Abstract
The effects of rare earth elements (REEs) not only on cell growth and flavonoid accumulation of Tetrastigma hemsleyanum suspension cells but also on the isoenzyme patterns and activities of related enzymes were studied in this paper. There were no significant differences in enhancement of flavonoid accumulation in T. hemsleyanum suspension cells among La3+, Ce3+, and Nd3+. Whereas their inductive effects on cell proliferation varied greatly. The most significant effects were achieved with 100 μM Ce3+and Nd3+. Under treatment over a 25-day culture period, the maximal biomass levels reached 1.92- and 1.74-fold and the total flavonoid contents are 1.45- and 1.49-fold, than that of control, respectively. Catalase, phenylalanine ammonia-lyase (PAL), and peroxidase (POD) activity was activated significantly when the REE concentration range from 0 to 300 μM, whereas no significant changes were found in superoxide dismutase activity. Differences of esterase isozymes under REE treatment only laid in expression level, and there were no specific bands. The expression level of some POD isozymes strengthened with increasing concentration of REEs within the range of 50–200 μM. When REE concentration was higher than 300 μM, the expression of some POD isozymes was inhibited; meanwhile, some other new POD isozymes were induced. Our results also showed REEs did not directly influence PAL activity. So, we speculated that 50–200 μM REEs could activate some of antioxidant enzymes, adjust some isozymes expression, trigger the defense responses of T. hemsleyanum suspension cells, and stimulate flavonoid accumulation by inducing PAL activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tetrastigma hemsleyanum Planch. ex Franch is a rare and endangered medicinal plant in China; the main bioactive constituents of which are flavonoids. The plant has been shown not only to treat hepatitis, rheumatic arthritis, and high fevers and regulate immune functions [1], but also to suppress a variety of cancers such as carcinoma of the lung, liver, stomach, and leukemia by inducing tumor cell apoptosis [2]. Because of overexploitation, environmental deterioration, and difficult cultivation, wild T. hemsleyanum plants are on the verge of extinction. To conserve resources and lower the production costs, suspension cultures are being explored as a very promising alternative for the rapid production of the bioactive components of this plant. Our research group has obtained the suspension cell system of T. hemsleyanum. The main problems encountered in the in vitro culture of T. hemsleyanum are low biomass and low flavonoid content [3].
Many biotic and abiotic elicitors have been widely used to stimulate the production of secondary metabolites in various plant cell cultures, for example, yeast extract [4], fungal elicitor [5], heavy metal salts [6], and so on. However, most elicitors decreased the cell biomass while stimulating secondary metabolites. In recent years, rare earth elements (REEs) have been explored for the elicitation of secondary metabolites [7, 8]. In addition, quite a lot of studies have shown that suitable concentrations and types of REEs could affect many plant physiological activities, from enhancing the chlorophyll content and improving the photosynthetic rate [9–11] to increasing the plant biomass and absorption of nutrients [12–14], thus promoting plant growth and cell proliferation distinctly [15, 16]. Moreover, REEs could increase the protective enzyme activity [17], maintain the structural stability of cell membranes, and induce plant resistance against diseases and a poor environment [18]. In general, an appropriate amount of REEs not only promotes the production of secondary metabolites, but also improves plant growth and plant resistance against stress. Although numerous physiological effects of REEs on plants have been known for some time, the essentiality of REEs on plants is still largely unknown despite the efforts of many researchers.
Esterase (EST) plays an important role in primary metabolism of plant cells; the increase in EST activity usually shows the more vigorous related metabolic activity, which is favorable to the growth and development of cells [19]. The synthesis of secondary metabolites in plants is widely believed to be part of the defense and stress responses of plants. Plant defense reactions can be triggered by elicitors and antioxidant defense systems are based on enzymes such as superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD) [20], so these enzymes are key enzymes involved in secondary metabolites.
Thus far, there are some papers related to the change of antioxidase activity in plant seedlings under REE treatment [21, 22], but few studies have focused on the influence of REEs on isoenzyme expression related to metabolism. In particular, there is scarce research directed at the change of isoenzymes in cultured cells in vitro under REE treatment. Analysis of isoenzyme variation has proven to be particularly useful in research on the metabolic regulation of organisms. Thus, further investigation of the impacts on isoenzyme expression may be significant in clarifying the regulatory mechanism of REEs.
