Abstract
Purpose
To compare treatment results between fractionated gamma knife radiosurgery (f-GKRS) and staged gamma knife radiosurgery (s-GKRS) for mid-to-large brain metastases (BMs).
Methods
We retrospectively analyzed data of patients with medium (4–10 mL) to large (> 10 mL) BMs who underwent s-GKRS or f-GKRS between March 2008 and September 2022. Patients were treated with (i) s-GKRS before May 2018 and (ii) f-GKRS after May 2018. Patients who underwent follow-up magnetic resonance imaging at least once were enrolled. Case-matched studies were conducted by applying propensity score matching to minimize treatment selection bias and potential confounding. Local control (LC) was set as the primary endpoint and overall survival (OS) as the secondary endpoint.
Results
This study included 129 patients with 136 lesions and 70 patients with 78 lesions who underwent s-GKRS and f-GKRS, respectively. Overall, 124 lesions (62 lesions in each group) were selected in the case-matched group. No differences were observed in the 6-month and 1-year cumulative incidences of LC failure between the s-GKRS and f-GKRS groups (15.6% vs. 15.9% at 6 months and 25.6% vs. 25.6% at 1 year; p = 0.617). One-year OS rates were 62.6% (95% confidence interval [CI]: 45.4–75.7%) and 73.9% (95% CI: 58.8–84.2%) in the s-GKRS and f-GKRS groups, respectively. The post-GKRS median survival time was shorter in the s-GKRS group than in the f-GKRS group (17 vs. 36 months), without significance (p = 0.202).
Conclusions
This is the first study to compare f-GKRS and s-GKRS in large BMs. Fractionation is as effective as staged GKRS for treating mid-to-large BMs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gamma knife radiosurgery (GKRS) was originally designed as a single-fraction irradiation with the Leksell frame fixation system, which contributes to treatment accuracy. It is a less invasive and safer treatment for brain metastases than surgical procedures. However, Leksell frame fixation is considered relatively invasive compared to conventional radiation therapy equipment with mask fixation, and its therapeutic effect is limited to large brain metastases, rather than smaller lesions [1]. In studies reporting treatment results for large metastases, the 1-year local control (LC) is reported to be 66.6–84.6%, with a high frequency of radiation necrosis (38.8–48.0%) [2,3,4,5]. Therefore, radiation-induced toxicity is a challenge in increasing tumor control rates. As a result, staged GKRS (s-GKRS) has been established to maintain therapeutic efficacy and reduce the risk of adverse events [6,7,8].
The Leksell Gamma Knife® Icon™ is designed with optional thermoplastic mask fixation. This novel technique allows frameless fractionated GKRS (f-GKRS) to be applied to larger lesions. Hypofractionated radiotherapy (HFRT) involves delivery of high cumulative radiation doses to large lesions while minimizing exposure to normal brain tissue. Pre-existing treatment equipment has been used to treat larger brain metastases using HFRT, and its efficacy and safety have been demonstrated [9]. Theoretically, this technique is expected to be adaptable with frameless f-GKRS for large brain metastases. However, only few publications have reported the preliminary outcomes of single-center studies [10,11,12], with small sample sizes and limited follow-up durations. While s-GKRS uses the tentative volume shrinkage of the preceding irradiation, f-GKRS uses the biological benefits of inter-fraction tissue repair in normal tissue injuries. Although these two different methods were designed to treat large brain metastases, their treatment outcomes have not been compared yet. This retrospective single-center study compared the treatment outcomes of s-GKRS and f-GKRS for large brain metastases using propensity score matching.
Methods
Data source and study population
An institutional database was used to investigate patient information and clinical outcomes. Patients with medium (4–10 mL) to large (> 10 mL) brain metastases who underwent s-GKRS or f-GKRS between March 2008 and September 2022 were identified. Patients were treated with (i) s-GKRS before Icon™ was installed in May 2018, and with (ii) f-GKRS after May 2018. Patients who underwent follow-up magnetic resonance imaging (MRI) at least once were included. Patients with (i) recurrent lesions previously treated with GKRS, (ii) a history of whole-brain radiotherapy, (iii) post-surgical lesions (tumor bed lesions), and (iv) those without post-GKRS follow-up images were excluded.
Age, sex, Karnofsky performance status scores, primary cancer site and status, extracranial metastases, neurological symptoms, tumor location, target volume, target maximum diameter, marginal dose, maximum dose, follow-up MRI images, adverse events, and status at the last visit were inspected.
