Abstract
Purpose
Fine root decomposition plays an essential role in the nutrient cycle and energy transfer in terrestrial ecosystems, and changes in decomposition induced by nitrogen (N) deposition have become a global concern. However, patterns of fine root decomposition with N application are still scattered, and the dominant factors regulating decomposition are still controversial. Here, we aimed to explore general patterns and key drivers of decomposition in temperate forests with N application.
Methods
From 20 studies, we synthesized 123 records of fine root decomposition in temperate forests where N was applied. We explored the overall effect of decomposition with N application and the variation in decomposition among N application rates, N forms, fertilization condition of root growth and decomposition (FF, from fertilized to fertilized conditions, and UFF, from unfertilized to fertilized conditions), tree functional types and soil depth. The dominant factors of decomposition were identified using regression.
Results
Our results showed that N application decreased fine root decomposition. Specifically, decomposition decreased at the application rate of 100–150 kg N ha‒1 yr‒1, under NH4NO3 application, in broadleaf trees and in deep layers, attributable to the inhibited microbial enzyme activity. Decomposition decreased in FF, likely resulting from home-field advantage (HFA) effects. Multiple regressions showed that initial lignin content was the most important factor determining decomposition.
Conclusion
Our results suggested that inhibited microbial enzymes were associated with decreased decomposition under N application in temperate forests. Additionally, our results confirmed the importance of initial root traits, such as lignin, in regulating decomposition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Inputs of atmospheric nitrogen (N) into terrestrial ecosystems have sharply increased in the past thirty years (Reay et al. 2008). For example, temperate forests received approximately 20 kg N ha‒1 yr‒1 during 2000–2010 (Ackerman et al. 2019; Yu et al. 2019). Studies indicate that N deposition can substantially affect carbon (C) flow, particularly decomposition in terrestrial ecosystems (Hou et al. 2020; Song et al. 2015). While extant observations of decomposition under N deposition have mainly focused on aboveground tissues (e.g., leaf litter), little effort was directed to fine roots (Dong et al. 2019; Li et al. 2019; Ye et al. 2019). Fine roots, usually referring to roots ≤ 2 mm in diameter (Chen and Brassard 2012; Wang et al. 2020), and are key contributors to the terrestrial C cycle (Kou et al. 2018). Studies of fine root decomposition under N deposition are essential to predict the impact of N deposition on C dynamics in terrestrial ecosystems (Fan and Guo 2010; Sun et al. 2015).
Research has shown both inhibitory and promotion effects of N application on fine root decomposition (Argiroff et al. 2019; Jiang et al. 2018; Li et al. 2016). Study has attributed the inhibitory effect of N application on decomposition to the role that N plays in dampening the release of root exudates to the rhizosphere (Sun et al. 2016). This appears to reduce the energy required by the decomposers to synthesize extracellular enzymes that degrade C components (Kuzyakov et al. 2007). In addition, studies report that the inhibitory effect of N application on decomposition may also relate to changes in substrate chemistry (Helmisaari et al. 2008; Tu et al. 2015). For example, increasing the P and decreasing the C:P ratio during decomposition that could not meet the needs of microbial populations may result in reduced decomposition (Jing et al. 2019). However, studies have also found that exogenous N can stimulate the decomposition rate of fine roots (Berg 2014; Dong et al. 2020). The promotional effect of N application on fine root decomposition may be due to changes in the soil microbial community structure, pH, available N and other soil characteristics (Dong et al. 2020; Sun et al. 2016). This, in turn, can stimulate the production of hydrolytic enzymes, which increases the degradation of hemicellulose and cellulose (Berg 2014; Waldrop et al. 2004). So far, we still do not have an overall understanding of the various factors driving N decomposition under N application, and that we need to better investigate how the driving factors interacts in a wide range of climatic and soil conditions.
Numerous studies have found that experimental factors can affect fine root decomposition under N deposition (Gholz et al. 2000; Jing et al. 2019; Silva et al. 2019). For example, the rate of N application can affect the decomposition of fine roots, with promotion and inhibition effects found at low (e.g., 30 kg N ha−1 yr−1) and high (e.g., 90 and 120 kg N ha−1 yr−1) N application rates, respectively (Jiang et al. 2018; Mao et al. 2011; Nadelhoffer 2000; Song et al. 2017). Additionally, the form of N could play an important role in study outcomes. Research has shown that inorganic N (e.g., NH4NO3) inhibits the decomposition of fine roots, which occurs as a result of its inhibitory effect on ligninolytic enzyme activity (Kou et al. 2018; Song et al. 2017; Tu et al. 2015). However, research shows that organic N (e.g., urea) mildly stimulates fine root decomposition as a result of its positive effect on the production and activity of hydrolytic enzymes (Dong et al. 2020; Hobbie et al. 2012). In addition, studies have found that the decomposition rate of fine roots is greatest when mixtures of organic and inorganic N forms are added (Dong et al. 2020). Compared to the application of only organic or inorganic N, the application of mixed N forms can meet the needs of a diverse decomposer community with special preferences (Hobbie 2005; 2015).
In addition to experimental factors, root decomposition under N application may also be affected by environmental factors, soil characteristics and root traits, such as the mean annual temperature (MAT), soil pH values, and root lignin content (Berg 2014; Castle et al. 2017; Peng et al. 2017). MAT positively affects root decomposition through its substantial impacts on microbial activities (Kirschbaum 2006; See et al. 2019). Moreover, MAT affects decomposition via its induced changes in TN and C:N in litter (Zhang et al. 2008). Soil pH positively correlates with decomposition, which may be linked to promoted soil enzyme activities with increasing pH (Castle et al. 2017; Sinsabaugh et al. 2008). Lignin, a group of complex aromatic polymers that exist in plant cell walls, usually acts as a structural barrier preventing microorganisms from obtaining labile C compounds and thus resisting enzymatic degradation (Austin and Ballare 2010). Nevertheless, the dominant factors regulating fine root decomposition under N application remain unclear.