In the present study, we studied the variability in activity and isoenzyme profiles of these key enzymes involved in primary and secondary metabolites under REE treatment in suspension cells of T. hemsleyanum. The aim of the study was to improve flavonoid production and cell growth of T. hemsleyanum cells under the induction of the suitable REE elicitors. More generally, such studies may contribute to the efforts to reveal the REE mechanism of promoting plant cell growth and secondary metabolism.
Materials and Methods
Chemicals
The reagents used for electrophoresis were purchased from Sigma-Aldrich Co. Ltd. (USA): Ammonium persulfate, Tetramethylethylenediamine, Acry, and Bis. NdCl3, Ce(NO3)3·6H2O, La(NO3)3·6H2O and others were purchased from Sinopharm Chemical Reagent Co. Ltd. (China). The chemicals and reagents used in this research were analytical grade
Cell Lines and Culture Conditions
Callus were inducted from the young stems of T. hemsleyanum in MS medium, supplemented with 0.2 mg/L naphthalene acetic acid (NAA), 2.0 mg/L 6-benzyladenine (6-BA), 30 g/L sucrose, and 8 g/L agar. The green–yellow callus was transferred into liquid MS containing 1.5 mg/L NAA, 1.0 mg/L 6-BA, and 30 g/L sucrose. The pH of the medium was adjusted to 5.8 before autoclaving. The inoculum density was approximately 60 g fresh weight per liter of the medium. The culture was incubated in a rotary shaker (150 rpm) under a 16-h photoperiod and at a temperature of 24 ± 2 °C. Autoclaved REE solution was added to the culture at day 10. The control group was inoculated with an equal volume of sterile double-distilled water. Samples were collected after another 15 days. The sampling time was defined through a preexperiment, as the logarithmic phase of T. hemsleyanum suspension cells was at the period of the 10th to 18th day [3].
Determination of Cell Growth and Total Flavonoids
Cell growth was determinated according to Peng et al. [3]. After 15 days of growth, the fresh weight of each sample was measured. The growth ratio (in percent) of each culture was obtained by dividing the difference between the fresh weight at the end and at the beginning of the growth period by the fresh weight at the beginning of the growth period. The cells were separated by filtration and then dried at 60 °C under vacuum to constant weight to obtain the dry cell weight. The total flavonoids were extracted and determined by the method of Zhishen [23]. The total flavonoid concentration was calculated by dividing the content of total flavonoids by the dry cell weight (in milligram per gram dry weight (DW)).
Enzyme Extraction and Assay
Suspension culture tissue (2 g) was ground in 1 ml of ice-cold 0.1 M Tris–HCl extraction buffer (pH 6.5) containing 70 mM 2-mercaptoethanol and 5 % polyvinylpyrrolidone. The homogenate was centrifuged at 15,000 rpm for 15 min at 4 °C, and the clear supernatants were collected for the determination of enzyme activity and the analysis of isoenzymes. The activity of enzymes was denoted in units/fresh cell weight (U g−1 FW).
POD activity was determined by the change in absorbance at 460 nm during incubation of the extracts at 25 °C with 10 mM guaiacol and 1 mM H2O2 in 0.05 M sodium acetate buffer (pH 5.4) in a total volume of 3 ml. An enzyme unit (U) represented a 0.1 increase in absorbance (460 nm) per second. This was based on the method of Donald [24], with some modifications.
CAT activity was determined by the change in absorbance at 240 nm during incubation of the extracts at 25 °C with 10 mM H2O2 in 0.05 M phosphate buffer (pH 7.8) in a total volume of 3 ml. An enzyme unit (U) represented a 0.1 decrease in absorbance (240 nm) per minute. This was based on the method of Wang [25], with some modifications.
Phenylalanine ammonia-lyase (PAL) activity was determined according to the method of Peng [26]. An enzyme unit (U) represented a 0.1 increase in absorbance (290 nm) per minute. SOD activity was determined according to the method of Beauchamp and Fridovich [27].
