Abstract
The rare earth elements are increasingly being used as trace supplements in different fields. In this study, subcellular distribution, the chemical forms and toxicity of cerium (Ce) were evaluated for Elodea canadensis. The effect of Ce (5–20 mg L−1) applied for 7 days was assessed by measuring changes in the nutrient elements, photosynthetic pigments, malondialdehyde and antioxidant systems. Ce accumulation was greatest in the cell walls, followed by the organelles and the soluble fraction. Ce levels were higher in cellulose and pectin than in other biomacromolecules. The toxic effects caused by Ce were shown by a reduction in photosynthetic pigments, disruption of nutrient elements, and increases in MDA content. E. canadensis shows Ce-induced oxidative stress by modulating antioxidant enzymes, such as guaiacol peroxidase and catalase. Elevated Ce levels may represent a potential risk for aquatic ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The rare earth elements (REEs) comprise a group of 15 trivalent metallic elements with similar chemical properties. REEs have been widely used in industry (electronics, petrochemical, metallurgy, machinery, energy, light industry, etc.) because of their diverse physical and chemical effects and fertilizers containing REEs (mainly La and Ce) have been used to improve crop production since the early 1990s in China. As a result, increasing quantities of REEs are discharged into the environment, which may then threaten environmental safety (Zhu et al. 1997). Although REEs are less toxic than other metals and their compounds, the long-term hazardous effect of REEs on human health is still serious (Zhu et al. 1996). REEs have also been classified as main environmental pollutants in China (National Natural Science Foundation of China 1996). Thus, it is imperative that we understand the environmental and ecological effects of REEs. Unfortunately, the results from limited laboratory studies on the effects of REEs on higher plants are inconsistent. For example, the addition of cerium nitrate (0.5–10 µM) or lanthanum nitrate (0.5–10 µM) to the culture medium significantly increased the primary root lengths of Arabidopsis thaliana (He and Loh 2000), although the mechanism behind its beneficial effects has not yet been clarified. In contrast, REEs in nutrient solutions at concentrations of 0.1–2 mg L−1 reduced root elongation in corn and mungbean (Diatloff and Smith 1995) and Hu et al. (2002) reported that 0.5–20 mg L−1 La or Ce inhibited primary root elongation in Triticum aestivum. This may due to Ce being a Ca2+ channel antagonist (Kobayashi et al. 2007). This might be attributed to plant species, growth medium, the distinct REEs and concentrations and time of action.
Increased concentrations of REEs have been detected in aquatic environments as a consequence of weathering, leaching and uncontrolled contrived release (Dia et al. 2000; Protano and Riccobono 2002; Zhang and Liu 2004). Therefore, more research emphasis needs to be placed on the distribution, bioavailability, bioaccumulation and environmental toxicity of REEs in aquatic organisms (Yang et al. 1999; Barry and Meehan 2000). However, as far as can be ascertained, very little information is available on the potential effects of REEs on aquatic plants (Yang et al. 1999; Wang et al. 2007; Xu et al. 2012).
Elodea canadensis (Waterweed), a submerged aquatic plant, is considered a model experimental system for metal accumulation and has been successfully used for pollution monitoring and phytotoxicity tests (Mal et al. 2002; Dalla Vecchia et al. 2005; Fritioff and Greger 2007; Thiébaut et al. 2010) because it is a widely distributed, well-studied species and there is evidence that it accumulates high levels of pollutants (Cd, Cu, Zn, and so on) from water and sediments (Kähkönen et al. 1997; Nyquist and Greger 2007; Mal et al. 2002). At present, no information is available on the accumulation capacity and environmental toxicity of REEs in E. canadensis. However, the acquisition of such information is essential if strategies are to be developed for the environmentally sustainable application of REES (Barry and Meehan 2000). In this study, the response of E. canadensis to cerium (Ce) was systematically investigated with reference to: (1) bioaccumulation of Ce; (2) the pattern of Ce subcellular distribution using tissue fractionation; (3) chemical forms, using sequential extraction with different solutions and (4) physiological responses to Ce-induced stress, particularly concerning oxidative stress protection and nutrient balance. The objective of this study was to elucidate the mechanisms behind Ce toxicity and to determine the potential hazards associated with the discharge of REEs into aquatic ecosystems.
Materials and methods
Plant material and experimental setup
Elodea canadensis Michaux. is native to the continental United States and Canada (Bowmer et al. 1995) and was introduced into China in the 1990s. Following its introduction, E. canadensis became widely distributed in lakes, ponds, springs and quiet streams throughout China.
