Abstract
Ginkgo suspension cells were used to investigate the mechanism that governs the shift between primary and secondary metabolism under NaCl elicitation. The production of three flavonol glycosides, chlorophyll fluorescence, ion content, the antioxidant system, and the cellular ultrastructure in the presence of NaCl doses from 5 to 175 mM were examined. At low salt doses (5–50 mM), cell growth and flavonol glycosides accumulation were stimulated without damaging cell structure or inducing oxidative stress by maintaining high K+ and chlorophyll content. At moderate salt doses (75–125 mM), the cells could withstand the salt stress without an impact on survival by changing internal cellular structure, maintaining high levels of K+ and Ca2+ and increasing anti-oxidative enzyme activities rather than flavonol glycosides to counteract the inhibition of the photosystem II, the accumulation of Na+ and hydrogen peroxide (H2O2) in the cells. This allowed cells to divert their metabolism from growth to defense-related pathways and tolerate NaCl stress. At higher salinity (150–175 mM), the cellular structure was damaged, and the high Na+ and low K+ content led to osmotic stress, and therefore, the stimulation of peroxidase (POD) and catalase (CAT) was not enough to cope with high H2O2 accumulation. The high production of flavonol glycosides may be a response of elicitation stimulation to serious damage at 175 mM NaCl. In conclusion, the use of 175 mM NaCl may be desirable for the induction of flavonol glycoside production in Ginkgo suspension cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ginkgo biloba L., known as a ‘living fossil’, is one of the oldest living tree species. Its leaf extract and leaf powder (e.g., EGb761) are popular botanical medicines because they contain two important bioactive constituents in their leaves, flavonol glycosides (e.g., quercetin, kaempferol and isorhamnetin) and terpene trilactones (e.g., ginkgolides A, B, C, J, M, K, L and bilobalide). However, it is not economical to use field-grown trees as a supply of Ginkgo leaves for continuous commercial-scale pharmacological extracts. Ginkgo cell culture provides an alternative way for biotechnological production of bioactive metabolites (Cybulska-Heinrich et al. 2012; Van Beek and Montoro 2009; Sabater-Jara et al. 2013; Singh et al. 2008).
The yields and production rates of secondary metabolites by plant cell culture are generally low and the commercialized production of secondary metabolites using cell cultures has not been widely adopted (Cai et al. 2012). To produce high quality and quantities of secondary compounds of commercial interest, many methods have been developed. The elicitation strategy is one of the most important methods for production of flavonol glycosides and ginkgolides (Hao et al. 2010; Kang et al. 2009). Use of this method reduces the time to attain a high yield of product and increases the yield of secondary compounds (Kang et al. 2006; Guo et al. 2011; Zhao et al. 2013). Elicitor is a substance which initiates and/or improves the biosynthesis of bioactive compounds found at a low concentration in a living cell system (Santamaria et al. 2011) and classified as biotic or abiotic. Biotic elicitors such as enzymes, fungi or bacteria originate from plant cells and microorganisms have been used in Ginkgo cell cultures for production of flavonoids (Hao et al. 2010). Abiotic elicitors are chemical or physical stresses, and include high salinity, heavy metals, rare-earth elements, ozone, ultraviolet irradiation, etc. (Cai et al. 2012; Yusuf et al. 2013). Inorganic salts that are readily available, economical and easy to use are regarded as an efficient method of elicitation for secondary metabolite production (Sabater-Jara et al. 2013). KCl and NaCl have been studied in plant stress physiology and secondary metabolism in several plant species at the cellular level (Niknam et al. 2011; Jeong and Park 2006; Zhao et al. 2009; Abrol et al. 2012). For example, NaCl treatment inhibited ginseng hairy root growth and increased the production of ginseng saponin (Jeong and Park 2006).
Although the presence of high salinity could stimulate secondary metabolite production in several plant cell cultures, it can also constrain the cell growth and inhibit cell viability. It is very difficult to promote cell growth and increase the production of secondary metabolites simultaneously. Furthermore, adding abiotic elicitors into a cell culture medium is a stress process and usually induces the generation of reactive oxygen species (ROS), and also induces a series of anti-oxidative defense systems synchronously. Flavonoid accumulation was reported in conditions of salt-induced oxidative stress (Yusuf et al. 2013; Abrol et al. 2012). However, the existing literature in Ginkgo cell culture mainly focuses on the yield of secondary metabolites. Kang et al. (2006, 2009, 2010) studied the production of bilobalide and ginkgolides using methyl jasmonate (MJ), salicylic acid (SA), bacteria and KCl as elicitors. Hao et al. (2009, 2010) investigated flavonoid accumulation induced by fungal endophytes and UV-B irradiation. But there is no information about flavonoid status under salt stress on the cellular level in G. biloba L. Especially little work has been performed on the association between flavonoid production and photosynthesis, ion homeostasis and antioxidant system under salt stress. For these reasons, an in-depth research is needed to elucidate the mechanism of the shift between primary and flavonoid metabolism in Ginkgo cell culture under NaCl elicitation.
The aim of this work is to (1) examine the responses of Ginkgo cells to different doses of NaCl, (2) investigate relationships between flavonol glycosides accumulation and primary metabolism (including chlorophyll fluorescence parameters, ion content, antioxidant systems and cellular ultrastructure), and (3) evaluate the defense strategies in a Ginkgo cell suspension culture system under salt stress. It will be beneficial to demonstrate the mechanism by which salt elicitation results in a response of secondary metabolite production via ion homeostasis, photosynthesis and antioxidant system. The results of this study should help to improve understanding how cells respond to growth and defense strategies during salt treatment, and be beneficial for studying the use of biosynthetic pathways of suspension cell culture for production of flavonol glycosides.
Materials and methods
Callus induction and suspension cell culture
Ginkgo cell lines were produced using our previous method (Chen 2007). The leaves of Ginkgo biloba L. cv., ‘Da Fozhi’ were collected in spring from the Ginkgo Garden of Nanjing Forestry University, China. Leaf pieces were cultured in MS (Murashige and Skoog 1962) medium supplemented with 1.0 mg L−1 NAA and 1.0 mg L−1 6-BA for 35 days to format calli, and then the calli were subcultured in MS medium with 1 mg L−1 NAA, and 0.5 mg L−1 KT. Afterwards, calli were subcultured every 3 weeks on the same medium.
Suspension cultures were generated from calli transferred to liquid MS medium containing 1 mg L−1 NAA and 0.5 mg L−1 KT on a rotary shaker at 100 rpm and were maintained by refreshing the medium every 12 days, at an initial cell density of 3 g fresh weigh per 50 mL of medium.
NaCl treatment and cell growth analysis
Ginkgo suspension cells were routinely maintained by subculturing every 12 days and by diluting 50 mL into 100 mL fresh medium in 250 mL Erlenmeyer flasks containing 1.0 mg L−1 NAA and 0.5 mg L−1 KT. On the 6th day after inoculation (the linear phase of cell development, as indicated by our previous experiment), 0 (control), 5, 10, 25, 50, 75, 100, 125, 150 and 175 mM NaCl (final concentrations, by autoclaving) was added into the medium, and the cells were harvested 7 days after the addition of NaCl.