EST and POD Isoenzyme Analysis
Polyacrylamide gel electrophoresis in a discontinuous buffer system was used to study the patterns of EST and POD isoenzymes. Table 1 shows the separating gel and the stacking gel. The electrode buffer was Tris–glycine (pH 8.3). The electrophoretic separations were performed at 4 °C. The initial current was 20 mA and was adjusted to 40 mA when 0.2 % bromophenol blue entered the separation gel. The duration of the electrophoresis time was 5–6 h. The sampling amounts of the crude enzymes for EST and POD isoenzyme analysis were 30 and 5 μg of total protein, respectively.
EST was stained according to Mandak [28]. The gel stained for POD activity was immersed for 2–5 min in 200 ml of 0.07 % ascorbic acid solution containing 2 g benzidine (dissolved in 18 ml acetic acid) and 1 ml of 30 % hydrogen peroxide (added before use). The gel was then submitted for analysis in a Bio-Rad Gel Doc XR+ imaging system (Bio-Rad Co. Ltd., USA).
Statistical Analysis
All experiments were carried out in triplicate, and the results were expressed as means ± standard deviation (SD). The data were subjected to one-way analysis of variance in SPSS 17.0 for Windows (SPSS Inc., USA), and the significance between treatments was assessed by Tukey's test at P < 0.05.
Results
Effect of Different Concentrations of REEs on Cell Proliferation and Flavonoid Accumulation
Table 2 shows that the optimal dosages of each REE had a clear positive influence on cell growth and total flavonoid yield. There was a steady increase in cell growth with increasing concentrations of REEs when the concentration ranged from 0 to 100 μM. When the concentration of REEs was higher than 100 μM, the stimulation was weaker. REEs even restrained cell proliferation markedly at 300 μM concentration. Cell growth was markedly increased by 100 μM Nd3+, Ce3+, and La3+ by 92.3, 74.2, and 49.1 %, respectively, compared to the control and the differences between them were significant (P < 0.05).
REEs caused a prominent increase flavonoid content with increasing concentrations of REEs when their concentration ranged from 0 to 200 μM. When the concentration was higher than 200 μM, the flavonoid content was lower. The total flavonoid content was markedly increased by 200 μM Nd3+, Ce3+, and La3+ by about 55, 58, and 41 %, respectively, compared to the control. The total flavonoid content in the 100-μM REE treatment group was slightly lower than that in the 200-μM treatment group, and the difference between them was not significant, but the fresh weight growth rate of the former was significantly higher than that of the latter. Thus, in terms of flavonoid production, the optimal concentration of REEs was 100 μM.
Effect of Different Concentrations of REEs on POD, CAT, SOD, and PAL Activity
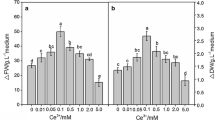
In view of their more efficient stimulation, Ce3+ and Nd3+ were selected for further experiments. Figure 1 shows that the effect of REEs on enzymes activity was consistent with the hormesis effect (low-dose stimulatory and high-dose inhibitory response). SOD activity did not differ significantly between the treated group and the control when REEs were added within 0–300 μM. PAL, CAT, and POD were distinctly activated when the REE concentration was within the range of 0–200 μM. CAT activity did not change with the REE concentration basically. However, PAL and POD activities were higher with the increase of the REE concentration. CAT, SOD, and PAL activity decreased significantly and dropped below that in the control when the REE concentration was higher than 300 μM, which indicated that, similarly to heavy metal ions, REEs could inhibit the enzyme system of plants when their concentration is beyond the critical value.
Effect of REEs on PAL (a), POD (b), CAT(c), and SOD (d) activity in T. hemsleyanum suspension culture. Black up-pointing triangle indicated a significant increase and black down-pointing triangle indicated a significant decrease compared with the control by LSD multiple comparison test (P < 0.05). The vertical bars denote ±SD, n = 3
Effect of Different Concentrations of REEs on the POD and EST Isoenzyme
Our above-mentioned results showed that the impact of REEs on POD activity was significant and dose dependent. Therefore, research into the influence of REEs on POD and EST isozymes would be helpful in revealing the antioxidation and metabolic regulation mechanism of REEs.
Figure 2 shows that the changes in the strip numbers and their color depths were consistent with the changes in their activity. There were no real fundamental distinctions in the POD isozyme patterns between the control and the 10-μM REE treatment group. The difference in the 50–200-μM treatment group was mainly in the intensity of the bands; no special bands appeared. The enzymatic expression levels of POD1 and POD2 were significantly increased. The expression of POD1 continued to intensify when the concentration of REEs ranged from 200 to 300 μM, and at the same time, the expression of POD2 weakened. Some new bands emerged between POD1 and POD2 when the concentration of REEs ranged from 300 to 500 μM.