Elodea canadensis was collected from Lake PiPa, Nanjing, China. Before metal treatment, plants were acclimatized for 10 day under laboratory conditions (114 μmol m−2 s−1 light irradiance, 14 h photoperiod and 25 °C/20 °C day/night) in 1/10 Hoagland solution. During exposure, eight 10-cm-long shoots without roots were kept in 2-L beakers containing different concentrations of Ce (5, 10, 15 and 20 mg L−1). The Ce solution was prepared from Ce(NO3)3·6H2O in 1/10 Hoagland solution (without KH2PO4 to avoid Ce3+ precipitation). KH2PO4 was replaced with an equivalent molar concentration of KCl (Wang et al. 2007; Hu et al. 2002). The levels of cerium nitrate selected in the present experiment were set according to the results of He and Loh (2000), Hu et al. (2002) and Wang et al. (2007). The control plants were grown in the same medium with neither Ce3+ nor PO4 3−, thus both experimental and control plants experienced the similar changes due to the lack of phosphates. Each treatment had three replicates (20 plants per replicate), and all of the solutions were changed every 2 days to maintain a rather constant Ce concentration directly available to the shoots and the plant nutrition requirements during the 7-day culture period. After harvesting, plants were washed with distilled water, and used for study of various parameters.
Tissue fractionation and Ce quantification
The subcellular distribution of Ce within the leaves was determined according to the method described by Xiong et al. (2009). In brief, plant tissues (2.0 g) were homogenized in pre-chilled extraction buffer containing 50 mM Hepes (pH 7.5), 500 mM sucrose, 1 mM dithiothreitol, 5 mM ascorbate and 1 % polyvinylpolypyrrolidone. The homogenate was centrifuged at 500g for 5 min to isolate the cell wall fraction. The supernatant of the first centrifugation step was then centrifuged at 20,000g for 45 min to sediment cell organelles and the resultant supernatant solution was referred to as soluble fraction. All steps were performed at 4 °C. The fractions of the samples were digested in a mixture of HNO3 and HClO4 (4:1 v/v) at 120 °C for at least 3 h. Concentrations of Ce in the fractions were determined by inductively coupled plasma atomic emission spectrometry (ICP-AES, Leeman labs, Prodigy, USA) after digestion with HNO3 and HClO4. The liquid standard reference material of Ce (GSB 04-1775-2004) was diluted and analyzed directly when assessing Ce content in leaves with the same conditions under strict control.
Chemical form extraction
The biomacromolecules were separated through chemical sequence extraction followed by ICP-AES analysis according to Lai et al. (2006). The first step involved grinding 2.0 g of dry plants to powder and extracting them with ether for 8 h (Soxhlet extraction) to provide a crude lipid fraction (F1). The second step involved extracting the residue generated with 30 mL boiling water for 3.5 h, and centrifuging the mixture at 800g for 10 min. The process was repeated twice and the supernatant solutions were combined after they were concentrated with a rotary evaporator under reduced pressure to generate the crude polysaccharide fraction (F2). The third step involved extracting the residue obtained from the second step with 20 mL of 0.1 M NaOH at 80 °C for 2 h, and centrifuging the mixture at 10,000g for 10 min. The process was repeated two times, and all of the supernatant solutions were combined. The supernatant solution generated is a crude protein extract (F3), and the residue comprises cellulose and pectin (F4). The concentrations of Ce in the biomacromolecules were determined using ICP-AES.
Mineral nutrient content determinations
After 7 days of treatment, leaves were washed thoroughly with 10 mmol L−1 EDTA solution at 4 °C for 30 min with stirring, followed by washing with double distilled water to remove superficial metal and nutrient ions. The contents of Ca, Mg, Fe, K and Mn were analyzed by ICP-AES.
Pigments’ concentration measurement
Chlorophylls (Chl) and carotenoids (Car) were extracted with 80 % acetone and absorbances at 470, 647, 663 and 740 nm recorded on a spectrophotometer (Thermo GENESYS 10). The contents of Chl a, Chl b and Car were estimated according to the equation \(1 2. 2 5 \; \times \; {\text{A}}_{ 6 6 3} {-} 2. 7 9\; \times \;{\text{A}}_{ 6 4 7} ,{ 21}. 50 \; \times \;{\text{A}}_{ 6 4 7} {-} 5. 10 \; \times \;{\text{A}}_{ 6 6 3}\) and \(\left( { 1,000 \; \times \; {\text{A}}_{ 4 70} {-} 1. 8 2 \; \times \;{\text{Chl }}a{-} 8 5.0 2 \; \times \;{\text{Chl }}b} \right)/ 1 9 8\), based on Lichtenthaler coefficients (Lichtenthaler 1987), respectively.
