Abstract
A dominant paradigm in ecology is that plants are limited by nitrogen (N) during primary succession. Whether generalizable patterns of nutrient limitation are also applicable to metabolically and phylogenetically diverse soil microbial communities, however, is not well understood. We investigated if measures of N and phosphorus (P) pools inform our understanding of the nutrient(s) most limiting to soil microbial community activities during primary succession. We evaluated soil biogeochemical properties and microbial processes using two complementary methodological approaches—a nutrient addition microcosm experiment and extracellular enzyme assays—to assess microbial nutrient limitation across three actively retreating glacial chronosequences. Microbial respiratory responses in the microcosm experiment provided evidence for N, P and N/P co-limitation at Easton Glacier, Washington, USA, Puca Glacier, Peru, and Mendenhall Glacier, Alaska, USA, respectively, and patterns of nutrient limitation generally reflected site-level differences in soil nutrient availability. The activities of three key extracellular enzymes known to vary with soil N and P availability developed in broadly similar ways among sites, increasing with succession and consistently correlating with changes in soil total N pools. Together, our findings demonstrate that during the earliest stages of soil development, microbial nutrient limitation and activity generally reflect soil nutrient supply, a result that is broadly consistent with biogeochemical theory.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrogen (N) and phosphorus (P) limit biological processes in many terrestrial ecosystems (Elser et al. 2007; Lebauer and Treseder 2008; Harpole et al. 2011) and the relative availability of each nutrient is a key determinant of ecosystem function. A large body of research indicates that plant primary production is most often N limited in newly developing ecosystems, whereas primary production in older, more developed ecosystems is often limited by P availability (Walker and Syers 1976; Vitousek et al. 1993; Richardson et al. 2004; Vitousek 2004; Wardle et al. 2004; Turner and Laliberté 2014; but see Laliberté et al. 2012). This shift in the nature of nutrient limitation reflects the fact that as soils develop, elements of atmospheric origin (like N) accumulate through biological processes, while pools of rock-derived mineral nutrients (like P) generally decline via chronic leaching during weathering (Walker and Syers 1976; Crews et al. 1995; Peltzer et al. 2010; Selmants and Hart 2010). While research increasingly suggests a key role of microbial communities in early soil formation and biogeochemical cycling (Reynolds et al. 2003; Nemergut et al. 2007; Schmidt et al. 2008; Brankatschk et al. 2011), the nature and predictability of microbial nutrient limitation during early ecosystem succession remains unclear.
During the earliest stages of primary succession, soil microbial communities undergo rapid compositional (e.g., Sigler and Zeyer 2004; Nemergut et al. 2007; Schmidt et al. 2008; Knelman et al. 2014) and functional (e.g., Ohtonen et al. 1999; Tscherko et al. 2003; Bardgett et al. 2007; Schmidt et al. 2008) shifts that may reflect subtle changes in soil nutrient availability (Knelman et al. 2014). Such patterns are likely the product of both the role of soil nutrients in structuring microbial communities and the role of microorganisms in mediating the availability of soil nutrients. Indeed, the elemental stoichiometry of soil microorganisms is well constrained (Cleveland and Liptzin 2007), and shifts in soil nutrient availability that deviate from a narrow stoichiometric range may favor certain soil microorganisms or physiological capacities over others (Craine et al. 2007; Ramirez et al. 2012; Leff et al. 2015). For example, some of the first microbial colonizers of newly exposed glacial till include autotrophic cyanobacteria and heterotrophic diazatrophs capable of fixing atmospheric N (Nemergut et al. 2007; Schmidt et al. 2008; Sattin et al. 2009; Brankatschk et al. 2011) and N fixation likely contributes to the rapid accumulation of N relative to P in newly developing soils even prior to plant colonization.
To date, assessments of microbial nutrient limitation in developing soils have shown somewhat equivocal results based on functional responses of soil microbial communities. Available P was found to limit heterotrophic microbial activity in early succession soils in the Peruvian Andes (Schmidt et al. 2011), the Rocky Mountains of Colorado (King et al. 2008), and central Alaska (Darcy and Schmidt 2016). By contrast, available N limited heterotrophic microbial activity in recently deglaciated soils in the Swiss Alps (Göransson et al. 2011) and the Arctic (Yoshitake et al. 2007). Yet of the studies that have been done, few have simultaneously tested the strength of N limitation relative to P (but see Göransson et al. 2011; Darcy and Schmidt 2016). In large part, our current understanding of nutrient limitation in early successional soil microbial communities has been confounded by the use of inconsistent methodology and sampling approaches among studies. However, it is also possible that nutrient limitation of soil microbial communities may not be easily captured using a single metric, partly because methods used to assess microbial-accessible nutrient pools often only reveal “operationally defined”—as opposed to biologically available—pools. Commonly employed functional metrics including respiration and decomposition (Cleveland et al. 2006; Cleveland and Townsend 2006; Bradford et al. 2008) or microbial growth (Bååth et al. 2001) responses following nutrient additions, or assays of extracellular enzyme activities (Sinsabaugh et al. 1993; Sinsabaugh and Moorhead 1994) each yield independent, but sometimes contradictory information about the nature of microbial nutrient limitation. For this reason, the use of consistent sampling approaches and simultaneous assessments of multiple nutrient cycling metrics may yield better insight into the nutrients most limiting to biological communities when comparing among ecosystems (Sullivan et al. 2014).
Here, we examined microbial nutrient limitation and its relationship to soil nutrient pools over the course of early soil development at three biogeochemically different glacial forefield sites—Easton, Mendenhall, and Puca Glaciers. At these sites, previous studies have independently documented shifts in soil nutrients, microbial communities (Nemergut et al. 2007; Schmidt et al. 2008; Sattin et al. 2009; Knelman et al. 2012, 2014; Castle et al. 2016b), and microbial processes (Schmidt et al. 2008; Sattin et al. 2009) during the first ~ 100 years of ecosystem succession. To logically establish a foundation for understanding the nutrient(s) most limiting to developing soil microbial communities, we tested three hypotheses. First, we hypothesized that as soils develop, the size of nutrient pools and the relative abundances of soil nutrients shift, such that increasing available N pools result in the widening of available N:P ratios (H1). Second, we hypothesized that microbial nutrient limitation would reflect both pools and relative availabilities of N and P in developing glacial soils. As such, we hypothesized that the respiratory activity of soil heterotrophs would increase following +N additions in soils with low available N:P ratios and with +P additions in soils with relatively high available N:P ratios (H2). Third, we hypothesized that soil extracellular enzyme activities would increase during primary succession, and that the ratios of N- to P-hydrolyzing enzymes would be inversely related to nutrient availability (H3). Theoretically, increasing soil organic carbon (C) concentrations would provide additional substrate for enzyme activity; thus, we predicted that activities of all enzymes would increase over these short successional sequence (Schmidt et al. 2008; Sattin et al. 2009). However, over much longer periods of ecosystem succession, N- and P-hydrolyzing enzymes have been shown to negatively correlate with soil nutrient availability among sites that occupy age gradients (Olander and Vitousek 2000; Allison et al. 2007). Although the research presented here represents a fairly short period of succession, we predicted that relative patterns of nutrient acquisition would be similar such that as soil N accumulation outpaces P through succession, N:P ratios of available nutrient pools will increase and the N:P ratio of enzyme investment would decrease.