Figure 3 shows that the differences of EST isozymes under REE treatment only laid in expression level, and there were no specific bands. EST activity could be improved with an increase in the expression level of EST1–EST4 when the REE concentration was within the range of 0–100 μM, whereas at a higher concentration of 200 μM, each spectrum signal weakened distinctly, indicating a decrease in the expression level. At this time, the cells might begin to be poisoned by REEs. EST expression was kept to a minimum in all treatments when the REE concentration was 500 μM, so that the spectrum signal near the cathode was almost undetectable. The results showed that EST activity was highest under 50–100 μM REE treatment. The present conclusion agrees with the earlier supposition that 50–100 μM REEs are clearly capable of promoting cell proliferation.
Discussion
Effect of REEs on Cell Proliferation and Secondary Metabolite Accumulation
In this work, the ionic radius of La3+, Ce3+, and Nd3+ decreased in turn and the stimulation of flavonoids showed no obvious difference, but the promotion of cell proliferation was obviously enhanced. The promotion of Arnebia euchroma (Royle) Johnst cell growth by Ce3+ and Nd3+ has previously been found to be superior to that by La3+ [29], which is consistent with our result. However, Ozaki et al. [30] reported that the uptake of REEs declined with a decrease in the ionic radius. As REEs perform its role by being absorbed into plant cells [31], the conclusion of Ozaki et al. contradicted our result. It is probably because that the REEs used in the experiment of Ozaki et al. were all heavy REEs except for Ce3+, while those used in our experiment were all light REEs. Light REEs and heavy REEs differ in their ion radius, solubility, and the ability to bind to chlorophylls [32]. These differentiations between them may lead to the differences in absorption features, for example, the absorption and transition efficiency of light REEs is much higher than that of heavy REEs in wheat seeds [33].
Cistanch deserticola cells have been found to be more sensitive to La3+ than to other REEs [34]. Saussurea medusa cells have been found to be more sensitive to Ce3+ than to La3+, mixture of REEs, and Nd3+ [35]. However, the mixture of REEs showed the most remarkable effects on the cell growth of A. euchroma and production of shikonin derivatives [7]. The contradictory findings may be due to the fact that the specific absorption of REEs varies in different plants and in different growth environments [36]. The significance of the stimulation also depends on the type of cells and the culture growth stage [37]. Therefore, treatment with REEs showed different effects. So, it should not be simply said that the effect of REE stimulus varied with their ionic radius.
The results of the present study indicated that an appropriate amount of REEs had positive effects on cell growth and flavonoid accumulation, whereas high doses of REEs evidently had negative effects, which are consistent with previous reports [7, 34, 37, 38]. It was proposed that REEs affected the plant physiology by initiating the Ca2+ signal transduction system [22]. Others argued that REEs could influence the metabolism of plants by replacing or competing with the metal ions such as Ca2+ and Mg2+ at their low concentration for binding sites in cell membrane and further affect the function of some enzymes and cell membrane and enhance the absorption, utilization, and transformation of nutrients, thereby leading to the fast growth of plant cells [34, 39, 40]. When the REE concentration was high enough, they possessed some properties of heavy metal ions, combined with membrane proteins, and inhibited the enzyme activities [7]. Likewise, Yang et al. [41] found that Ce3+ at the low or high concentration could directly interact with the proteins on/in the plasma membrane of horseradish. The interaction improved or destroyed the structure of the proteins on/in the plasma membrane and thus promoted or decreased the intra-/extracellular substance exchange, and then, the growth of cells is accelerated or damaged.
Effect of REEs on the Related Enzyme Activities of Metabolism
Previous studies have shown that REEs enhanced root morphogenesis and the growth of S aussurea involucrate by acting as a mild abiotic stress to stimulate POD and SOD activities [42]. REEs have also been found to alleviate the oxidative damage induced by UV-B radiation and to reduce the content of reactive oxygen species (ROS) and MDA [43, 44]. Our results basically agree with the data reported by Shi et al. [45], which showed that low concentrations of La3+ (0.002–0.02 mM) promoted plant growth in cucumber seedlings but did not affect the activity of antioxidant enzymes, whereas higher concentrations (0.2−2 mM) stimulated these activities but suppressed plant growth. However, the maximum tolerant dose we found is different from that in the above-mentioned study; this is probably because the REE uptake and usage efficiency vary among seedlings and in vitro culture systems.
