Abstract
Cadmium (Cd2+) is a toxic heavy metal that is widespread throughout the environment. It is a subject of interest to environmental scientists because of its toxicity to plants, animals, and humans. The current work aims to evaluate the effects of Cd2+ on the production of phenolic compounds and morphological, physiological and biochemical responses of Lupinus albus L. plants exposed to Cd2+ at 0, 10, 20, 50, 100, and 150 μM CdCl2 for 7 days. Cd2+ induced negative effect on growth especially at the dose of 150 µM. Cd2+ also induced chlorosis and reduced photosynthetic activity. Besides, the metal increased the level of malondialdehyde (MDA). Under Cd2+ toxicity (50, 100, and 150 µM), the superoxide dismutase (SOD) and catalase (CAT) activities were increased or not significantly affected, while at 150 µM Cd2+ affected the activity of these enzymes. At the highest Cd2+ level (150 µM), proline and total polyphenol and flavonoid contents were markedly increased in leaves and roots of L. albus. Our results suggest that L. albus plants produced phenolic compounds with reducing capacity as a selective mechanism triggered by the highest activity of Cd2+.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Environmental problems have always received great attention from scientists since they threaten seriously human health and agricultural production. Heavy metals such as cadmium (Cd2+), lead (Pb), copper (Cu), and zinc (Zn), which are at the top of the list of environmental toxicants threatening nature. These pollutants contamination problem is becoming vital, and necessities essential and practical solutions to decrease the dangers to the maximum extent [1]. Moreover, Cd2+ is classified as one of the most toxic heavy metals, representing a potential hazard through bioaccumulation along the food chain. This heavy metal is taken up by plants in significant quantities from contaminated water and soil. Furthermore, accumulated Cd2+ in crop tissues results in various structural, biochemical and physiological transformations. Cd2+ disturbs photosynthesis by hindering photoactivation of photosystem II. Besides, it alters respiration, converted carbohydrate metabolism. In addition, Cd2+ upsets the nutrients and water uptake in plants, delay opening and closing of stomata [2] and prevents the activity of Calvin cycle enzymes. Cd2+ also alters antioxidants activity and decays the yield of crop plants.
Hence, an effective approach such phytoremediation for Cd2+ remediation from contaminated environments is crucial to avoid harmful damage in plants and to reduce or to prevent Cd2+ accumulation in plant tissues because conventional physiochemical approaches are highly expensive and may affect the soil. Phytoremediation is important and efficient since it is economically feasible and eco-friendly technology [3].
Phytoremediation approach includes several types of distinct strategies such as phytoextraction, phytostabilization, phytovolatilization, phytodegradation and phytofiltration. Thus, some plants may these strategies and grow on soils contaminated by heavy metals such as Brassica juncea [4], Lactuca sativa L. [5], Sesuvium portulacastrum [6]. In fact, plants develop specific mechanisms to detoxify the metal or to make it unavailable.
Lupinus albus or white lupin is a very interesting species. According to Sujak et al. [7] Lupin seeds are rich in proteins and bioactive compounds and fibers [8]. So, it would be very interesting to understand the adaptive response of white lupin to Cd2+, and to evaluate the metal tolerance in order to propose it as species of interest to cleaning up soils contaminated by Cd2+. Indeed, phytoextraction of heavy metals on marginal areas represents a very relevant and promising approach to be part of the rehabilitation and enhancement of abandoned marginal areas in order to increase forage or food resources. In this context, the objective of our research is to investigate the response of white lupine under the effect of different concentrations of Cd2+ by determining morphological, physiological and biochemical parameters.
MATERIAL AND METHODS
Plant material and growth conditions. White lupin (Lupinus albus subsp albus. L.) from Egypt was investigated. The seeds were sterilized and then germinated on moistened filter paper in Petri dishes at 25°C in the dark. After 4 days, seedlings were transferred to a growth chamber: 25°C (150 µmol mPAR) with 60% relative humidity during the day and 20°C at night; photoperiod 14 h daily with an intensity of 110 µmol photon/(m2 s) at the canopy level. Seedlings were grown on stainless net floating on the continuously aerated nutrient solution in pots, each of them being composed of 2 L nutrient solution and 8 seedlings. After germination, plants were grown hydroponically for eleven days in pots filled with 4.5 L of continuously aerated Hoagland’s nutrient solution (5 plants per pot). The Hoagland’s solution consisted of 5 mM Ca(NO3)2, 5 mM KNO3, 1 mM KH2PO4, 50 µM H3BO3, 1 mM MgSO4, 4.5 µM MnCl2, 3.8 µM ZnSO4, 0.3 µM CuSO4, 0.1 mM (NH4)6Mo7O24 and 10 µM Fe-EDTA, and was adjusted to a pH 5.8. The total volume of the solution was kept constant by adding deionized water to compensate for water lost through plant transpiration, sampling and evaporation. Solutions were changed every 3 days and pH was adjusted daily.
The plants were then transplanted into a nutrient medium supplemented with increasing doses of Cd2+ (0, 10, 20, 50, 100, and 150 μM CdCl2). After 7 days of CdCl2 treatment, plants were harvested for analysis to study the effect of CdCl2 on growth parameters and the physiological and biochemical behavior of white lupin.
Growth analysis. The different parts of the white lupin plants (leaves, stems and roots) were measured using a graduated scale and the results are expressed in mm.