Cell growth was primarily measured by cell fresh weight (FW) and dry weight (DW). The cells and media were gently pressed on filter paper by a Büchner funnel to remove excess medium and then fresh weight was determined. Half of the fresh cells were weighed and stored at −70 °C for an active compound assay, and the other half was dried at 60 °C for 12 h until a constant weight (DW) was obtained for determination of flavonoids and mineral elements.
Culture medium and culture conditions
All media mentioned above were supplemented with 30 g L−1 sucrose and adjusted to pH 5.8 with 1 M KOH or 1 M HCl (prior to the addition of 7 g L−1 agar (Sinopharm, CNCM LTD) for callus induction and culture, no agar in liquid culture), then autoclaved for 20 min at 121 °C. All plant growth regulators were filter sterilized prior to addition into the medium by autoclaving. Cultures were incubated at 25 ± 1 °C with a 14 h photoperiod using 55 µmol m−2 s−1 fluorescent illumination.
Phenylalanine ammonia-lyase (PAL) activity and flavonoid content
The activity of phenylalanine ammonia-lyase (PAL) was measured by the method of Koukol and Conn (1961) with some modifications. Cells (500 mg) were homogenized in 50 mM borate buffer (pH 8.8) containing 10 mM β-mercaptoethanol, and 1 % polyvinyl pyrrolidone (PVP). The homogenate was centrifuged at 10,000×g at 4 °C for 20 min, and the supernatant was collected for the enzyme assay. The reaction mixture (5 mL) containing 50 mM boric acid buffer (pH 8.8), 20 mM l-phenylalanine, and enzyme extract was incubated at 37 °C for 30 min. The reaction was terminated by 1 M HCl. The production of cinnamic acid correlating with the PAL activity was measured at 290 nm.
The flavonoid extraction was performed by the method of Upton (2003). The powder (1.0 g DW) was sieved through a 200 mesh sieve and incubated in methanol containing 25 % (v/v) HCl (4:1, v/v) for 1 h at 95 °C. Afterwards, the acid-hydrolyzed extracts were filtered, and the supernatant re-suspended in the above-mentioned extract solution. Samples were filtered through a PTFE filter (Φ 0.22 µM) prior to HPLC analysis.
HPLC was performed according to Hao et al. (2010) using an Agilent 1200 HPLC series(Agilent Inc., USA) equipped with a quaternary gradient pump and spectrophotometric photodiode array detector (DAD) and connected to a C18 column (ODS, 4.6 × 250 mm, 5.0 μm, and an Agilent 1200 automatic injector). The mobile phase was methanol and 0.4 % phosphoric acid in H2O (55:45, v/v) at 1.0 mL min−1, the column temperature was 30 °C and the detection was performed at 360 nm. Three flavonol glycosides (quercetin, kaempferol, isorhamnetin) contained were selected as standards (Van Beek and Montoro 2009). The extraction samples were prepared and analyzed three times.
Total flavonoid (mg g−1 DW) = quercetin × 2.51 + kaempferol × 2.64 + isorhamnetin × 2.39
Total flavonoid yield (mg DW L−1 medium) = Total flavonol glycosides (mg g−1 DW) × g L−1 medium × 1,000/150; g: DW of flavonol glycosides per flask medium volume (150 mL).
Pigment content and chlorophyll fluorescence
Fresh cells (300 mg) were submerged in 8 mL of a mixed solution containing acetone: ethanol: water (4.5:4.5:1) overnight. Absorbance of chlorophyll a (Chl a) and chlorophyll b (Chl b) were measured at 645 and 663 nm according to Holden (1965), carotenoids were estimated at 470 nm by the method of Davies (1976).
Chlorophyll fluorescence measurements were performed according to the method of Breia et al. (2013) and Osorio et al. (2013) using a chlorophyll fluorescence imager (CF Imager, Technologica, UK). To determine the minimum (F o, dark fluorescence) and maximum (F m) fluorescence, a 5 mm thick aliquot of suspension cells was placed on black paper maintained in the dark for 30 min before measuring the chlorophyll fluorescence. The maximum fluorescence yield, F m, was determined using a saturating pulse of 6,000 μmol m−2 s−1 photosynthetic photon fluorescence rate (PFD) for 1 s. The maximum light adapted fluorescence (F m′) was determined in the presence of an actinic illumination of 500 μmol m−2 s−1. Chlorophyll fluorescence parameters such as the maximal quantum yield of PSII photochemistry (F v/F m), photochemical efficiency of PSII in the light (F v′/F m′), the photochemical quenching coefficient q p, and the non-photochemical quenching coefficient NPQ (F m/F m′ − 1) were calculated using the software supplied by the manufacturer. The effective quantum yield (Yield) was defined as qp × F v′/F m′.
Ion concentrations
The ion content was measured according to the method of Maria et al. (2012). Dry sample (200 mg) was digested in 0.1 M HNO3 in a closed microwave system. The determination of ions was performed using an ICS-900 (Dionex, Sunnyvale, CA, USA) spectrometer.
Membrane permeability, lipid peroxidation and H2O2
Membrane permeability was measured by quantifying the relative electrical conductivity (REC) according to Sun et al. (2010). REC (%) was defined as S 1/S 2 × 100. S 1 was the conductivity of fresh cells which were immersed into double distilled water at 37 °C for 1 h and S 2 was the conductivity of fresh cells which were heated at 100 °C for 10 min. The malondialdehyde (MDA) content used to determine the extent of lipid peroxidation was determined by the method of Rao and Sresty (2000). The absorbance of the supernatant was measured at 532 and 600 nm using a spectrophotometer (Unico, USA). The MDA concentration was calculated using an extinction coefficient of 155 mM−1 cm−1. The H2O2 content was measured according to the method described by Sekmen et al. (2012). The absorbance was measured at 410 nm and the content of H2O2 was calculated using a standard curve. The range of H2O2 concentrations used for the calibration curve was 0–100 μmolL−1.
Analysis of antioxidant enzymes
The enzyme extract method was modified as described by Knorzer et al. (1996). Fresh cell samples (500 mg) were grounded with liquid nitrogen and homogenized in 50 mM phosphate buffer solution (pH 7.0), containing 0.1 mM ethylene diamine tetra acetic acid (EDTA), and 2 mM dithiothreitol (DTT). The homogenate was centrifuged at 10,000×g for 20 min at 4 °C. The supernatant was used for the analysis of antioxidant enzymes.
SOD (EC 1.15.1.1) activity was assayed according to the method of Beyer and Fridovich (1987). One unit of SOD was defined using inhibited NBT photo reduction by 50 % at 560 nm. CAT (EC 1.11.1.6) activity was estimated according to the method of Mittal et al. (2012). The activity was recorded as the linear rate of decrease of H2O2 absorbance at 240 nm and defined 1 μmol H2O2 decomposed in 1 min as 1 unit of CAT. POD (EC1.11.1.7) activity (guaiacol peroxidase) was measured using a modified method of Dong et al. (2010). The absorbance was monitored at 470 nm for 1 and 2 min in the presence and absence of enzyme extract. One unit of POD activity was expressed as nmol H2O2 decomposed in 1 min.
