Abstract
Fresh tree root decomposition induced by tillage is an important source of soil nutrients in agroforestry systems. Here we examined the effects of tree species, root size and soil N enrichment on fresh root decomposition under laboratory conditions. Fresh roots with two diameters (<2 and 2–5 mm) of Populus euramericana cv. ‘N3016’ (poplar) and Pinus tabulaeformis (pine) collected from agroforestry systems in Northeast China were used in the experiment. For each root treatment, four N levels (0, 50, 100 and 150 μg N g−1 soil) were added. We recognized N concentration and C/N ratio as the root quality variables, and determined decomposition rates as cumulative CO2 production and mass loss. Poplar roots had higher N concentration and lower C/N ratio and decomposed faster than pine roots, and smaller roots decomposed faster than the corresponding larger roots. The effect of N addition on root decomposition varied from positive to negligible to negative, and depended on root quality and N addition rates. Increased N availability did not accelerate and even suppressed poplar root decomposition, whereas generally stimulated pine root decomposition. Our results suggest that root quality should be incorporated into the design of agroforestry systems. Moreover, the differential responses of N addition on decomposition of fresh roots with different quality provide insights into soil nutrient management in agroforestry practices.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant root decomposition is a critical component of C and nutrient cycling in terrestrial ecosystems, and hence plays an important role in maintaining soil fertility and plant productivity (McClaugherty et al. 1984; Gill and Jackson 2000; Silver and Miya 2001). Root decomposition depends on both biotic and abiotic factors; the most important is root quality (Silver and Miya 2001). Root quality generally varies with soil nutrient conditions (Cotrufo and Ineson 1995), plant species (Lehmann et al. 1995; Gholz et al. 2000) and root diameter (Scheu and Schauermann 1994; King et al. 1997).
In agroforestry systems, tree can increase organic matter input and improve soil nutrient availability through root and leaf litter decomposition (Lehmann and Zech 1998; Jose et al. 2000; Jose 2009). Munoz and Beer (2001) found that, in Costa Rica, tree fine root turnover contributed from 6% to 13% and from 3% to 6% of total nutrient input to soils in the Cordia alliodora– and Erythrina poeppigiana–Theobroma cacao agroforestry systems, respectively. Moreover, Jose et al. (2000) observed that tree fine roots may play a more significant role in nutrient cycling in temperate agroforestry systems, due to their faster nutrient release as compared to tree leaf litters. However, most of the previous studies about root decomposition in agroforestry systems have focused on naturally senesced tree roots, and there is little information on decomposition dynamics of fresh tree roots (Schroth 2003). In agroforestry systems, fresh tree root decomposition usually occurs when superficial tree roots are destroyed by tillage at the beginning of a cropping season. Tree roots cut off during soil tillage may have higher nutrient contents than roots that die naturally (Schroth 2003). Therefore, knowledge about the decomposition pattern of fresh tree roots can guide our decisions on tree species selection and nutrient management in agroforestry systems.
Root decomposition is generally affected by soil N availability, which can alter root N concentration and decomposer activity and abundance (Fog 1988; Manning et al. 2008). While the correlation between initial root N concentration and decay rates has been well documented (Berg 1984; Silver and Miya 2001), the relationship between root decomposition and external N availability is still unclear. Several studies have found significantly faster decay rates under increased N availability (Van der Krift et al. 2001; King et al. 2002), whereas some other studies have observed no significant change (King et al. 1997; Ludovici and Kress 2006). In agroforestry systems, mineral N fertilizer application is widely used as the conventional agronomic management to improve soil N status and increase system productivity (Jose et al. 2004). Since soil microbial growth and activity are generally limited by N (Schimel and Weintraub 2003), increased soil N availability following fertilization may alter the decomposer activity and impact fresh root decomposition in agroforestry systems.
In semiarid regions of Northeast China, poplar (Populus spp.) and pine (Pinus spp.) are widely adopted in agroforestry systems. In this paper, we used fresh tree roots (<2 and 2–5 mm in diameter) from poplar (Populus euramericana cv. ‘N3016’)- and Chinese pine (Pinus tabulaeformis)-based agroforestry systems in the laboratory decomposition experiment. The poplar is a fast-growing tree with a rotation period of 20–25 years, while Chinese pine is a comparatively slow-growing tree with a rotation of over 60 years. We recognized initial N concentration and C/N ratio as the main root quality variables. The specific objectives of our study were (1) to assess the effect of tree species on fresh root quality and thus decomposition rates. We hypothesized (hypothesis one) that poplar fresh roots would have higher quality (high N concentration and low C/N ratio) than pine fresh roots, and decompose faster; (2) to examine the effect of root diameter on root quality and decomposition dynamics. We hypothesized (hypothesis two) that smaller roots would have higher quality than larger roots and decompose more rapidly; and (3) to investigate the responses of fresh tree root decomposition to soil N enrichment. We hypothesized (hypothesis three) that N addition to soils would stimulate fresh tree root decomposition due to the N limitation during decomposition.