Roots were immediately dipped in a cold 0.01 M HCl solution to eliminate externally adsorbed Cd2+, then washed 3 times with cold distilled water and blotted with filter paper. Fresh weight (FW) was determined immediately, and dry weight (DW) was measured after 48 h of desiccation at 65°C.
Content of photosynthetic pigments. Chlorophyll (Chl) content is determined by spectrophotometry according to the method of McKinney [9]. The aerial part of the fresh plant is immersed in acetone and stored overnight at 4°C. The plant material is then ground in a mortar with 80% acetone. After centrifugation (5 min at 3000 g), the Chl content in the supernatant is determined by measuring the absorbance at 645, 663, 652, and 460 nm, using a Lambda1A spectrophotometer (Perkin-Elmer, United States). The concentrations of chlorophyll a, b, total and carotenoids (Carot), expressed in µg/(g FW), are determined using the following formulas:
Measurement of water content. The water content (WC), expressed in mL/(g DW), was calculated according to the following equation:
where, FW is the weight measured at final harvest, DW is the weight measured after drying at 80°C.
The sensitivity index (SI) corresponds to the difference in DW production between treated and control plants. It was calculated using the ratio:
Determination of cadmium content. Dried samples (about 25 mg) were grounded to a fine powder and processed in 4 : 1 (v/v) HNO3/HClO4 (20 mL) mixture. After cooling, the residues were solubilized with a 7% solution of HNO3 and then filtered. The Cd2+ concentrations were determined by atomic absorption spectrometry Spectra AA 220 FS (Gemini BV, United States).
Soluble sugars, proline and MDA content. 25 mg of DW was extracted in the presence of 5 mL of 80% ethanol. The samples were placed in a water bath at 70°C for 30 min and, then, centrifuged at 6000 g for 15 min. Subsequently, 25 μL of supernatant was removed, and added to 5 mL of anthrone solution under fume hood. The tubes were placed in a water bath at 100°C for 10 min, and then placed on ice. The absorbance was measured at 640 nm [10].
The determination of proline is a colorimetric assay based on the proline-ninhydrin complex. 100 mg of dry material was mixed with 1.5 mL of sulfosalicylic acid. The mixture was centrifuged at 12 000 g for 20 min at 4°C. Then, 500 μL of the extract was homogenized with 500 μL of sulfosalicylic acid, 1 mL of ninhydrin reagent and 1 mL of concentrated acetic acid. The extracts are incubated in a water bath at 100°C for 1 h and then cooled at 4°C. The mixture catalyzes the proline-ninhydrin reaction. To separate the phases, 2 mL of toluene is added. The absorbance was determined at 520 nm and the proline concentration was expressed in μg/(mg DW) [11].
Malondialdehyde determination is based on the reaction between MDA and two molecules of thiobarbituric acid (TBA). 250 mg of fresh leaves ground in liquid nitrogen were suspended in 5 mL of 5% trichloroacetic acid (TCA) (w/v) and centrifuged at 12 000 g for 10 min. 2 mL of the obtained extract was mixed with 2 mL of TBA (0.67%) and the mixture was kept in a water bath at 100°C for 30 min followed by ice bath. Then the mixture was centrifuged for 1 min at 6000 g. The absorbance of the supernatant was measured at 532 and 600 nm. The amount of MDA is calculated according to Lambert Beer’s law [12]:
where ε is molar extinction coefficient of 155 mM–1 cm–1.
Soluble total protein. This assay was carried out according to the method of Bradford [13]. 10 µL of protein extract was mixed with 200 µL of Bradford reagent. After 5 min of incubation at room temperature in the dark, the absorbance was measured at 595 nm and compared with that of a standard range of bovine serum albumin.
Ammonium (\({\mathbf{NH}}_{{\mathbf{4}}}^{{\mathbf{ + }}}\)) assay. A mass of 0.5 to 1.0 g of the plant material, previously frozen in liquid nitrogen, was ground at 4°C in the presence of 2 mL of H2SO4 and 0.5% of polyclart AT. The ground material was centrifuged at 30 000 g for 15 min. The obtained supernatant was placed at 37°C for 30 min and the absorbance was measured at 620 nm [14].
Protease activity. 100 mg of fresh plant material already stored in liquid nitrogen was mixed with 1 mL of buffer containing 50 mM Tris-HCl, pH 7.5, 1% PVP and 5% β-mercaptoethanol. Centrifugation was carried out at 14 000 g for 30 min at 5°C. An incubation of 3 h at 37°C was carried out and reaction was stopped by the addition of 320 μL TCA (5%). The mixture was centrifuged at 15 000 g for 10 min at 5°C and the absorbance was read at 330 nm.
Phytochemical Analysis
Determination of total phenolic contents (TPC). Total phenolics content (TPC) of the methanolic extracts of different parts of white lupin was determined by using the Folin-Ciocalteu method with slight modifications [15]. Initially, 125 µL of diluted extract was dissolved in 60 µL of distilled water and 15 µL of Folin-Ciocalteu reagent. The mixture was vortexed before adding 150 mL of 7% Na2CO3. After incubation in the dark for 1 h, the absorbance at 760 nm was measured against the prepared blank. Total phenolic content was expressed as mg of gallic acid equivalents per gram of dry weight (mg GAE/g DW) using a calibration curve with gallic acid as standard (0.001–0.01 µg/mL).