Transmission electron microscopy
The cell ultrastructure was assayed according to Chen et al. (2006) and Li et al. (2013). Suspension cells were fixed in a solution of 5 % glutaraldehyde in 0.1 M sodium phosphate buffer, pH 7.0. After 4 h at 0–4 °C and three rinses with buffer, the samples were post-fixed in 2 % osmic acid (OsO4), then rinsed again with the same buffer. Thereafter, the samples were dehydrated in an ethanol series and embedded in Epon 812 resin. The samples were cut into thin sections of approximately 70 μm with a LKB-V ultra-microtome (Leica CM3050S, GER). The thin sections were stained with lead citrate and uranyl acetate before being examined using a JEM-1400 (JEOL, JPN) transmission electron microscope operated at 75 kV.
Statistical analysis
Two independent series of experiments were performed and each treatment had three replications (flasks) within each experiment. Each parameter was measured twice with three replicates, respectively. Error bars indicate the standard deviation of the mean value (mean ± SD). The data from six measurements (n = 6) were analyzed using one-way ANOVA and the data generated was subjected to statistical analysis using SPSS version 17.0 (SPSS Inc., IL, USA) and Duncan’s multiple range test (DMRT) (Duncan 1955).
Results
Cell growth, flavonoid content, and PAL activity response to varying NaCl doses
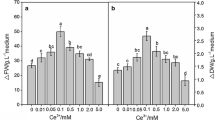
The influence of the NaCl dose on suspension cell growth was evaluated on the 7th day after NaCl was added to the medium (Fig. 1). The ΔFW (increase in FW) and ΔDW (increase in DW) in the presence of all NaCl treatments appeared to first increase from 5 to 25 mM NaCl, then dramatically decreased from 50 to 175 mM NaCl in ΔFW but slightly decreased from 50 to 100 mM, then sharply decreased to 175 mM in ΔDW. Moreover, the highest ΔFW and ΔDW were both noted for the 25 mM treatment, which was higher by 399 and 109 % compared with the 175 mM treatment and 105 and 49.5 % compared with the control treatment, respectively. Furthermore, the ΔFW (NaCl doses ≥125 mM) and the ΔDW (NaCl doses ≥150 mM) were less than the control (p < 0.05), particularly for the 175 mM NaCl treatment where the ΔFW was 41 % and ΔDW was 71 % of the control (Fig. 1). This indicated that the high dose NaCl treatment (150–175 mM) could suppress Ginkgo cell growth and in fact, the cells appeared brown in the presence of 175 mM NaCl compared with the green color observed in the presence of 0–125 mM NaCl (Fig. 3).
The influence of NaCl on Ginkgo cell growth at the 7th day after addition of different NaCl doses to the medium. Values are mean ± SD from two independent experiments (n = 6). Means followed by different letters above the bars indicate significant differences (p < 0.01) among treatments according to the Duncan’s multiple range test. ΔFW final FW − initial FW, ∆DW final DW − initial DW
The production of total flavonoids and flavonoid glycosides was also influenced by the NaCl doses (Table 1). The total flavonoid levels in all NaCl treatments were significantly higher than in the control treatment (p < 0.01). Furthermore, the flavonoid levels apparently increased from 25 to 50 mM (low NaCl dose) and from 125 to 175 mM NaCl (high NaCl dose). The total flavonoids peaked at 25 and 175 mM NaCl at 2.3-fold (0.95 mg g−1 DW) and 3.1-fold higher (1.31 mg g−1 DW) than the control, respectively. By comparison, at 5 and 10 mM NaCl (low dose) and 75 and 100 mM NaCl (moderate dose) low flavonoid levels were observed. A similar trend was detected in total flavonoid yields (Table 1).
Three flavonol glycosides (quercetin, kaempferol and isorhamnetin) were measured in Ginkgo biloba cell extracts by HPLC in 7 day old NaCl-treated cells. These three flavonol glycosides were observed to first increase from 5 to 25 mM NaCl, decrease from 50 to 75 mM NaCl, and then increase again at NaCl concentrations above 100 mM. The higher 1.57-fold and 1.83-fold increments in quercetin, and 1.72-fold and 4.15-fold increments in kaempferol were observed at 25 and 175 mM NaCl treatments compared with the control (p < 0.01), respectively. The highest content of isorhamnetin was found for the 50 and 175 mM NaCl treatments. Quercetin was the main flavonol glycoside (38.2–58.6 %) in Ginkgo cells, and was observed in higher quantities at the low NaCl doses than at the high doses. Conversely, the kaempferol (16.3–35.4 %) content was higher at high NaCl doses while the isorhamnetin (14.4–38.6 %) was observed at the moderate doses (50, 75 mM NaCl) (Table 1).
The effect of the NaCl dose on the PAL activity is shown in Fig. 2. Small increments in the PAL activity was observed at 5 and 10 mM NaCl, but significantly increased at 25 mM, then decreased from 50 to 75 mM, and then again increased from 100 to 175 mM NaCl compared with the control. The maximum PAL activity was observed at concentrations of 25 and 150 mM of NaCl, at 1.67-fold and 1.90-fold of the control, respectively (p < 0.01).
The influence of NaCl on PAL activity in Ginkgo suspension cells at the 7th day after addition of different NaCl doses to the medium. Values are mean ± SD from two independent experiments (n = 6). Means followed by different letters above the bars indicate significant differences (p < 0.01) among treatments according to the Duncan’s multiple range test
Pigment content, chlorophyll fluorescence, and ion content response to varying NaCl doses
The cell color changed with the salinity levels. Increasing salinity doses caused the cell color to change from light green (control) to deep green (25 mM), then to yellow green (100–150 mM) and last to brown (175 mM). The cells cultured in the presence of 10–25 mM NaCl were the most green of the nine NaCl treatment levels (Fig. 3). The color changes of cells were positively correlated with the fresh biomass variations and the NaCl dose
The effect of the NaCl dose on the content of total chlorophylls (Chl t) and carotenoids (Car), the ratio of Chl a/b and Chl t/Car in Ginkgo cells are shown in Table 2. The Chl t and Car first increased (from 5 to 25 mM NaCl) then decreased (from 50 to 175 mM NaCl). The maxima were observed at 25 mM NaCl followed by 10 mM NaCl (p < 0.01). Exposure to NaCl levels lower than 75 mM led to increases in Chl t and Car content, whereas the Chl t decreased by 15.0 and 45.0 %, and the Car content decreased by 33.7 and 38.0 % at 150 and 175 mM NaCl, compared with the control, respectively (p < 0.01). Similar trends were observed for the Chl a and Chl b levels except at the 100 and 150 mM salinity levels, where the ratios of Chl a/b and Chl t/Car first increased then decreased with increasing NaCl levels (Table 2).