Yuan et al. [46] have shown that CAT and SOD activities in suspension cultures of Taxus cuspidata were activated by Ce4+, whereas POD activity was restrained strongly. In the present study, no significant changes were found in SOD activity when REEs were added within 0–300 μM, but CAT and POD activity was activated significantly, and POD activity even amounted to threefold of that in control when the REE concentration was 100 μM, and the REE dosage also had a strong influence on POD activity. Our research found that SOD activity did not change significantly under REE treatment. However, the activities of CAT and POD were enhanced with an increase of REE concentration. The result of the current study is very similar to that of Wang et al. [47]; they reported that the activities of CAT, POD, and SOD in Lepidium meyenii shoots were enhanced to different extents by La3+, Ce3+, and Nd3+. In conclusion, an appropriate concentration of REEs could activate the antioxidase activity and strengthen the ability of scavenging radicals in plant cells, but each antioxidase changes differently among various kinds of plant cells.
The phenylpropanoid metabolic pathway plays a very important role in plant secondary metabolism, which is a main mechanism of flavonoid formation [48, 49]. The key enzyme in the first stage of the phenylpropanoid transition is PAL [50]. Previous studies have shown that PAL activity responds to various abiotic stimuli and therefore enhances the biosynthesis of flavonoid [51, 52]. Our results showed that 50–200 μM REEs clearly enhanced PAL activity, and the effect was more remarkable with the increase in concentration, which agreed with the observed influence of REEs on flavonoid content. To test the way in which REEs influence PAL activity, we conducted a pilot trial; different concentrations of Ce 3+ and Nd3+ were added into the reaction solution for PAL enzyme activity determination, and the PAL enzyme activity of cells untreated by REEs was then determined. The results showed that 50–200 μM Ce3+ and Nd3+ had no activation function on PAL activity, instead the inhibitory effect was strengthened with the increase in the REE concentration (not shown). Thus, it is speculated that REEs did not directly influence PAL, but instead acted indirectly by regulating its metabolic pathway.
In light of these data and previous results, we speculated that 50–200 μM REEs could activate some of antioxidant enzymes, trigger the defense responses of T. hemsleyanum suspension cells, and stimulate flavonoid accumulation by inducing PAL activity, although more data are needed to support this hypothesis.
Effect of REEs on the Regulation of Related Isoenzyme Expression
Many studies have suggested that isoenzymes are closely related to the antistress quality and the synthesis of plant secondary metabolism products. EST and POD exist in all parts and at different development stages of plants. They participate in many important physiological activities. Their varieties play a major role in plant metabolism regulation and environmental stress response [53]. Some researches have shown that environmental factors can result not only in changes in EST and POD activity, but also in their isoenzyme expression, for example insect violation [54], disease [55], radiation [56], air temperature [57], and so on.
Previous studies have reported that no obvious changes were observed in the band number of CAT patterns of Lemna minor L. except for varying intensities under 5 mM REE treatment [21]. Similarly, in our research, no obvious changes were observed in the band number of POD and EST patterns except for varying intensities of some bands due to increasing concentrations of REEs when their concentration was within the range of 0–200 μM. However, several new POD bands emerged when more than 300 μM of REEs were supplemented. At the same time, some EST bands weakened and vanished. It might be because the response to stress was various with the sorts of enzyme. On the other hand, the researchers leading the above trial adopted seedlings in vivo rather than cultures in vitro as study object; suspension culture cells in vitro were more sensitive to the changed outside culture conditions. Moreover, L. minor L. itself was a “highly REE-tolerant” plant species. Treatments at concentrations up to 5 mM RE did not cause either visible symptoms on L. minor L. seedling or significant effects on ROS production and lipid peroxidation [21]; however, 200 μM REE had a clear negative influence on cell growth of T. hemsleyanum in our study.