To address these hypotheses, we employed a twofold approach designed to directly assess the role of soil nutrients in regulating microbial function in the first ~ 100 years after glacial retreat. First, we used a laboratory-based nutrient fertilization experiment to identify the relative extent of N and P limitation to decomposition (Eviner et al. 2000; Sullivan et al. 2014), a key process performed by heterotrophic microbial communities in developed (e.g. Cleveland et al. 2006; Vitousek and Farrington 1997; Hobbie and Vitousek 2000) and in developing ecosystems (e.g., Göransson et al. 2011; Yoshitake et al. 2007). Second, we assayed the activities (Sinsabaugh et al. 2002; Stursova et al. 2006) and calculated ratios of N- and P-hydrolyzing enzymes (Sinsabaugh and Follstad Shah 2012).
Methods and materials
Study sites and sample collection
We conducted research at three previously characterized glacial forefields spanning a broad range of initial soil biogeochemistries: Easton Glacier (Castle et al. 2016a, b); Mendenhall Glacier (Nemergut et al. 2007; Sattin et al. 2009; Knelman et al. 2014), and Puca Glacier (Schmidt et al. 2008, 2012). Easton Glacier is located in Mt. Baker-Snoqualmie National Forest, Washington USA (48°44 N, 121°50 E, 1637 m.a.s.l.) and the underlying geology is volcanic andesite (Tabor et al. 2003). The Easton Glacier site is characterized by relatively high precipitation (1984–2009 mean: 198 cm year−1, NADP Site WA19) and moderate temperatures (1961–1990 mean annual minimum/maximum temperature: 3.9 °C/13.6 °C, Western Regional Climate Center ID 458715). The Mendenhall Glacier located in the Tongass National Forest of southeast Alaska, USA (58°26 N, 134°33 E; 75 m.a.s.l.) and the underlying metamorphic geology is composed of plagioclase, quartz and horneblend minerals (Alexander and Burt 1996). Mendenhall’s climate is characterized as a perhumid maritime climate with relatively high rainfall (2004–2009 mean precipitation: 153 cm year−1, NADP Site AK02) and mild temperatures (1965–1980 mean annual minimum/max temperature: − 1 °C/8 °C, Western Regional Climate Center ID 504110). Puca Glacier is located in the Laguna Sibinacocha Basin in the Cordillera Vilcanota of the Peruvian Andes (13°46 S, 71°04 W; 5000 m.a.s.l.). The geology of this site is composed of quartz and calcite rich sedimentary rock (Nemergut et al. 2007). Puca Glacier receives ~ 100 cm of annual precipitation and soil surface temperatures range from − 5 to 25 °C in the dry season (Nemergut 2004; Nemergut et al. 2007). Previous studies have detected photoautotrophic microorganisms (i.e., cyanobacteria) in soils of Easton and Mendenhall Glaciers in relatively low abundances (< 5%; Castle et al. 2016b); thus, these sites were categorized as heterotrophic successional sequences (Fierer et al. 2010). However, it is important to point out that only bacterial sequences have been assayed at these sites and we note it was recently shown that microscopic algae dominate soil communities of an Alaskan glacial chronosequence with geology similar to the Mendenhall Glacier (Darcy and Schmidt 2016). By contrast, Puca Glacier has relatively high abundances of cyanobacteria (Schmidt et al. 2008) and has been characterized as having a predominantly autotrophic soil microbial community.
In 2010 and again in 2011, we sampled from four transects positioned parallel to the terminus of each glacier (Knelman et al. 2014; Castle et al. 2016a, b). To account for different rates of glacial retreat across sites, transects were field-located on the basis of time since glacial retreat and degree of plant cover. The field transects were characterized as follows: transect 1 was located nearest to the glacial terminus and consisted of newly exposed glacial till that was less a year old; transect 2 was ~ 5 years old; transect 3 included some element of soil crust cover (lichens, mosses, cyanobacterial crust); and transect 4 was characterized by 25–50% plant cover. Plant cover was visually estimated in the field.
In 2010, soils were collected from each of the three sites within the snow-free growing season (August through September). We collected nine samples per transect, with the exception of Puca Glacier where only five samples were collected. For Easton and Mendenhall Glaciers, we created three independent pooled samples by systematically combining three individual samples of equal masses into a single sample. For Puca Glacier, we pooled samples by combining two individual samples at equal masses and the third sample was left un-pooled. For both years, ~ 500 to 1000 g of soil was collected into polyethylene bags from the soil surface (0–5 cm). Collection trowels were sterilized with ethanol and wiped clean with paper towels between every contact with the soil. Soils were transported on ice to the lab where they were refrigerated for several days prior to processing. Samples were homogenized and picked free of rocks and roots greater than 4 mm × 4 mm and subsamples were processed for soil chemical and physical analyses or stored frozen (− 4 °C) for extracellular enzyme analyses.
In July–September of 2011, transects from each site were relocated in the field by handheld GPS. Along each transect and at approximately the same sampling locations, six samples were collected using the before mentioned methods. From the six samples, we created three independent composites by pooling three groups of two samples. Fresh soils were sieved (4 mm × 4 mm) and stored under refrigeration for several days prior to the start of the nutrient addition incubation.