Total flavonoid content (TFC). Total flavonoid content (TFC) of each methanolic extract, was estimated using a colorimetric assay developed by Ben Mansour et al. [15]. An aliquot (250 mL) of the samples was mixed with 75 mL NaNO2 solution (5%; w/v) for 6 min, followed by the addition of 150 mL AlCl3·6H2O (10%; w/v). After 5 min at room temperature (RT), 500 mL of 1 M NaOH was added. The final volume was adjusted to 2.5 mL with H2O and thoroughly mixed. The absorbance of the mixture was determined at 510 nm and converted to flavonoid concentration from a catechin standard curve and expressed as mg catechin equivalents per gram of dry weight (mg CE/g DW).
Total condensed tannins (TCT). The condensed tannins content was determined according to the method of Ben Mansour et al. [15]. 50 mL of extract was mixed with 3 mL of 4% vanillin (w/v). Then, 1.5 mL 2 M hydrochloric acid was added to the mixture. After incubation for 15 min at RT, the absorbance was measured at 500 nm. The results were expressed as mg catechin equivalents per gram of dry weight (mg CE/g DW).
Antioxidant enzymes assay. For protein extraction, 100 mg of fresh plant material already preserved in liquid nitrogen was mixed with 700 μL of extraction buffer containing 50 mM phosphate buffer (pH 7.0), 1 mM Na-EDTA, 20 mM MgCl2, 50 mM KCl, and 0.5 mM PMSF. Centrifugation was carried out at 14000 g for 30 min at 4°C. The supernatant obtained contains the soluble proteins and is used for protein determination and for enzymatic activity assays.
Superoxide dismutase activity. SOD (EC 1.15.1.1) was assayed according to the method of Boudali et al. [16] and the absorbance was measured at 560 nm.
Catalase activity. CAT (EC 1.11.1.6) was assayed according to the Boudali et al. [16]. The reaction mixture contains a volume of 50 mM phosphate buffer (pH 7.0), 20 μL H2O2 and 20 μL of plant extract. The assay was performed by measuring the absorbance at 240 nm and the activity was expressed in mmol/min/mg FW.
Statistical analysis. Data shown are means of 20 replicates for each treatment. Analysis of variance between treatments means was carried out with the SPSS 10.0 program. Means were compared using the Duncan’s test at P < 0.05.
RESULTS
Plant Morphology and Growth
Cd2+ induced negative effect on growth especially at the dose of 150 µM (Supplementary Fig. S1). We noticed that this effect increased with increasing cadmium concentration in the culture medium. Cd2+ concentration had significant effect on growth parameters (Table 1). Growth reduction is observed from the lowest applied dose (10 µM) and decreased considerably under high Cd2+ concentration (100 and 150 µM). Furthermore, Cd2+ significantly decreased the DW in Lupin plants, especially in roots (Fig. 1). The effect of cadmium is more pronounced with the higher Cd2+ concentration (100 and 150 µM). Leaf DW decreased by about 39 and 60% at 100 and 150 μM Cd2+, respectively, compared to the control. For the root, DW decreased by about 70 and 78% compared to the control under 100 and 150 μM Cd2+, respectively. However, Cd2+ treatment showed no significant effect on stem DW.
In addition, the data from Table 1 shows a reduction in the length of the aerial part of about 7, 14, and 31% at 50, 100, and 150 μM Cd2+, respectively, compared to the control. This effect is more significant in the roots (41, 42, and 49% at 50, 100, and 150 μM Cd2+, respectively, compared to the control). It is noticeable that the root system remained more sensitive to Cd2+ than leaves and stems.
The estimation of the water content of various plant tissues (leaves, roots and stems) grown under Cd2+ exposure was shown in Fig. 2. In the shoot system (leaves and stems), WC decreased proportionally to the Cd2+ dose. However, root WC was almost constant for all doses applied.
Cadmium Accumulation
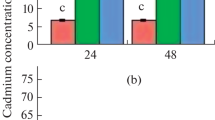
In treated plants, Cd2+ concentrations in leaves, stems and roots increased with increasing concentration of CdCl2 in the culture medium (Fig. 3). At all concentrations of CdCl2, the Cd2+ concentration in root tissues were higher than in the leaves and stems. Examination of the proportions of Cd2+ retained in the roots and transported to the leaves and stems, in relation to the total amount of Cd2+ accumulated, showed that the roots accumulated the major fraction of the absorbed metal.
Photosynthetic Pigments Contents
CdCl2 application induced a significant decrease in leaf chlorophyll content (Table 2). Under 150 µM Cd2+ treatment, the total chlorophyll content decreased by about 32% compared to the control plant. Treatment with Cd2+ caused a decrease in Chl a and Chl b contents of 30 and 37%, respectively, under 100 and 150 µM Cd Cl2 compared to the control plant.
Moreover, the stem chlorophyll content also decreased significantly with the higher concentration of Cd2+. Thus, under 150 µM of CdCl2 treatment, total chlorophyll decreased by about 62% referring to the control. Chl a and b contents also decreased, by 22 and 85%, respectively, under 150 μM CdCl2 treatment. These results allowed to conclude that Cd2+ treatment, even at moderate concentration, decreased the chlorophyll contents.
Regarding carotenoids, the results presented in Table 2 showed a significant increase in carotenoid levels under 10 µM CdCl2. Moreover, in leaves, under 100 μM CdCl2 treatment, the carotenoids level increased about 10 times compared to control plants; while in stems it was about 2 times compared to the control.