The chlorophyll fluorescence parameters, the F v/F m ratio, yield, q P and NPQ, varied with the NaCl doses compared with the control (Table 3). Similar trends in the F v/F m ratio, yield, q P values were observed. As the NaCl increased from 5 to 25 mM, the values increased, then gradually decreased from 50 to 125 mM and then they sharply decreased at 150 and 175 mM NaCl. The maximum values were observed at 25 mM NaCl where they were 101.7, 57.8, 29.7 and 105.2 % higher than at 175 mM NaCl, respectively (p < 0.05). A secondary maximum for all the values appeared at 50 mM NaCl. However, the NPQ value significantly increased at levels of NaCl less than 100 mM but significantly decreased >125 mM NaCl (p < 0.01) (Table 3).
The ions content and ratio of K+/Na+ in the suspension cells cultured at different NaCl doses were analyzed (Table 4). Increasing the levels of NaCl resulted in a steady increase in the content of Na+. The maximum Na+ content was observed at 150 and 175 mM NaCl, at 32.8- and 30.1-fold higher than the control, respectively. The K+ concentration increased at NaCl levels ≥50 mM, then declined with further increases in the NaCl levels. The maximum K+ content was observed at 50 mM NaCl where it was about 2.3-fold higher than the control. Except for the cells treated at 175 mM NaCl, the K+ concentrations were higher compared with the control. Therefore, the K+/Na+ ratio significantly decreased with increasing NaCl levels and it was lower than the control for all NaCl treatments, and was only 2.93 % of the control at 175 mM NaCl (p < 0.01) (Table 4).
The Mg2+ and Ca2+ concentrations in the suspension cells showed similar trends. Mg2+ concentrations changed very little at salinity ≤100 mM, but were always lower than the control. The Ca2+ content did not significantly differ compared with the control at 5 and 10 mM NaCl and from 25 to 125 mM NaCl where it was maintained between 2.14 and 2.43 mg g−1 DW. Significant reductions in Ca2+ were observed at 150 and 175 mM NaCl where it decreased by 96.7 % at 175 mM NaCl compared with the control (p < 0.05) (Table 4).
Membrane potential, lipid peroxidation, H2O2 response to varying NaCl doses
Relative conductivity (REC) is a parameter that can be used to assess the membrane potential (MP) of a plant and judge whether the cell membrane has undergone damage owing to environment stress. The REC of cells cultured in the presence of NaCl decreased from 5 to 50 mM compared with the control, however, it sharply increased and was higher than the control from 125 to 175 mM NaCl, reaching a peak (2.2-fold of the control) at 175 mM NaCl (Fig. 5a). There were significant changes in REC from 5 to 175 mM NaCl (p < 0.01).
Lipid peroxidation in cells was assessed by measuring the MDA content (Fig. 5b). Salt treatment caused almost no increases in MDA content from 5 to 25 mM NaCl. At 50–100 mM NaCl, the MDA values ranged from 24.0 to 25.0 µmol g−1 FW. The maximum accumulation of MDA (3.63-fold of the control) was observed at 150 mM NaCl, while a higher salt dose (175 mM NaCl) resulted in a MDA content lower than that observed for 150 mM NaCl (p < 0.01).
In contrast, the H2O2 content was higher than the control in all NaCl-treated cells, increasing from 5 to 150 mM NaCl, then decreasing at 175 mM NaCl. Moreover, at 125 and 150 mM NaCl the H2O2 content was 3.3- and 3.5-fold of the control, respectively (p < 0.01) (Fig. 5c).
Antioxidant enzyme activities response to varying NaCl doses
The changes in the antioxidant enzymes SOD, POD and CAT are shown in Fig. 5. SOD activity did not vary significantly at NaCl concentrations ≤100 mM. At NaCl doses >100 mM, SOD activity was inhibited with decreases of 13.8 and 16.3 % at 150 and 175 mM NaCl, respectively, compared with the control (p < 0.01) (Fig. 5d). The CAT activities gradually increased up to 75 mM NaCl (2.88-fold of the control), then were unchanged at 100–125 mM, and then significantly decreased from 150 to 175 mM NaCl (Fig. 5e). The CAT activity was higher than the control at NaCl levels ≤150 mM, but was apparently reduced at 175 mM NaCl (70 % of the control). In contrast to SOD and CAT, all NaCl treatments resulted in higher POD activity than the control where it increased gradually up to 100 mM NaCl, sharply increased at 125 mM (2.3-fold of the control), then remained steady to 175 mM (Fig. 5f).
Ultrastructure of Ginkgo suspension cells
After a 7-day culture, many mitochondria, central vacuoles, small vacuoles, and starch granules appeared in the control and 25 mM NaCl treatment cells as observed by transmission electron microscopy (TEM) micrographic images (Fig. 6a–c), and peripherally situated protoplasmic compartments (Fig. 6a, d). The thylakoids formed by lamellae and the chloroplast membrane were also distinctly observed (Fig. 6c, f). There was a significant difference in the shape of the chloroplasts between the two treatments, the chloroplast become more rounded in 25 mM NaCl-treated cells than in the control, where it was long and pointy (Fig. 6c, f).
The plastid membrane disappeared when the suspension cells were cultured in the presence of 100 and 175 mM NaCl, and many osmiophilic droplets (Ph) and starch granules (at 100 mM NaCl) were observed in the chloroplast lamellae area (Fig. 6i, l). However, the chloroplast lamellaes were distinctly observed in 100 mM-treated cells, and damaged granules were observed in the 175 mM-treated cells (where only starch granules were observed). In addition, the shape of the starch granules in the chloroplast varied between the two treatments (Fig. 6i, l).
When the cells were cultured in the presence of 175 mM NaCl, the plasma and vacuole membranes were destroyed and showed clear signs of degradation, particularly for the nucleus and cytoplasm (Fig. 6j, k). The most peculiar feature of these cells compared with the control and the 25 mM treatment was the increase in microscopic accretion on the outside of the cell wall at 100 and 175 mM NaCl (Fig. 6g, j, direction of the arrows). Moreover, many small bubbles on the inside of the cell wall and many small secretory vacuoles near plasma membrane were evident in the cells treated at 100 mM NaCl (Fig. 6g, h). These features suggested that high doses of NaCl might promote the secretion of secondary substances.
Discussion
The use of elicitation strategies to enhance the biosynthesis of secondary metabolites (for example flavonol and bilobalide) in cell cultures has been reported (Hao et al. 2010; Sun et al. 2012; Niknam et al. 2011). In the present work, nine NaCl concentrations were classified as low level (5–50 mM), moderate level (75–125 mM) and high level (150–175 mM) doses according to various cellular response parameters. Low salinity doses (5–50 mM) enhanced the cell growth, whereas high salinity doses (≥150 mM) restrained cell growth and damaged the cellular structure of the Ginkgo cells. However, the moderate salinity doses did not significantly inhibit cell growth and did not apparently affect cellular structure. This was similar to the result of Kang et al. (2010), where 800 mM KCl severely inhibited the growth of Ginkgo cells and other similar species (Niknam et al. 2011; Al-Khayri Jameel 2002). Moreover, two peaks of flavonoid production were observed at 25 NaCl (where cell growth was stimulated) and 175 mM NaCl (where cell structure was damaged). Three flavonoid glycosides were also shown to vary with the NaCl dose. The accumulation of quercetin, kaempferol and isorhamnetin mainly occurred at low NaCl doses, high NaCl doses, and moderate doses, respectively.