Materials and methods
Root and soil sampling and analysis
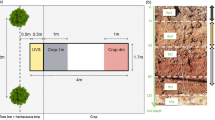
Fresh tree root samples and soil used for the incubation experiment were collected from a farm in Taipingzhuang Town (41°47'N and 119°15'E, 632 m above sea level), Jianping County, Liaoning Province, Northeast China. The study site belongs to the temperate, semiarid continental monsoon climate, with a mean annual temperature of 6.5°C, precipitation of 467 mm (more than 60% falling between June and August), and the frost-free period of 148 days. The poplar- and Chinese pine-based agroforestry systems were established in April 2004 and 1995, respectively. The spatial arrangement of tree and crop rows in agroforestry systems is shown in Fig. 1. All the agroforestry systems were tilled before seeding.
In order to avoid the occurrence of home-field advantage during fresh tree root decomposition, soil samples were collected at 0–15 cm layer in maize (Zea mays)-based sole cropping systems in April 2009 when fields were fallow. During the periods of soil sampling, crop stubs were remained in the field, and would be removed from the systems during tillage. After removing roots, macrofauna and visible debris, the soil was sieved (2 mm) and was stored at 4°C for one week until the laboratory incubation experiment commenced. The soil was a sandy loam with pH in a 1:2.5 (weight:volume) water suspension of 8.26; organic C of 5.01 mg g−1; total N of 0.47 mg g−1; and C/N ratio of 10.7.
In April 2009, fresh tree root samples were collected by excavating soil (0–15 cm depth) from agroforestry systems, separated by sieving and carefully washed. In the present study, we only collected small roots <5 mm in diameter, because coarse roots >5 mm in diameter were scarce in 0–15 cm soil layer. Fresh roots were separated into two diameter size classes (<2 mm and 2–5 mm in diameter) and cut into 2 cm in length. Fresh roots were mixed carefully and divided into two subsamples for each size class. The first group of subsample was stored at 4°C for 2 days and used for the incubation experiment. The second group of subsample was analyzed for initial water content and chemical composition. The subsamples were weighed, oven-dried to a constant mass at approximately 65°C for 48 h, reweighed, and milled (<0.25 mm) for measurement of organic C and N concentration. Organic C was determined using the K2Cr2O7–H2SO4 wet oxidation method (Walkley and Black 1934). To determine the total N, the samples were first mineralized using the Kjeldahl method (Bremner 1996). The total N concentration in the digested solution was assessed using a continuous-flow autoanalyzer (AutoAnalyzer III, Bran+Luebbe GmbH, Germany).
Experimental design
Two tree species—poplar and Chinese pine were selected with two root size classes (<2 and 2–5 mm) and four N addition levels (0, 50, 100 and 150 μg N g−1 soil) for a total of 16 treatments. An unamended treatment (containing only soil) was included as a control. Each treatment was replicated four times. In addition, four containers without soil and fresh tree roots were considered as blanks.