Total Soluble Sugar, Proline and MDA Contents
Exposure to CdCl2 increased the soluble sugar content in roots, stems, and leaves of white lupin plant (Fig. 4a). The results showed that under 150 μM CdCl2, the sugar content level in leaves, stems and roots increased by 456, 151, and 259%, respectively, compared to control plants.
Effect of different Cd2+ concentrations on metabolites production in white lupin seedlings. (a) Total sugar content, (b) proline content, and (c) MDA content, where (1) leaves; (2) stems; (3) roots. Data are the means of 20 replicates. Mean values followed by the same letter are not significantly different at P ≤ 0.05.
A significant accumulation of proline was observed (Fig. 4b). This accumulation depended on the concentration of CdCl2 in the culture medium and in the plant organ. Thereby, the proline accumulation was higher in aerial parts (especially leaves) than in roots and this effect increased with increasing the concentration of CdCl2 in the medium. Consequently, the increase in proline level reached 263% in the leaves compared to the control. While this increase reached 174 and 145%, respectively, in the stems and roots compared to the control.
Addition of Cd2+ significantly increased the production of MDA, an indicator of the membrane lipid peroxidation. The results showed that the increase in MDA level was proportional to the concentration of CdCl2in the medium. The more important increase as compared to control plants was obtained mainly in roots. Under 150 µM CdCl2 treatment the MDA content was about 1456% in roots, 351% in leaves, and 253% in stems compared to control plants (Fig. 4c).
Secondary Metabolites Production
Figure 5a illustrated the effect of different cadmium concentrations on total polyphenol contents in white lupin seedling. Results obtained confirmed that the methanolic extract of the roots was richer in polyphenols compared to leaves and stems. Accordingly, Cd2+ treatment induced a strong increase in TPC in all plant organs compared to the control plants. TPC in leaves increased only at 150 µM CdCl2 (192%). However, for roots the increase of TPC was observed from the lowest dose applied (182% at 10 µM CdCl2). For stem, TPC increase was about 429% under 150 μM CdCl2 treatments. In control plants, TFC were quite close in different plant organs. However, a significant increase of TFC was observed with increasing Cd2+ concentration in the culture medium (Fig. 5b). TFC was more significant in roots than in stems and leaves. In fact, under 100 μM CdCl2 treatment, TFC increased by 190% in leaves and stems. In contrast, the TFC in the roots reached 346% of the value measured in the control plants. In addition, Cd2+ induced a significant increase of the TCT in the different organs of the plant (Fig. 5c). Results showed a significant increase in leaves, stems and roots by about 174, 171, and 167%, respectively, under 150 µM CdCl2 treatment compared to control plants.
Effect of different Cd2+ concentrations on secondary metabolites production in white lupin seedlings. (a) Total polyphenol contents, (b) total flavonoid content, and (c) total condensed tannins content, where (1) leaves; (2) stems; (3) roots. Data are the means of 20 replicates. Mean values followed by the same letter are not significantly different at P ≤ 0.05.
Antioxidant Enzymes Activities
In leaves, CAT activity increased under Cd2+ treatment (Fig. 6a). Nevertheless, CAT activity increase remained constant independently of different CdCl2 concentration in the culture medium. CAT activity increased about 26% from 10 μM to 150 μM CdCl2. While in stems and roots, CAT activity enhancement was more significant under the high cadmium concentration. Hence, at 150 μM CdCl2, CAT activity increase about 77 and 61.9%, respectively, in stems and roots compared to control plants.
Effect of different Cd2+ concentrations on the activities of antioxidant enzymes in white lupin seedlings. (a) Catalase activity and (b) superoxide dismutase activity, where (1) leaves; (2) stems; (3) roots. Data are the means of 20 replicates. Mean values followed by the same letter are not significantly different at P ≤ 0.05.
SOD increased under 150 μM CdCl2 in leaves compared to control plants. Whereas, no significant enhancement in SOD activity was observed in roots (Fig. 6b).
Soluble Protein, Ammonium Contents and Protease Activity in Plant
In control seedlings, the total soluble protein (SP) content varied between the 3 studied organs. Thus, SP content decreased by about 18.4, 46.2, and 83.8% in leaves, stems, and roots, respectively, at 150 μM CdCl2 compared to control plants (Table 3). The decrease in SP content could be related to the stimulation of the proteolytic pathway.
Ammonium (\({\text{NH}}_{4}^{ + }\)) content was higher in the stems (0.875 µmol/g FW), than in the roots (0.562 µmol/g FW) and leaves (0.312 µmol/g FW) for control plants (Table 3). In stressed condition, \({\text{NH}}_{4}^{ + }\) content increased by about 481, 30, and 289% in leaves, stems and roots, respectively, at 150 μM of CdCl2 of that measured in control plants.
The increase of \({\text{NH}}_{4}^{ + }\) content could be explained by the activation of protease pathways and the disruption of \({\text{NH}}_{4}^{ + }\) incorporation. In control plants, the protease activity in aerial parts appeared to be greater than in roots. Under 150 μM CdCl2 the protease activity increased by about 122.7, 30.1, and 85.8% in leaves, roots and stems, respectively, as compared with control plants (Table 3).