PAL is a key inducible enzyme that links primary and secondary metabolism. Many stress factors (such as temperature, salinity) or elicitors (e.g., SA, MJ) can enhance PAL activity (MacDonald and D’ Cunha 2007; Zhao et al. 2009). Two peaks of PAL activity were observed for Salvia miltiorrhiza cells (Dong et al. 2010) with elicitor induction time, but the first one coincided with the peak of a phenolic compound while the second one coincided with the shikonin peak. However, there was no direct correlation between the peaks of PAL activity and the shikonin production peak under l-phenylalanine treatments in Arnebia euchroma (Sykłowska-Baranek et al. 2012). In our study, the peak of PAL activity was observed at 25 mM (low dose), 150 and 175 mM (high dose) NaCl treatments and coincided with the peak of flavonoid production (Table 1), while the lower PAL activity and flavonoid content was observed at 75 mM NaCl. These results were similar to Abrol et al. (2012) and suggested that plants might divert their metabolism towards secondary metabolite production by consuming more energy in the presence of low NaCl concentrations. However, the lower PAL activity and flavonoid content observed for the 75 mM dose suggested that at moderate salinity, adaption or defense mechanisms exist to combat oxidative stress in cells.
Chlorophyll is an antenna which absorbs and transfers light energy to the reaction center. When plants suffer biotic or abiotic stress, Chl and F v/F m values decrease (Boughalleb et al. 2009). In the present work, the content of Chl t, chl a, and chl b increased at low NaCl levels (≤50 mM), and decreased under moderate and high NaCl levels (≥75 mM) compared with the control (Table 2). Furthermore, the increase of the Chl a/b ratio from 75 to 125 mM NaCl hinted that moderate salt treatment primarily reduced the content of chlorophyll b rather than chlorophyll a, perhaps as a photo-protective strategy. A lower Chl a/b ratio and higher Chl a content at low NaCl levels should be beneficial to LHCII to capture high light energy, while a lower Chl a/b ratio and lower Chl a content could lead to serious photoinhibition at higher NaCl levels (≥150 mM). Carotenoids can facilitate the non-radiative dissipation of excitation energy and increase antioxidant protection (Stahl and Sies 2003), in the present work, carotenoids content was reduced at ≥50 mM NaCl, but the Chl t/Car ratio did not decrease in the presence of different NaCl levels except at 100 and 175 mM NaCl in contrast to another report, where Chl t/Car ratio decreased in Ginkgo tree leaves under open-field condition compared with shade enclosure condition (Wang et al. 2014). Furthermore, the Chl t/Car ratio in different rice genotypes had different responses to water stress (Gauthami et al. 2014). This indicated that chlorophyll content was more affected than that of carotenoids under abiotic stress, and it seems that the level of Chl t/Car ratio was different between cell level and the whole plant level, and a species or genus.
Chlorophyll changes could be demonstrated by examining the effects of the salt treatment on the chlorophyll fluorescence parameters. The variations in chlorophyll directly coincided with the chlorophyll fluorescence parameters (F v/F m, yield, q p, Table 3; Fig. 4) and indicated that photosystem II of the Ginkgo cells was promoted at low NaCl levels and inhibited at high NaCl levels (≥150 mM) as a result of salinity stress. Similar to our work, low F v/F m was reported to be associated with Na+ dose and plant species under conditions of NaCl stress (James et al. 2006; Naidoo et al. 2008). NPQ acts in a photo-protective capacity to protect the plant against damage caused by excess energy. In our work, the higher value of NPQ was observed at 75 and 100 mM NaCl, and supported the conclusion that Ginkgo cells were well adapted to moderate salt conditions by employing a photo-protective strategy.
In general, salinity can reduce plant growth or damage plants by osmotic effects, toxic effects of ions and creating an imbalance in the uptake of essential nutrients (Ho 2003; Sun et al. 2012). Wang et al. (2003) reported that Ginkgo trees could grow at 0.3 % (51 mM) soil salinity and growth would be restrained at conditions higher than 0.3 % soil salinity. In our work, the Na+ and K+ content in the Ginkgo suspension cells both increased with increasing NaCl levels (≤50 mM). However, the Na+ content continuously increased but the K+ content markedly declined when the NaCl dose was higher than 75 mM. Furthermore, the K+/Na+ ratio, and Mg2+ and Ca2+ concentrations decreased with increasing NaCl levels for all NaCl treatments (Table 4). This suggested that Ginkgo cells could regulate homeostasis between K+, Ca2+, Mg+ and Na+ ions to balance the osmotic pressure by maintaining a high K+ concentration and K+/Na+ ratio, and cell growth would be unaffected at low NaCl levels (≤50 mM), and would withstand salt stress at moderate NaCl levels (75–100 mM) as a result of higher Ca2+ and Mg2+ concentrations. The brown and damaged cells observed at 175 mM NaCl was directly related to the higher Na+ concentrations, and significant declines in the K+, Ca2+, and Mg+ levels and K+/Na+ ratio. Similar results were obtained for Populus euphratica callus (Sun et al. 2010), a potato cell line (Queiros et al. 2011), date palm callus (Al-Khayri Jameel 2002), and Gypsophila oblanceolata seedlings (Sekmen et al. 2012).
Presently, the roles played by antioxidant enzymes in maintaining salt tolerance in cells are still unclear. Antioxidant enzymes are known to detoxify the excess reactive oxygen species (ROS) and reduce oxidative damage. However, high antioxidant enzyme activities may be regarded as symptoms of oxidative damage (Hao et al. 2012). As reported herein, SOD activity was up-regulated at 25 and 50 mM NaCl, down-regulated from 125 to 175 mM NaCl (Fig. 5d). However, higher levels of REC (membrane permeability), MDA, and H2O2 were found at 125–175, 150, 125–150 mM NaCl treatments, respectively (Fig. 5a–c). These results indicated that SOD was rapidly induced in the presence of low salt level. In plants, SOD could convert O2 •− to H2O2 and O2 by dismutation. There are several SOD-mediated routes of H2O2 formation in plants: H2O2 is generated by SOD dismutation O2 •− which is produced in PSI and PSII of chloroplastic electron transport chain (ETC), or in complex I and complex III of mitochondrial ETC by ‘electron leakage’; produced in peroxisome membranes by NADH/NADPH-dependent small ETC, or in peroxisomes matrix by xanthine oxidase; produced in cytoplasm and endoplasmic reticulum by cytochrome P450; and produced in apoplast by peroxidases, germin-like oxalate oxidase and amine oxidases (Abogadallah 2010; Gill and Tuteja 2010; Demidchik 2014). In present study, CAT and POD activities were activated in Ginkgo cells at 50–125 mM and 125–175 mM NaCl levels, respectively. Furthermore, higher CAT and POD activities at 125 mM NaCl did not prevent the H2O2 accumulation (Fig. 5e, f). Thus, it was likely that a low level of H2O2 played a signaling role under stress, and therefore, the role of CAT or POD was to control cellular H2O2 concentrations rather than working to remove it completely (Abogadallah et al. 2009). Hence, the result indicated that Ginkgo cells depend on an increase in the activity of CAT at 50–125 mM NaCl and POD at ≥125 mM NaCl to control H2O2 and MDA levels. This may be because POD was required to assist with the fine regulation of H2O2 while CAT was involved in mass scavenging of H2O2. This conclusion was in agreement with reports by Sarkar et al. (2013), Tounekti et al. (2011) and Noreen and Ashraf (2009).
