Abstract
Intercropping of upland rice with short-duration grain legumes has major advantages in increasing crop yields and soil productivity. However, the contribution of arbuscular mycorrhizas, the common mutualistic symbiosis between most crops and mycorrhizal fungi, is not fully understood in intercropping systems. We assayed the contribution of inoculation of the arbuscular mycorrhizal fungus (AMF) Glomus caledonium on nutrient acquisition and biomass yield. Using the method of plastic film and nylon net partition and tracing 15N transferred between the intercropped upland rice (Oryza sativa ssp. Japonica Nipponbare) and mung bean (Vigna radiata L. Chuanyuan), we compared the intercropping, with separation of the whole root systems by a plastic film, with and without a barrier of nylon net to allow penetration of the fungal hyphae. Intercropping significantly improved the formation of arbuscular mycorrhizas, particularly in the upland rice roots. The improved formation of mycorrhizas by the intercropping increased total P uptake by 57% in rice, total P and N acquisition by 65% and 64% respectively in mung bean, and nodulation by 54% in mung bean. The percentage of total 15N transfer from mung bean to rice leaves was increased from 5.4% to 15.7% by inoculation with AMF. In contrast, there was only 2.7% of 15N transfer from rice to mung bean and no AMF effect on N transfer. It is concluded that cereal and legume crop intercropping increase mycorrhiza formation, which in turn improves nodulation, N and P acquisition and N transfer in the legumes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cereal–legume intercropping is a widely used agricultural system with significant advantage in yield and a high rate of resource utilization, and is considered now as an important practice in sustainable agriculture development (Ghosh et al. 2007; Kwabiah 2005; Laxminarayana and Munda 2004; Sarkar and Sanyal 2000; Sarr et al. 2008; Toomsan et al. 2000). Rice (Oryza sativa L.) and mung bean (Vigna radiata L.) are important food crops, especially in tropical and subtropical regions. Traditional waterlogged rice cultivation consumes huge amounts of fresh water (Bouman et al. 2007; Mikkelsen and DeDatta 1991) and is claimed to be a main source of methane emission (Allen et al. 2003; Chakraborty et al. 2000; Fumoto et al. 2008). Aerobic upland rice is now being considered as a good water-saving model in agriculture (Bouman et al. 2007). Therefore, studies on aerobic rice and legume intercropping systems are important for sustainable development of agriculture and global climate change.
Arbuscular mycorrhizal fungi (AMF) can form symbioses (arbuscular mycorrhizas) with the majority of land plants (Smith and Read 1997). When in symbiosis, AMF promote plant water and nutrient uptake, especially of insoluble soil phosphate (Pi) fraction (Clark and Zeto 2000; Marschner and Dell 1994). The fungi in return benefit from the supply of carbohydrates derived from photosynthesis (Barker et al. 1998; Harrison 1999; Johnson et al. 1997). Inoculation of mycorrhizal fungi to rice grown under upland condition could improve both growth and nutrient acquisition (Gao et al. 2007; Herdler et al. 2008; Purakayastha and Chhonkar 2001; Zhang et al. 2005), as well as regulate the expression of many relevant genes (Güimil et al. 2005; Paszkowski et al. 2002; Sawers et al. 2008). It is therefore imperative to investigate the effect of intercropping on mycorrhizal colonization and the effect of mycorrhizas on rice and mung bean intercropping.
Legumes can transfer fixed N to intercropped cereals during their joint growing period and this N is an important resource for the cereals (Af Geijersstam and Mårtensson 2006; Bethlenfalvay et al. 1991; Chalk 1998; Hamel et al. 1992; Jensen 1996; Shen and Chu 2004). Intercropping with legumes was found to increase protein content in cereal grains or shoots (Lauk and Lauk 2008; Lithourgidis et al. 2007; Martin et al. 1998). Some studies have indicated that N is being transferred from legumes to cereals via hyphal linkage (Johansen and Jensen 1996; Martins and Cruz 1998). Others have claimed that there was no obvious effect of hyphal linkage on N transfer (Hamel et al. 1991; Ikram et al. 1994; Reeves 1992). Plant growth and N transfer for AMF inoculated intercropped rice and mung bean may differ from the earlier report without AMF inoculation that the possible transfer of N from mung bean to rice did not increase total N uptake of rice (Aggarwal et al. 1992).
To evaluate the contribution of AMF in a rice and mung bean intercropping system, we inoculated AMF to intercropped rice and mung bean. By utilizing the 15N isotope, the interactions among AMF, rice and mung bean were studied to test the hypothesis that AMF could improve the efficiency of biological N fixation by the legume plants, and utilization of N and P and total plant yield for the two crops.
Materials and methods
Plant growth medium
A mixture of river sand, laboratory quartz sand and peat at the proportion 7:2:1 (w/w) was used in the experiments. The culture medium contained 36.4 g kg−1 of organic matter, 0.38 g kg−1 of total N, 0.13 g kg−1 of total P, 10.6 mg kg−1 of Olsen-P and pH of 6.78 (1:2.5 soil-to-water). The mixture was steam-sterilized at 121°C for 2 h, and 3.5 kg of the mixture was then put into each plastic pot (21 cm in diameter and 25 cm in depth) for the greenhouse experiment.