Fresh root decomposition
Root decomposition was studied in laboratory microcosms using a modified method based on that described by Cotrufo and Ineson (1995) and Robinson et al. (1999), and we determined root decomposition rates as cumulative CO2 production and mass loss. Fresh soil (80-g, oven-dried), previously stored at 4°C, was placed in a 500 mL glass flask and N was added as (NH4)2SO4 solution for N addition treatments. Distilled water was added to each microcosm to adjust its moisture content to 60% water-holding capacity (Wilke 2005). Three grams of roots were placed on the surface of fresh soil in the flask. A plastic vial containing 20 mL 1 mol L−1 NaOH solution was placed in each flask to trap the evolved CO2 and the total weight of the glass flask containing the incubation soil and the CO2 traps was recorded. The flasks were incubated at 25 ± 1°C in darkness for 119 days (approximately equivalent to the length of a complete growing season). After 2, 7, 14, 28, 42, 56, 84 and 119 days of incubation, the evolved CO2 trapped in NaOH was determined by back titration with 0.5 mol L−1 HCl after precipitating the carbonate with 1 mol L−1 BaCl2 solution. After the CO2 traps were taken out, the flasks were left open for 4 h to allow the air in the flasks to be replenished. In order to maintain at 60% water-holding capacity throughout the incubation, soil moisture content was checked by weighing the flask every 3–5 days and adjusted by adding distilled water when necessary. After 119 days, the roots were separated from the soil, oven-dried to a constant mass at approximately 65°C and weighed. For each fresh tree root treatment, the CO2 production was calculated as the difference between the CO2 produced from the treatment (soil containing fresh roots) and that from the control (soil without fresh roots), and expressed as mg CO2-C g−1 root. Mass loss was calculated as the difference between the initial and final root mass, and expressed as a percentage of initial root mass.
Kinetic models and statistical analyses
Cumulative CO2 production kinetics were fitted with a simple compartment negative exponential model (Stanford and Smith 1972):
where C t is the cumulative CO2 production at time t, C 0 is the potential cumulative CO2 production, k is the decomposition rate constant, and t is time in days.
Data were statistically analyzed using SPSS (v. 13.0) for Windows software package (SPSS Inc. 2004), and the accepted significance level was α = 0.05. Standard errors of the treatment means were calculated from the one-way analysis of variance (ANOVA). Three-way ANOVA was used to examine the effects of tree species, root diameter and N addition rate on cumulative CO2 production, potential cumulative CO2 production, decomposition rate constant and mass loss. Multiple comparisons among means of cumulative CO2 production, potential cumulative CO2 production, decomposition rate constant and mass loss of different treatments were performed with Tukey’s HSD (Honestly Significant Difference) test. Data were tested for normality using the Kolmogorov-Smirnov test (SPSS Inc. 2004), and all data were conformed to a normal distribution (data not shown).
Results
The initial chemical composition of fresh tree roots
Poplar fresh roots had higher N concentration and lower C/N ratio than pine fresh roots, and for each tree species, <2 mm fresh roots had higher N concentration and lower C/N ratio than 2–5 mm fresh roots (Table 1). For all fresh tree roots, <2 mm poplar fresh roots had the highest N concentration (9.75 mg g−1) and the lowest C/N ratio (46.1), while 2–5 mm pine fresh roots had the lowest N concentration (3.44 mg g−1) and the greatest C/N ratio (148.5) (Table 1).
Effect of tree species and root diameter size on fresh root decomposition
During 119 days of laboratory incubation, tree species, root diameter size and their interaction had significant effects on cumulative CO2 production (Table 2) and mass loss (Table 3). Poplar fresh roots decomposed faster than pine fresh roots, and for both poplar and pine, <2 mm fresh roots decomposed faster than the corresponding 2–5 mm fresh roots (Fig. 2). At the end of incubation (day 119), cumulative CO2 production (305 mg CO2-C g−1 root) and mass loss (38.9%) of <2 mm poplar roots were 92% and 86% higher than those of the corresponding pine ones, respectively (Table 4). Similarly, cumulative CO2 production (228 mg CO2-C g−1 root) and mass loss (30.4%) of 2–5 mm poplar roots were 74% and 99% greater than those of the corresponding pine ones, respectively (Table 4). In addition, <2 mm poplar roots had 34% and 28% greater cumulative CO2 production and mass loss than 2–5 mm ones, respectively; and <2 mm pine roots had 21% and 37% higher cumulative CO2 production and mass loss than 2–5 mm ones, respectively (Table 4).
Cumulative CO2 production during the decomposition of fresh roots with two diameters (<2 and 2–5 mm) of two tree species Populus euramericana cv. ‘N3016’ (poplar) and Pinus tabulaeformis (pine) under different N addition levels (0, 50, 100 and 150 μg N g−1 soil) in a microcosm study. Error bars represent±SE (n = 4)
Tree species, root diameter size and their interaction produced significant effects on potential cumulative CO2 production and decomposition rate constant (Table 3). Poplar roots had greater potential cumulative CO2 production than pine roots, and <2 mm roots had greater potential cumulative CO2 production than the corresponding 2–5 mm roots (Tables 3 and 4). For all tree roots, <2 mm poplar roots had the greatest potential cumulative CO2 production (320 mg CO2-C g−1 root), whereas 2–5 mm pine roots had the lowest (143 mg CO2-C g−1 root) (Table 4). However, decomposition rate constant showed no consistent trend with tree species and root diameter. For the poplar, <2 mm roots (0.0243 day−1) had higher decomposition rate constant than 2–5 mm roots (0.0145 day−1), while the decomposition rate constant of pine showed a reverse trend (Table 4).