ROS (H2O2 and O ·−2 ) content assay
The rate of O ·−2 generation was measured as described by Wang and Luo (1990). In brief, fresh shoots (0.5 g) were ground with potassium phosphate buffer solution (pH 7.8, 50 mM). The extracts were centrifuged at 10,000g for 20 min at 4 °C. The supernatant (0.5 mL) was mixed with 0.5 mL 50 mM potassium phosphate buffer solution (pH 7.8) and 0.1 mL 10 mM hydroxylamine hydrochloride, and incubated at 25 °C for 1 h. 17 mM sulfanilamide and 7 mM naphthylamine were then added to the mixture, followed by 20 min incubation at 25 °C. The absorbance was measured at 530 nm with spectrophotometer (Thermo GENESYS 10). The level of H2O2 was determined using a H2O2 testing kit purchased from Nanjing Jiancheng Bioengineering Institute of Jiangsu Province, China.
Lipid peroxidation
Lipid peroxidation was estimated by determining the malondialdehyde (MDA) content according to Heath and Packer (1968). The amount of MDA present was calculated using an extinction coefficient of 155 mM cm−1. The concentration of MDA was expressed in nmol g−1 FW.
Enzyme measurement
After treatment, plant material (1 g) was put in a pre-cooled mortar, in which 0.05 M PBS buffer was added. After a grinding in ice bath, solid phase was centrifuged at 12,000g for 20 min at 4 °C. Supernatant was used to measure the activities of enzymes.
Superoxide dismutase (SOD) (EC 1.15.1.1) was measured by the modified nitrite method (Ōyanagui 1984). Superoxide anion generated by hypoxanthine and xanthine oxidase was changed to nitrite ion by hydroxylamine. The nitrite ion was measured by color densitometry at 550 nm with the aid of a coloring reagent. The degree of reduction in superoxide anion was used to represent SOD activity. 50 % inhibition of superoxide production was represented as 1 unit of SOD activity.
The guaiacol peroxidase (GPX) (EC 1.11.1.7) activity was determined using the guaiacol and hydrogen peroxide method (extinction coefficient 26.6 mM−1 cm−1) (Kraus and Fletcher 1994). The reaction mixture contained 1 mL of 200 mM NaH2PO4/Na2HPO4 buffer (pH 6.5), 25 μL of plant extract and 10 μL of 10 % (w/v) hydrogen peroxide. The increase in A 470 was followed for 2 min.
Catalase (CAT) (EC 1.11.1.6) activity was measured by assaying the H2O2-forming stable complex with ammonium molybdate (Góth 1991). One volume of extract was incubated in five volumes of reaction mixture containing 65 mM hydrogen peroxide in a 60-mM sodium–potassium phosphate buffer, pH 7.4 at 25 °C for 4 min. The enzymatic reaction was stopped with one volume of 32.4 mM ammonium molybdate and the dose of the yellow complex of molybdate and hydrogen peroxide was measured at 405 nm. One unit of CAT activity was defined as the amount of enzyme needed to decompose 1 μmol hydrogen peroxide per minute per gram fresh weight.
The activity of ascorbate peroxidase (APX) (EC 1.11.1.11) was determined according to the method of Nakano and Asada (1981) by estimating the decrease in absorbance at 290 nm (an absorbance coefficient of 2.8 mM−1 cm−1) as ascorbate was oxidized. The reaction mixture contained 50 mM potassium phosphate buffer (pH 7.0), 0.5 mM ascorbate, 0.1 mM H2O2 and 0.1 mM EDTA and 0.1 mL of enzyme extract in a total volume of 1 mL, and the enzyme at 25 °C. One unit of enzyme activity was defined as 1 mmol of ascorbate oxidized min−1 g−1 fresh weight.
Glutathione reductase (GR) (EC 1.1.4.2) activity was measured spectrophotometrically by monitoring the decrease in absorbance caused by NADPH oxidation (E = 6.2 mM−1 cm−1) at 340 nm (Tanaka et al. 1994). The reaction mixture contained 25 mM potassium phosphate buffer (pH 7.8, having 0.2 mM EDTA), 0.5 mM glutathione disulfide (GSSG), 0.12 mM NADPH and the plant extract aliquot.