Seeding, transplanting and inoculation
Seeds of rice (Oryza sativa L. ssp. Japonica Nipponbare) and mung bean (Vigna radiata L. Chuanyuan) were sterilized by immersion in 10% H2O2 for 30 minutes before germination. Rice seedlings were transplanted at the three leaf stage, while mung beans were directly sown in the pots after inoculation of the growth medium with 100 ml suspension containing 106 CFU of Rhizobium leguminosarum (kindly supplied by Jiangsu Academy of Agricultural Sciences, China) and with 350 g of inoculum of the AM fungus Glomus caledonium 90036 containing 5,000–6,000 spores (courtesy of Prof. Xiangui Lin, Nanjing Soil Science Institution, CSA). Because the inoculum consisted of the AM fungal spores, sand and colonized root fragments, the non-AM inoculation control was amended with steam-sterilized inoculum.
In each pot, two holes (4 cm in diameter and 15 cm in depth) for rice seedlings and two holes for mung bean seeds were arranged. Each hole was planted with four seedlings of rice or four seeds of mung beans in a glasshouse. The pots were regularly watered to maintain soil moisture at 70% of field water holding capacity. One week after germination two relative uniform seedlings were kept in each hole for further growth.
Experimental design
In the experiment, three separation arrangements of the rice compartments from mung bean compartment were designed, i.e., (Fig. 1) (1) plastic-film separation, which was a total separation not allowing any material to cross, (2) a 30 μm aperture nylon net separation, which allowed only the hyphae to cross (Li et al. 1991), and (3) no separation, allowing contact between roots of the two plant species. Each arrangement had an AM fungus treatment and a control with steam-sterilized inoculum. Each treatment and control had six replicates. All the pots were placed randomly. Pots were watered from below and fed weekly with 20 ml of a half-strength Hoagland solution without phosphate (Hoagland and Arnon 1950).
Isotopic tracing
One month after planting, an isotopic labeling experiment was conducted utilizing (15NH4)2SO4, enriched with 42.11 atom % of 15N and produced by Shanghai Chemical Engineering Academy. Before labeling, mung beans in each pot were placed in a PVC cylindrical membrane, and a plastic film with three layers of filter paper on it was set on the surface of the growth medium to prevent pollution from isotopic N. Mung bean leaves were then labeled with 10 ml of 1.5% (15NH4)2SO4 solution. The shoots of the mung beans were sealed until the labeling solution dried, one day after labeling. Contamination of other plants and of the growth medium by the 15N fertilizer was prevented by this process. Rice leaves were also labeled three times with the same amount of the solution as described above. Each labeling was replicated three times. Monocultured rice or mung bean was planted at the same time as the controls to examine the natural 15N abundance.
Sampling and analysis
Plants were harvested 20 days after labeling in total 50 days after inoculation. Shoots were firstly cut off at ground level and the whole culture medium in the pot was taken out and placed on a sieve with 0.2 mm mesh. The medium attached on the roots was removed gently by running tap water. The roots of rice and mung bean were immersed in tap water and separated phenologically by their different colors and rooting patterns. The fresh root fractions of each sample were weighed immediately after separation. All the root nodules in each pot were removed from the mung bean roots and weighed as fresh weight immediately after numbers were being counted. Fresh roots were cut into segments about 1 cm long and mixed thoroughly. A randomly selected 0.5-g sample of fresh root samples per pot was used to detect mycorrhizal root colonization (Phillip and Hayman 1970; Trouvelot et al. 1986). The computer program MYCOCALC (www.dijon.inra.fr/mychintec/Mycocalc-prg/download.html) was used to calculate the percentage of root length colonized by AMF.
The shoots and remaining fresh roots were dried at 70°C for 48 h to a constant weight after killing enzymatic activity at 105°C for 0.5 h. Plant samples were digested with H2SO4·H2O2 methods for N and P analysis. Total N and P in plant digests were determined by the micro-Kjeldahl procedure (Bremner 1965) and the molybdenum blue colorimetric method (John 1970), respectively. The 15N abundance of shoots was determined using a MAT-251 isotope mass spectrometer.
N transfer was calculated as described by Hardarson et al. (1988) and Ofosu-Budu et al. (1995):
where NT% indicates N transfer rate, NR indicates atom% 15N excess in recipient crop, and ND indicates atom% 15N excess in donor crop;
where NE indicates atom% 15N excess, NC indicates N content, and DW indicates crop dry weight;
where ANT indicates amount of N transferred and NCD indicates N content of donor plant;
where NTFD% indicates the N percentage transferred from donor crop to recipient plant and TNCR indicates the total N content in recipient crop;
where NNT indicates net N transferred, ANTM indicates amount of N transferred with mung bean as 15N donor plant and ANTR indicates amount of N transferred with rice as 15N donor plant.
Statistical analysis
Statistical analysis was carried out using SPSS11.5 software. Data were analyzed by ANOVA (GLM-procedure) for a completely randomized factorial design model. If required, data were %-transformed to maintain homogeneity of variance. The difference of the treatments was compared using least significant difference (LSD) test at the 0.05 probability level.