With increasing N deposition, temperate forests have been proven to be the strongest C sink in temperate terrestrial ecosystems (Galloway et al. 2008; Yu et al. 2014). Despite the essential roles of fine roots in the C cycles, our understanding of the C budget of temperate forests based on fine root decomposition under N application remains unclear (Kou et al. 2018). Here, we conducted a meta-analysis to study the effect of N application on fine root decomposition in temperate forests using data from 20 peer-reviewed publications. Based on the fertilization condition of root growth and decomposition, we partitioned decomposition into two groups: FF (from fertilized to fertilized conditions) and UFF (from unfertilized to fertilized conditions). Roots were harvested either under UF (unfertilized) or F (fertilized) conditions; then, for decomposition, UF roots were incubated under either UF (UF UF) or F conditions (UF F) (first: growing condition; second: decomposition condition), whereas F roots were incubated only under F conditions (F F). For FF, N was added under both growth and decomposition conditions, while for UFF, N was added only under decomposition conditions. We aimed to (1) examine the general patterns of the responses of fine root decomposition to N application in temperate forests; (2) explore how different N application rates, N forms, FF vs. UFF, tree functional types and soil depth influence fine root decomposition in response to N application; and (3) identify the key drivers of fine root decomposition. We hypothesized that (1) N application reduces the decomposition of fine roots, resulting from inhibited activity of ligninolytic enzymes (Song et al. 2017; Weand et al. 2010) or decreased microbial biomass and fungi to bacteria ratio (Cheng et al. 2019), and that (2) experimental factors have large impacts on fine root decomposition. For example, medium N application rates (100–150 kg N ha−1 yr−1) may have a significant effect on decomposition because of altered acid-unhydrolysable residue (AUR) (Kou et al. 2015); and (3) initial root traits, such as lignin content, which regulate decomposition. Lignin is related to structural protection from microbial degradation, which generally slows the decomposition process (Austin and Ballare 2010).
Methods
Data sources
We compiled 123 independent data points from 20 studies that were published between 2004 and 2019 using Web of Science, Google Scholar and China National Knowledge Infrastructure (CNKI) (supplementary material). We used the search string: (fine root OR fine roots) AND (decomposition OR decay OR breakdown) AND (simulated N deposition OR N application OR N additions). Observations that met the following criteria were selected in our analysis (Fig. S1): (1) experiments were conducted in temperate forest ecosystems with all kinds of roots having diameters ≤ 2.0 mm (Figs. 1 and S2) (Ferreira et al. 2015); (2) both fertilized and unfertilized plots were established with consistent biotic and abiotic conditions; (3) at least one of the variables was measured and did not include modelled values; (4) only unfertilized and N fertilized data were selected (other experimental factors were excluded); (5) either the decomposition rate or mass loss of fine roots over a known duration was reported; and (6) data were collected once if reported by multiple publications. Studies were considered independent based on the following criteria: (1) studies conducted at different sites and with different N application rates; (2) discrete studies at the same site (LeBauer and Treseder 2008; Liu and Greaver 2010); (3) different species in a study; or (4) a study including several experiments under various abiotic conditions, such as different locations, N application rates and soil layers (Chen et al. 2019). Our 123 records met at least one of the above criteria and thus were assumed to be independent. The 123 records are from the upper midwestern part of the USA or in China. There may be some latitudinal and possibly phylogenetic bias, which needs further study with more data in temperate forests.
We collected four categories of factors: environmental (including MAT, mean annual precipitation (MAP), latitude and longitude), experimental factors (including soil depth, decomposition duration, N application rate, fertilization frequency, mesh size and initial root mass), soil characteristics (including initial pH, N:P, TN and TP), and initial root traits (including lignin, N, P, N:P, C:P, C:N and cellulose). When the data were reported graphically, we used GetData Graph Digitizer v.2.24 (http://getdata-graph-digitizer.com/) to extract data values.
We either directly collected MAT and MAP when they were reported or indirectly extracted them if not reported from the Global Climate database (http://www.worldclim) using the latitudes and longitudes of the study areas. MAT ranged from 2.0 to 17.9 °C, and MAP ranged from 66 to 1490 mm in these studies. N application rates varied from 5 to 300 kg N ha‒1 yr‒1, and we grouped them into < 50, 50–100, 100–150 and ≥ 150 kg N ha‒1 yr‒1 (Tian and Niu 2015). Different forms of N were applied, including NH4NO3, NH4Cl, urea and NaNO3. In the lnRR calculation, for UF F, lnRR = ln (X UF F/X UF UF) = ln (X UF F) − ln (X UF UF), and for F F, lnRR = ln (X F F/X UF UF) = ln (X F F) − ln (X UF UF). The same initial roots were incubated in the F and UF treatments. Tree functional types were divided into broadleaf and conifer trees based on leaf morphological and phenological traits (Wu et al. 2017). Soil depth was categorized into surface (0–10 cm) and deep layers (0–20, 0–30 and > 30 cm).
Statistical analysis
We estimated decomposition coefficients based on the fine root mass remaining during the decomposition period if the data were not directly reported. Negative exponential models were used for coefficient estimation (Berg 2014; Olson 1963): mt/m0 = e−k t, where mt is the residual mass of fine roots at time t (years), m0 is the fine root mass at the beginning of the experiment, and k is the decomposition coefficient (per year). We used the natural log response ratio (lnRR) as the effect size to assess the response of fine root decomposition to N application (Zheng et al. 2019): lnRR = ln (Xt/Xc) = ln (Xt) − ln (Xc), where Xt and Xc are the mean of the fertilized and unfertilized groups, respectively. We estimated the linear relationship between lnRR and continuous predictors by comparing linear and log-linear responses (Chen et al. 2019). However, in our database, sampling variances were not reported in 4 of the 20 studies. More importantly, weighting based on sampling variances would assign extreme importance to some individual observations (Ma and Chen 2016). Similar to previous research (Pittelkow et al. 2015), we used the number of replicates for calculating weighting: Wr = Nt Nc/(Nt + Nc), where Wr is the weight for each observation and Nt and Nc are the replicates of observations in fertilized and unfertilized groups, respectively. We compared linear and log-linear responses to assess the assumption of linearity between continuous predictors and lnRR.