Soil biogeochemical characterization
Ammonium (NH4 +) and nitrate (NO3 −) were extracted from field-moist soil using 2M KCl (Mulvaney 1994) and concentrations were assessed colorimetrically (Weatherburn 1967; Doane and Horwath 2003) using a microplate reader (Biotek Inc., Winooski, VT, USA). Inorganic nitrogen is presented as the sum of NH4 +–N and NO3 −–N. Bio-available P (PO4 3−–P) concentrations were measured on air-dried soil by extracting 3–5 g of soil with 0.5 M sodium bicarbonate for 30 min. Extracts were filtered and analyzed colorimetrically using the ammonium molybdate–malachite green method adapted for microplate analysis (Jeannotte et al. 2004). Total C and N were measured on dried (48 h at 60 °C), pulverized samples using a CHN elemental analyzer 1110 (CE Instruments, Wigan, UK). Inorganic carbon was measured with a coulometer (UIC Inc., Joliet, IL, USA). Organic carbon was inferred as the difference between total carbon and inorganic carbon. Microbial biomass C and N were measured using a 0.5 M K2SO4 extraction on chloroform fumigated and unfumigated field-moist soils (Horwath and Paul 1994) and extracts were analyzed on a Shimadzu TOC-VCPN (Shimadzu, Columbia, MD, USA). Soil pH was determined using a digital pH meter (Fisher Scientific, Pittsburgh, USA) using a 1:2 ratio of air-dried soil to water. All soil chemical properties are expressed in terms of dry soil mass.
Nutrient addition incubation experiment
We assessed the nature of nutrient limitation (N, P, or N/P co-limitation) for soil microbes using a full-factorial nutrient addition microcosm experiment. We measured respiratory responses following nutrient amendments and calculated response ratios based on CO2 produced in nutrient treatments relative to no-nutrient controls. Microcosms consisted of a 10 g subsample of field soil in a cylindrical gas-tight glass incubation vial fitted with Teflon septa. To each microcosm, we added solutions of NH4NO3–N, KH2PO4–P, the two combined, or neither to create four different treatments (+N, +P, +NP, no-nutrient control). Nutrients were added in a 10:1 ratio of N to P with absolute quantities scaled to reflect the average amounts of soil microbial biomass at each successional stage (Ley et al. 2004). Transects 1 and 2 received low nutrient additions (5 μg N and 0.5 μg P dry soil−1), transect 3 received intermediate nutrient (10 μg N and 1 μg P dry soil−1), and transect 4 received high nutrient additions (20 μg N and μg 2 μg P dry soil−1). Water without nutrients was added to the no-nutrient controls treatment vials. All solutions were prepared with sterile, deionized water (autoclaved 120 °C for 1 h). For the duration of the experiment, microcosms were maintained at ~ 50% water holding capacity and were incubated at 13 °C, a temperature representative of field conditions, in dark incubation chambers in order to isolate the activity of soil heterotrophs.
On the first day of the experiment, nutrient solutions were added and microcosms equilibrated for a period of 6h prior to the first CO2 flux measurement. Subsequent respiration measurements were made on the second, fourth, and ninth days following nutrient additions. For each sampling event, microcosms were capped and incubated for 4–6 h in the dark before the headspace was mixed and sampled with a gas-tight needle and syringe. We immediately measured gas samples using gas chromatography (Shimadzu GC 2014, Columbia, USA). CO2 flux rates were calculated for each sampling event and using a basic numerical integration technique, cumulative CO2 fluxes were calculated across all experimental time points. Response ratios to nutrient additions represent CO2 produced in nutrient treatments (+N, +P, +NP) relative to no-nutrient controls.
Extracellular enzyme assays
Soils were assayed for three hydrolytic enzymes involved in N (β-1,4-N-acetylglucosaminidase; leucine amino peptidase) and P mineralization (phosphatases). Assays were conducted using established protocols (Saiya-Cork et al. 2002) as follows. Due to the sensitivity of enzyme activities to environmental pH conditions (German et al. 2011), assays were conducted at site-specific pH values. In short, soil slurries were made with ~ 2 g field soil and 125 mL of 50 mM sodium acetate buffer solution (pH ~ 5) for Easton Glacier soils and 50 mM sodium hydroxide (pH ~ 8) buffer for Mendenhall and Puca Glacier soils. An immersion blender was used to homogenize the soil and buffer solution. Sample slurries were stirred using a magnetic stirrer while samples were transferred to a 96-well plate. Samples and the appropriate controls, standards, and blanks were plated as described by Weintraub et al. (2007). We did not add NaOH to stop reactions (see methodological discussion by German et al. 2011). All assays were incubated at 13 °C for an average time of 24 h which is appropriate for cold, oligotrophic soils (Weintraub et al. 2007). Fluorometric measurements were made at 365 nm excitation and 450 nm emission. Enzyme activities were calculated per gram dry soil (nmol activity h−1 g soil−1). Finally, we examined relative nutrient acquisition by calculating ratios of enzyme acquisition of N relative to P-hydrolyzing enzymes (Sinsabaugh et al. 2008).
Statistical analyses
All data were tested for normality and homogeneity of variances. If necessary, soil characteristics, respiration, and enzyme data were log transformed prior to statistical analyses. Significance was determined at α = 0.05. Analyses were completed using R (R Core Team 2014). Differences in soil characteristics between sites and successional stages were tested using a multivariate analysis of variance (MANOVA) with the MASS package (Venables and Ripley 2002) in R. When significant interactions between site and transect were present, differences were explored using subsequent one-way ANOVAs followed by Tukey HSD post hoc tests.
Response ratios of microbial respiration following nutrient addition (+N, +P, +NP) were analyzed by a full-factorial ANOVA with site, transect, and nutrient addition as fixed effects using the lm function in R with a weighted least squares approach (Neter et al. 1996). To specify contrasts relevant to our study questions, we performed simultaneous inferences using the functions glht and cftest within the R package MULTCOMP V 1.3-7 (Hothorn et al. 2008). Extracellular enzyme activities and enzyme ratios were analyzed by separate one-way ANOVAs followed by TukeyHSD post hoc tests. Pearson’s correlation coefficients were calculated between enzyme activities, respiration responses, and soil chemical properties using the cor.test function in R on log transformed data.
Results
Soil biogeochemistry
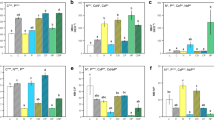
Over the short chronosequences considered in this study, the soil biogeochemical conditions at each glacial forefield developed in a unique way regardless of successional stage (site × transect interaction: P < 0.001). Easton Glacier soils had relatively high concentrations of available P and low concentrations of N, Mendenhall soils had relatively low concentrations of both available N and available P, and Puca Glacier soils had relatively high concentrations of available N and low concentrations of available P (Fig. 1; Table S1).