Results
AM fungus colonization
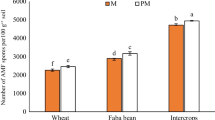
Intercropping significantly increased AM fungal colonization of roots (Fig. 2). When rice and mung bean plants were separated by a plastic film, no more than 4% of the rice roots were colonized by the AM fungus, while about 48.6% of mung bean roots were colonized. When the separation was absent or replaced by the nylon-net with 30 μm diameter apertures (that prevented root crossover), about 33% of the rice and 65% of the mung bean roots were colonized. The significant increase in total AM fungal colonization resulted in a significant increase of formation of arbuscules (Fig. 2).
AM fungal colonization of rice (a) and mung bean (b) roots after inoculation with Glomus caledonium: plastic-film separation (PFS), nylon-net separation (NNS) and no separation (NS). The white columns represent total AM fungal colonization; the black columns represent arbuscular colonization. AM fungal colonization in plants was determined 50 days after inoculation. Different letters on the columns of different separation arrangements either for arbuscular or total AM infection indicate significant difference at P < 0.05 level. Means±SD of three replicates
No difference in colonization was found between the nylon-net separation and no separation treatments (Fig. 2).
Biomass yield
The biomass of mung bean shoots with AM fungal inoculation intercropped with plastic-film separation, nylon-net separation, and no separation, increased by 56%, 76% and 82%, respectively, as compared to non-inoculation treatments (Fig. 3). The biomass of rice shoots (Fig. 3) was not significantly different among all the treatments, either in the inoculation of the AM fungus or in intercropping with mung bean plants. Separating rice from mung bean by a plastic film, significantly decreased the biomass of mung bean plants, but not that of rice plants treated with AM inoculation (Fig. 3b). No significant difference of mung bean biomass between treatments of the nylon-net separation and no separation was found (Fig. 3a,b). There were small but not significant increases of total root biomass weight of both the rice and mung bean in the whole pot by either intercropping or AMF colonization (Fig. 3c,d).
Biomass of rice (a and c) and mung bean (b and d) with and without inoculation with Glomus caledonium: plastic-film separation (PFS), nylon-net separation (NNS) and no separation (NS). Both rice and mung bean were four plants per pot. Biomass in plants was determined 50 days after inoculation. Different letters above columns within an inoculation treatment indicate significant difference at P < 0.05 level. The white column represents −AMF, the black column represents +AMF. Means±SD of three replicates
P and N uptake
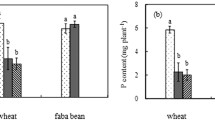
Intercropping of rice and mung bean with AMF inoculation significantly increased P concentrations in rice shoots, while no difference was observed in rice N concentrations (Fig. 4). The intercropping and AMF inoculation did not significantly affect either N or P concentrations in mung bean (Fig. 4). Intercropping with AM fungal inoculation significantly enhanced the content of N and P in mung bean shoots (Fig. 5).
N and P concentrations in shoots of rice (a and c) and mung bean (b and d) with and without inoculation with Glomus caledonium: plastic-film separation (PFS), nylon-net separation (NNS) and no separation (NS). The white column represents −AMF, the black column represents +AMF. N and P concentrations in shoots were determined 50 days after inoculation. Different letters above columns within an inoculation treatment indicate significant difference at P < 0.05 level. Means±SD of three replicates
N and P content of shoots of rice (a and c) and mung bean (b and d) after inoculation with Glomus caledonium: plastic-film separation (PFS), nylon-net separation (NNS) and no separation (NS). The white columns represent −AMF, the black columns represent +AMF. N and P concentrations in shoots were determined 50 days after inoculation. Different letters above columns within an inoculation treatment indicate significant difference at P < 0.05 level. Means±SD of three replicates
Nodulation of mung bean
In comparison with the non-inoculated treatments, the AM fungal inoculation increased the total root nodule numbers per pot by 20%, 62% and 54% in the intercropping with plastic-film separation, nylon-net separation, and no separation treatments, respectively (Fig. 6a). It also increased the total fresh weight of the nodules by 80%, 107% and 130%, respectively (Fig. 6b). The AMF colonization and intercropping with rice also significantly increased the root nodule number, particularly the root nodule fresh weight of the unit weight of fresh roots (Fig. 6c,d).
Nodulation in mung bean intercropped with rice, with or without inoculation with Glomus caledonium: Plastic-film separation (PFS), nylon-net separation (NNS) and no separation (NS). a and c Numbers of root nodules; b and d Fresh weight (FW) of root nodules. The white columns represent −AMF; the black columns represent +AMF. Different letters above columns within an inoculation treatment indicate a significant difference at the P < 0.05 level. Means±SD of three replicates
N transfer
The results of 15N labeling (Table 1) show that inoculation of the AM fungus in intercropping enhanced the net nitrogen transfer from mung bean to rice. The amounts of N transferred from mung bean to rice exceeded that from rice to mung bean (Table 1). However, there was no significant difference in N transfer between nylon-net separation and no separation treatments.