Our analyses used stepwise multiple regressions to identify the relationship between the fine root decomposition rate and the four factor categories. In cases where the number of common points in each category was insufficient, factors that significantly correlated with the log response ratio were used in a stepwise regression of each category except environmental factors (including MAT, MAP, latitude and longitude). The stepwise multiple regression analysis had two steps: (1) factors in each category were contained in the analysis (Models A1–4), and (2) we ran analyses of all variables included in Model A (Model B). We used the Akaike information criterion (AIC), which provides information about the likelihood of a model being significant for the given data and its parameterization, to compare the likelihood of competing models. When comparing two alternative models, a lower AIC is more likely (Bond-Lamberty et al. 2018; Manning et al. 2008). All statistical analyses were performed in R 3.5.1.
Results
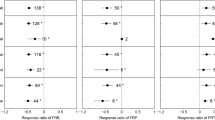
In general, our analyses showed that the exogenous N application decreased the decomposition of fine roots (p < 0.01; Fig. 2a). Decomposition decreased substantially at the application rate of 100–150 kg N ha−1 yr−1 (p < 0.01; Fig. 2b), and it was not significantly affected by the other application rates (all p > 0.05; Fig. 2b). NH4NO3 reduced the decomposition of fine roots (p < 0.05; Fig. 2c), but the effects of the other forms of N fertilizers on decomposition were not significant (all p > 0.05; Fig. 2c).
The overall effect of N application on the fine root decomposition rate (a) and the effects of N application on decomposition changes with N application rate (b) and N form (c). The N application rates were < 50, 50–100, 100–150 and ≥ 150 kg N ha−1 yr−1. N forms include NH4NO3, NH4Cl, urea and NaNO3. The sample size is indicated next to each attribute, and error bars indicate 95% confidence intervals. *: p < 0.05, **: p < 0.01
Decomposition decreased in FF (p < 0.01; Fig. 3a) but did not significantly change in UFF (p > 0.05; Fig. 3a). Among the tree functional types, broadleaf trees experienced a decrease in fine root decomposition with N application (p < 0.05; Fig. 3b), but conifer trees did not experience a decrease (p > 0.05; Fig. 3b). While N application did not change fine root decomposition in the surface soil layers (p > 0.05; Fig. 3c), it decreased decomposition in the deep soil layers (p < 0.01; Fig. 3c). The decomposition bag and mesh sizes did not significantly affect decomposition (all p > 0.05; Fig. 4).
Effect of N application on fine root decomposition rate for FF vs. UFF (a), different tree functional types (b), and soil layers (c). FF: from fertilized conditions to fertilized conditions and UFF: from unfertilized conditions to fertilized conditions. The sample size is indicated next to each attribute, and error bars indicate 95% confidence intervals. *: p < 0.05, **: p < 0.01
Fine root decomposition in response to N application was influenced by both environmental and experimental factors and initial soil characteristics and root traits (measured before N application in the decomposition experiments). The response ratios of fine root decomposition were positively correlated with MAT (p < 0.05; Fig. 5a) and initial soil pH (p < 0.001; Fig. 5d) and negatively correlated with soil depth (p < 0.001; Fig. 5b), decomposition duration (p < 0.05; Fig. 5c), initial soil N:P (p < 0.05; Fig. 5e), initial root lignin (p < 0.01; Fig. 5f), N (p < 0.01; Fig. 5g) and P (p < 0.01; Fig. 5h). We found no significant correlations of the response ratios of fine root decomposition with MAP, N application rate, frequency, mesh size, initial root mass, initial soil total N (TN), soil total P (TP), initial root N:P, C:P, C:N, cellulose, the response ratios of root N, P or initial root mass per unit area of bags (all p > 0.05; Fig. S3).
The relationships between the response ratios of fine root decomposition and environmental factors: (a) MAT, experimental factors: (b) soil depth, and (c) decomposition duration; soil characteristics: (d) initial soil pH and (e) initial soil N:P; and initial root traits: (f) lignin, (g) N and (h) P in N application experiments. In Fig. 5f-h, others indicate herbs. *: p < 0.05, **: p < 0.01, ***: p < 0.001
Multiple regression analyses showed that the fine root decomposition response to N application was affected by MAT (p < 0.05; Table 1), initial soil pH (p < 0.01; Table 1), soil depth (p < 0.001; Table 1) and initial root lignin (p < 0.01; Table 1) within each category. Further analysis showed that initial root lignin was the most important factor regulating fine root decomposition responses to the N application (p < 0.01; Table 1).
Discussion
Effects of N application on fine root decomposition
Consistent with previous findings (Carreiro et al. 2000; Gholz et al. 2000), our results showed that N application decreased fine root decomposition in temperate forest ecosystems. Decreases mainly resulted from the suppression of lignin degradation — the activity of ligninolytic enzymes was inhibited (Song et al. 2017; Tu et al. 2015). Phenol oxidase, a critical lignin-degrading enzyme (Hobbie et al. 2012; Kellner et al. 2008), was found to significantly decrease under N application (Fig. 6). Moreover, we found that phenol oxidase was positively correlated with the root decomposition rate (Fig. 6). Fine roots are usually composed of lignin-rich substances in temperate forests (Rasse et al. 2005; Xia et al. 2015). Therefore, the inhibition of lignin degradation by N application leads to a negative effect of N application on fine root decomposition in temperate forests. These results indicated that fine root decomposition significantly changed under N application experiments, which likely affected ligninolytic enzymes, but further research is needed to fully understand the impact of N application on ligninolytic enzymes, especially phenol oxidase. Additionally, under conditions that support nitrification, N input could significantly increase the loss of soil TN, NO3− and cations from the soil solution and increase H+ in the soil through NH4+ nitrification (Conn and Day 1996), leading to a significant decrease in the root decomposition rate (Kou et al. 2018; Manning et al. 2008).