Effect of N addition on fresh root decomposition
During the incubation, N addition affected cumulative CO2 production and mass loss, and the effects varied with root quality and N addition level (Tables 2 and 3). For <2 mm poplar roots, N addition caused a 14% reduction in mass loss only at a rate of 150 μg N g−1, and had no effects on cumulative CO2 production and mass loss in other cases (Table 4). Cumulative CO2 production of 2–5 mm poplar roots decreased by 12% when 150 μg N g−1 was added, while N addition at 100 and 150 μg N g−1 caused 16% and 22% declines in mass loss, respectively. During the entire incubation period, cumulative CO2 production of <2 mm pine roots increased by 10% and 17% with the N addition at 100 and 150 μg N g−1, respectively, and mass loss increased by 19% and 18%, respectively (Table 4). However, N addition increased cumulative CO2 production and mass loss of 2–5 mm pine roots, irrespective of addition levels (Table 4). For 2–5 mm pine roots, N addition at 50, 100 and 150 μg N g−1 caused 24%, 31% and 39% increases in cumulative CO2 production, respectively, and 35%, 25% and 42% in mass loss (Table 4).
Tree species, root diameter size, N addition level and their interaction affected potential cumulative CO2 production and decomposition rate constant (Table 3). N addition level had no effects on potential cumulative CO2 production of <2 mm poplar and pine roots, whereas N addition decreased potential cumulative CO2 production of 2–5 mm poplar roots, and increased potential cumulative CO2 production of 2–5 mm pine roots (Table 4). However, for all tree roots, N addition generally increased decomposition rate constant (Table 4).
Discussion
Our data supported the first hypothesis that poplar fresh roots decomposed faster than pine fresh roots during the incubation period, due to the higher root quality. Our result confirmed the observations of Silver and Miya (2001), who used a global data-set of root decomposition and reviewed that, root chemical composition, especially root C/N ratio and Ca concentration was the main controller of root decomposition. High N concentration and low C/N ratio in poplar roots may stimulate microbial growth and hence root decomposition rates, whereas pine roots with high C/N ratio had low decomposition rates due to the great amounts of structural woody materials as well as low available N for decomposer organisms (Silver and Miya 2001). In temperate agroforestry systems, root litters with high quality can decompose rapidly and stimulate nutrient cycling and replenish soil fertility (Jose et al. 2000). Therefore, root quality should be considered in the design of agroforestry systems in temperate regions from the viewpoint of sustaining soil fertility. Moreover, our results suggest that poplar can enhance nutrient cycling through decomposition of high quality roots, and have an advantage over Chinese pine during the species selection in semiarid agroforestry systems of Northeast China.
According to our second hypothesis, for both poplar and pine, <2 mm fresh roots had higher quality than the corresponding 2–5 mm fresh roots, and hence decomposed more rapidly. Previous studies also found that root decomposition rates decreased with increased diameter size (e.g. Camiré et al. 1991; Ludovici and Kress 2006). However, some studies observed that larger diameter roots decomposed faster than smaller ones albeit lower N concentration and higher C/N ratio (McClaugherty et al. 1984; Lin et al. 2011), because high level of N concentration in roots may stimulate the formation of N-lignin complexes and slow decomposition rates (Camiré et al. 1991). In our study, high levels of N in smaller tree roots may stimulate the growth of microorganisms and thus enhance decomposition rates (Camiré et al. 1991; Silver and Miya 2001). In addition, slower leaching rates of water-soluble compounds (Fahey et al. 1988), a longer time required for fungal hyphae penetration (Berg 1984), or increased proportions of resistant organic substances and structural mass (King et al. 1997; Silver and Miya 2001) may suppress decomposition of larger diameter fresh roots. The greater decay rates of smaller tree roots imply that fine roots play an important role in nutrient cycling in temperate agroforestry systems.