Influence of different NaCl treatments on the REC (a), MDA (b), H2O2 (c) content and SOD (d), CAT (e) and POD (f) activities of Ginkgo biloba L. suspension cells at the 7th day after addition of different NaCl doses to the medium. Values are mean ± SD from two independent experiments (n = 6). Means followed by different letters above the bars indicate significant differences (p < 0.01) in among treatments according to the Duncan’s multiple range test
The initial defense strategies of plants under oxidative stress involve the induction of antioxidant enzymes or production of secondary metabolites (Abrol et al. 2012). The lower flavonoid content and PAL activity at 75 mM NaCl may be interpreted to indicate that the cellular metabolism was diverted to increase anti-oxidative enzyme production rather than secondary metabolites. This was likely to be an optimal defense strategy for cellular allocation of resources by reducing metabolic cost only after some degree of initial damage has emerged under conditions of serious stress (Lattanzio et al. 2009).
The changes in structure of intracellular components (e.g., photosynthetic apparatus, cell walls, membranes, mitochondria and chloroplasts) were tightly associated with photochemical efficiency and antioxidant stress under salt stress (Fidalgo et al. 2004; Queiros et al. 2011). Wang et al. (2008) found that chloroplasts lamellae in Ginkgo trees disappeared and many osmiophilic droplets were observed under the soil water level in 20 % of the field capacity. Our previous study in Ginkgo callus showed that the callus color was associated with cell ultrastructure changes, flavonoid content and protein (Chen et al. 2006). In cucumber, the chloroplast envelope was destroyed and the plastoglobuli number in thylakoid membranes increased at 75 mM NaCl (Shu et al. 2013). Salinity could not only affect the cellular structural integrity and membrane structure, but also increased the number of starch grains in potato cell plastids at 100 mM NaCl (Queiros et al. 2011). In the present study, the integrity of the cellular and membrane structure were maintained in the control and at 25 mM NaCl treatment (Fig. 6a–f). Although the photosynthetic apparatus were affected, many osmiophilic droplets, starch grains in plastid lamellae area, and many small secretory vacuoles near the plasma membrane at 100 mM (Fig. 6g–i) were observed. The cellular damage and higher REC was found when the cells were cultured in the presence of 175 mM NaCl. (Fig. 6j–l) This result was similar to observations for a citrus cell line where increases in the number of amyloplasts, lipid bodies and starch were observed in acclimated citrus cells at 100 mM NaCl (Ferreira and Lima-Costa 2008). High dose NaCl stress might promote the secretion of secondary cellular substances, furthermore Ginkgo cells were able to adapt to a certain extent to NaCl stress at concentrations less than 100 mM.
Transmission electron microscopy (TEM) micrographic images of Ginkgo biloba L. cells in the presence of various NaCl doses for 7 days. a–c Control cells; d–f cells treated with 25 mM NaCl; g–i cells treated with 100 mM NaCl; j–l cells treated with 175 mM NaCl. The arrows indicated small bubbles in h, microscopic accretions in g and j. Ch chloroplast, S starch grain, CW cell wall, Ph osmiophilic droplets, V vacuole, M mitochondria, N cell nucleus, ER endoplasmic reticulum
In conclusion, at low salt doses (5–50 mM NaCl), the salt appears to stimulate cell growth and promote flavonol glycoside production in Ginkgo cells. This appears to be primarily as a result of accumulating K+, and maintaining ion homeostasis and a higher K+/Na+ ratio and second, by achieving a higher chlorophyll content.
The defense and tolerance to salinity exhibited at moderate salt levels (75–125 mM NaCl) can be attributed to the higher CAT activity, high K+ content and accompanied starch grain accumulation in the plastids rather than flavonol glycosides to counteract the inhibition of the PSII, the increasing Na+ content and ROS generation.
High salinity, in particular 175 mM NaCl, damaged the cellular structure because of high Na+ and low K+ content and loss of ion homeostasis and inhibition of PSII and high membrane permeability.
The highest flavonol glycoside accumulation was shown at 175 mM NaCl. Therefore, induction at 175 mM NaCl would be desirable for in vitro production of flavonol glycosides in Ginkgo cells.
Author contribution
This present study was accomplished with the collaboration of all authors. Y. Chen obtained cell cultures system, performed flavonoids study and was a writer of the manuscript; F. Z. Lin experimented chlorophyll fluorescence and pigments; H. Yang did ion analyses; L. Yue and F. Hu performed antioxidant system; J. L. Wang and Y. Y. Luo was responsible for cell ultrastructure; F. L. Cao, Project supervisor was responsible for experimental design, verification of the paper.