Effects of the N application rate and N form on decomposition
Our results demonstrated the inhibition of decomposition when the N application rate was 100–150 kg N ha−1 yr−1, but no significant inhibition or promotion effects occurred at other greater or lesser application rates. Kou et al. (2018) suggested that the rate of N application is one of the factors affecting fine root decomposition. Inhibition effects at an application rate of 100–150 kg N ha−1 yr−1 might result from inhibited microbial enzyme activity (Sun et al. 2016) and increased concentrations of AUR in fine roots that reduced decomposition (Kou et al. 2015). Our results showed that fine root decomposition decreased when the N application rate was 100–150 kg N ha‒1 yr‒1, considering that the globally relevant range of N deposition values is from 0 to 50 kg N ha‒1 yr‒1, which may not affect fine root decomposition in terrestrial ecosystems (for example, temperate forests). However, with a continuous increase in N deposition, the decomposition of fine roots may decrease in future terrestrial ecosystems. On the other hand, our results showed that N application decreased fine root decomposition when NH4NO3 was applied, while other forms of N had no significant effect on decomposition. Previous studies have proposed that the application of NH4NO3 reduces fine root decomposition through (1) the suppression of ligninolytic enzyme activity (Song et al. 2017; Tu et al. 2015) and (2) an increase in the combination of inorganic N ions with AUR in decomposing roots (Jiang et al. 2018). Other forms of N fertilizers had no significant effects on decomposition, but this was likely due to the limited number of observations leading to high variation (low statistical power).
Effects of N application on decomposition differed with fertilization condition of root growth and decomposition
The lnRR of fine root decomposition showed a decrease in FF but not in UFF. Evidence is growing that litter usually decomposes faster in its native habitat than in other habitats (Freschet et al. 2012). The vast majority of these studies indicate that home-field advantage (HFA) effects exist in leaf litter decomposition (Gholz et al. 2000; Lin et al. 2020). However, some studies show that a HFA also exists in fine root decomposition (Freschet et al. 2012; Jacobs et al. 2018), which may also be applicable to our results. With respect to FF, the decomposition in the fertilized groups decreased with N application, while decomposition in the unfertilized groups occurring under their original conditions changed little, leading to a general decrease in lnRR. For UFF, decomposition decreased in not only the fertilized group but also the unfertilized group, resulting in nonsignificant effects of N application on fine root decomposition in UFF. Another possible reason for this outcome is that fertilized roots have a different initial chemical composition than that of unfertilized roots since N application would increase the N content and decrease the C content (Li et al. 2015). N application can increase nitrate availability to roots, which causes the roots to absorb more N and store it in fine root tissue (Nadelhoffer 2000; Reay et al. 2008). This, in turn, can inhibit decomposition (Li et al. 2015). Additionally, cost–benefit theory indicates that less C is devoted to roots when soil resource availability is high, which may lead to a decrease in decomposition under N application (Gough et al. 2004; Wang et al. 2012).
Effects of N application on decomposition in relation to tree functional types and soil depth
We found that N application reduced decomposition in broadleaf trees but had no significant impacts on decomposition in conifer trees. Fine roots of broadleaf trees usually have a high N content compared with that of conifer trees (Silver and Miya 2001). Previous studies have shown that a high N content may inhibit the synthesis and activity of ligninolytic enzymes (e.g., phenol oxidase) or convert lignin into other compounds that are resistant to degradation, ultimately inhibiting decomposition (Kou et al. 2018; Tu et al. 2015). Additionally, our results showed that N application inhibited fine root decomposition in the deep soil layers but not in the surface soil layers. Phenol oxidase in the deep soil layer is inhibited under N application (Jian et al. 2016). This slows lignin degradation, ultimately leading to a decrease in the rate of decomposition in deep soil layers (Hobbie et al. 2012; Sinsabaugh et al. 2009).
Effects of N application on decomposition in relation to mesh and bag sizes
Neither bag size nor mesh size had significant impacts on the response ratios of decomposition. Mesh size affects decomposition by interfering with decomposition processes (Heinemeyer et al. 2007; Maillard et al. 2021). For example, larger organisms (e.g., microarthropods) that carry microbes on their body and soil fauna (e.g., macro- and meso-invertebrates) are excluded by small mesh and thus cannot bring microbial decomposers inside (Beidler and Pritchard 2017; Kampichler and Bruckner 2009). In our study, mesh size did not affect decomposition. This result may be attributable to the low quality (e.g., high in lignin) of the fine roots in temperate forests (Rasse et al. 2005; Xia et al. 2015), which diminished the differences in decomposition rates associated with mesh sizes. Additionally, we found no significant correlations between the response ratios of decomposition and initial root mass per unit area of bags (Fig. S3n), probably indicating nonsignificant bag-size effects on decomposition.
Factors regulating decomposition under N application
Substrate quality is one of the important factors controlling the responses of litter decomposition to N application (Knorr et al. 2005). Our results found that the highest decrease in root decomposition was as high as 42.99% when roots were rich in lignin, N and P (Fig. 5f-h). Because of its structural irregularity, lignin has a strong resistance to decomposition and generally slows the decomposition process (David et al. 1988; Fogel and Kermit 1977). High root N concentrations may enhance the reaction between N and intermediate products of lignin degradation and slow decomposition processes (Tu et al. 2015). Root P negatively affected root decomposition, which may have resulted from a high P content inhibiting microbial activity (Jing et al. 2019; Sinsabaugh et al. 1993). N application can change substrate quality, such as increasing lignin, N, and P concentrations in fine roots, thereby inhibiting decomposition (Tu et al. 2015). However, we found no significant correlations between the lnRR of root decomposition and the lnRR of root traits (e.g., N, P). Possible reasons for this result may include (but not limited to): (1) the fine root decomposition that decreased under N application may have been related to suppressed lignin degradation rather than to altered root traits (Berg and Laskowski 2006; Fang et al. 2007; Hobbie 2008), and (2) the number of studies was limited, leading to no significant correlations being found.