DISCUSSION
The investigation described in this study examines the response of white lupin to Cd2+. Cadmium treatments reduced the growth of lupin plants as has been reported by other works [17, 18]. In addition, it was demonstrated roots from white lupin were the preferential organs for Cd2+ accumulation [19] since the roots are probably the first to suffer Cd2+ injury. Cd2+ uptake is related to endodermal apoplasmic barriers which may restrict or derestrict the Cd2+ loading into xylem. Additionally, Cd2+ concentration in the roots tissues was higher than in the leaves and stems. Similar results have been observed in Ageratum conyzoides L. [20]. In addition, it has been proven that roots appear as trap organs, ensuring the accumulation of high part of the metal, thus limiting its export to stem and leaf [21]. Root Cd2+ accumulation could be explained by the parietal retention of Cd2+and the strong precipitation of the metal in apoplasmic spaces or in xylem. Another work showed that endocellular sequestration of the pollutant could be carried out by sulfur peptides [22]. The present results showed that the high concentration of Cd2+ develop significant physiological and metabolic disorders in white lupin seedling. In fact, many visual symptoms of toxicity were detected in white lupin plants. These symptoms were manifested by necrosis and chlorosis spots in leaves and by browning in roots. These effects were also associated with leaf drop especially for the doses 100 and 150 μM CdCl2. Therefore, these symptoms might be attributed to a mineral deficiency including manganese, magnesium and iron [23]. The plant growth was significantly reduced in the presence of high Cd2+ concentration; this effect was linked to ionic Cd2+ toxicity in shoots and roots [24]. Another work explained the reduction of biomass by the loss of cell turgescence and by a nutritional deficiency caused by the heavy metal [25].
These negative effects could also be in related to balance disturbances of some growth hormones notably auxin and the deterioration on the cell walls composition [26]. This study showed that the growth reduction of white lupin was accompanied by dehydration especially in shoots. In fact, the reduction of the root absorption surface following the fall of root growth caused an imbalance between the shoot water demand and the root absorption capacity [27]. This effect may be due also to the inhibition of the xylem cell division and elongation by cadmium.
Cd2+ treatment induced content of total Chl, Chl a and Chl b by reduction. The reduction of chlorophyll is one of the primary events in plants exposed to metal stress and results from the inhibition of the enzymes responsible for chlorophyll biosynthesis. A research focused on Brassica napus showed chlorosis is due to decrease in the density of chloroplasts and an increase in the size of mesophylls cells [28]. Photosynthesis decrease could be related to a block in photosystem II (PSII) acceptor and donor side electron transport under Cd2+ treatment and/or a decrease in quantum yield of PSII [29]. On the other hand, the MDA content in white lupin seedlings increased proportionally to CdCl2 concentration in the nutrient solution. This effect is an indicator of lipids membrane peroxidation. MDA levels increased simultaneously with membrane instability and decrease in membrane integrity followed by a stimulation of the lipoxygenase activity. Indeed, another study showed that lipid peroxidation was accompanied by hydroxyl and peroxide accumulation, hence the destruction of the membrane and cellular components [30]. Furthermore it has been shown also that MDA production is followed by a change in the activity of antioxidant enzymes such as CAT and/or SOD [31].
To tolerate and reduce oxidative damage, plants set up enzymatic (CAT, SOD) and non-enzymatic antioxidant defense systems (carotenoids and polyphenols). These systems play an important role in regulating ROS concentrations in plant. CAT and SOD enzymes allowed the destruction of free radical species by catalyzing the transformation of the \({\text{O}}_{2}^{{\bullet - }}\) radical into H2O2. Our results revealed a stimulation of SOD and CAT activities in leaves, stems and roots of white lupin under Cd2+ treatment. These results were confirmed by Bashir et al. [32] who showed a significant increase in SOD activity in Cd2+-treated plants. Furthermore, several studies showed that ascorbate peroxidase (APX) act as H2O2 scavenging enzyme. Also, it is indispensable for the protection of chloroplasts and other cell constituents. Heavy metals stimulated the expression of APX in plants. In fact, Cd2+ stress enhanced leaf APX activity in Brassica juncea [33] and T. aestivum [34]. On the other hand, high Cd2+ concentrations induced the increase of the levels of non-enzymatic antioxidant molecules such as carotenoids and phenolic compounds in different parts of the plant. This effect in carotenoid levels provided protection against oxidative stress due to its ability to trap and neutralize peroxyl radicals. In addition, the presence of phenolic compounds prevents Reactive Oxygen Species (ROS) production, either by inactivating of chlorophylls or by dissipating excess energy through the xanthophyll cycle. In Cd2+-treated seedlings, the polyphenol content was explained by the presence of defense enzymes involved in phenolic metabolism. Indeed, phenolic compounds could chelate transition metal ions, eliminate ROS and thus inhibit lipid peroxidation. Similar results, including an increase in TPC were reported in olive tree (Olea europaea) and in Cakile maritimum subjected to water deficit and salt stress [35]. In addition, the present study showed an increase in proline concentration in different organs of plant. In fact, proline played a major role in plant osmoregulation and osmotolerance to various abiotic constraints. Recent studies showed proline improve growth and yield in Cd2+-treated common bean plants and improve enzymatic and non-enzymatic antioxidant activities [36]. Another work showed that proline could protect enzymes from inactivation and also induced mechanism of Cd2+ chelation and detoxification in tomato plants, by forming a non-toxic Cd2+-proline complex [37]. Proline accumulation could lead to direct impact manifested by the improvement of photosynthetic activity and mineral nutrition, while the indirect impact of this metabolite resulted from improved tolerance. Cd2+ stress induced a significant increase in soluble sugar levels in shoots and roots of treated lupin seedlings. Various metals caused an increase in soluble sugar content of Lemna polyrrhiza plants [38]. In the same tendancy, soluble sugars accumulation is positively correlated with metal tolerance in Synara scolymus plants [39]. The accumulation of soluble sugars leads to the improved permeability of the membrane, the homeostasis of the maintained water and the reinforced antioxidant defense mechanisms. Another work on pea (P. sativum) showed that soluble sugars act as an osmoprotectants, which maintained turgor and induced tolerance in stressed plants [40].