Statistical analyses
All values presented were expressed as means of three replications and their standard deviations (SD). One-way analysis of variance (ANOVA) was performed with SPSS 17.0 to determine the significance of differences among means. Different letters indicate significantly different values at P < 0.05. Multiple comparisons were based on Fisher’s least significant difference (LSD) procedure.
Results
Subcellular distribution of Ce
The Ce contents of the different subcellular fractions increased significantly as the levels of Ce in the medium rose, except in the organelle fraction, which had the lowest Ce levels at the highest concentrations of exogenous Ce (Table 1). A large amount of Ce (75–81 %) was associated with the cell wall. The proportions of the total Ce found in the organelle fraction and in the soluble fraction were about 13–20 % and about 4–6 %, respectively.
Ce content in various biomolecules
As shown in Table 2, the highest Ce contents were bound to cellulose and pectin. As exogenous Ce levels rose from 5 to 20 mg L−1, the Ce contents in crude lipid, crude polysaccharides, cellulose and pectin increased significantly (P F1 < 0.01; P F2 < 0.05; P F4 < 0.05). The amount of Ce in cellulose and pectin accounted for about 84–98 % of the total Ce, which was much higher than that found in the other fractions. However, the Ce content in crude protein peaked at 10 mg L−1 and then declined gradually to levels that were lower than the control.
Nutrient elements
Increasing Ce levels in the nutrient medium led to significant reductions in Ca and Mg contents (P Ca < 0.05; P Mg < 0.05), with a maximum decrease of 43 and 34 %, respectively, when the plants were exposed to 20 mg L−1 Ce for 7 days (Table 3). Fe content tended to decline in response to increasing external Ce levels and the maximum decline was 35 % in plant leaves when they were exposed to 10 mg L−1. However, K and Mn concentrations slightly decreased up to 10 mg L−1, which were then followed by significant increases at higher concentrations, when compared to the controls.
Photosynthetic pigments
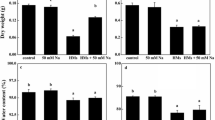
Exposure to all levels of Ce caused a sharp reduction in the contents of both chlorophyll a (Chl a) and chlorophyll b (Chl b) (Fig. 1). When the Ce concentration was 20 mg L−1, the contents of Chl a and Chl b were 21 and 29 % of that in the control plants, respectively. In addition, the carotenoids (Car) content peaked at 5 mg L−1 Ce and then showed a linear decline at higher Ce concentrations. Statistical analysis showed highly significant negative correlations between chlorophyll levels and Ce concentrations (P Chl a < 0.01; P Chl b < 0.01).
Levels of ROS (O ·−2 and H2O2)
At all Ce concentrations, the rate of O ·−2 generation was substantially higher than the value for the control treatment (Fig. 2a), with the maximum rate of O ·−2 generation being 850 % higher than the value under the control conditions. Statistical analysis showed a high significant positive correlation between the rate of O ·−2 generation and the Ce concentrations (P < 0.01). The H2O2 content increased gradually as the Ce concentration rose and peaked at 15 mg L−1 Ce (Fig. 2b). Although the H2O2 content tended to decrease at the highest concentration tested, it was still higher than the levels seen in the controls.
Lipid peroxidation
The MDA content in E. canadensis increased as the Ce levels rose (Fig. 3). When the Ce concentration reached 20 mg L−1, the MDA content was 85 % higher than under the control conditions. There was a positive linear correlation between MDA content and Ce concentration in the medium (R = 0.97, P < 0.01).
Antioxidant enzymes
The antioxidant enzymes examined in this study were: SOD, GPX, APX, CAT and GR. Enzyme activity in Ce-treated plants exhibited different responses when exposed to different levels of Ce (Table 4).
A significant concentration-dependent decline was observed in the activity of SOD, APX and GR in the leaves of E. canadensis at all concentrations after exposure for 7 days, with the maximum inhibition (44, 51 and 74 %, respectively), compared to their respective controls, occurring when the plants were exposed to 20 mg L−1 Ce (P SOD < 0.01; P APX < 0.01; P GR < 0.05).
In contrast to SOD, APX and GR activities, the activity of GPX and CAT increased progressively, compared to their respective controls, as the Ce levels rose in the culture medium (P GPX < 0.01; P CAT < 0.01). The maximum increase was recorded as 41 and 11 %, respectively, when the plants were treated with 20 mg L−1 Ce for 7 days.
Discussion
The bioaccumulation of REEs in aquatic plants is often accompanied by the induction of a variety of cellular and physiological changes, some of which directly contribute to metal tolerance capacity of the plants (Wang et al. 2007; Ippolito et al. 2010; Xu et al. 2012). In this study, Ce accumulation in E. canadensis also resulted in considerable plant physiological changes, which were observed using several biomarkers.