In addition to initial root traits, MAT, soil depth and pH also affected decomposition. High temperatures increase decomposition by enhancing microbial activity (Davidson and Janssens 2006; Petraglia et al. 2018). The impacts of the soil layer and pH on decomposition may be linked to soil enzyme activities (Hobbie et al. 2012; Sinsabaugh et al. 2008). Phenol oxidase in the deep layer is inhibited, leading to decreased decomposition (Jian et al. 2016). A high pH can stimulate the production of hydrolytic enzymes, thereby increasing the degradation of hemicellulose and cellulose, leading to increased decomposition (Berg 2014; Sun et al. 2015). N application affects decomposition by changing soil characteristics (Tu et al. 2015). For example, in long-term N application experiments, with the decrease in soil N:P, microbial activity increased and decomposition accelerated (Ashraf et al. 2020; Geisseler and Scow 2014). Nevertheless, because of the limited number of data points for the soil variables (e.g., soil TN = 6, TP = 1 and pH = 9), we did not test the relationships between the lnRR of root decomposition and the lnRR of soil variables.
Overall, our study contributed to understanding and predicting the impacts of N application on fine root decomposition in temperate forest ecosystems. However, the decomposition of fine roots in response to N application may differ among different ecosystems (Fig. S2), and more attention should be given to belowground impacts. Moreover, functional differences between absorptive and transport fine roots cause them to differ in structural development and nutrient concentration (McCormack et al. 2015). The effects of N application on decomposition should be related to the type of fine roots (Kou et al. 2015; Sun et al. 2015). Further study is needed to fully understand the impact of root types on decomposition under N application.
References
Ackerman D, Millet DB, Chen X (2019) Global Estimates of Inorganic Nitrogen Deposition Across Four Decades. Global Biogeochem Cycles 33:100–107. https://doi.org/10.1029/2018gb005990
Argiroff WA, Zak DR, Upchurch RA, Salley SO, Grandy AS (2019) Anthropogenic N deposition alters soil organic matter biochemistry and microbial communities on decaying fine roots. Glob Change Biol 25:4369–4382. https://doi.org/10.1111/gcb.14770
Ashraf MN, Hu C, Wu L, Duan Y, Zhang W, Aziz T, Cai A, Abrar MM, Xu M (2020) Soil and microbial biomass stoichiometry regulate soil organic carbon and nitrogen mineralization in rice-wheat rotation subjected to long-term fertilization. J Soils Sediments 20:3103–3113. https://doi.org/10.1007/s11368-020-02642-y
Austin AT, Ballare CL (2010) Dual role of lignin in plant litter decomposition in terrestrial ecosystems. Proc Natl Acad Sci USA 107:4618–4622. https://doi.org/10.1073/pnas.0909396107
Beidler KV, Pritchard SG (2017) Maintaining connectivity: understanding the role of root order and mycelial networks in fine root decomposition of woody plants. Plant Soil 420:19–36. https://doi.org/10.1007/s11104-017-3393-8
Berg B (2014) Decomposition patterns for foliar litter – A theory for influencing factors. Soil Biol Biochem 78:222–232. https://doi.org/10.1016/j.soilbio.2014.08.005
Berg B, Laskowski R (2006) Litter decomposition: A guide to carbon and nutrient turnover. Elsevier Ltd, Burlington
Bond-Lamberty B, Bailey VL, Chen M, Gough CM, Vargas R (2018) Globally rising soil heterotrophic respiration over recent decades. Nature 560:80–83. https://doi.org/10.1038/s41586-018-0358-x
Carreiro MM, Sinsabaugh RL, Repert DA, ParkhurstSource DF (2000) Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition. Ecology 81(9):2359–2365
Castle SC, Sullivan BW, Knelman J, Hood E, Nemergut DR, Schmidt SK, Cleveland CC (2017) Nutrient limitation of soil microbial activity during the earliest stages of ecosystem development. Oecologia 185:513–524. https://doi.org/10.1007/s00442-017-3965-6
Chen HYH, Brassard BW (2012) Intrinsic and Extrinsic Controls of Fine Root Life Span. Crit Rev Plant Sci 32:151–161. https://doi.org/10.1080/07352689.2012.734742
Chen C, Chen HYH, Chen X, Huang Z (2019) Meta-analysis shows positive effects of plant diversity on microbial biomass and respiration. Nat Commun 10:1332. https://doi.org/10.1038/s41467-019-09258-y
Cheng Y, Wang J, Chang SX, Cai Z, Muller C, Zhang J (2019) Nitrogen deposition affects both net and gross soil nitrogen transformations in forest ecosystems: A review. Environ Pollut 244:608–616. https://doi.org/10.1016/j.envpol.2018.10.054
Conn CE, Day FP (1996) Response of root and cotton strip decay to nitrogen amendment along a barrier island dune chronosequence. Can J Bot 74(2):276–284
David P, Pete S, Maria DN (1988) Soil Conditions and Plant Growth. Longmans, New York
Davidson EA, Janssens IA (2006) Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440:165–173. https://doi.org/10.1038/nature04514
Dong L, Sun T, Berg B, Zhang L, Zhang Q, Wang Z (2019) Effects of different forms of N deposition on leaf litter decomposition and extracellular enzyme activities in a temperate grassland. Soil Biol Biochem 134:78–80. https://doi.org/10.1016/j.soilbio.2019.03.016
Dong L, Berg B, Sun T, Wang Z, Han X (2020) Response of fine root decomposition to different forms of N deposition in a temperate grassland. Soil Biol Biochem 147:107845. https://doi.org/10.1016/j.soilbio.2020.107845