Results obtained in the present work showed that plant subjected to Cd2+ accumulated high levels of ammonium in their organs. This result confirmed that Cd2+ affect nitrogen metabolism in white lupin seedling. The accumulation of \({\text{NH}}_{4}^{ + }\) correlated with a decrease of protein content and an increase of proteolytic activity in different organs of plant. Other works showed a correlation between protein degradation and proteolytic activity with respect to oxidative stress has also been proposed, suggesting an increase in protein degradation as an index of oxidative stress. Cadmium treatment induced ammonium accumulation in several plant species such as Medicago sativa [22]. In addition, toxic accumulation of ammonium in plant is linked to the disruption of the incorporation of \({\text{NH}}_{4}^{ + }\) in the carbon skeletons by the decrease of enzymatic activities of glutamine synthetase (GS) and glutamate synthase (GOGAT) in white lupin plant subjected to cadmium.
CONCLUSIONS
The present results indicate that Lupinus albus L. was affected by high doses of Cd2+ (100 and 150 µM CdCl2). Cd2+ exposure mainly reduced plant growth parameters and photosynthetic activity. In addition, cadmium generated nutritional deficiencies, toxic accumulation of ammonium, and increased the activities of antioxidant enzymes such CAT and SOD in L. albus plants. Nevertheless, white lupin could survive at high Cd2+ doses and could tolerate moderate Cd2+ doses by the increase of cellular metabolites like soluble sugar and proline to overcome the ionic and osmotic effect of Cd2+. In addition, to defend oxidative stress, Cd2+ induced the production of secondary metabolites such as phenolic compounds.
ABBREVIATIONS AND NOTATION
APX | ascorbate peroxidase |
CAT | catalase |
Cd2+ | cadmium |
Chl | chlorophyll |
DW | dry weight |
FW | fresh weight |
MDA | malondialdehyde |
\({\text{NH}}_{4}^{ + }\) | ammonium |
ROS | reactive oxygen species |
SP | soluble protein |
SOD | superoxide dismutase |
TPC | total phenolic contents |
TFC | total flavonoid content |
TCT | total condensed tannins |
WC | water content |
REFERENCES
Suhani, I., Sahab, S., Srivastava, V., and Singh, R.P., Impact of cadmium pollution on food safety and human health, Curr. Opin. Toxicol., 2021, vol. 27 p. 1. https://doi.org/10.1016/j.cotox.2021.04.004
Nazar, R., Iqbal, N., Masood, A., Khan, M.I.R., Syeed, S., and Khan, N.A., Cadmium toxicity in plants and role of mineral nutrients in its alleviation, Am. J. Plant Sci., 2012, vol. 3, p. 1476. https://doi.org/10.4236/ajps.2012.310178
Mahawar, L., Popek, R., Shekhawat, G. S., Alyemeni, M. N., and Ahmad, P., Exogenous hemin improves Cd2+ tolerance and remediation potential in Vigna radiata by intensifying the HO-1 mediated antioxidant defence system, Sci. Rep., 2021, vol. 11, p. 2811. https://doi.org/10.1038/s41598-021-82391-1
Wang, J., Liang, S., Xiang, W., Dai, H., Duan, Y., Kang, F., and Chai, T., A repeat region from the Brassica juncea HMA4 gene BjHMA4R is specifically involved in Cd2+ binding in the cytosol under low heavy metal concentrations, BMC Plant Biol., 2019, vol. 19, p. 89. https://doi.org/10.1186/s12870-019-1674-5
Dawuda, M.M., Liao, W., Hu, L., Yu, J., Xie, J., Calderón-Urrea, A., Jin, X., and Wu, Y., Root tolerance and biochemical response of Chinese lettuce (Lactuca sativa L.) genotypes to cadmium stress, Peer J., 2019, vol. 7, p. 7530. https://doi.org/10.7717/peerj.7530
Mnasri, M., Ghabriche, R., Fourati, E., Zaier, H., Sabally, K., Barrington, S., Lutts, S., Abdelly, C., and Ghnaya, T., Cd and Ni transport and accumulation in the halophyte Sesuvium portulacastrum: Implication of organic acids in these processes, Front. Plant Sci., 2015, vol. 6, p. 156. https://doi.org/10.3389/fpls.2015.00156
Sujak, A., Kotlarz, A., and Strobel, W., Compositional and nutritional evaluation of several lupin seeds, Food Chem., 2006, vol. 98, p. 711. https://doi.org/10.1016/j.foodchem.2005.06.036
Bähr, M., Fechner, A., Hasenkopf, K., Mittermaier, S., and Jahreis, G., Chemical composition of dehulled seeds of selected lupin cultivars in comparison to pea and soya bean, LWT–Food Sci. Technol., 2014, vol. 59, p. 587. https://doi.org/10.1016/j.lwt.2014.05.026
McKinney, G., Absorption of light by chlorophyll solutions, J. Biol. Chem., 1941, vol. 140, p. 315.