In conclusion, along with the increase in the REE concentration, EST and POD activity initially increased and then later decreased. In the meantime, the isozyme expression was regulated. The expression quantity of some bands was increased. When REE concentration was higher than the critical value, they could make abiotic environmental stresses and inhibit the expression of some isozymes, and some new “protective enzymes” were induced to resist the damage, at the same time. This finding matched the typical stress reaction characteristics of plants. A plant will adopt all kinds of measures to improve its resistance so as to adapt to a poor environment. However, when the stress level surpasses the tolerance limit of the plant, its resistance measures are weakened, and the plant will eventually die.
Abbreviations
- REEs:
-
Rare earth elements
- PAL:
-
Phenylalanine ammonia-lyase
- SOD:
-
Superoxide dismutase
- NAA:
-
Naphthalene acetic acid
- EST:
-
Esterase
- FW:
-
Fresh weight
- CAT:
-
Catalase
- ROS:
-
Reactive oxygen species
- POD:
-
Peroxidase
- 6-BA:
-
6-Benzyladenine
- DW:
-
Dry weight
References
Xu CJ, Ding GQ, Fu JY et al (2008) Immunoregulatory effects of ethyl-acetate fraction of extracts from Tetrastigma hemsleyanum Diels et. Gilg on immune functions of ICR mice. Biomed Environ Sci 21(4):325–331
He FG (2010) Research progress in anticancer effect of Tetrastigma hemsleyanum Diels et Gilg and its mechanism. J Oncol 16:75–77 (in Chinese)
Peng X, Zhang J, He JY (2012) Comparison on accumulation of flavonoids in loose and compact callus suspension cell culture of Tetrastigma hemsleyanum. Chin Tradit Herb Drugs 43:577–580 (in chinese)
Karwasara VS, Jain R, Tomar P et al (2010) Elicitation as yield enhancement strategy for glycyrrhizin production by cell cultures of Abrus precatorius Linn. In Vitro Cell Dev Biol Plant 46:354–362
Chakraborty A, Chattopadhyay S (2008) Stimulation of menthol production in Mentha piperita cell culture. In Vitro Cell Dev Biol Plant 44:518–524
Karwasara VS, Dixit VK (2012) Culture medium optimization for improved puerarin production by cell suspension cultures of Pueraria tuberosa (Roxb. ex Willd.) DC. In Vitro Cell Dev Biol Plant 48:189–199
Ge F, Wang XD, Zhao B, Wang YC (2006) Effects of rare earth elements on the growth of Arnebia euchroma cells and the biosynthesis of shikonin. Plant Growth Regul 48:283–290
Yuan XF, Zhao B, Wang YC (2005) Application of rare earth elements in medicinal plant cell and tissue culture. Chin Bull Bot 22:115–120
Zhou M, Gong X, Wang Y et al (2011) Improvement of cerium of photosynthesis functions of maize under magnesium deficiency. Biol Trace Elem Res 142:760–772
Liu XQ, Ze YG, Liu C et al (2009) Effects of Ce3+ on improvement of spectral characteristics and function of chloroplasts damaged by linolenic acid in spinach. J Rare Earths 27:288–293
Huang H, Chen L, Liu XQ et al (2008) Absorption and transfer of light and photoreduction activities of spinach chloroplasts under calcium deficiency: promotion by cerium. Biol Trace Elem Res 122:157–167
Huang G, Wang L, Zhou Q (2012) Lanthanum (III) regulates the nitrogen assimilation in soybean seedlings under ultraviolet-B radiation. Biol Trace Elem Res. doi:10.1007/s12011-012-9528-0
Olivares E, Aguiar G, Colonnello G (2011) Rare earth elements in vascular plants: a review. Interciencia 36(5):331–340
Diatloff E, Smith FW, Asher CJ (2008) Effects of lanthanum and cerium on the growth and mineral nutrition of corn and mungbean. Ann Bot 101(7):971–982
d’Aquino L, Massimo M, Carboni MA (2009) Effect of some rare earth elements on the growth and lanthanide accumulation in different Trichoderma strains. Soil Biol Biochem 41:2406–2413