Abbreviations
- ROS:
-
Reactive oxygen species
- Chl:
-
Chlorophyll
- MDA:
-
Malondialdehyde
- SOD:
-
Superoxide dismutase
- CAT:
-
Catalase
- POD:
-
Guaiacol peroxidase
- PAL:
-
Phenylalanine ammonia-lyase
- Chl a/b :
-
Chlorophyll a/b ratio
- Car:
-
Carotenoid
- Chl t/Car:
-
Rate of total chlorophyll to Carotenoid
- PSII:
-
Photosystem II
- PSI:
-
Photosystem I
- F v/F m :
-
Maximum PSII photochemical efficiency
- Yield:
-
Actual PSII quantum yield
- q P :
-
Photochemical quenching coefficient
- NPQ:
-
Non-photochemical quenching coefficient
- NAA:
-
Naphthaleneacetic acid
- BA:
-
Benzyladenine
- KT:
-
Kinetin
References
Abogadallah GM (2010) Antioxidative defense under salt stress. Plant Signal Behav 5(4):369–374
Abogadallah GM, Serag MM, Quick WP (2009) Fine and coarse regulation of reactive oxygen species in the salt tolerant mutants of barnyard grass and their wild-type parents under salt stress. Physiol Plant 138:60–73
Abrol E, Vyas D, Koul S (2012) Metabolic shift from secondary metabolite production to induction of anti-oxidative enzymes during NaCl stress in Swertia chirata Buch.-Ham. Acta Physiol Plant 34:541–546
Al-Khayri Jameel M (2002) Growth, proline accumulation, and ion content in sodium chloride-stressed callus of date palm. In Vitro Cell Dev Biol Plant 38:79–82
Beyer WF, Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161:559–566
Boughalleb F, Mounir DM, Tiba BB (2009) Photosystem II photochemistry and physiological parameters of three fodder shrubs, Nitraria retusa, Atriplex halimus and Medicago arborea under salt stress. Acta Physiol Plant 31:463–476
Breia R, Vieira S, da Silva JM, Geros H, Cunha A (2013) Mapping grape berry photosynthesis by chlorophyll fluorescence imaging: the effect of saturating pulse intensity in different tissues. Photochem Photobiol 89:579–585
Cai ZZ, Kastell A, Knorr D, Smetanska I (2012) Exudation: an expanding technique for continuous production and release of secondary metabolites from plant cell suspension and hairy root cultures. Plant Cell Rep 31:461–477
Chen Y, Cao FL (2007) Leave source from callus induction and flavonoid content in callus from different tissues of five Ginkgo biloba L. cultivars. J Zhejiang For Coll 24(2):150–155 (in Chinese)
Chen Y, Cao FL, Gan XH (2006) Protein analysis in Ginkgo calli with different colors by electrophoresis and electron microscopy. Acta Bot Boreal Occident Sin 26(11):2239–2243 (in Chinese)
Cybulska-Heinrich AK, Mozaffarieh M, Flammer J (2012) Ginkgo biloba: an adjuvant therapy for progressive normal and high tension glaucoma. Mol Vis 18:390–402
Davies BH (1976) Carotenoids. In: Good win TW chemistry and biochemistry of plant pigments (vol 2). Academic Press Inc, London, pp 38–165
Demidchik V (2014) Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. Environ Exp Bot. doi:10.1016/j.envexpbot.2014.06.021
Dong JN, Wan GW, Liang ZS (2010) Accumulation of salicylic acid induced phenolic compounds and raised activities of secondary metabolic and antioxidative enzymes in Salvia miltiorrhiza cell culture. J Biotechnol 148:99–104
Duncan DB (1955) Multiple range and multiple F test. Biometrics 11:1–42
Ferreira AL, Lima-Costa ME (2008) Growth and ultrastructural characteristics of Citrus cells grown in medium containing NaCl. Biolgia Plantarum 52(1):129–132
Fidalgo F, Santos A, Santos I, Salema R (2004) Effects of long-term salt stress on antioxidant defence systems, leaf water relations and chloroplast ultrastructure of potato plants. Ann Appl Biol 145:185–192
Gauthami P, Subrahmanyam D, Padma V, Kiran TV, Rao YV, Rao PR, Voleti SR (2014) Variation in leaf photosynthetic response of rice genotypes to post-anthesis water deficit. Ind J Plant Physiol. doi:10.1007/s40502-014-0086-7
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Guo ZG, Liu Y, Xing XH (2011) Enhanced catharanthine biosynthesis through regulation of cyclooxygenase in the cell suspension culture of Catharanthus roseus (L.). Process Biochem 46:783–787
Hao GP, Du XH, Zhao FX, Shi RJ, Wang JM (2009) Role of nitric oxide in UV-B-induced activation of PAL and stimulation of flavonoid biosynthesis in Ginkgo biloba callus. Plant Cell Tissue Organ Cult 97:175–185
Hao GP, Du XH, Zhao FX, Ji HW (2010) Fungal endophytes-induced abscisic acid is required for flavonoid accumulation in suspension cells of Ginkgo biloba. Biotechnol Lett 32:305–314
Hao L, Zhao Y, Jin DD, Zhang L, Bi XH, Chen HX, Xu Q (2012) Salicylic acid-altering Arabidopsis mutants response to salt stress. Plant Soil 354:81–95
Ho LC (2003) Interactions between root and shoot environmental factors on crop yield and quality. Acta Hortic 609:121–126
Holden M (1965) Chlorophylls: chemistry and biochemistry of plant pigments. Academic Press, London, p 461
James RA, Munns R, Caemmerer S, Trejo C, Miller C, Condon T (2006) Photosynthetic capacity is related to the cellular and subcellular partitioning of Na+, K+ and Cl− in salt affected barley and durum wheat. Plant, Cell Environ 29:2185–2197
Jeong GT, Park DH (2006) Enhanced secondary metabolite biosynthesis by elicitation in transformed plant root system. Appl Biochem Biotechnol 130(1):436–446
Kang SM, Min JY, Kim YD, Kang YM, Park DJ, Jung HN, Kim SW, Choi MS (2006) Effects of methyl jasmonate and salicylic acid on the production of bilobalide and ginkgolides in cell cultures of Ginkgo biloba. In Vitro Cell Dev Biol Plant 42:44–49
Kang SM, Min JY, Kim YD, Karigar CS, Kim SW, Goo GH, Choi MS (2009) Effect of biotic elicitors on the accumulation of bilobalide and ginkgolides in Ginkgo biloba cell cultures. J Biotechnol 139:84–88
Kang SM, Min JY, Park DJ, Jeong MJ, Song HJ, Heo CM, Kim HG, Yang JK, Lee CH, Karigar CS, Choi MS (2010) Potassium chloride elicits enhancement of bilobalide and Ginkgolides production by Ginkgo biloba cell cultures. For Sci Technol 6:49–54
Knorzer OC, Durner J, Boger P (1996) Alterations in the anti-oxidative system of suspension-cultured soybean cells (Glycine max) induced by oxidative stress. Physiol Plant 97:388–396
Koukol J, Conn EE (1961) The metabolism of aromatic compounds in higher plants. Purification and properties of the l-phenylalanine de aminase of Hordeum vulagare. J Biol Chem 236:2692–2698
Lattanzio V, Cardinali A, Ruta C, Fortunato IM, Lattanzio VMT, Linsalata V, Cicco N (2009) Relationship of secondary metabolism to growth in oregano (Origanum vulgare L.) shoot cultures under nutritional stress. Env Exp Bot 65:54–62
Li CY, Liu B, Li CH, Zeng Q, Hao MZ, Han ZM, Zhu XG, Shen WJ (2013) Leaf morphology and ultrastructure responses to elevated O3 in transgenic Bt (cry1Ab/cry1Ac) rice and oxidative conventional rice under fully open-air field conditions. PLoS One 8(12):e82199