Inconsistent with our third hypothesis, N addition had variable effects on the fresh root decomposition rates: varying from positive via negligible to negative (Fig. 2 and Table 4). Indeed, the effect of soil N enrichment on decomposer activity was yet contradictory. Previous studies found that increased N availability had positive (Allen and Schlesinger 2004), negligible (Keeler et al. 2009) or negative (Hu et al. 2010) effects on soil decomposer activity. Consequently, N addition either resulted in an increase in root decay (Van der Krift et al. 2001; King et al. 2002) or had no significant effect (King et al. 1997; Ludovici and Kress 2006). In addition, Laiho et al. (2004) found that root decomposition rates did not vary systematically with soil nutrient levels in boreal peatlands. Therefore, in our study, the various responses of fresh root decomposition to N addition may be resulted from the differences in fresh root quality and fertilization rate.
Based on a meta-analysis, Knorr et al. (2005) found that increased external N input generally stimulates the decomposition of leaf litters containing low lignin and other recalcitrant compounds, while inhibiting the decay of leaf litters with high lignin concentration. Although we did not measure tree root lignin concentration, Berg and McClaugherty (2008) pointed that the lignin content of deciduous species was generally lower than that of evergreen species. However, in our study, increased N availability did not accelerate and even suppressed the decomposition of poplar fresh root, but generally stimulated the decay of pine fresh roots. These inconsistent results illustrate that the effect of N addition on decomposition of fresh roots may be different from the leaf litters. For poplar roots with low C/N ratio, increased N availability may aggravate C-limitation for microbial degradation (Schimel and Weintraub 2003), depress microbial activity (Fog 1988) and form recalcitrant complexes (Camiré et al. 1991), and hence slow decomposition rates. However, for pine roots with high C/N ratio, N addition may stimulate microbial growth and activity and increase root decay rates (Schimel and Weintraub 2003). In addition, the effect of N addition on fresh root decomposition may depend not only on chemical quality, but also on physical quality (e.g. tissue architecture) (Lindedam et al. 2009). Still now, we cannot fully explain such contrasting effects of increased N availability on decomposition of fresh roots with different quality. Further studies are needed to better understand the interactive effects between N addition and root quality on fresh root decomposition, due to the poor knowledge of the mechanism behind these processes and the lack of adequate related studies. The differential responses of decomposition of fresh tree roots with different quality to N addition may be benefit to improve our understanding of nutrient cycles in agroforestry systems.
During the period of fresh tree root decomposition, in some cases, the effect of N addition on cumulative CO2 production was different from that on mass loss (Table 4). For example, N addition at 100 μg N g−1 decreased mass loss of 2–5 mm poplar fresh roots, but did not affect cumulative CO2 production (Table 4). No changes in cumulative CO2 production may be attributed to the enhanced microbial C utilization efficiency (Schimel and Weintraub 2003) and altered microbial community composition and abundance (Hu et al. 2010). These contradictory data also suggest that, during fresh tree root decomposition, N addition causes a shift in pathways of decay from cumulative CO2 production to mass loss through leaching and loss as fine particulate matter (Robinson et al. 1999; Zeng et al. 2010). Increased mass loss through leaching and fragmentation under N addition caused greater decomposition rates of fresh tree roots, although there was no significant change in cumulative CO2 production.
Generally, incorporation of fresh tree roots into soils may produce a priming effect, the stimulation of soil organic C mineralization induced by the addition of organic substances (Kuzyakov et al. 2000). During the incubation period, we did not separate the soil-derived CO2 from the root-derived CO2, and distinguish the sources of CO2 production. Consequently, we cannot examine the influence of priming effect on CO2 production during fresh tree root decomposition. We acknowledge that priming effect would exaggerate the amount of CO2 release derived from the decomposition of tree fresh roots. However, we expected that CO2 produced from both root decomposition and priming effect would be the overall result of tree fresh root addition to soils. In the further studies, application of 14C-labelled tree roots to soils should be used to trace the source of CO2 and assess the priming effect induced by fresh root addition in the decomposition process.
In the present study, we investigated fresh tree root decomposition rates under constant soil humidity and incubation temperature using laboratory-based methodology. This approach can accurately examine the effects of root quality and N addition rates on fresh tree root decomposition in the absence of anthropogenic and natural disturbance. However, laboratory-based methodology does not mimic many abiotic factors controlling root decomposition in the field, such as periodic drying and wetting of the soil. Moreover, fresh tree root decomposition rates in the laboratory may be greater than those in the field in the semiarid temperate regions, due to the higher soil humidity and incubation temperature. Further studies are needed to examine the effects of root quality and N addition on fresh tree root decomposition and nutrient release in the field.