Boyko A, Matsuoka A, Kovalchuk I (2011) Potassium chloride and rare earth elements improve plant growth and increase the frequency of the Agrobacterium tumefaciens-mediated plant transformation. Plant Cell Rep 30:505–518
Wang CT, Shi GX, Xu QS (2005) Toxic effect of Cd2+ on Potamogeton crispus alleviated by exogenous Nd3+. J Rare Earths 23(6):752–755 (in chinese)
Liu YJ, Wang Y, Wang FB (2008) Control effect of lanthanum against plant disease. J Rare Earths 26:115–120
Osorio S, Alba R, Damasceno CM et al (2011) Systems biology of tomato fruit development: combined transcript, protein, and metabolite analysis of tomato transcription factor and ethylene receptor mutants reveals novel regulatory interactions. Plant Physiol 157(1):405–425
Bowler C, Camp WV, Montagy MV et al (1994) Superoxide dismutase in plants. Crit Rev Plant Sci 13(3):199–218
Ippolito MP, Fasciano C, d’Aquino L et al (2010) Responses of antioxidant systems after exposition to rare earths and their role in chilling stress in common duckweed (Lemna minor L.): a defensive weapon or a boomerang? Arch Environ Contam Toxicol 58:42–52
Fl Z, Tian HE, Wang ZP et al (2003) Effect of rare earth element europium on amaranthin synthesis in Amarathus caudatus seedlings. Biol Trace Elem Res 93:271–282
Zhishen JT, Mengcheng T, Jianming W (1999) Research on antioxidant activity of flavonoids from natural materials. Food Chem 64:555–559
Cipollini DF Jr (1998) The induction of soluble peroxidase activity in bean leaves by wind-induced mechanical perturbation. Am J Bot 85(11):1586–1591
Wang YS, Tian SP, Xu Y et al (2004) Changes in the activities of pro- and anti-oxidant enzymes in peach fruit inoculated with Cryptococcus laurentii or Penicillium expansum at 0 or 20°C. Postharvest Biol Technol 34:21–28
Peng X, Jin WT, Ling QZ (2012) Changes of phenolics content and related enzymatic activities in Fritillaria thunbergii Miq. and their relationships with dormancy release. Med Plant 3(6):44–47
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assay applicable to acrylamide gels. Anal Biochem 44:276–287
Mandak B, Bimova K, Pysek P (2005) Isoenzyme diversity in Reynoutria (Polygonaceae) taxa: escape from sterility by hybridization. Plant Syst Evol 253:219–230
Huang WH (2011) Biological effect of rare earth element and genetic transformation on Arnebia euehroma (Royle) Johnst. cell. Dissertation, University of Kunming Science and Technology (in Chinese)
Ozaki T, Enomoto S, Minai Y (2000) Beneficial effect of rare earth elements on the growth of Dryopteris erythrosora. J Plant Physiol 156:330–334
Gao YS, Zeng FL, Yi A (2003) Research of the entry of rare earth elements Eu3+ and La3+ into plant cell. Biol Trace Elem Res 91:253–265
Wei ZG, Hong FS, Yin M et al (2005) Structural differences between light and heavy rare earth element binding chlorophylls in naturally grown fern Dicranopteris linearis. Biol Trace Elem Res 106:279–297
Liang T, Yan BZ, Zhang S (2001) Contents and the biogeochemical characteristics of rare earth elements in wheat seeds. Biogeochemistry 54:41–49
Ouyang J, Xiaodong W, Bing Z et al (2003) Effects of rare earth elements on the growth of Cistanch deserticola cells and the production of phenylethanoid glycosides. J Biotechnol 102:129–134
Yuan XF, Wang Q, Zhao B et al (2002) Improved cell growth and total flavonoids of Saussurea medusa on solid culture medium supplemented with rare earth elements. Biotechnol Lett 24:1889–1892
Jing F, Bei W, Shan XQ et al (2007) Evaluation of bioavailability of light rare earth elements to wheat (Triticum aestivum L.)under field conditions. Geoderma 141:53–59
Wu JY, Wang CG, Mei XG (2001) Stimulation of taxol production and excretion in Taxus spp cell cultures by rare earth chemical lanthanum. J Biotechnol 85:67–73
Zhou J, Fang L, Li X et al (2012) Jasmonic acid (JA) acts as a signal molecule in LaCl3-induced baicalin synthesis in Scutellaria baicalensis seedlings. Biol Trace Elem Res 148:392–395