Fan P, Guo D (2010) Slow decomposition of lower order roots: a key mechanism of root carbon and nutrient retention in the soil. Oecologia 163:509–515. https://doi.org/10.1007/s00442-009-1541-4
Fang H, Mo J, Peng S, Li Z, Wang H (2007) Cumulative effects of nitrogen additions on litter decomposition in three tropical forests in southern China. Plant Soil 297:233–242. https://doi.org/10.1007/s11104-007-9339-9
Ferreira V, Castagneyrol B, Koricheva J, Gulis V, Chauvet E, Graca MA (2015) A meta-analysis of the effects of nutrient enrichment on litter decomposition in streams. Biol Rev Camb Philos Soc 90:669–688. https://doi.org/10.1111/brv.12125
Fogel R, Kermit CJ (1977) Effect of habitat and substate quality on douglas fir litter decomposition in western oregon. Can J Bot 55:1632–1640
Freschet GT, Rien A, Johannes HCC (2012) Multiple mechanisms for trait effects on litter decomposition: moving beyond home-field advantage with a new hypothesis. J Ecol 100:619–630. https://doi.org/10.1111/j.1365-2745.2011.01943.x
Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai ZC, Freney JR, Martinelli LA, Seitzinger SP, Sutton MA (2008) Transformation of the Nitrogen Cycle: Recent Trends, Questions, and Potential Solutions. Science 320:889–892
Geisseler D, Scow KM (2014) Long-term effects of mineral fertilizers on soil microorganisms – A review. Soil Biol Biochem 75:54–63. https://doi.org/10.1016/j.soilbio.2014.03.023
Gholz HL, Wedin DA, Smitherman SM, Harmon ME, Parton WJ (2000) Long-term dynamics of pine and hardwood litter in contrasting environments: toward a global model of decomposition. Glob Change Biol 6:751–765
Gough CM, Seller JR, Maier CA (2004) Short-term effects of fertilization on loblolly pine (Pinus taeda L.) physiology. Plant, Cell Environ 27:876–886
Heinemeyer A, Hartley IP, Evans SP, Carreira De La Fuente JA, Ineson P (2007) Forest soil CO2 flux: uncovering the contribution and environmental responses of ectomycorrhizas. Glob Change Biol 13:1786–1797. https://doi.org/10.1111/j.1365-2486.2007.01383.x
Helmisaari H-S, Saarsalmi A, Kukkola M (2008) Effects of wood ash and nitrogen fertilization on fine root biomass and soil and foliage nutrients in a Norway spruce stand in Finland. Plant Soil 314:121–132. https://doi.org/10.1007/s11104-008-9711-4
Hobbie SE (2005) Contrasting Effects of Substrate and Fertilizer Nitrogen on the Early Stages of Litter Decomposition. Ecosystems 8:644–656. https://doi.org/10.1007/S10021-003-0110-7
Hobbie SE (2008) Nitrogen effects on decomposition: a five-year experiment in eight temperate sites. Ecology 89(9):2633–2644
Hobbie SE (2015) Plant species effects on nutrient cycling: revisiting litter feedbacks. Trends Ecol Evol 30:357–363. https://doi.org/10.1016/j.tree.2015.03.015
Hobbie SE, Eddy WC, Buyarski CR, Adair EC, Ogdahl ML, Weisenhorn P (2012) Response of decomposing litter and its microbial community to multiple forms of nitrogen enrichment. Ecol Monogr 82(3):389–405
Hou SL, Hattenschwiler S, Yang JJ, Sistla S, Wei HW, Zhang ZW, Hu YY, Wang RZ, Cui SY, Lu XT, Han XG (2020) Increasing rates of long-term nitrogen deposition consistently increased litter decomposition in a semi-arid grassland. New Phytol. https://doi.org/10.1111/nph.16854
Jacobs LM, Sulman BN, Brzostek ER, Feighery JJ, Phillips RP (2018) Interactions among decaying leaf litter, root litter and soil organic matter vary with mycorrhizal type. J Ecol 106:502–513. https://doi.org/10.1111/1365-2745.12921
Jian S, Li J, Chen J, Wang G, Mayes MA, Dzantor KE, Hui D, Luo Y (2016) Soil extracellular enzyme activities, soil carbon and nitrogen storage under nitrogen fertilization: A meta-analysis. Soil Biol Biochem 101:32–43. https://doi.org/10.1016/j.soilbio.2016.07.003
Jiang L, Kou L, Li S (2018) Alterations of early-stage decomposition of leaves and absorptive roots by deposition of nitrogen and phosphorus have contrasting mechanisms. Soil Biol Biochem 127:213–222. https://doi.org/10.1016/j.soilbio.2018.09.037
Jing H, Zhang P, Li J, Yao X, Liu G, Wang G (2019) Effect of nitrogen addition on the decomposition and release of compounds from fine roots with different diameters: the importance of initial substrate chemistry. Plant Soil 438:281–296. https://doi.org/10.1007/s11104-019-04017-w
Kampichler C, Bruckner A (2009) The role of microarthropods in terrestrial decomposition: a meta-analysis of 40 years of litterbag studies. Biol Rev Camb Philos Soc 84:375–389. https://doi.org/10.1111/j.1469-185X.2009.00078.x
Kellner H, Luis P, Zimdars B, Kiesel B, Buscot F (2008) Diversity of bacterial laccase-like multicopper oxidase genes in forest and grassland Cambisol soil Samples. Soil Biol Biochem 40(3):638–648. https://doi.org/10.1016/j.soilbio.2007.09.013
Kirschbaum M (2006) The temperature dependence of organic-matter decomposition—still a topic of debate. Soil Biol Biochem 38:2510–2518. https://doi.org/10.1016/j.soilbio.2006.01.030
Knorr M, Frey SD, Curtis PS (2005) Nitrogen additions and litter decomposition: a meta-analysis. Ecology 86(12):3252–3257
Kou L, Chen WW, Zhang XY, Gao WL, Yang H, Li DD, Li SG (2015) Differential responses of needle and branch order based root decay to nitrogen addition dominant effects of acid unhydrolyzable residue and microbial enzymes. Plant Soil 394(1–2):1–13. https://doi.org/10.1007/s11104-015-2517-2