Due to a very thin cuticle, E. canadensis, which is a plant that remains completely submerged, can effectively absorb metals directly from the aquatic medium through its wide leaf surfaces (Dalla Vecchia et al. 2005). According to Thiébaut et al. (2010), the bioconcentration factor provides an index of a plant’s ability to accumulate a metal with respect to the metal concentration in the substrate. The calculated ratio of the accumulated Ce content in dry weight to the initial metal concentration in the water ranged from 840 (at an initial Ce concentration of 20 mg L−1) to 1,450 (at an initial Ce concentration of 5 mg L−1) in this study, which confirmed that, in the observed concentration ranges, Ce could accumulate to significant levels in E. canadensis. According to Cho et al. (1994), a fast metabolism-independent surface reaction and a slow metabolism-dependent cellular uptake are associated with the separation of dissolved metals from water using aquatic plant biomass. The former has been modeled as a diffusion process that ends when the soluble metal ions bind to the outer cell wall of the biomass and can separate significant amounts of metal within minutes, whereas the latter has modeled as a mass transfer process from the outer cell wall to the cell or cell wall interior, which may take hours or days (Axtell et al. 2003). Guo et al. (2007) find that Ce(III) can penetrate cell membranes and enter into the cells via apoplastic and symplastic channels in the leaf or via plasmodesmata, and both extra- and intracellular deposition occurs. In the present study, the proportion of Ce in different subcellular fractions remains fairly constant for all treatments, which suggests that both diffusion process and mass transfer process probably play major roles in the uptake of Ce by E. canadensis. Bioaccumulation and bioavailability of REEs are dependent on the quantities of REEs applied, the mode of application, their propensity to migrate or leach from soils and their movement in water (Barry and Meehan 2000).
In Elodea, more than half of the accumulated Cd has been reported to be in the cell wall fraction (Nyquist and Greger 2007; Dalla Vecchia et al. 2005). In the present study, the distribution patterns of Ce in the subcellular components indicated that the Ce was primarily located in the cell walls (75–81 %) (Table 1), which suggested that the cell wall played an important role in Ce accumulation and metal stress avoidance, a finding consistent with those of previous studies by Shan et al. (2003) and Lai et al. (2006), and the uptake of Ce was largely expected to be passive due to high cell wall accumulation (Lai et al. 2006; Fritioff and Greger 2007). It is well known that the cell wall can afford functional groups such as carboxylic (galacturonic acids in pectin) and hydroxyl (in cellulose) which can strongly bind metal cations in aqueous solution (Iqbal et al. 2009; Rakhshaee et al. 2009) through complexation, co-ordination, chelation and ion exchange, etc. (Keskinkan et al. 2003). In fact, the results of cytochemical tests have confirmed that the cell wall of E. canadensis was rich in hydrophilic matrix polysaccharides with high acidity degree and, in particular, in pectic substances (Dalla Vecchia et al. 2005). In leaves of E. canadensis, these chemical features of the cell walls explain the possibility to bind high amounts of Ce cations to the negative charges. Consistently, our investigation showed that cellulose and pectin had a much higher propensity to accumulate Ce than other compounds (Table 2). In addition, it has also been found that a small amount of Ce (20–25 %) can reach the cytoplasm via active transport by competing with other metals for the membrane transporters and ion channels (such as Ca2+) (Hu et al. 2002), which leads to the disturbance of a number of metabolic processes, as shown by increased lipid peroxidation (Fig. 3) and decreased Ca and Mg content (Table 3), and these responses might also result from Ce-induced P deficiency as well (Ruíz-Herrera et al. 2012).
In this study, Ce exposure resulted in a significant drop in Ca and Mg content (Table 3), which confirmed the results of Hu et al. (2002), who reported that Ca and Mg decreased with increasing concentrations of Ce in Triticum aestivum seedlings. In a previous study, La treatment also caused a decreased uptake of Ca and Mg in Hydrocharis dubia (Xu et al. 2012). The inhibition may result from the destruction of membrane stability (Hu et al. 2004) and the inhibition of H+-ATPase and Ca2+-ATPase activities in the plasma membrane in the presence of elevated concentrations of exogenous Ce (Li et al. 2003; Wang et al. 2008). With regard to Ca, because Ce has a similar ionic radius to Ca and a higher valence, Ce could have become bound to superficially located Ca absorption sites in a less reversible manner than Ca (Hu et al. 2002). As a consequence of this, Ce may interact with many Ca-dependent biological systems, resulting in toxicity or impaired function (Barry and Meehan 2000).