Yemm, E.W. and Willis, A.J., The estimation of carbohydrates in plant extracts by anthrone, Biochem. J., 1954, vol. 57, p. 508. https://doi.org/10.1042/bj0570508
Bates, L.S., Waldren, R.P., and Teare, I.D., Rapid determination of the free proline for water-stress studies, Plant Soil, 1973, vol. 39 p. 205. https://doi.org/10.1007/BF00018060
Hodges, D.M., Delong, J.M., Forney, C.F., and Prange, R.K., Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds, Planta, 1999, vol. 207, p. 604. https://doi.org/10.1007/s004250050524
Bradford, M.M., A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the protein-day binding, Anal. Biochem., 1976, vol. 72, p. 248. https://doi.org/10.1006/abio.1976.9999
Weatherburn, M.W., Phenol-hypochlorite reaction for determination of ammonia, Anal. Chem., 1967, vol. 39, p. 971. https://doi.org/10.1021/ac60252a045
Ben Mansour, R., Wasli, H., Serairi-Beji, R., Bourgou, S., Dakhlaoui, S., Selmi, S., Khamessi, S., Hammami, M., Ksouri, R., and Megdiche-Ksouri, W., In vivo gastroprotective effect and biological potentialities of six Tunisian medicinal plants using multivariate data treatment, Plant Biosyst. Int. J. Deal. All Asp. Plant Biol., 2020, vol. 156, p. 152. https://doi.org/10.1080/11263504.2020.1845840
Boudali, G., Ghnaya, T., and Ben-Abdallah, S., Zincum Metallicum, a homeopathic drug, alleviates Zn-induced toxic effects and promotes plant growth and antioxidant capacity in Lepidium sativum L., Environ. Sci. Pollut. Res., 2022, vol. 29, p. 338724. https://doi.org/10.1007/s11356-022-18633-0
Zornoza, P., Vázquez, S., Esteban, E., Fernández-Pascual, M., and Carpena, R., Cadmium-stress in nodulated white lupin: Strategies to avoid toxicity, Plant Physiol. Biochem., 2002, vol. 40, p. 1003. https://doi.org/10.1016/s0981-9428(02)01464-x
Vázquez, S., Agha, R., Granado, A., Sarro, M.J., Esteban, E., and Peñalosa Carpena, R.O., Use of white lupin plant for phytostabilization of Cd and as polluted acid soil, Water, Air, Soil Pollut., 2006, vol. 177, p. 349. https://doi.org/10.1007/s11270-006-9178-y
Zornoza, P., Sánchez-Pardo, B., and Carpena, R.O., Interaction and accumulation of manganese and cadmium in the manganese accumulator Lupinus albus, J. Plant Physiol., 2010, vol. 167, p. 1027. https://doi.org/10.1016/j.jplph.2010.02.011
Zhu, G., Xiao, H., Guo, Q., Zhang, Z., Zhao, J., and Yang, D., Effects of cadmium stress on growth and amino acid metabolism in two Compositae plants, Ecotoxicol. Environ. Saf., 2018, vol. 158, p. 300. https://doi.org/10.1016/j.ecoenv.2018.04.045
Pereira, G.I.G., Molina, S.M.G., and Azevedo, R.A., Activity of antioxidant enzymes in response to cadmium in Crotalaria juncea, Plant Soil, 2002, vol. 239, p. 123. https://doi.org/10.1023/A:1014951524286
Yang, S., Zu, Y., Li, B., Bi, Y., Jia, L., He, Y., and Yuan Li, Y., Response and intraspecific differences in nitrogen metabolism of alfalfa (Medicago sativa L.) under cadmium stress, Chemosphere, 2019, vol. 220, p. 69. https://doi.org/10.1016/j.chemosphere.2018.12.101
Ben Ammar, W., Nouairi, I., Zarrouk, M., and Jemal, F., The effect of cadmium on lipid and fatty acid biosynthesis in tomato leaves, Biologia, 2008, vol. 63, p. 86. https://doi.org/10.2478/s11756-008-0002-6
Zorrig, W., Rouached, A., Shahzad, Z., Abdelly, C., Davidian, J.C., and Berthomieu, P., Identification of three relationships linking cadmium accumulation to cadmium tolerance and zinc and citrate accumulation in lettuce, J. Plant Physiol., 2010, vol. 167, p. 1239. https://doi.org/10.1016/j.jplph.2010.04.012
Gouia, H., Suzuki, A., Brulfert, J., and Ghorbal, M.H., Effects of cadmium on the co-ordination of nitrogen and carbon metabolism in bean seedlings, J. Plant Physiol., 2003, vol. 160, p. 367. https://doi.org/10.1078/0176-1617-00785
Chaoui, A. and El Ferjani, E., Effects of cadmium and copper on antioxidant capacities, lignification and auxin degradation in leaves of pea (Pisums ativum L.) seedlings, C. R. Biol., 2005, vol. 328, p. 23. https://doi.org/10.1016/j.crvi.2004.10.001
Chamseddine, M., Wided, B.A., Guy, H., Marie-Edith, C., and Fatma, J., Cadmium and copper induction of oxidative stress and antioxidative response in tomato (Solanum lycopersicon) leaves, Plant Growth Regul., 2009, vol. 57, p. 89. https://doi.org/10.1007/s10725-008-9324-1