Discussion
The beneficial effects of intercropping systems have been well reported (Hamel et al. 1991; Hauggaard-Nielsen and Jensen 2005; Mårtensson et al. 1998; Reeves 1992; Sieverding and Leihner 1984). In the present study, we demonstrated that rice grown under upland conditions acquired more P and also improved intercropped mung bean N and P acquisition, root nodulation and growth. We present evidence that the beneficial effects of intercropping are, at least in part, due to the contribution of AM fungal hyphae.
Intercropping with legume improved mycorrhizal fungal colonization of roots of both upland rice and mung bean
Although most legume crops, e.g. mung bean, are known as excellent hosts of many AM fungi (Haugen and Smith 1992; Kasiamdari et al. 2002; Khasa et al. 1992; Lin. et al. 2001; Sprent and James 2007), mycorrhizal colonization on rice roots, and hence promotion of the rice growth, might depend on the rice cultivar, growth conditions and fungal strains. Some positive results with rice (Gao et al. 2007; Purakayastha and Chhonkar 2001; Solaiman and Hirata 1997; Zhang et al. 2005), are being challenged by reports that show no influence on rice growth when only AMF were inoculated (Herdler et al. 2008; Raimam et al. 2007). The combination of AM fungal with bacterial inoculants was suggested to have the best effect on plant growth (Dhillion 1992; Raimam et al. 2007).
In a previous unpublished study, we inoculated five different species of AMF on rice roots grown alone under aerobic conditions. In both pot and field experiments, we observed that the total AMF colonization was commonly less than 20% of root length, much lower than that of legume and Solanaceae plants. However, we observed that when rice was intercropped with legume crops such as mung bean (Vigna radiata L.), peanut (Arachis hypogaea L.) or white clover (Trifolium repens L.), the AMF colonization on rice roots could reach 30–80%. The results in this paper show that intercropping upland rice with mung bean could promote colonization on the roots of both rice and mung bean by Glomus caledonium (Fig. 2). In general, many factors such as root density, root exudates and nutrient status of the rhizosphere, contribute to regulate colonization by AMF (Smith and Read 1997). The intercropping system did not significantly increase root density in this study (Fig. 3c,d), however, the diffusion of root exudates to the adjacent root compartment was relative free both in the nylon-net separation and no separation treatments. Since root exudates govern signaling between AMF and their host plants (Harrison 1999; Hause and Fester 2005), the additional exudates from intercropped roots would stimulate the establishment of AMF symbiosis. In addition, the intercropping enhanced depletion of N, particularly P in the culture medium due to increase of total uptake by the two plants (Fig. 5b–d). It is commonly observed that the inoculation rate of AMF on the plant roots is negatively related to the status of P availability in the rhizosphere (Smith and Read 1997; Sawers et al. 2008). Moreover, the small difference in colonization between the nylon-net separation treatment and the no separation treatment in this study suggests that traversed AM hyphae could possibly multiply the infection rates in the intercropping system.
The significant increase of mycorrhizal mung bean biomass (Fig. 3), which was about two fold more than that of the rice biomass, indicates that the mung bean may be an important route for the colonization process of the intercropping system. There might be competition between rice and mung bean during their common growth stage in the intercropping system for water and nutrients in the rhizosphere, and possible adverse effect on their photosynthesis rate due to shading. However, intercropping of upland rice with mung bean, a short-duration grain legume, has major advantages. Early stage rice offers the potential to better utilize space and nutritional resources to mung bean. During the mung bean growth stage, its ability to fix N2 led directly to a competitive advantage under N limiting condition. After mung bean was harvested, extricated space and nutritional resources, particularly N released by legume roots, could in turn improve the development of rice at the late growth stage.
Mycorrhizal hyphae play an important role not only in P acquisition, but also in transferring N in intercropping root system from mung bean to rice
It has been reported that AM fungal hyphae are the dominant routes of inorganic P acquisition from soil (or culture medium) whether the plant growth or total P uptake have been improved or not (Li et al. 2006; Smith et al. 2003, 2004). In the intercropping systems of this study, the P concentrations were significantly increased in shoots of the mycorrhizal rice, while there was no change in the shoots of the mycorrhizal mung bean (Fig. 4). The intercropping significantly enhanced P accumulation in shoots of both mycorrhizal rice and mung bean (Fig. 5).
The arbuscular mycorrhizal symbiosis can effectively transfer large amounts of nitrogen from soil to plant roots (Ames et al. 1983; Govindarajulu et al. 2005; He et al. 2003; Johansen et al. 1993). However, some earlier studies by labeling 15N in the culture medium showed that AMF colonization did not result directly in the transfer of fixed N or soil N from legume to non-legume plants, despite an AMF-stimulated increase in N fixation in legume crops (Hamel et al. 1992; Ikram et al. 1994; Reeves 1992). Hence more accurate experiments are needed to prove whether N is transferred directly or indirectly through the soil by common mycorrhizal networks (He et al. 2003; Johansen and Jensen 1996). The present study used two-way 15N-transfer through 15N shoot labeling and compartments with three different separation methods to explore the mycorrhizal function in N transfer. We showed that N-transfer in the root systems of mung bean and rice intercropping is by a two-way mechanism: from the legume to the non-legume and from the non-legume to the legume plants but with a net flux from the legume to the non-legume plant (Table 1).