In this study, after 7 days of exposure to various concentrations of Ce, chlorotic areas were observed on E. canadensis leaves, which were associated with a reduction in chlorophyll content (Fig. 1). Loss of photosynthetic pigments is a common response by aquatic plants to REE treatment, which has been observed in several aquatic plants, such as Hydrilla verticillata (Wang et al. 2007), Lemna minor (Ippolito et al. 2010) and Hydrocharis dubia (Xu et al. 2012). They attributed the reduction in the chlorophyll content to the REE-induced changes in chloroplast ultrastructure (Xu et al. 2012) and the disturbance in chlorophyll biosynthesis or degradation caused by lipid peroxidation (Wang et al. 2007; Xu et al. 2012). These results were also confirmed by the results obtained in this study. They showed that Ce treatment resulted in a significant Mg2+ deficiency (Table 3), which is required for the synthesis of chlorophylls and lipid peroxidation (Fig. 3). The dose-dependent relationship between chlorophyll and heavy metals has been used to determine the 50 % effective concentration (EC50) and the maximum permissible concentration (MPC) of a given toxicant (Mohan and Hosetti 2006). In this investigation, the calculated EC50 and MPC of Ce, with regard to E. canadensis, were 3.6 and 0.36 mg L−1, respectively, after 7 days of exposure.
Malondialdehyde, a decomposition product of polyunsaturated fatty acids, has been utilized as a suitable biomarker for lipid peroxidation (Mittler 2002). In this study, the significant increase in MDA content indicated that Ce had initiated lipid peroxidation in the leaves of E. canadensis (Fig. 3). As Ce is not a redox transition metal that leads to oxidative damage directly (such as Cu, Zn and Fe), the oxidative stress induced by Ce in the leaves of E. canadensis could be an indirect result of the over-production of ROS levels (Fig. 2) and the inhibition of antioxidant enzymes (SOD, APX and GR) (Table 4). These results are in agreement with those observed in Hydrilla verticillata (Wang et al. 2007), Lemna minor (Ippolito et al. 2010) and Hydrocharis dubia (Xu et al. 2012).
In the present study, various antioxidant enzymes showed different change in their activity. A remarkable increase in GPX and CAT was evident in E. canadensis plants at all Ce concentrations, which was in contrast to the significant inhibition of SOD, APX and GR, which might be attributed to the inhibition or/and even damage of APX and SOD (probably the Cu/Zn and Fe-SOD) protein by ROS in high concentration of Ce. The stimulation of some antioxidants (e.g., GPX, GR or CAT) has been noted in several aquatic plants subjected to REEs and is commonly considered as a stress marker without any positive physiological role in the stress responses (Wang et al. 2007; Ippolito et al. 2010; Xu et al. 2012). Indeed, Ce did cause significant oxidative damage, according to some stress indicator levels, such as increased lipid peroxidation levels (Fig. 3) and lower chlorophyll contents (Fig. 1). These results suggest that the tolerance capacity of E. canadensis to the toxic metals depends on the equilibrium between the production of ROS and the quenching activity of the antioxidants. However, in the case of ROS, our results are much higher than that of earlier studies by Wang et al. (2007) and Ippolito et al. (2010), which can be attributed to the different methods used by different researchers.
In summary, the results of this study suggested that Ce could enter into the cytoplasm of E. canadensis via the plasma membrane. This subsequently causes a series of alterations to different metabolic pathways, such as photosynthesis, nutrient balance or oxidative mechanisms. Ce bioaccumulation in E. canadensis resulted in significant oxidative stress (increase in MDA) and nutrient imbalance (Ca and Mg). This indicated that the toxic effects of REEs were similar to those of other trace metals. In the present study, the MPC of Ce for E. canadensis relative to environmental safety was suggested to be 0.36 mg L−1 in aquatic environment.
Author contribution
W. Y. Chu and S. J. Cai designed the experiment, W. Y. Chu and S. J. Cai performed tissue fractionation, chemical forms’ extraction and Ce quantification. W. Y. Chu analyzed data and wrote the manuscript. W. Y. Chu and H. Qiu performed mineral nutrient content determination experiment. S. J. Cai, F. F. Li and T. Xu performed enzyme, ROS content and pigments’ concentration assay experiment. Q. S. Xu designed the experiment, supervised the research and revised the manuscript before submitting it to the journal.