Liu C, Cao WQ, Lu Y et al (2009) Cerium under calcium deficiency influence on the antioxidative defense system in spinach plants. Plant and Soil 323:285–294
Ze YG, Zhou M, Luo LY et al (2009) Effects of cerium on key enzymes of carbon assimilation of spinach under magnesium deficiency. Biol Trace Elem Res 131(2):154–164
Yang GM, Sun ZG, Lv XF et al (2012) Living target of Ce(III) action on horseradish cells: proteins on/in cell membrane. Biol Trace Elem Res. doi:10.1007/s12011-012-9514-6
Guo B, Xu L, Guan ZJ et al (2012) Effect of lanthanum on rooting of in vitro regenerated shoots of Saussurea involucrata Kar. et Kir. Biol Trace Elem Res 147:334–340
Wang L, Huang X, Zhou Q (2009) Protective effect of rare earth against oxidative stress under ultraviolet-B radiation. Biol Trace Elem Res 128:82–93
Paola M, Paciolla C, d'Aquino L et al (2007) Effect of rare earth elements on growth and antioxidant metabolism in Lemna minor L. Caryologia 60:125–128
Shi P, Chen GC, Huang ZW (2005) Effects of La3+ on the active oxygen scavenging enzyme activities in cucumber seedling leaves. Russ J Plant Physiol 52:294–297
Yuan YJ, Li JC, Ge ZQ et al (2002) Superoxide anion burst and taxol production induced by Ce4+ in suspension cultures of Taxus cuspidata. J Mol Catal B: Enzym 18:251–260
Wang YL, Wang XD, Zhao B et al (2007) Reduction of hyperhydricity in the culture of Lepidium meyenii shoots by the addition of rare earth elements. Plant Growth Regul 52:151–159
Camm EL, Towers GHN (1973) Review article: phenylalanine ammonia lyase. Phytochemistry 12:961–973
Lister CE, Lancaster JE, Walker JRL (1996) Phenylalanine ammonia-lyase (PAL) activity and its relationship to anthocyanin and flavonoid levels in New Zealand-grown apple cultivars. J Am Soc Hortic Sci 12(2):281–285
Creasy LL (1971) Role of phenylalanine in the biosynthesis of flavonoids and cinnamic acids in strawberry leaf disks. Phytochemistry 10(11):2705–2711
Eichholza I, Rohn S, Gamm A et al (2012) UV-B-mediated flavonoid synthesis in white asparagus (Asparagus offcinalis L.). Food Res Int 48:196–201
Huang J, Gu M, Lai Z et al (2010) Functional analysis of the Arabidopsis PAL gene family in plant growth, development, and response to environmental stress. Plant Physiol 153(4):1526–1538
Hossain Z, Mandal AK, Datta SK et al (2006) Development of NaCl tolerant strain in Chrysanthemum morifolium Ramat. through in vitro mutagenesis. Plant Biol 8(4):450–461
Muarlidharan J, John E, Channamma L et al (1996) Change in esterases in response to blast infection in fingermillet seedlings. Phytochemistry 43:1151–1155
Sahoo MR, Dasgupta M, Kole PC et al (2007) Antioxidative enzymes and isozymes analysis of taro genotypes and their implications in Phytophthora blight disease resistance. Mycopathologia 163(4):241–248
Roy S, Begum Y, Chakraborty A et al (2006) Radiation-induced phenotypic alterations in relation to isozymes and RAPD markers in Vigna radiate (L) Wilczek. Int J Radiat Biol 82(11):823–832
Bogdanovic J, Milosavic N, Prodanovic R et al (2007) Variability of antioxidant enzyme activity and isoenzyme profile in needles of Serbian spruce (Picea omorika (Panc.) Purkinye). Biochem Syst Ecol 35:263–273
Acknowledgments
This work was supported by the Natural Science Foundation of Ningbo City (grant no. 2012A610179) and the New Shoot Talents Program of Zhejiang Province (grant no. 2010R433006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xin, P., Shuang-Lin, Z., Jun-Yao, H. et al. Influence of Rare Earth Elements on Metabolism and Related Enzyme Activity and Isozyme Expression in Tetrastigma hemsleyanum Cell Suspension Cultures. Biol Trace Elem Res 152, 82–90 (2013). https://doi.org/10.1007/s12011-013-9600-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-013-9600-4