Soil characteristics of Easton, Mendenhall, and Puca Glaciers during four stages of early ecosystem succession. Points represent means (± 1 SE; n = 3) for each field transect. a Inorganic N (NH4 +–N + NO3 −–N), b available P (PO4 3−) are presented as μg g soil−1. c Organic C and d total N are presented as mg g soil−1. e Microbial biomass C and f N are presented as μg g soil−1. Stages of succession are indicated by transect numbers: 1 (0- to 1-year-old soil); 2 (5-year-old soil); 3 (biological soil crust cover); 4 (25–50% plant cover)
Though total soil N increased with increasing successional stage at all sites, biologically available N (NH4 +–N and NO3 −–N) only increased during succession at Mendenhall and Puca Glaciers (Fig. 1; Table S1). Available P (PO4 3−) did not vary consistently among sites, but we measured up to 70 times more available P in Easton Glacier soils compared to other sites (Fig. 1; Table S1). Differences in available N and P pools resulted in unique available N:P ratios across sites (Fig. 2; Table S2). At Easton Glacier, initially low available N:P ratios did not change with succession. At Mendenhall, available N:P ratios were significantly greater at the latest stage of succession compared to the younger soils (P = 0.0019). Lastly, in Puca Glacier soils initial N:P ratios were low, but increased to a maximum at the second transect before declining to a consistent value at the third and fourth transects. However, the N:P ratios in transects 2–4 were significantly greater than the ratio in transect one (P = 0.05).
Natural log ratios of soil available N:P (N [NH4 +–N + NO3 −–N] to P [PO4 3−]) for Easton, Mendenhall, and Puca Glaciers. Points represent means (± 1 SE; n = 3) for each field transect. Stages of succession indicated by transect numbers: 1 (0- to 1-year-old soil); 2 (5-year-old soil); 3 (biological soil crust cover); 4 (25–50% plant cover)
Nutrient limitation assays
Soil microbial respiration rates were generally higher when nutrients were added compared to unfertilized control samples, regardless of glacial site or successional status (Fig. 3). Overall, patterns of nutrient limitation at each site depended on the nutrient added (site × addition interaction: P < 0.001; Table 1), but could be characterized as predominantly N limited at the Easton Glacier, N- or N/P co-limited at the Mendenhall Glacier, and P limited at the Puca Glacier. However, the difference in microbial respiration response ratios among glacial sites depended on the successional stage of soil (site × transect interaction: P < 0.001). For Easton Glacier, +N additions elicited the greatest respiration responses in the youngest soils, and in later successional stages, +N, either alone or in combination with +P (Fig. 3; Fig. S1) drove the highest rates of respiration. For Mendenhall Glacier soils, additions of +NP produced greater respiration responses than additions either +N or +P alone, with the exception of the latest successional stage where no differences were observed. At Puca Glacier and over all successional stages, soil respiration responses were significantly higher in the presence of +P, alone or in combination with +N, than when fertilized with +N only.
Response ratios to nutrient additions (RRx) of heterotrophic soil respiration for full-factorial N × P fertilization of soil from Easton, Mendenhall and Puca Glaciers. For each nutrient, RRx was calculated as CO2 produced in nutrient treatments (+N, +P, +NP) relative to no-nutrient controls. Bars represent mean response ratios (± 1 SE; n = 3) of nutrient additions relative to a no-nutrient control. Stages of succession indicated by transect number: 1 (0- to 1-year-old soil); 2 (5-year-old soil); 3 (biological soil crust cover); 4 (25–50% plant cover). For each site, letters represent significant differences (P < 0.05) between the three bars
Extracellular enzyme activities
Generally, P-hydrolyzing extracellular enzyme activities were initially low and increased during succession at Easton (F = 15.53; P = 0.001), Mendenhall (F = 10.92; P = 0.003), and Puca Glaciers (F = 58.15; P < 0.0001), although absolute activities differed among sites (Fig. 4). By contrast, the activity of N-hydrolyzing enzymes increased continuously during succession at Mendenhall (F = 5.79; P = 0.021) and Puca Glaciers (F = 34.10; P < 0.0001). At Easton Glacier, N-hydrolyzing enzyme activities in the youngest soils were not significantly different from activity in later succession soils.
a Extracellular enzyme activities (EEA) of N-(β-1,4-N-acetylglucosaminidase + leucine amino peptidase) and P-hydrolyzing (phosphatase) enzymes. Bars represent mean activities (± 1 SE; n = 3) for each successional age. For each site, letters represent significant differences (P < 0.05) between the four bars. b N- to P-hydrolyzing extracellular enzyme ratios. Points represent mean ratios (± 1 SE; n = 3) for samples collected at each transect. Stages of succession indicated by transect numbers: 1 (0–1-year-old soil); 2 (5-year-old soil); 3 (biological soil crust cover); 4 (25–50% plant cover)
Across all three glacial sites, the N:P enzyme ratios were greatest in young successional soils. However, ratios were an order of magnitude lower at Easton Glacier relative to the other sites. While N:P enzyme ratios were unchanged during through succession at Easton Glacier, we observed a sharp decrease in the N:P ratio of hydrolyzing enzymes from young to old soils at Mendenhall (F = 5.56; P = 0.023) and Puca Glaciers (F = 25.57; P = 0.0002), as increases in P-hydrolyzing enzyme activities outpaced N-hydrolyzing enzymes through time. This decline was most pronounced in the first three stages of succession at Mendenhall and Puca. Activities of N- and P-hydrolyzing enzymes and N:P enzyme ratios were not systematically correlated with available soil nutrients across sites (Table 2). Rather, N- and P-hydrolyzing enzymes were only consistently positively correlated with soil total N among all three sites (Table 2).
Discussion
While studies of primary succession have typically focused on vegetated chronosequences where plant–soil feedbacks result in generally consistent patterns of nutrient accumulation (Matthews 1992; Walker and del Moral 2003), our data show that soil biogeochemistry is much more variable in the predominantly unvegetated stages of early succession. Nonetheless, variation in biogeochemical conditions among sites did play a strong role in structuring microbial activity in the early stages of soil development. We found idiosyncratic patterns of N and P limitation among sites that broadly reflected site-level differences in the relative availability of soil nutrients. Additionally, soil extracellular enzyme activities generally increased during succession, suggesting a progressive and increasing microbial reliance on organic matter derived nutrients as soils developed.
