plantlet in vitro. Plant Cell Tiss Organ Cult 120:571–584
Wang X, Lin YH, Liu DG, Xu HJ, Liu T, Zhao FY (2012) Cerium toxicity, uptake and translocation in Arabidopsis thaliana seedlings. J Rare Earth 30(6):579–585
Wang J, Qian J, Yao LY, Lu YH (2015) Enhanced production of flavonoids by methyl jasmonate elicitation in cell suspension culture of Hypericum perforatum. Bioresour Bioprocess 2:5. doi:10.1186/s40643-014-0033-5
Wu J, Wang C, Mei X (2001) Stimulation of taxol production and excretion in Taxus spp. Cell cultures by rare earth chemical Lanthanum. J Biotechnol 85:67–73
Xu QM, Chen H (2011) Antioxidant responses of rice seedling to Ce4+ under hydroponic cultures. Ecotox Environ Safe 74:1693–1699
Yang S, Lu SH, Yuan YJ (2008) Lipidomic analysis reveals differential defense responses of Taxus cuspidate cells to two elicitors, methyl jasmonate and cerium (Ce4+). Biochim Biophys Acta 1781:123–134
Yang S, Lu SH, Yuan YJ (2009) Cerium elicitor-induced phosphatidic acid triggers apoptotic signaling development in Taxus cuspidata cell suspension cultures. Chem Phys Lipids 159:13–20
Yuan YJ, Li JC, Ge ZQ, Wu JC (2002a) Superoxide anion burst and taxol production induced by Ce4+ in suspension cultures of Taxus cuspidate. J Mol Catal B-Enzym 18:251–260
Yuan XF, Wang Q, Zhao B, Wang YC (2002b) Improved cell growth and total flavonoids of Saussurea medusa on solid culture medium supplemented with rare earth elements. Biotechnol Lett 24:1889–1892
Ze YG, Zhou M, Luo LY, Ji Z, Liu C, Yin ST, Duan YM, Li N, Hong FS (2009) Effects of cerium on key enzymes of carbon assimilation of spinach under magnesium deficiency. Biol Trace Elem Res 131(2):154–164
Zhang M, Tang SH, Huang X, Zhang FB, Pang YW, Huang QY, Qiong Y (2014) Selenium uptake, dynamic changes in selenium content and its influence on photosynthesis and chlorophyll fluorescence in rice (Oryza sativa L.). Env Exp Bot 107:39–45
Zhao J, Zhu WH, Hu Q (2000) Promotion of indole alkaloid production in Catharanthus roseus cell cultures by rare-earth elements. Biotechnol Lett 22:825–828
Zhao LJ, Peralta-Videa JR, Peng B, Bandyopadhyay S, Corral-Diaz B, Osuna-Avila P, Montes MO, Keller AA, Gardea-Torresdey JL (2014) Alginate modifies the physiological impact of CeO2 nanoparticles in corn seedlings cultivated in soil. J Environ Sci 26:382–389
Acknowledgments
Funding for this work is provided by the Program for Priority Academic Program Development of Jiangsu Province of China (PAPD); the Program of twelfth Five-year National Science & Technology Support Project (2012BAD21B05); the National Natural Science Foundation of China (31170627); the Program for Innovative Research Team in Universities of Educational Department (PCSIRT, China).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Y., Luo, Y., Qiu, N. et al. Ce3+ induces flavonoids accumulation by regulation of pigments, ions, chlorophyll fluorescence and antioxidant enzymes in suspension cells of Ginkgo biloba L.. Plant Cell Tiss Organ Cult 123, 283–296 (2015). https://doi.org/10.1007/s11240-015-0831-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-015-0831-2