In intercropping with mycorrhizal plants, N-transfer is likely to occur via re-absorption of the donor plant root released N by the roots and mycorrhizal hyphae of N-receiver plant, and via acquisition and translocation of N by a common mycorrhizal network (Johansen and Jensen 1996; He et al. 2003). In this study, the separation in the pot by 30 µm nylon-net basically prevented the direct contact of the roots but allowed hyphae penetration and linkages between the two plant species. With no AMF present, separation of the root system by the nylon-net still resulted in transfer of about 5.4% and 2.3% of 15N fed to the leaves of mung bean and rice to their intercropped counterpart, respectively (Table 1). This showed that there was N released from the both plant roots and decomposition of dead nodules and roots in the present intercropping system. The donor-released 15N diffused across the nylon net and was taken up by intercropped receiver roots. Inoculation of AMF in the rice roots did not give a significant increase of the 15N transfer from rice to mung bean, however, the mycorrhizal symbioses in the mung bean resulted in a four-fold increase of net 15N transfer from mung bean to rice (Table 1). The lack of a significant difference of N transfer between the no separation and nylon-net separation treatments (Table 1) indicates that the increased amount of N transfer resulted from AM fungus hyphal links rather than direct root-to-root connections in the intercropping system. The contribution of the N released from mung bean roots to be taken up by intercropped rice roots needs to be clarified in future by monitoring changes of labeled 15N in the root exudates. In addition, the intercropping with rice increased the total N uptake by mycorrhizal mung bean (Fig. 5b). This increase might be due to the improved N fixation by the increased numbers and nodule weight (Fig. 6).
Why did the intercropping improved growth of mung bean, but not of rice?
In the present study, AMF stimulated the growth of mung bean more than that of rice under the same cultivation condition (Fig. 3). This result coincides with the results of a recent study that showed that cowpea was the dominant crop in a rice-cowpea intercrop (Oroka and Omoregie 2007). In leguminous plants, the efficiency of N-fixation depends on root-nodule development. P has specific roles in nodule initiation, growth, and functioning (Israel 1987; Israel 1993; Olivera et al. 2004). The higher P requirement for symbiotic N fixation is internal rather than being associated with differences in the ability of roots to regulate nodule number and mass (Israel 1987). Transport of P into host plants and its release to root cells is an important function of AMF (Ryan et al. 2007; Smith et al. 2001). Previous studies showed inoculation of AMF increased nodule number, nodule weight and acetylene reduction activity of nodules and N fixation (Kawai and Yamamoto 1986; Newbould and Rangeley 1984). Our results also showed that the number of root-nodule and fresh weight in mycorrhizal mung bean were much higher than in non-mycorrhizal mung bean (Fig. 6). This shows that the formation of mycorrhizas is beneficial to formation of nodules. Growth promotion of AMF to mung bean might be ascribed to the improved plant nutrition by increasing uptake of soil P and enhancing the activity of N fixation.
In the mung bean–rice intercropping system, AM fungal inoculation increased the biomass of mung bean at the expense of rice, reducing the rice/legume dry mass ratio. This phenomenon may suggest that the establishment of AMF mycelia needs a sequestration of carbon of leaf photosynthates relatively more from rice than from mung bean (Johnson et al. 2002a; Johnson et al. 2002b). Since mung bean is a fast-growing short-life crop that last about 60–75 days and is easily colonized by AMF, the intercropping system with AM inoculation may play important roles in sequestration of elevated atmospheric CO2, activation of inactive soil P and biosynthesis of N especially in tropical and subtropical regions of the world (Gavito et al. 2000). The integrated effects of AMF inoculation in the mung bean–rice intercropping system on the yields of grains and acquisition of nutrients other than N and P need to be further clarified for continuous planting in the field.