We hypothesized that soil nutrient pools and the abundance of N relative to P would change during soil development (H1), consistent with rapid N accumulation due to the free-living N-fixation often reported during early succession (Duc et al. 2009; Brankatschk et al. 2011) and symbiotic N-fixing shrubs and forbs in later succession (e.g., Halvorson et al. 1992). This hypothesis was partially supported by our results. Though absolute concentrations of soil total N varied among the three sites, our findings were consistent; total soil N, but not available soil N, was greater in the oldest successional stage compared to younger stages at all three of the sites (Fig. 1). However, we did not observe consistent patterns in soil available N, P and thus N:P ratios as we had predicted. Though not assessed directly in this study, parent geologies (i.e., sedimentary vs. volcanic) can vary widely in total P concentrations (e.g., Castle and Neff 2009), possibly leading to the disparities in soil available P that we observed among young proglacial sites. Further, mineralogy and soil pH (Frossard et al. 2000) have known controls on weathering rates and available P pools and may have contributed to site-level differences in available P concentrations. For example, previous studies at Puca Glacier have shown that although unweathered minerals contain high concentrations of P (610 μg/g; Schmidt et al. 2011), available P is very low (0.8 μg/g; Schmidt et al. 2011) in the relatively neutral-to-basic (pH ~ 8.0) substrates. Therefore, while the predicted increases in soil N occurred, neither the concentration of soil available N and P, nor the soil N:P ratio, changed consistently among the three chronosequences we measured. Though these measurements represent only a broad snapshot of soil nutrient pools without taking important seasonal, temporal, or microscale biogeochemical dynamics into account, which could also be important for microbial process and the development of microbial nutrient limitation, these results provide evidence for variable biogeochemical conditions during primary succession.
Microbial nutrient limitation during primary succession
While soil N:P ratios did not consistently change as predicted during early primary succession, the initial soil available N:P ratio seemed to be a reliable indicator of nutrient limitation thresholds of soil respiration. Decomposition was limited by N at the site with the lowest soil N:P ratio (Easton Glacier), whereas decomposition was limited by P at the site with the highest N:P ratio (Puca Glacier). At the site with an intermediate soil N:P ratio (Mendenhall Glacier), decomposition appeared to be co-limited by N and P. In that sense, our findings were broadly consistent with biogeochemical theory, though they may also reflect compositional and physiological differences in microbial communities among sites. For example, previous work at the Puca Glacier has demonstrated that the predominance of cyanobacteria (Nemergut et al. 2007; Schmidt et al. 2008; Knelman et al. 2014) and high rates of free-living N fixation (Schmidt et al. 2008) quickly lead to high soil N concentrations, widening available soil N:P ratios, and strong P limitation even in the youngest soils. Complimentary studies at the Mendenhall and Easton Glaciers have shown that autotrophic cyanobacteria and other heterotrophic diazotrophs exist in young soils, though at substantially lower relative abundances (Sattin et al. 2009; Knelman et al. 2012; Castle et al. 2016b). However, relatively high rates of free-living N-fixation have also been documented within the first 6 years of succession at Mendenhall Glacier (Sattin et al. 2009; Knelman et al. 2012). Thus, it appears that despite the observed variability in soil nutrient dynamics in these early stages of succession, N-fixing microorganisms may be consistent members of the early successional community contributing to the increasing N pools across geochemically distinct primary succession gradients. Such findings would suggest an important role for free-living N-fixing microorganisms in early succession community assembly, akin to the role that N-fixing plant species play in the facilitation of plant species that colonize later in succession (Halvorson et al. 1992; Menge and Hedin 2009).
Broadly, the patterns of nutrient limitation across glacial sites reflected differences in the relative availability of soil nutrients, but the nature of nutrient limitation at each site did not change through succession as we had originally hypothesized (H2). Instead, consistent patterns of nutrient limitation through succession may reflect the relatively subtle shifts in pools of soil N and P that were observed. Regardless, these findings from the earliest stages of succession contrast with the community-wide shifts in soil microbial nutrient limitation often observed over longer timescales of ecosystem development (e.g., Reed et al. 2011). Interestingly, results presented here are unlike those from other studies, which have shown a heterotrophic response to nutrients necessarily depends on the addition of labile C (e.g., Yoshitake et al. 2007; Göransson et al. 2011) and suggest that C may be an overarching constraint on heterotrophic microbial community activity during succession. Here, the addition of nutrients alone elicited strong respiratory responses of soil microorganisms even in the low soil organic C conditions associated with early primary succession conditions (Fig. 1). While low in concentration, the chemically diverse soil organic C present in unvegetated glacial till (Castle et al. 2016b) may alleviate C constraints on the early successional soil microbial community as previous studies have suggested (Bardgett et al. 2007; Anesio et al. 2008). Regardless, our study suggests that decomposition of such organic matter can be enhanced when N and P are added.
Microbial nutrient acquisition during primary succession
In general, extracellular enzyme activity increased through succession, with the greatest enzyme activities coinciding with successional stages that had the greatest plant cover. In addition, with the exception of Mendenhall Glacier, enzyme activities also increased with increasing soil organic C concentrations (H3). The rise in soil enzyme activity is consistent with the idea that belowground decomposers increasingly rely on the mineralization of organically bound nutrients as organic matter content of soil increases (Parfitt et al. 2005; Sinsabaugh et al. 2008), and has been observed over both short and long time scales of soil development (Allison et al. 2007; Schmidt et al. 2008; Sattin et al. 2009). It is possible that shifts in enzyme activities are the result of increasing microbial biomass, changes in microbial composition (Waldrop et al. 2000; Gallo et al. 2004) and associated shifts in the physiological capacity of decomposer communities. In the present study, we observed increases in microbial biomass C with succession at all sites. Further, previous evidence from these glacial sites has shown strong successional shifts in soil bacterial community composition (Sattin et al. 2009; Nemergut et al. 2007; Schmidt et al. 2008; Knelman et al. 2014; Castle et al. 2016b). Though not yet characterized for these sites, it has been observed that bacterial populations dominate newly exposed glacial sediments, while fungi become dominant in later succession (i.e., fungal to bacterial ratios increase through succession as plant colonization supplies organic matter to soil; e.g., Allison et al. 2007; Fernández-Martínez et al. 2017). Along with such shifts microbial community structure, we would predict changes in enzyme production and allocation as others have observed in secondary succession ecosystems (e.g., Cline and Zak 2015).