Baryla, A., Carrier, P., Franck, F., Coulomb, C., Sahut, C., and Havaux, M., Leaf chlorosis in oilseed rape plants (Brassica napus) grown on cadmium-polluted soil: Causes and consequences for photosynthesis and growth, Planta, 2001, vol. 212, p. 696. https://doi.org/10.1007/s004250000439
Chu, J., Zhu, F., Chen, X., Liang, H., Wang, R., Wang, X., and Huang, X., Effects of cadmium on photosynthesis of Schima superba young plant detected by chlorophyll fluorescence, Environ Sci Pollut Res., 2018, vol. 25, p. 10679. https://doi.org/10.1007/s11356-018-1294-x
Manquián-Cerda, K., Escudey, M., Zúñiga, G., Arancibia-Miranda, N., Molina, M., and Cruces, E., Effect of cadmium on phenolic compounds, antioxidant enzyme activity and oxidative stress in blueberry (Vaccinium corymbosum L.) plant lets grown in vitro, Ecotoxicol. Environ. Saf., 2016, vol. 133 p. 316. https://doi.org/10.1016/j.ecoenv.2016.07.029
Fidalgo, F., Freitas, R., Ferreira, R., Pessoa, A.M., and Teixeira, J., Solanum nigrum L. antioxidant defense system isozymes are regulated transcriptionally and posttranslationally in Cd-induced stress, Environ. Exp. Bot., 2011, vol. 72, p. 312. https://doi.org/10.1016/j.envexpbot.2011.04.007
Bashir, S., Zhu, J., and Fu, Q., Cadmium mobility, uptake and anti-oxidative response of water spinach (Ipomoea aquatic) under rice straw biochar, zeolite and rock phosphate as amendments, Chemosphere, 2018, vol. 194 p. 579. https://doi.org/10.1016/j.chemosphere.2017.11.162
Ahmad, P., Nabi, G., and Ashraf, M., Cadmium-induced oxidative damage in mustard [Brassica juncea (L.) Czern. & Coss.] plants can be alleviated by salicylic acid, S. Afr. J. Bot., 2011, vol. 77, p. 36. https://doi.org/10.1016/j.sajb.2010.05.003
Khan, N.A., Anjum, N.A., Nazar, R., and Iqbal, N., Increased activity of ATP-sulfurylase and increased contents of cysteine and glutathione reduce high cadmium-induced oxidative stress in mustard cultivar with high photosynthetic potential, Russ. J. Plant Physiol., 2009, vol. 56, p. 670. https://doi.org/10.1134/S1021443709050136
Ksouri, R., Megdiche, W., Debez, A., Falleh, H., Grignon, C., and Abdelly, C., Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritime, Plant Physiol. Biochem., 2007, vol. 45, p. 244. https://doi.org/10.1016/j.plaphy.2007.02.001
Rady, M., Elrys, A.S., El-Maatic, M.F.A., and Desoky, E.S.M., Interplaying roles of silicon and proline effectively improve salt and cadmium stress tolerance in Phaseolus vulgaris plant, Plant Physiol Biochem., 2019, vol. 139, p. 558. https://doi.org/10.1016/j.plaphy.2019.04.025
Hayat, S., Hasan, S.A., and Ahmed, A., Growth, nitrate reductase activity and antioxidant system in cadmium stressed tomato (Lycopersion escudentum Mill) cultivars, Biotechnol., Agron., Soc. Environ., 2011, vol. 15, p. 401. https://doi.org/1780-4507/index.php?id=7681
John, R., Ahmad, P., Gadgil, K., and Sharma, S., Effect of cadmium and lead on growth, biochemical parameters and uptake in Lemna polyrrhiza L, Plant Soil Environ., 2008, vol. 54, p. 262. https://doi.org/10.17221/2787-PSE
Karimi, L.N., Khanahmadi, M., and Moradi, B., Accumulation and phytotoxicity of lead in Cynara scolymus, Ind. J. Sci. Technol., 2012, vol. 5, p. 3634. https://doi.org/10.17485/ijst/2012/v5i11.17
Shahid, M.A., Balal, R.M., Pervez, M.A., Abbas, T., Aqeel, M.A., Javaid, M.M., and Garcia-Sanchez, F., Exogenous proline and proline-enriched Lolium perenne leaf extract protects against phytotoxic effects of nickel and salinity in Pisum sativum by altering polyamine metabolism in leaves, Turk. J. Bot., 2014, vol. 38, p. 914. https://doi.org/10.3906/bot-1312-13
Funding
This work was conducted in the Laboratory of Vegetable Productivity and Environmental Constraints, Faculty of Sciences of Tunis (LR18ES04) and supported by grants from the Tunisian Ministry of Higher Education, Scientific Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This work does not contain any studies involving human and animal subjects.
CONFLICT OF INTEREST
The authors of this work declare that they have no conflicts of interest.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
M’rah, S., Ben Mansour, R., Guesmi, L. et al. Interactive Effects of Cadmium on Phenolic Compounds, Antioxidant Enzyme Activity and Oxidative Stress in Lupinus albus L. Grown in Vitro. Russ J Plant Physiol 71, 111 (2024). https://doi.org/10.1134/S1021443724604786
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S1021443724604786