References
Af Geijersstam L, Mårtensson A (2006) Nitrogen fixation and residual effects of field pea intercropped with oats. Acta Agricult Scand B 56:186–196
Aggarwal PK, Garrity DP, Liboon SP, Morris RA (1992) Resource use and plant interactions in a rice-mungbean intercrop. Agron J 84:71–78
Allen LH Jr, Albrecht SL, Colon-Guasp W, Covell SA, Baker JT, Pan D et al (2003) Methane emissions of rice increased by elevated carbon dioxide and temperature. J Environ Qual 32:1978–1991
Ames RN, Reid CPP, Porter LK, Cambardella C (1983) Hyphal uptake and transport of nitrogen from two 15N-labelled sources by Glomus mosseae, a vesicular–arbuscular mycorrhizal fungus. New Phytol 95:381–396 doi:10.1111/j.1469-8137.1983.tb03506.x
Barker SJ, Tagu D, Delp G (1998) Regulation of root and fungal morphogenesis in mycorrhizal symbioses. Plant Physiol 116:1201–1207 doi:10.1104/pp.116.4.1201
Bethlenfalvay GJ, Reyes-Solis MG, Camel SB, Ferrera-Cerrato R (1991) Nutrient transfer between the root zones of soybean and maize plants connected by a common mycorrhizal mycelium. Physiol Plant 82:423–432 doi:10.1111/j.1399-3054.1991.tb02928.x
Bouman BAM, Humphreys E, Tuong TP, Baker RB (2007) Rice and water. Adv Agron 92:187–237 doi:10.1016/S0065-2113(04)92004-4
Bremner JM (1965) Chapter 83: total nitrogen. In: Black CA (ed) Methods of soil analysis: part 2. chemical and microbiological properties. American Society of Agronomy, Madison, WI, pp 1149–1178
Chakraborty N, Sarkar GM, Lahiri SC (2000) Methane emission from rice paddy soils, aerotolerance of methanogens and global thermal warming. Environmentalist 20:343–350 doi:10.1023/A:1006734101607
Chalk PM (1998) Dynamics of biologically fixed N in legume–cereal rotations: a review. Aust J Agric Res 49:303–316 doi:10.1071/A97013
Clark RB, Zeto SK (2000) Mineral acquisition by arbuscular mycorrhizal plants. J Plant Nutr 23:867–902
Dhillion SS (1992) Dual inoculation of pretransplant stage Oryza sativa L. plants with indigenous vesicular–arbuscular mycorrhizal fungi and fluorescent Pseudomonas spp. Biol Fertil Soils 13:147–151 doi:10.1007/BF00337340
Fumoto T, Kobayashi K, Li C, Yagi K, Hasegawa T (2008) Revising a process-based biogeochemistry model (DNDC) to simulate methane emission from rice paddy fields under various residue management and fertilizer regimes. Glob Change Biol 14:382–402
Gao X, Kuyper TW, Zou C, Zhang FS, Hoffland E (2007) Mycorrhizal responsiveness of aerobic rice genotypes is negatively correlated with their zinc uptake when nonmycorrhizal. Plant Soil 290:283–291 doi:10.1007/s11104-006-9160-x
Gavito ME, Curtis PS, Mikkelsen TN, Jakobsen I (2000) Atmospheric CO2 and mycorrhiza effects on biomass allocation and nutrient uptake of nodulated pea (Pisum sativum L.) plants. J Exp Bot 51:1931–1938 doi:10.1093/jexbot/51.352.1931
Ghosh PK, Bandyopadhyay KK, Wanjari RH, Manna MC, Misra AK, Mohanty M et al (2007) Legume effect for enhancing productivity and nutrient use-efficiency in major cropping systems—an Indian perspective: a review. J Sustain Agric 30:61–86 doi:10.1300/J064v30n01_07
Govindarajulu M, Pfeffer PE, Jin H, Abubaker J, Douds DD, Allen JW et al (2005) Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 435:819–823 doi:10.1038/nature03610
Güimil S, Chang HS, Zhu T, Sesma A, Osbourn A, Roux C, Ioannidis V, Oakeley EJ, Docquier M, Descombes P, Briggs SP, Paszkowski U (2005) Comparative transcriptomics of rice reveals an ancient pattern of response to microbial colonization. Proc Natl Acad Sci USA 102:8066–8070
Hamel C, Furlan V, Smith DL (1991) N2-fixation and transfer in a field grown mycorrhizal corn and soybean intercrop. Plant Soil 133:177–185
Hamel C, Furlan V, Smith DL (1992) Mycorrhizal effects on interspecific plant competition and nitrogen transfer in legume-grass mixtures. Crop Sci 32:991–996
Hardarson G, Danso SKA, Zapata F (1988) Dinitrogen fixation measurements in alfalfa-ryegrass swards using nitrogen-15 and influence of the reference crop. Crop Sci 28:101–105
Harrison MJ (1999) Molecular and cellular aspects of the arbuscular mycorrhizal symbiosis. Annu Rev Plant Bio 50:361–389
Haugen LM, Smith SE (1992) The effect of high temperature and fallow period on infection of mung bean and cashew roots by the vesicular–arbuscular mycorrhizal fungus Glomus intraradices. Plant Soil 145:71–80
Hauggaard-Nielsen H, Jensen ES (2005) Facilitative root interactions in intercrops. Plant Soil 274:237–250
Hause B, Fester T (2005) Molecular and cell biology of arbuscular mycorrhizal symbiosis. Planta 221:184–196
He XH, Critchley C, Bledsoe C (2003) Nitrogen transfer within and between plants through common mycorrhizal networks (CMNs). Crit Rev Plant Sci 22:531–567
Herdler S, Kreuzer K, Scheu S, Bonkowski M (2008) Interactions between arbuscular mycorrhizal fungi (Glomus intraradices, Glomeromycota) and amoebae (Acanthamoeba castellanii, Protozoa) in the rhizosphere of rice (Oryza sativa). Soil Biol Biochem 40:660–668