Kou L, Jiang L, Fu X, Dai X, Wang H, Li S (2018) Nitrogen deposition increases root production and turnover but slows root decomposition in Pinus elliottii plantations. New Phytol 218:1450–1461. https://doi.org/10.1111/nph.15066
Kuzyakov Y, Hill PW, Jones DL (2007) Root exudate components change litter decomposition in a simulated rhizosphere depending on temperature. Plant Soil 290:293–305. https://doi.org/10.1007/s11104-006-9162-8
LeBauer DS, Treseder KK (2008) Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 89:371–379
Li WB, Jin CJ, Guan DX, Wang QK, Wang AZ, Yuan FH, Wu JB (2015) The effects of simulated nitrogen deposition on plant root traits: A meta-analysis. Soil Biol Biochem 82:112–118. https://doi.org/10.1016/j.soilbio.2015.01.001
Li Y, Ning Z, Cui D, Mao W, Bi J, Zhao X (2016) Litter Decomposition in a Semiarid Dune Grassland: Neutral Effect of Water Supply and Inhibitory Effect of Nitrogen Addition. PLoS ONE 11:e0162663. https://doi.org/10.1371/journal.pone.0162663
Li Y, Bezemer TM, Yang J, Lü X, Li X, Liang W, Han X, Li Q (2019) Changes in litter quality induced by N deposition alter soil microbial communities. Soil Biol Biochem 130:33–42. https://doi.org/10.1016/j.soilbio.2018.11.025
Lin DM, Dou PP, Yang GR, Qian SH, Wang HJ, Zhao L, Yang YC, Mi XC, Ma KP, Fanin N (2020) Home-field advantage of litter decomposition differs between leaves and fine roots. New Phytol 227(4):1–6
Liu L, Greaver TL (2010) A global perspective on belowground carbon dynamics under nitrogen enrichment. Ecol Lett 13:819–828. https://doi.org/10.1111/j.1461-0248.2010.01482.x
Ma Z, Chen HYH (2016) Effects of species diversity on fine root productivity in diverse ecosystems: a global meta-analysis. Glob Ecol Biogeogr 25:1387–1396. https://doi.org/10.1111/geb.12488
Maillard F, Kennedy PG, Adamczyk B, Heinonsalo J, Buee M (2021) Root presence modifies the long-term decomposition dynamics of fungal necromass and the associated microbial communities in a boreal forest. Mol Ecol 00:1–15. https://doi.org/10.1111/mec.15828
Manning P, Saunders M, Bardgett RD, Bonkowski M, Bradford MA, Ellis RJ, Kandeler E, Marhan S, Tscherko D (2008) Direct and indirect effects of nitrogen deposition on litter decomposition. Soil Biol Biochem 40:688–698. https://doi.org/10.1016/j.soilbio.2007.08.023
Mao R, Zeng DH, Li LJ (2011) Fresh root decomposition pattern of two contrasting tree species from temperate agroforestry systems effects of root diameter and nitrogen enrichment of soil. Plant Soil 347(1–2):115–123. https://doi.org/10.1007/s11104-011-0830-y
McCormack ML, Dickie IA, Eissenstat DM, Fahey TJ, Fernandez CW, Guo D, Helmisaari HS, Hobbie EA, Iversen CM, Jackson RB, Leppalammi-Kujansuu J, Norby RJ, Phillips RP, Pregitzer KS, Pritchard SG, Rewald B, Zadworny M (2015) Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol 207:505–518. https://doi.org/10.1111/nph.13363
Nadelhoffer KJ (2000) The potential effects of nitrogen deposition on fine-root production in forest ecosystems. New Phytol 147:131–139
Olson JS (1963) Energy-storage and balance of producers and decomposers in ecological-systems. Ecology 44(2):322–331
Peng Y, Guo D, Yang Y (2017) Global patterns of root dynamics under nitrogen enrichment. Glob Ecol Biogeogr 26:102–114. https://doi.org/10.1111/geb.12508
Petraglia A, Cacciatori C, Chelli S, Fenu G, Calderisi G, Gargano D, Abeli T, Orsenigo S, Carbognani M (2018) Litter decomposition: effects of temperature driven by soil moisture and vegetation type. Plant Soil 435:187–200. https://doi.org/10.1007/s11104-018-3889-x
Pittelkow CM, Liang X, Linquist BA, van Groenigen KJ, Lee J, Lundy ME, van Gestel N, Six J, Venterea RT, van Kessel C (2015) Productivity limits and potentials of the principles of conservation agriculture. Nature 517:365–368. https://doi.org/10.1038/nature13809
Rasse DP, Rumpel C, Dignac M-F (2005) Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant Soil 269:341–356. https://doi.org/10.1007/s11104-004-0907-y
Reay DS, Dentener F, Smith P, Grace J, Feely RA (2008) Global nitrogen deposition and carbon sinks. Nat Geosci 1(7):430–437
See CR, Mccormack M, L, Hobbie SE, Flores‐Moreno H, Silver WL, Kennedy PG (2019) Global patterns in fine root decomposition: climate, chemistry, mycorrhizal association and woodiness. Ecol Lett 22:946–953. https://doi.org/10.1111/ele.13248
Silva HMSd, Jr JCBD, Silveira ML, Santos MVFd, Freitas EVd, Lira MdA (2019) Root decomposition of grazed signalgrass in response to stocking and nitrogen fertilization rates. Crop Sci 59:1–8. https://doi.org/10.2135/cropsci2018.08.523
Silver WL, Miya RK (2001) Global patterns in root decomposition: comparisons of climate and litter quality effects. Oecologia 129:407–419. https://doi.org/10.1007/s004420100740
Sinsabaugh RL, Antibus RK, Linkins AE, Mcclaugherty CA, Rayburn L, Repert D, Weiland T (1993) Wood decomposition: nitrogen and phosphorus dynamics in relation to extracellular enzyme activity. Ecology 74(5):1586–1593