References
Axtell NR, Sternberg SPK, Claussen K (2003) Lead and nickel removal using Microspora and Lemna minor. Bioresour Technol 89:41–48
Barry MJ, Meehan BJ (2000) The acute and chronic toxicity of lanthanum to Daphnia carinata. Chemosphere 41:1669–1674
Bowmer KH, Jacobs SWL, Sainty GR (1995) Identification, biology and management of Elodea canadensis, Hydrocharitaceae. J Aquat Plant Manag 33:13–19
Cho DY, Lee S, Park S, Chung A (1994) Studies on the biosorption of heavy metals onto Chlorella vulgaris. J Environ Sci Health A 29:389–409
Dalla Vecchia F, Rocca NL, Moro I, De Faveri S, Andreoli C, Rascio N (2005) Morphogenetic, ultrastructural and physiological damages suffered by submerged leaves of Elodea canadensis exposed to cadmium. Plant Sci 168:329–338
Dia A, Gruau G, Olivié-Lauquet G, Riou C, Molénat J, Curmi P (2000) The distribution of rare earth elements in groundwaters: assessing the role of source-rock composition, redox changes and colloidal particles. Geochim Cosmochim Acta 64:4131–4151
Diatloff E, Smith FW (1995) Rare earth elements and plant growth: I. Effects of lanthanum and cerium on root elongation of corn and mungbean. J Plant Nutr 18:1963–1976
Fritioff Å, Greger M (2007) Fate of cadmium in Elodea canadensis. Chemosphere 67:265–375
Góth L (1991) A simple method for determination of serum catalase and revision of reference range. Clin Chim Acta 196:143–152
Guo XS, Zhou Q, Lu TH, Dang M, Huang XH (2007) Distribution and Translocation of 141Ce (III) in horseradish. Ann Bot 100:1459–1465
He YW, Loh CS (2000) Cerium and lanthanum promote floral initiation and reproductive growth of Arabidopsis thaliana. Plant Sci 159:117–124
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts I. Kinetic and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hu X, Ding ZH, Chen YJ, Wang XR, Dai LM (2002) Bioaccumulation of lanthanum and cerium and their effects on the growth of wheat (Triticum aestivum L.) seedlings. Chemosphere 48:621–629
Hu Z, Richter H, Sparovek G, Schnug E (2004) Physiological and biochemical effects of rare earth elements on plants and their agricultural significance: a review. J Plant Nutr 27:183–220
Ippolito MP, Fasciano C, d’Aquino L, Morgana M, Tommasi F (2010) Responses of antioxidant systems after exposition to rare earths and their role in chilling stress in common duckweed (Lemna minor L.): a defensive weapon or a boomerang? Arch Environ Contam Toxicol 58:42–52
Iqbal M, Saeed A, Zafar SI (2009) FTIR spectrophotometry, kinetics and adsorption isotherms modeling, ion exchange, and EDX analysis for understanding the mechanism of Cd2+ and Pb2+ removal by mango peel waste. J Hazard Mater 164:161–171
Kähkönen MA, Pantsar-Kallio M, Manninen PKG (1997) Analysing heavy metal concentrations in the different parts of Elodea canadensis and surface sediment with PCA in two boreal lakes in southern Finland. Chemosphere 35:2645–2656
Keskinkan O, Goksu MZL, Yuceer A, Basibuyuk M, Forster CF (2003) Heavy metal adsorption characteristics of a submerged aquatic plant (Myriophyllum spicatum). Process Biochem 39:179–183
Kobayashi Y, Ikka T, Kinura K, Yasuda O, Koyama H (2007) Characterisation of lanthanum toxicity for root growth of Arabidopsis thaliana from the aspect of natural genetic variation. Funct Plant Biol 34:984–994
Kraus TE, Fletcher RA (1994) Paclobutrazol protects wheat seedlings from heat and paraquat injury. Is detoxification of active oxygen involved? Plant Cell Physiol 35:45–52
Lai Y, Wang QQ, Yang LM, Huang BL (2006) Subcellular distribution of rare earth elements and characterization of their binding species in a newly discovered hyperaccumulator Pronephrium simplex. Talanta 70:26–31
Li YH, Yan CL, Liu JC, Chen YH, Hu J, Xue B (2003) Effects of La3+ on ATPase activities of plasma membrane vesicles isolated from Casuarina equisetifolia seedlings under acid rain stress. J Rare Earth 21:675–679
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic membranes. Method Enzymol 148:350–382
Mal TK, Adorjan P, Corbett AL (2002) Effect of copper on growth of an aquatic macrophyte, Elodea canadensis. Environ Pollut 120:307–311