Hoagland DL, Arnon DI (1950) The water culture method of growing plants without soil. Cali Agri Experi Station Cir 347
Ikram A, Jensen ES, Jakobsen I (1994) No significant transfer of N and P from Pueraria phaseoloides to Hevea brasiliensis via hyphal links of arbuscular mycorrhiza. Soil Biol Biochem 26:1541–1547
Israel DW (1987) Investigation of the role of phosphorus in symbiotic dinitrogen fixation. Plant Physiol 84:835–840
Israel DW (1993) Symbiotic dinitrogen fixation and host-plant growth during development of and recovery from phosphorus deficiency. Physiol Plant 88:294–300
Jensen ES (1996) Grain yield, symbiotic N2 fixation and interspecific competition for inorganic N in pea-barley intercrops. Plant Soil 182:25–38
Johansen A, Jensen ES (1996) Transfer of N and P from intact or decomposing roots of pea to barley interconnected by an arbuscular mycorrhizal fungus. Soil Biol Biochem 28:73–81
Johansen A, Jakobsen I, Jensen ES (1993) Hyphal transport by a vesicular–arbuscular mycorrhizal fungus of N applied to the soil as ammonium or nitrate. Biol Fertil Soils 16:66–70
John MK (1970) Colorimetric determination of phosphorus in soil and plant material with ascorbic acid. Soil Sci 11:214–220
Johnson NC, Graham JH, Smith FA (1997) Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytol 135:575–585
Johnson D, Leake JR, Ostle N, Ineson P, Read DJ (2002a) In situ 13CO2 pulse-labelling of upland grassland demonstrates a rapid pathway of carbon flux from arbuscular mycorrhizal mycelia to the soil. New Phytol 153:327–334
Johnson D, Leake JR, Read DJ (2002b) Transfer of recent photosynthate into mycorrhizal mycelium of an upland grassland: Short-term respiratory losses and accumulation of 14C. Soil Biol Biochem 34:1521–1524
Kasiamdari RS, Smith SE, Smith FA, Scott ES (2002) Influence of the mycorrhizal fungus, Glomus coronatum, and soil phosphorus on infection and disease caused by binucleate Rhizoctonia and Rhizoctonia solani on mung bean (Vigna radiata). Plant Soil 238:235–244
Kawai Y, Yamamoto Y (1986) Increase in the formation and nitrogen fixation of soybean nodules by vesicular–arbuscular mycorrhiza. Plant Cell Physiol 27:399–405
Khasa P, Furlan V, Fortin JA (1992) Response of some tropical plant species to endomycorrhizal fungi under field conditions. Trop Agri 69:279–283
Kwabiah AB (2005) Biological efficiency and economic benefits of pea–barley and pea–oat intercrops. J Sustain Agri 25:117–128
Lauk R, Lauk E (2008) Pea-oat intercrops are superior to pea–wheat and pea–barley intercrops. Acta Agricult Scand B 58:139–144
Laxminarayana K, Munda GC (2004) Performance of rice (Oryza sativa) and maize (Zea mays)-based cropping systems under mid-hills of Mizoram. Indian J Agro 49:230–232
Li XL, George E, Marschner H (1991) Extension of the phosphorus depletion zone in VA-mycorrhizal white clover in a calcareous soil. Plant Soil 136:41–48
Li H, Smith SE, Holloway RE, Zhu Y, Smith FA (2006) Arbuscular mycorrhizal fungi contribute to phosphorus uptake by wheat grown in a phosphorus-fixing soil even in the absence of positive growth responses. New Phytol 172:536–543
Lin XG, Shuguang W, Yaqin S (2001) Tolerance of VA mycorrhizal fungi to soil acidity. Pedosphere 11:105–113
Lithourgidis AS, Dhima KV, Vasilakoglou IB, Dordas CA, Yiakoulaki MD (2007) Sustainable production of barley and wheat by intercropping common vetch. Agron Sustain Dev 27:95–99
Marschner H, Dell B (1994) Nutrient uptake in mycorrhizal symbiosis. Plant Soil 159:89–102
Mårtensson AM, Rydberg I, Vestberg M (1998) Potential to improve transfer of N in intercropped systems by optimising host-endophyte combinations. Plant Soil 205:57–66
Martin RC, Astatkie T, Cooper JM (1998) The effect of soybean variety on corn-soybean intercrop biomass and protein yields. Can J Plant Sci 78:289–294
Martins MA, Cruz AF (1998) The role of the external mycelial network of arbuscular mycorrhizal fungi: III. A study of nitrogen transfer between plants interconnected by a common mycelium. Rev Microbiol 29:289–294
Mikkelsen DS, DeDatta SK (1991) Rice culture. In: Luh BS (ed) In rice production. Van Nostrand Reinhold, New York, pp 103–186
Newbould P, Rangeley A (1984) Effect of lime, phosphorus and mycorrhizal fungi on growth, nodulation and nitrogen fixation by white clover (Trifolium repens) grown in UK hill soils. Plant Soil 76:105–114
Ofosu-Budu KG, Noumura K, Fujita K (1995) N2 fixation, N transfer and biomass production of soybean cv. bragg or its supernodulating nts1007 and sorghum mixing-cropping at two rates of N fertilizer. Soil Biol Biochem 27:311–317
Olivera M, Tejera N, Iribarne C, Ocana A, Lluch C (2004) Growth, nitrogen fixation and ammonium assimilation in common bean (Phaseolus vulgaris): effect of phosphorus. Physiol Plant 121:498–505