While total enzyme activity increased through succession, our hypothesis that the relative allocation to N- and P-hydrolyzing enzymes would reflect patterns in soil nutrient availability was not supported at all sites. For example, while soil nutrient pools and results from the nutrient addition experiment suggest N limitation in Easton Glacier soils, N-hydrolyzing enzymes were surprisingly low here relative to the other two sites. It is possible that the relatively low N-hydrolyzing activity in Easton Glacier soils throughout succession is caused by a strong influence of pH on enzyme activities (Dick and Tabatabai 1987; Allison 2006; Sinsabaugh et al. 2008). While leucine amino peptidase, a N-hydrolyzing enzyme assayed in this study, performs optimally under basic (pH ~ 9.0) conditions, the average soil pH of Easton Glacier was low throughout succession (pH ~ 4.2 to 5.5). However, we note that past studies measuring N acquiring enzymes along retreating glaciers at pH conditions similar to what we present here (Allison et al. 2007), have seen no relationship over time with available N concentrations. Thus, this discrepancy between available nutrients and enzyme activities in mineral soil may be consistent with the notion that the soil physical properties may be a proximate limitation to microbial processes beyond nutrient supply (Schimel and Schaffer 2012). If so, this would suggest that enzymes, particularly N-acquiring enzymes, are a less reliable predictor of microbial nutrient limitation as has been suggested in the past (McGill and Cole 1981).
In more developed ecosystems, ratios of enzymatic nutrient acquisition have been used as an indicator of the resource(s) most limiting to decomposer communities (Sinsabaugh et al. 2009). In this study, decreasing enzyme N:P ratios from young to old glacial soils at Mendenhall and Puca Glaciers (and, to a lesser degree Easton Glacier) reflected P-hydrolyzing enzyme activities that increased more through succession relative to N-hydrolyzing enzymes. Regardless, we note that the enzyme N:P ratios measured in the latest stage of succession for Mendenhall (N:P = 0.54) and Puca Glaciers (N:P = 0.94) approach the global value (N:P = 0.44) presented by Sinsabaugh et al. (2009) in a synoptic study of more developed soils.
Conclusions
Here, we highlight how early stages of ecosystem succession may display wider variation in edaphic characteristics than more developed vegetated soils, resulting in idiosyncratic patterns of microbial nutrient limitation among sites. Importantly, our work challenges the assumption that belowground patterns of limitation follow aboveground patterns in the earliest stages of primary succession. Although we suggest that nutrient limitation of microbial communities in early succession may not be simply generalized across successional environments, as has more often been the case with plant communities, our work supports the role of soil nutrient capital as well as microbial metabolic diversity in providing mechanistic explanations of patterns in microbial community activity through succession.
References
Alexander EB, Burt R (1996) Soil development on moraines of Mendenhall Glacier, southeast Alaska. 1. The moraines and soil morphology. Geoderma 72:1–17
Allison SD (2006) Soil minerals and humic acids alter enzyme stability: implications for ecosystem processes. Biogeochemistry 81:361–373
Allison VJ, Condron LM, Peltzer DA, Richardson SJ, Turner BL (2007) Changes in enzyme activities and soil microbial community composition along carbon and nutrient gradients at the Franz Josef chronosequence, New Zealand. Soil Biol Biochem 39:1770–1781
Anesio AM, Hodson AJ, Fritz A, Psenner R, Sattler B (2008) High microbial activity on glaciers: importance to the global carbon cycle. Glob Change Biol 15:955–960
Bardgett RD, Richter A, Bol R, Garnett MH, Bäumler R, Xu X, Lopez-Capel E, Manning DA, Hobbs PJ, Hartley IR, Wanek W (2007) Heterotrophic microbial communities use ancient carbon following glacial retreat. Biol Lett 3:487–490
Bååth E (2001) Estimation of fungal growth rates in soil using 14C-acetate incorporation into ergosterol. Soil Biol Biochem 33:2011–2018
Bradford MA, Fierer N, Reynolds JF (2008) Soil carbon stocks in experimental mesocosms are dependent on the rate of labile carbon, nitrogen and phosphorus inputs to soils. Funct Ecol 22:964–974
Brankatschk R, Toewe S, Kleineidam K, Schloter M, Zeyer J (2011) Abundances and potential activities of nitrogen cycling microbial communities along a chronosequence of a glacier forefield. ISME J 5:1025–1037
Castle SC, Neff JC (2009) Plant response to nutrient availability across bedrock geologies. Ecosystems 12:101–113
Castle SC, Lekberg Y, Affleck D, Cleveland CC (2016a) Soil abiotic and biotic controls on plant performance during primary succession in a glacial landscape. J Ecol 104:1555–1565
Castle SC, Nemergut DR, Grandy AS, Leff JW, Graham EB, Hood E, Schmidt SK, Wickings K, Cleveland CC (2016b) Biogeochemical drivers of microbial community convergence across actively retreating glaciers. Soil Biol Biochem 101:74–84
Chapin FS, Walker LR, Fastie CL, Sharman LC (1994) Mechanisms of primary succession following deglaciation at Glacier Bay, Alaska. Ecol Monogr 64:149–175
Cleveland CC, Townsend AR (2006) Nutrient additions to a tropical rain forest drive substantial soil carbon dioxide losses to the atmosphere. Proc Nat Acad Sci 103:10316–10321
Cleveland C, Liptzin D (2007) C:N: P stoichiometry in soil: is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 85:235–252
Cleveland CC, Reed SC, Townsend AR (2006) Nutrient regulation of organic matter decomposition in a tropical rain forest. Ecology 87:492–503
Cline LC, Zak DR (2015) Soil microbial communities are shaped by plant-driven changes in resource availability during secondary succession. Ecology 96:3374–3385
Craine JM, Morrow C, Fierer M (2007) Microbial nitrogen limitation increases decomposition. Ecology 88:2105–2113
Crews TE, Kitayama K, Fownes JH, Riley RH, Herbert DA, Mueller-Dombois D, Vitousek PM (1995) Changes in soil-phosphorus fractions and ecosystem dynamics across a long chronosequence in Hawaii. J Ecol 76:1407–1424
Darcy JL, Schmidt SK (2016) Nutrient limitation of microbial phototrophs on a debris-covered glacier. Soil Biol Biochem 95:156–163