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Mohan BS, Hosetti BB (2006) Phytotoxicity of cadmium on the physiological dynamics of Salvinia natans L. grown in macrophyte ponds. J Environ Biol 27:701–704
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
National Natural Science Foundation of China (1996) Environmental chemistry. Science Press, Beijing
Nyquist G, Greger M (2007) Uptake of Zn, Cu and Cd in metal loaded Elodea canadensis. Environ Exp Bot 60:219–226
Ōyanagui Y (1984) Reevaluation of assay methods and establishment of kit for superoxide dismutase activity. Anal Biochem 142:290–296
Protano G, Riccobono F (2002) High contents of rare earth elements (REEs) in stream waters of a Cu–Pb–Zn mining area. Environ Pollut 117:499–514
Rakhshaee R, Giahi M, Pourahmad A (2009) Studying effect of cell wall’s carboxyl–carboxylate ratio change of Lemna minor to remove heavy metals from aqueous solution. J Hazard Mater 163:165–173
Ruíz-Herrera LF, Sánchez-Calderón L, Herrera-Estrella L, López-Bucio J (2012) Rare earth elements lanthanum and gadolinium induce phosphate-deficiency responses in Arabidopsis thaliana seedlings. Plant Soil 353:231–247
Shan XQ, Wang HO, Zhang SZ, Zhou HF, Zheng Y, Yu H, Wen B (2003) Accumulation and uptake of light rare earth elements in a hyperaccumulator Dicranopteris dichotoma. Plant Sci 165:1343–1353
Tanaka K, Sano T, Ishizuka K, Kitta K, Kawamura Y (1994) Comparison of properties of leaf and root glutathione reductases from spinach. Physiol Plant 91:353–358
Thiébaut G, Gross Y, Gierlinski P, Boiché A (2010) Accumulation of metals in Elodea canadensis and Elodea nuttallii: implications for plant-macroinvertebrate interactions. Sci Total Environ 408:5499–5505
Wang AG, Luo GH (1990) Quantitative relation between the reaction of hydroxylamine and superoxide anion radicals in plants. Plant Physiol Commun 6:55–57
Wang X, Shi GX, Xu QS, Xu BJ, Zhao J (2007) Lanthanum- and cerium-induced oxidative stress in submerged Hydrilla verticillata plants. Russ J Plant Physl 54:693–697
Wang LH, Huang XH, Zhou Q (2008) Effects of rare earth elements on the distribution of mineral elements and heavy metals in horseradish. Chemosphere 73:314–319
Xiong J, An LY, Lu H, Zhu C (2009) Exogenous nitric oxide enhances cadmium tolerance of rice by increasing pectin and hemicellulose contents in root cell wall. Planta 230:755–765
Xu QS, Fu YY, Min HL, Cai SJ, Sha S, Cheng GY (2012) Laboratory assessment of uptake and toxicity of lanthanum (La) in the leaves of Hydrocharis dubia (Bl.) Backer. Environ Sci Pollut Res 19:3950–3958
Yang XY, Yin DQ, Sun H, Wang XR, Dai LM, Chen YJ, Cao M (1999) Distribution and bioavailability of rare earth elements in aquatic microcosm. Chemosphere 39:2443–2450
Zhang J, Liu CQ (2004) Major and rare earth elements in rainwaters from Japan and East China Sea: natural and anthropogenic sources. Chem Geol 209:315–326
Zhu WF, Xu SQ, Zhang H, Shao PP, Wu DS, Yang WJ, Feng J (1996) Investigation on the intelligence quotient of children in the areas with high REE background (I)-REE bioeffects in the REE-high areas of southern Jiangxi province. Chin Sci Bull 41:1977–1981
Zhu W, Kennedy M, de Leer EWB, Zhou H, Alaerts GJFR (1997) Distribution and modeling of rare earth elements in Chinese river sediments. Sci Total Environ 204:233–243
Acknowledgments
This research was supported by the National Natural Science Foundation of China (No. 31170162), A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and sponsored by Qing Lan Project. Ce samples were analyzed by Nanjing Normal University Center for Analysis and Testing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Krolicka.
Rights and permissions
About this article
Cite this article
Chu, W.Y., Cai, S.J., Fu, Y.Y. et al. The toxicity of cerium nitrate to Elodea canadensis: subcellular distribution, chemical forms and physiological effects. Acta Physiol Plant 36, 2491–2499 (2014). https://doi.org/10.1007/s11738-014-1622-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-014-1622-9