Oroka FO, Omoregie AU (2007) Competition in a rice–cowpea intercrop as affected by nitrogen fertilizer and plant population. Scientia Agricola 64:621–629
Paszkowski U, Kroken S, Roux C, Briggs SP (2002) Rice phosphate transporters include an evolutionarily divergent gene specifically activated in arbuscular mycorrhizal symbiosis. Proc Natl Acad Sci USA 99:13324–11329
Phillip JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular arbuscular mycorrhizal fungi for rapid assessment of infection. T Brit Myco Soc 55:158–160
Purakayastha TJ, Chhonkar PK (2001) Influence of vesicular–arbuscular mycorrhizal fungi (Glomus etunicatum L.) on mobilization of zinc in wetland rice (Oryza sativa L.). Biol Fertil Soils 33:323–327
Raimam MP, Albino U, Cruz MF, Lovato GM, Spago F, Ferracin TP, Lima DS, Goulart T, Bernardi CM, Miyauchi M, Nogueira MA, Andrade G (2007) Interaction among free-living N-fixing bacteria isolated from Drosera villosa var. villosa and AM fungi (Glomus clarum) in rice (Oryza sativa). Appl Soil Ecol 35:25–34
Reeves M (1992) The role of VAM fungi in nitrogen dynamics in maize–bean intercrops. Plant Soil 144:85–92
Ryan MH, McCully ME, Huang CX (2007) Relative amounts of soluble and insoluble forms of phosphorus and other elements in intraradical hyphae and arbuscules of arbuscular mycorrhizas. Funct Plant Biol 34:457–464
Sarkar RK, Sanyal SR (2000) Production potential and economic feasibility of sesame (Sesamum indicum)-based intercropping system with pulse and oilseed crops on rice fallow land. Indian J Agro 45:545–550
Sawers RJH, Gutjahr C, Paszkowski U (2008) Cereal mycorrhiza: an ancient symbiosis in modern agriculture. Trends Plant Sci 13:93–97
Sarr PS, Khouma M, Sene M, Guisse A, Badiane AN, Yamakawa T (2008) Effect of pearl millet-cowpea cropping systems on nitrogen recovery, nitrogen use efficiency and biological fixation using the 15N tracer technique. Soil Sci Plant Nutr 54:142–147
Shen QR, Chu GX (2004) Bi-directional nitrogen transfer in an intercropping system of peanut with rice cultivated in aerobic soil. Biol Fertil Soils 40:81–87
Sieverding E, Leihner DE (1984) Influence of crop rotation and intercropping of cassava with legumes on VA mycorrhizal symbiosis of cassava. Plant Soil 80:143–146
Smith SE, Read DJ (1997) The symbionts forming VA mycorrhizas. In Mycorrhizal symbiosis, 2nd edn. Academic, London, pp 11–32
Smith SE, Dickson S, Smith FA (2001) Nutrient transfer in arbuscular mycorrhizas: How are fungal and plant processes integrated? Funct Plant Biol 28:683–694
Smith SE, Smith FA, Jakobsen I (2003) Mycorrhizal fungi can dominate phosphate supply to plants irrespective of growth responses. Plant Physiol 133:16–20
Smith SE, Smith FA, Jakobsen I (2004) Functional diversity in arbuscular mycorrhizal (AM) symbioses: The contribution of the mycorrhizal P uptake pathway is not correlated with mycorrhizal responses in growth or total P uptake. New Phytol 162:511–524
Solaiman MZ, Hirata H (1997) Responses of directly seeded wetland rice to arbuscular mycorrhizal fungi inoculation. J Plant Nutr 20:1479–1487
Sprent JI, James EK (2007) Legume evolution: where do nodules and mycorrhizas fit in? Plant Physiol 144:575–581
Toomsan B, Cadisch G, Srichantawong M, Thongsodsaeng C, Giller KE, Limpinuntana V (2000) Biological N2 fixation and residual N benefit of pre-rice leguminous crops and green manures. Neth J Agr Sci 48:19–29
Trouvelot A, Kough JL, Gianinazzi-Pearson V (1986) Mesure de taux de mycorhization VA d’un systeme radiculaire. Recherche de méthodes d’estimation ayant une signification fonctionnelle. In: Gianinazzi-Pearson V, Gianinazzi S (eds) Physiological and genetical aspects of mycorrhizae. INRA, Paris, pp 217–221
Zhang XH, Zhu YG, Chen BD, Lin AJ, Smith SE, Smith FA (2005) Arbuscular mycorrhizal fungi contribute to resistance of upland rice to combined metal contamination of soil. J Plant Nutr 28:2065–2077
Acknowledgement
This work was supported by China 863 program (2006AA10Z134), National Natural Science Foundation of China. We thank Ms. Juan Zhu and Mr. Guiyun Zhang for technical support, Dr. Xiaolin Li from China Agricultural University and Dr. Xiangui Lin from Nanjing Soil Science Institute, CAS for providing mycorrhizal fungal inoculum, Dr. Uzi Kafkafi for revising the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: F. Andrew Smith.
Rights and permissions
About this article
Cite this article
Li, Y., Ran, W., Zhang, R. et al. Facilitated legume nodulation, phosphate uptake and nitrogen transfer by arbuscular inoculation in an upland rice and mung bean intercropping system. Plant Soil 315, 285–296 (2009). https://doi.org/10.1007/s11104-008-9751-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-008-9751-9