Dick WA, Tabatabai MA (1987) Kinetics and activities of phosphatase-clay complexes. Soil Sci 143:5–15
Doane T, Horwath W (2003) Spectrophotometric determination of nitrate with a single reagent. Anal Lett 36:2713–2722
Duc L, Noll M, Meier BE, Burgmann H, Zeyer J (2009) High diversity of diazotrophs in the forefield of a receding alpine glacier. Microb Ecol 57:179–190
Elser JJ, Bracken ME, Cleland EE, Gruner DS, Harpole WS, Hillebrand H, Ngai JT, Seabloom EW, Shurin JB, Smith JE (2007) Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett 10:1135–1142
Eviner VT, Chapin FS III, Vaughn CE (2000) Nutrient manipulations in terrestrial ecosystems. In: Sala OE, Jackson RB, Mooney HA, Howarth RW (eds) Methods in ecosystem science. Springer, New York, pp 291–307
Fernández-Martínez MA, Pérez-Ortega S, Pointing SB, Green TA, Pintado A, Rozzi R, Sancho LG, de los Ríos A (2017) Microbial succession dynamics along glacier forefield chronosequences in Tierra del Fuego (Chile). Polar Biol 40:1939–1957
Fierer N, Nemergut DR, Knight R, Craine JM (2010) Changes through time: integrating microorganisms into the study of succession. Res Microbiol 161:635–642
Frossard E, Condron LM, Oberson A, Sinaj S, Fardeau JC (2000) Processes governing phosphorus availability in temperate soils. J Environ Qual 29:15–23
Gallo ME, Amonette R, Lauber C, Sinsabaugh RL, Zak DR (2004) Microbial community structure and oxidative enzyme activity in Nitrogen-amended north temperate forest soils. Microb Ecol 48:218–229
German DP, Weintraub MN, Grandy AS, Lauber CL, Rinkes ZL, Allison SD (2011) Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol Biochem 43:1387–1397
Göransson H, Venterink HO, Bååth E (2011) Soil bacterial growth and nutrient limitation along a chronosequence from a glacier forefield. Soil Biol Biochem 43:1333–1340
Halvorson JJ, Franz EH, Smith JL, Black RA (1992) Nitrogenase activity, nitrogen fixation, and nitrogen inputs by lupines at Mount St Helens. Ecology 73:87–98
Harpole WS, Ngai JT, Cleland EE, Seabloom EW, Borer ET, Bracken ME, Elser JJ, Gruner DS, Hillebrand H, Shurin JB, Smith JE (2011) Nutrient co-limitation of primary producer communities. Ecol Lett 14:852–862
Hobbie SE, Vitousek PM (2000) Nutrient limitation of decomposition in Hawaiian forests. Ecology 81:1867–1877
Horwath W, Paul E (1994) Microbial biomass. In: Weaver RW, Angle JS, Bottomley PS (eds) Methods of soil analysis, part 2: microbiological and biochemical properties. Soil Science Society of America, Fitchburg, pp 754–760
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363
Jeannotte R, Sommerville DW, Hamel C, Whalen JK (2004) A microplate assay to measure soil microbial biomass phosphorus. Biol Fertil Soils 40:201–205
King AJ, Meyer AF, Schmidt SK (2008) High levels of microbial biomass and activity in unvegetated tropical and temperate alpine soils. Soil Biol Biochem 40:2605–2610
Knelman JE, Legg TM, O’Neill SP, Washenberger CL, González A, Cleveland CC, Nemergut DR (2012) Bacterial community structure and function change in association with colonizer plants during early primary succession in a glacier forefield. Soil Biol Biogeochem 46:172–180
Knelman JE, Schmidt SK, Lynch RC, Darcy JL, Castle SC, Cleveland CC, Nemergut DR (2014) Nutrient addition dramatically accelerates microbial community succession. PloS One 9: e102609. doi:10.1371/journal.pone.0102609
Laliberté E, Turner BL, Costes T, Pearse SJ, Wyrwoll K-H, Zemunik G, Lambers H (2012) Experimental assessment of nutrient limitation along a 2-million year dune chronosequence in the south-western Australia biodiversity hotspot. J Ecol 100:631–642
Lebauer DS, Treseder KK (2008) Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 89:371–379
Leff JW, Jones SE, Prober SM, Barberán A, Borer ET, Firn JL, Harpole WS, Hobbie SE, Hofmockel KS, Knops JM, McCulley RL (2015) Consistent responses of soil microbial communities to elevated nutrient inputs in grasslands across the globe. Proc Natl Acad Sci 112(35):10967–10972
Ley R, Williams M, Schmidt S (2004) Microbial population dynamics in an extreme environment: controlling factors in talus soils at 3750 m in the Colorado Rocky Mountains. Biogeochemistry 68:313–335
Matthews JA (1992) The ecology of recently-deglaciated terrain: a geoecological approach to glacier forelands and primary succession. Cambridge University Press, Cambridge
McGill WB, Cole CV (1981) Comparative aspects of cycling of organic C, N, S and P through soil organic matter. Geoderma 26:267–286
Menge DNL, Hedin LO (2009) Nitrogen fixation in different biogeochemical niches along a 120,000-year chronosequence in New Zealand. Ecology 90:2190–2201
Mulvaney RL (1994) Nitrogen—inorganic forms. In: Sparks DL (ed) Methods of soil analysis, part 3: chemical methods. Soil Science Society of America, Fitchburg, pp 1129–1131
Nemergut, DR (2004) Evolution and ecology of high altitude soil microbial communities. Ph.D. dissertation, University of Colorado, Boulder, CO
Nemergut DR, Anderson SP, Cleveland CC, Martin AP, Miller AE, Seimon A, Schmidt SK (2007) Microbial community succession in an unvegetated recently deglaciated soil. Microb Ecol 53:110–122
Neter J, Kutner MH, Nachtsheim CJ, Wasserman W (1996) Applied linear statistical models, 4th edn. Irwin McGraw-Hill, Chicago
Ohtonen R, Fritze H, Pennanen T, Jumpponen A, Trappe J (1999) Ecosystem properties and microbial community changes in primary succession on a glacier forefront. Oecologia 119:239–246
Olander LP, Vitousek PM (2000) Regulation of soil phosphatase and chitinase activity by N and P availability. Biogeochemistry 49:175–191
Parfitt RL, Ross DJ, Coomes DA, Richardson SJ, Smale MC, Dahlgren RA (2005) N and P in New Zealand soil chronosequences and relationships with foliar N and P. Biogeochemistry 75:305–328
Peltzer D, Wardle D, Allison V, Baisden W (2010) Understanding ecosystem retrogression. Ecol Monogr 80:509–529
R Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Ramirez KS, Craine JM, Fierer N (2012) Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Glob Change Biol 18:1918–1927
Reed SC, Vitousek PM, Cleveland CC (2011) Are patterns in nutrient limitation belowground consistent with those aboveground: results from a 4 million year chronosequence. Biogeochemistry 106:323–336