Sinsabaugh RL, Lauber CL, Weintraub MN, Ahmed B, Allison SD, Crenshaw C, Contosta AR, Cusack D, Frey S, Gallo ME, Gartner TB, Hobbie SE, Holland K, Keeler BL, Powers JS, Stursova M, Takacs-Vesbach C, Waldrop MP, Wallenstein MD, Zak DR, Zeglin LH (2008) Stoichiometry of soil enzyme activity at global scale. Ecol Lett 11:1252–1264. https://doi.org/10.1111/j.1461-0248.2008.01245.x
Sinsabaugh RL, Hill BH, Follstad Shah JJ (2009) Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 462:795–798. https://doi.org/10.1038/nature08632
Song X, Zhou G, Gu H, Qi L (2015) Management practices amplify the effects of N deposition on leaf litter decomposition of the Moso bamboo forest. Plant Soil 395:391–400. https://doi.org/10.1007/s11104-015-2578-2
Song X, Li Q, Gu H (2017) Effect of nitrogen deposition and management practices on fine root decomposition in Moso bamboo plantations. Plant Soil 410:207–215. https://doi.org/10.1007/s11104-016-2997-8
Sun T, Dong L, Mao Z (2015) Simulated Atmospheric Nitrogen Deposition Alters Decomposition of Ephemeral Roots. Ecosystems 18:1240–1252. https://doi.org/10.1007/s10021-015-9895-4
Sun T, Dong LL, Wang ZW, Lü XT, Mao ZJ (2016) Effects of long-term nitrogen deposition on fine root decompositionand its extracellular enzyme activities in temperate forests. Soil Biol Biochem 93:50–59. https://doi.org/10.1016/j.soilbio.2015.10.023
Tian D, Niu S (2015) A global analysis of soil acidification caused by nitrogen addition. Environ Res Lett 10(2):1714–1721. https://doi.org/10.1088/1748-9326/10/2/024019
Tu L-h, Peng Y, Chen G, Hu H-l, Xiao Y-l, Hu T-x, Liu L, Tang Y (2015) Direct and indirect effects of nitrogen additions on fine root decomposition in a subtropical bamboo forest. Plant Soil 389:273–288. https://doi.org/10.1007/s11104-014-2353-9
Waldrop MP, Zak DR, Sinsabaugh RL (2004) Microbial community response to nitrogen deposition in northern forest ecosystems. Soil Biol Biochem 36:1443–1451. https://doi.org/10.1016/j.soilbio.2004.04.023
Wang C, Han S, Zhou Y, Yan C, Cheng X, Zheng X, Li MH (2012) Responses of fine roots and soil N availability to short-term nitrogen fertilization in a broad-leaved Korean pine mixed forest in northeastern China. PLoS ONE 7:e31042. https://doi.org/10.1371/journal.pone.0031042
Wang P, Huang K, Hu S (2020) Distinct fine-root responses to precipitation changes in herbaceous and woody plants: a meta-analysis. New Phytol 225:1491–1499. https://doi.org/10.1111/nph.16266
Weand MP, Arthur MA, Lovett GM, Mcculley RL, Weathers KC (2010) Effects of tree species and N additions on forest floor microbial communities and extracellular enzyme activities. Soil Biol Biochem 42(12):2161–2173. https://doi.org/10.1016/j.soilbio.2010.08.012
Wu H, Xiang W, Fang X, Lei P, Ouyang S, Deng X (2017) Tree functional types simplify forest carbon stock estimates induced by carbon concentration variations among species in a subtropical area. Sci Rep 7:4992. https://doi.org/10.1038/s41598-017-05306-z
Xia M, Talhelm AF, Pregitzer KS (2015) Fine roots are the dominant source of recalcitrant plant litter in sugar maple-dominated northern hardwood forests. New Phytol 208:715–726. https://doi.org/10.1111/nph.13494
Ye XM, Zhang Y, Chen FS, Wang GG, He P (2019) The effects of simulated deposited nitrogen on nutrient dynamics in decomposing litters across a wide quality spectrum using a 15N tracing technique. Plant Soil 442:141–156. https://doi.org/10.1007/s11104-019-04158-y
Yu G, Chen Z, Piao S, Peng C, Ciais P, Wang Q, Li X, Zhu X (2014) High carbon dioxide uptake by subtropical forest ecosystems in the East Asian monsoon region. Proc Natl Acad Sci U S A 111:4910–4915. https://doi.org/10.1073/pnas.1317065111
Yu G, Jia Y, He N, Zhu J, Chen Z, Wang Q, Piao S, Liu X, He H, Guo X, Wen Z, Li P, Ding G, Goulding K (2019) Stabilization of atmospheric nitrogen deposition in China over the past decade. Nat Geosci 12:424–429. https://doi.org/10.1038/s41561-019-0352-4
Zhang DQ, Hui DF, Luo YQ, Zhou GY (2008) Rates of litter decomposition in terrestrial ecosystems: global patterns and controlling factors. Journal of Plant Ecology 1(2):85–93. https://doi.org/10.1093/jpe/rtn002
Zheng M, Zhou Z, Luo Y, Zhao P, Mo J (2019) Global pattern and controls of biological nitrogen fixation under nutrient enrichment: A meta-analysis. Glob Change Biol 25:3018–3030. https://doi.org/10.1111/gcb.14705
Acknowledgements
This study is financially supported by the National Science Foundation of China (31700376), the Natural Science Key Fund for Colleges and Universities of Jiangsu Province of China (17KJA180006), the Six Talent Peaks Program of Jiangsu Province (JY-041& TD-XYDXX-006), and the “5151” Talent Program of Nanjing Forestry University.
Author information
Authors and Affiliations
Contributions
All authors contributed intellectual input, provided study assistance and prepared the manuscript. X.X. conceived the idea and designed the study. F. X. collected and analysed the data with help from C.X., Q. G. and X.X. F.X. wrote the manuscript with input from all authors.
Corresponding author
Additional information
Responsible Editor: Amandine Erktan.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fu, X., Xu, C., Geng, Q. et al. Effects of nitrogen application on the decomposition of fine roots in temperate forests: a meta-analysis. Plant Soil 472, 77–89 (2022). https://doi.org/10.1007/s11104-021-05176-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-021-05176-5