This study, reporting on fresh tree root decomposition induced by tillage, found that root quality was the primary factor controlling fresh tree root decomposition in temperate agroforestry systems. Therefore, root quality should be incorporated into the design of agroforestry systems in temperate regions. In semiarid regions of Northeast China, poplar can produce high quality root litters and have an advantage over Chinese pine during the design process of agroforestry systems. In addition, the effect of N addition on fresh tree root decomposition rates varied from positive via negligible to negative, and depended on root quality and N addition rates. This may help us make better decisions on nutrient management and sustain system productivity in temperate agroforestry practices. Our results also highlight the complex of the interactive effects of N addition and root quality on fresh root decomposition. In order to reveal the mechanism of root decomposition response to increased N availability, further studies should focus on the dynamics of decomposer community composition and activity during root decomposition.
References
Allen AS, Schlesinger WH (2004) Nutrient limitations to soil microbial biomass and activity in loblolly pine forests. Soil Biol Biochem 36:581–589
Berg B (1984) Decomposition of root litter and some factors regulating the process: long-term root litter decomposition in a Scots pine forest. Soil Biol Biochem 16:609–617
Berg B, McClaugherty C (2008) Plant litter: decomposition, humus formation, carbon sequestration. Springer, Berlin, pp 53–83
Bremner JM (1996) Nitrogen-total. In: Sparks DL, Page AL, Helmke PA, Loeppert RH, Soltanpour PN, Tabatabai MA, Johnston CT, Sumner ME (eds) Methods of soil analysis. Part 3. Chemical Methods. Soil Science Society of America Book Series, Number 5. Wisconsin, USA, pp 1085–1122
Camiré C, Côté B, Brulotte S (1991) Decomposition of root of black alder and hybrid poplar in short-rotation plantings: nitrogen and lignin control. Plant Soil 138:123–132
Cotrufo MF, Ineson P (1995) Effects of enhanced atmospheric CO2 and nutrient supply on the quality and subsequent decomposition of fine roots of Betula pendula Roth. and Picea sitchensis (Bong.) Carr. Plant Soil 170:267–277
Fahey TJ, Hughes JW, Pu M, Arthur MA (1988) Root decomposition and nutrient flux following whole-tree harvest of northern hardwood forest. Forest Sci 34:744–768
Fog K (1988) The effect of added nitrogen on the rate of decomposition of organic matter. Biol Rev 63:433–462
Gholz HL, Wedin DA, Smitherman SM, Harmon ME, Parton WJ (2000) Long-term dynamics of pine and hardwood litter in contrasting environments: toward a global model of decomposition. Global Change Biol 6:751–765
Gill RA, Jackson RB (2000) Global patterns of root turnover for terrestrial ecosystems. New Phytol 147:13–31
Hu YL, Zeng DH, Liu YX, Zhang YL, Chen ZH, Wang ZQ (2010) Responses of soil chemical and biological properties to nitrogen addition in a Dahurian larch plantation in Northeast China. Plant Soil 333:81–92
Jose S (2009) Agroforestry for ecosystem services and environmental benefits: an overview. Agroforest Syst 76:1–10
Jose S, Gillespie AR, Seifert JR, Mengel DB, Pope PE (2000) Defining competition vectors in a temperate alley cropping system in the midwestern USA. 3. Competition for nitrogen and litter decomposition dynamics. Agroforest Syst 48:61–77
Jose S, Gillespie AR, Pallardy SG (2004) Interspecific interactions in temperate agroforestry. Agroforest Syst 61:237–255
Keeler BL, Hobbie SE, Kellogg LE (2009) Effects of long-term nitrogen addition on microbial enzyme activity in eight forested and grassland sites: implications for litter and soil organic matter decomposition. Ecosystems 12:1–15
King JS, Allen HL, Dougherty P, Strain BR (1997) Decomposition of roots in loblolly pine: effects of nutrient and water availability and root size class on mass loss and nutrient dynamics. Plant Soil 195:171–184
King JS, Albaugh TJ, Allen HL, Buford M, Strain BR, Dougherty P (2002) Below-ground carbon input to soil is controlled by nutrient availability and fine root dynamics in loblolly pine. New Phytol 154:389–398