Reynolds H, Packer A, Bever J, Clay K (2003) Grassroots ecology: plant-microbe-soil interactions as drivers of plant community structure and dynamics. Ecology 84:2281–2291
Richardson SJ, Peltzer DA, Allen RB, McGlone MS, Parfitt RL (2004) Rapid development of phosphorus limitation in termperate rainforest along the Franz Josef chronosequence. Oecologia 139:267–276
Saiya-Cork K, Sinsabaugh R, Zak D (2002) The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol Biochem 34:1309–1315
Sattin S, Cleveland C, Hood E, Reed S (2009) Functional shifts in unvegetated, perhumid, recently-deglaciated soils do not correlate with shifts in soil bacterial community composition. J Microbiol 47:673–681
Schimel JP, Schaffer SM (2012) Microbial control over carbon cycling in soil. Front Microbiol 3:1–11
Schmidt SK, Reed SC, Nemergut DR, Grandy AS, Cleveland CC, Weintraub MN, Hill AW, Costello EK, Meyer AF, Neff JC, Martin AM (2008) The earliest stages of ecosystem succession in high-elevation (5000 metres above sea level), recently deglaciated soils. Proc R Soc B Biol Sci 275:2793–2802
Schmidt SK, Nemergut DR, Sowell P, Reed SC, Cleveland CC (2011) Estimating phosphorus availability for microbial growth in an emerging landscape. Geoderma 163:135–140
Schmidt SK, Nemergut DR, Todd BT, Lynch RC, Darcy JL, Cleveland CC, King AJ (2012) A simple method for determining limiting nutrients for photosynthetic crusts. Plant Ecol Divers. doi:10.1080/175508742012738714
Selmants P, Hart S (2010) Phosphorus and soil development: does the Walker and Syers model apply to semiarid ecosystems? Ecology 91:474–484
Sigler WV, Zeyer J (2004) Colony-forming analysis of bacterial community succession in deglaciated soils indicates pioneer stress-tolerant opportunists. Microb Ecol 48:316–323
Sinsabaugh RL (1994) Enzymic analysis of microbial patterns and process. Biol Fertil Soils 17:69–74
Sinsabaugh RL, Follstad Shah JJ (2012) Ecoenzymatic stoichiometry and ecological theory. Annu Rev Ecol Evol Syst 43:313–343
Sinsabaugh RL, Antibus RK, Linkins AE, Rayburn L, Repert D, Weiland T (1993) Wood decomposition: nitrogen and phosphorus dynamics in relation to extracellular enzyme activity. Ecology 74:1586–1593
Sinsabaugh RL, Carreiro MM, Repert DA (2002) Allocation of extracellular enzymatic activity in relation to litter composition, N deposition, and mass loss. Biogeochemistry 60:1–24
Sinsabaugh RL, Lauber CL, Weintraub MN, Ahmed B, Allison SD, Crenshaw C, Contosta AR, Cusack D, Frey S, Gallo ME, Gartner TB (2008) Stoichiometry of soil enzyme activity at global scale. Ecol Lett 11:1252–1264
Sinsabaugh RL, Hill BH, Follstad Shah JJ (2009) Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 462:795–798
Stursova M, Crenshaw CL, Sinsabaugh RL (2006) Microbial responses to long-term N deposition in a semiarid grassland. Microb Ecol 51:90–98
Sullivan BW, Alvarez-Clare S, Castle SC, Porder S, Reed SC, Schreeg L, Townsend AR, Cleveland CC (2014) Assessing nutrient limitation in complex forested ecosystems: alternatives to large-scale fertilization experiments. Ecology 95:668–681
Tabor RW, Haugerud RA, Hildreth W, Brown EH (2003) Geologic map of the Mount Baker 30 × 60 minute quadrangle. Washington US Geological Survey Map I-2660, scale 1:100,000
Tscherko D, Rustemeier J, Richter A, Wanek W, Kanedler E (2003) Functional diversity of the soil microflora in primary succession across two glacier forelands in the Central Alps. Eur J Soil Sci 54:685–696
Turner BL, Laliberté E (2014) Soil development and nutrient availability along a 2 million-year coastal dune chronosequence under species-rich mediterranean shrubland in southwestern Australia. Ecosystems 100:631–642
Venables WN, Ripley BD (2002) Modern applied statistics with S, 4th edn. Springer, New York
Vitousek PM (2004) Nutrient cycling and limitation: Hawai’i as a model system. Princeton University Press, Princeton
Vitousek P, Farrington H (1997) Nutrient limitation and soil development: experimental test of a biogeochemical theory. Biogeochemistry 37:63–75
Vitousek PM, Walker LR, Whiteaker LD, Matson PA (1993) Nutrient limitations to plant growth during primary succession in Hawaii Volcanoes National Park. Biogeochemistry 23:197–215
Waldrop MP, Balser TC, Firestone MK (2000) Linking microbial community composition to function in a tropical soil. Soil Biol Biochem 32:1837–1846
Walker L, del Moral R (2003) Primary succession and ecosystem rehabilitation. Cambridge University Press, New York
Walker T, Syers J (1976) Fate of phosphorus during pedogenesis. Geoderma 15:1–19
Wardle D, Bardgett R, Klironomos J, Setälä H, van der Putten W, Wall D (2004) Ecological linkages between aboveground and belowground biota. Science 304:1629–1633
Weatherburn MW (1967) Phenol-hypochlorite reaction for determination of ammonia. Anal Chem 39:971–974
Weintraub M, Scott-Denton L, Schmidt S, Monson R (2007) The effects of tree rhizodeposition on soil exoenzyme activity, dissolved organic carbon, and nutrient availability in a subalpine forest ecosystem. Oecologia 154:327–338
Yoshitake S, Uchida M, Koizumi H, Nakatsubo T (2007) Carbon and nitrogen limitation of soil microbial respiration in a High Arctic successional glacier foreland near Ny-Ålesund, Svalbard. Polar Res 26:22–30
Acknowledgements
We would like to thank R. Callaway, S. Dobrowski, A. Larson, Y. Lekberg, A. Marklein, M. Nasto, and three anonymous reviewers for comments on early drafts of this manuscript. Authors declare no conflict of interest. This work was supported by a National Science Foundation Grant (NSF DEB-0922306) made to CC, EH, DN, and SS.
Author information
Authors and Affiliations
Contributions
SCC and CCC conceived and designed the experiments. SCC performed the experiments and analyzed the data. SCC, BWS, JK, and CCC wrote the manuscript; other authors provided editorial advice and all authors approved of the final version of the manuscript.
Corresponding author
Additional information
Communicated by Jason P. Kaye.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Castle, S.C., Sullivan, B.W., Knelman, J. et al. Nutrient limitation of soil microbial activity during the earliest stages of ecosystem development. Oecologia 185, 513–524 (2017). https://doi.org/10.1007/s00442-017-3965-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-017-3965-6