MacDonald MJ, D’ Cunha GB (2007) A modern view of phenylalanine ammonialyase. Biochem Cell Biol 85:273–282
Maria F, Stanisław W, Halina M, El Zbieta RS, Apolonia S, Jolanta BK (2012) Membrane permeability and micro-and macroelement accumulation in spring wheat cultivars during the short-term effect of salinity and PEG induced water stresses. Acta Physiol Plant 34:985–995
Mittal S, Kumari N, Sharma V (2012) Differential response of salt stress on Brassicajuncea: photosynthetic performance, pigment, proline, D1 and antioxidant enzymes. Plant Physiol Biochem 54:17–26
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–494
Naidoo G, Rita S, Premila A (2008) Morphological and physiological responses of the halophyte, Odyssea paucinervis (Staph) (Poaceae), to salinity. Flora 203:437–447
Niknam V, Meratan AA, Ghaffari SM (2011) The effect of salt stress on lipid peroxidation and antioxidative enzymes in callus of two Acanthophyllum species. In Vitro Cell Dev Biol Plant 47:297–308
Noreen Z, Ashraf M (2009) Assessment of variation in antioxidative defense system in salt-treated pea (Pisum sativum) cultivars and its putative use as salinity tolerance markers. J Plant Physiol 116:1764–1774
Osorio ML, Goncalves S, Coelho N, Osorio J, Romano A (2013) Morphological, physiological and oxidative stress markers during acclimatization and field transfer of micropropagated Tuberaria major plants. Plant Cell Tiss Organ Cult 115:85–97
Queiros F, Rodrigues JA, Almeida JM, Almeida Domingos PF, Fidalgo F (2011) Differential responses of the antioxidant defence system and ultrastructure in a salt-adapted potato cell line. Plant Physiol Biochem 49:1410–1419
Rao KVM, Sresty TVS (2000) Antioxidative parameters in the seedlings of pigeon pea (Cajanus cajan L. Millspaugh) in response to Zn and Ni stresses. Plant Sci 157:113–128
Sabater-Jara AB, Souliman-Youssef S, Novo-Uzal E, Almagro L, Belchı-Navarro S, Pedreno MA (2013) Biotechnological approaches to enhance the biosynthesis of ginkgolides and bilobalide in Ginkgo biloba. Phytochem Rev 12:191–205
Santamaria AR, Mulinacci N, Valletta A, Innocenti M, Pasqua G (2011) Effects of elicitors on the production of resveratrol and viniferins in cell cultures of Vitis vinifera L. cv Italia. J Agric Food Chem 59(17):9094–9101
Sarkar RK, Mahata KR, Singh DP (2013) Differential responses of antioxidant system and photosynthetic characteristics in four rice cultivars differing in sensitivity to sodium chloride stress. Acta Physiol Plant 35:2915–2926
Sekmen AH, Turkan I, Ozgecan TZ, Ceyda O, Dinc A (2012) Different antioxidant defense responses to salt stress during germination and vegetative stages of endemic halophyte Gypsophila oblanceolata Bark. Environ Exp Bot 77:63–76
Shu S, Yuan LY, Guo SR, Sun J, Yuan YH (2013) Effects of exogenous spermine on chlorophyll fluorescence, antioxidant system and ultrastructure of chloroplasts in Cucumis sativus L. under salt stress. Plant Physiol Biochem 63:209–216
Singh B, Kaur P, Gopich, Singh RD, Ahuja PS (2008) Biology and chemistry of Ginkgo biloba. Fitoterapia 79:401–418
Solecka D, Kacperska A (2003) Phenylpropanoid deficiency affects the course of plant acclimation to cold. Physiol Plantarum 119:253–262
Stahl W, Sies H (2003) Antioxidant activity of carotenoids. Mol Aspects Med 24:345–351
Sun J, Li LS, Liu MQ, Wang MJ, Ding MQ, Deng SR, Lu CF, Zhou XY, Shen X, Zheng XJ, Chen SL (2010) Hydrogen peroxide and nitric oxide mediate K+/Na+ homeostasis and antioxidant defense in NaCl-stressed callus cells of two contrasting poplars. Plant Cell Tiss Organ Cult 103:205–215
Sun LN, Su H, Zhu Y, Xu MJ (2012) Involvement of abscisic acid in ozone-induced puerarin production of Pueraria thomsnii Benth. suspension cell cultures. Plant Cell Rep 31:179–185
Sykłowska-Baranek K, Pietrosiuk A, Naliwajski M, Kawiak A, Jeziorek M, Wyderska S, lojkowska E, Chinou I (2012) Effect of l-phenylalanine on PAL activity and production of naphthoquinone pigments in suspension cultures of Arnebia euchroma (Royle) Johnst. In Vitro Cell Dev Biol Plant 48:555–564
Tounekti T, Vadel AM, Onate M, Khemira H, Munné-Bosch S (2011) Salt-induced oxidative stress in rosemary plants: damage or protection? Environ Exp Bot 71:298–305
Upton R (ed) (2003) Ginkgo leaf–Ginkgo leaf dry extract—Ginkgo biloba L.—standards of analysis, quality control, and therapeutics. American herbal pharmacopoeia, Scotts Valley
Van Beek TA, Montoro P (2009) Chemical analysis and quality control of Ginkgo biloba leaves, extracts, and phytopharmaceuticals. J Chromatogr A 1216:2002–2032
Wang GB, Cao FL, Zhang WX (2003) A study on salt tolerance of Ginkgo biloba varieties. Scientia silvae Sinicae 39(5):168–172 (in Chinese)
Wang GB, Cao FL, Jing M, Hao MZ (2008) The effect of water stress on mesophyll cell ultrastructure of Ginkgo. J Nanjing For Univ (Nat Sci Ed) 32(5):65–70 (in Chinese)
Wang YW, Sun LG, Xu JY, Zhang JJ, Wu M, Ma J, Chen GX (2014) Physiological and growth characteristics of Ginkgo biloba L. exposed to open field and shade enclosure during the reproductive stage. Acta Physiol. doi:10.1007/s11738-014-1638-1
Yusuf NA, Annuar MSM, Khalid N (2013) Physical stress for over production of biomass and flavonoids in cell suspension cultures of Boesenbergia rotunda. Acta Physiol Plant 35:1713–1719
Zhao X, Tan HJ, Liu YB, Li XR, Chen GX (2009) Effect of salt stress on growth and osmotic regulation in Thellungiella and Arabidopsis callus. Plant Cell Tiss Organ Cult 98:97–103
Zhao B, Agblevor FA, Ritesh KC, Jelesko JG (2013) Enhanced production of the alkaloid nicotine in hairy root cultures of Nicotiana tabacum L. Plant Cell Tiss Organ Cult 113:121–129
Acknowledgments
Funding for this work is provided by the Program for Priority Academic Program Development of Jiangsu Province of China (PAPD); the National Natural Science Foundation of China (31170627); the Program for Innovative Research Team in Universities of Educational Department (PCSIRT, China).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L. A. Kleczkowski.
Rights and permissions
About this article
Cite this article
Chen, Y., Lin, F., Yang, H. et al. Effect of varying NaCl doses on flavonoid production in suspension cells of Ginkgo biloba: relationship to chlorophyll fluorescence, ion homeostasis, antioxidant system and ultrastructure. Acta Physiol Plant 36, 3173–3187 (2014). https://doi.org/10.1007/s11738-014-1684-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-014-1684-8