Knorr M, Frey SD, Curtis PS (2005) Nitrogen additions and litter decomposition: a meta-analysis. Ecology 86:3252–3257
Kuzyakov Y, Friedel JK, Stahr K (2000) Review of mechanism and quantification of priming effects. Soil Biol Biochem 32:1485–1498
Laiho R, Laine J, Trettin CC, Finér L (2004) Scots pine litter decomposition along drainage succession and soil nutrient gradients in peatland forests, and the effects of inter-annual weather variation. Soil Biol Biochem 36:1095–1109
Lehmann J, Zech W (1998) Fine root turnover of irrigated hedgerow intercropping in Northern Kenya. Plant Soil 198:19–31
Lehmann J, Schroth G, Zech W (1995) Decomposition and nutrient release from leaves, twigs and roots of three alley-cropped tree legumes in central Togo. Agroforest Syst 29:21–36
Lin C, Yang Y, Guo J, Chen G, Xie J (2011) Fine root decomposition of evergreen broadleaved and coniferous tree species in mid-subtropical China: dynamics of dry mass, nutrient and organic fractions. Plant Soil 338:311–327
Lindedam J, Magid J, Poulsen P, Luxhøi J (2009) Tissue architecture and soil fertility controls on decomposer communities and decomposition of roots. Soil Biol Biochem 41:1040–1049
Ludovici KH, Kress LW (2006) Decomposition and nutrient release from fresh and dried pine roots under two fertilizer regimes. Can J Forest Res 36:105–111
Manning P, Saunders M, Bardgett RD, Bonkowski M, Bradford A, Ellis RJ, Kandeler E, Marhan S, Tscherko D (2008) Direct and indirect effects of nitrogen deposition on litter decomposition. Soil Biol Biochem 40:688–698
McClaugherty CA, Aber JD, Melillo JM (1984) Decomposition dynamics of fine roots in forested ecosystems. Oikos 42:378–386
Munoz F, Beer J (2001) Fine root dynamics of shaded cacao plantations in Costa Rica. Agroforest Syst 51:119–130
Robinson CH, Kirkham JB, Littlewood R (1999) Decomposition of root mixtures from high arctic plants: a microcosm study. Soil Biol Biochem 31:1101–1108
Scheu S, Schauermann J (1994) Decomposition of roots and twigs: effects of wood type (beech and ash), diameter, site of exposure and macrofauna exclusion. Plant Soil 163:13–24
Schimel JP, Weintraub MN (2003) The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: a theoretical model. Soil Biol Biochem 35:549–563
Schroth G (2003) Decomposition and nutrient supply from biomass. In: Schroth G, Sinclair FL (eds) Trees, crops and soil fertility: concepts and research methods. CAB International, Wallingford, pp 131–150
Silver WL, Miya RK (2001) Global patterns in root decomposition: comparisons of climate and litter quality effects. Oecologia 129:407–419
SPSS Inc (2004) SPSS 13.0 base users guide. SPSS Inc, Chicago
Stanford G, Smith SJ (1972) Nitrogen mineralization potentials of soils. Soil Sci Soc Am Proc 36:465–472
Van der Krift TAJ, Kuikman PJ, Möller F, Berendse F (2001) Plant species and nutritional-mediated control over rhizodeposition and root decomposition. Plant Soil 228:191–200
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci 37:29–38
Wilke BM (2005) Determination of chemical and physical soil properties. In: Margesin R, Schinner F (eds) Manual for soil analysis: monitoring and assessing soil bioremediation. Springer, Berlin, pp 47–96
Zeng DH, Mao R, Chang SX, Li LJ, Yang D (2010) Carbon mineralization of tree leaf litter and crop residues from poplar-based agroforestry systems in Northeast China: a laboratory study. Appl Soil Ecol 44:133–137
Acknowledgments
This work was funded by the National Key Technologies R&D Program of China (Nos. 2011BAD38B02 and 2006BAD03A0502). We thank three anonymous reviewers and the section editor (Dr. Johannes Lehmann) for their helpful remarks on an earlier version of this manuscript, He-Ming Lin and Gui-Yan Ai for laboratory analyses, and Qing Zhang and Zhan-Peng Liu for the field work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Johannes Lehmann.
Rights and permissions
About this article
Cite this article
Mao, R., Zeng, DH. & Li, LJ. Fresh root decomposition pattern of two contrasting tree species from temperate agroforestry systems: effects of root diameter and nitrogen enrichment of soil. Plant Soil 347, 115–123 (2011). https://doi.org/10.1007/s11104-011-0830-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-011-0830-y