Radiosurgical indications and techniques
As treatment equipment model, f-GKRS was performed using Icon™ and s-GKRS using Perfexion™.
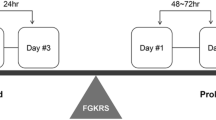
For s-GKRS, in principle, two-staged GKRS was selected for medium tumors and three-staged GKRS for large tumors. However, this was not always the case, depending on the patient’s condition and treatment schedule of the primary lesion. On the day before treatment, gadolinium-enhanced thin-slice T1-weighted and T2-weighted MRI images were obtained. The Leksell frame G (Elekta Instruments AB) was applied for patient immobilization. The frame was placed on the patient’s head under local anesthesia. Stereotactic contrast-enhanced MRI covering the whole brain was routinely used as a reference. This study followed the radiosurgical technique criteria of the study conducted by the Japanese Leksell Gamma Knife Society (JLGK1601) [7]. The prescription dose of each fraction was set to 11.8–14.2 Gy for two-staged GKRS and to 9.0–11.0 Gy for three-staged GKRS. The treatment intervals were completed within 6 weeks, with ≥ 12 days between each fraction (Supplemental Digital Content 1a). The planning target volume was designed to corresponded with the gross total volume defined on gadolinium-enhanced thin-slice T1-weighted images. For f-GKRS, in principle, more than five fractions (3.0–5.0 Gy/fraction) were selected for patients with relatively large tumors and five or fewer fractions (6.0–9.5 Gy/fraction) for mid-sized tumors. The number of fractions was adjusted according to the patient’s condition and treatment schedule. The IconTM-specific thermoplastic mask was molded 1 day before the first fraction and used for patient immobilization during treatment. MRI (gadolinium-enhanced thin-slice T1-weighted and T2-weighted MRI images) was performed within 3 days before the first fraction and used to plan the treatment. Irradiation was performed on consecutive days. The threshold of high definition motion management was set to 1.0-1.5 mm in all cases. Interfractional evaluation by MRI was performed when the treatment continued for > 1 week or more than five fractions, and the treatment plan was modified if necessary, as reported previously [13]. According to a previous report of large brain metastases treated using linear accelerator (LINAC)-based devices [14], the prescribed doses were determined within 40.0–60.0 Gy in biologically effective doses (BED), using a linear-quadratic model with an alpha-beta ratio of 10 (BED10) [15]. The treatment was fractionated into 3–15 fractions (Supplemental Digital Content 1b). The planning target volume was determined by adding 0.5-2.0 mm margin to the gross total volume defined on gadolinium-enhanced thin-slice T1-weighted images. All treatment plans were meticulously created and revised using Leksell GammaPlan (Elekta Instruments AB) by the same senior physician (A.A.).
Endpoints and post-GKRS follow-up
Follow-up MRI was performed 1–3 months after treatment and every few months thereafter. LC was set as the primary endpoint, and radiological deterioration of the treated lesion was considered LC failure. LC failure was defined as ≥ 20% enlargement of the targeted lesion’s diameter on contrast-enhanced areas on T1-weighted images.
The secondary endpoint was overall survival (OS). Adverse events were evaluated using the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Radiation necrosis was defined as LC failure with additional neuroimaging confirmation, such as positron emission tomography, single-photon emission computed tomography, specific MRI sequences (magnetic resonance perfusion or arterial spin label), and pathological confirmation.
Statistical analysis
Participants’ baseline characteristics are summarized as frequencies and proportions for categorical data and as medians and ranges for continuous variables. The baseline characteristics of the two groups were compared using Fisher’s exact test. LC and OS were analyzed using Kaplan–Meier curves, and OS rates were compared using the log-rank test. Case-matched studies were conducted by applying propensity score matching to minimize treatment selection bias and potential confounding. A one-to-one nearest neighbor matching algorithm was used without replacement within caliper widths of 0.2. Based on clinical knowledge and previous reports, 11 possible covariates were selected for their potential association. In addition, the Fine and Gray test was performed to calculate the cumulative incidences of LC failure, and death was considered a competing risk factor. As subgroup analysis, cumulative incidences of LC failure of s-GKRS vs. f-GKRS (≤5 fraction), and s-GKRS vs. f-GKRS (>5 fraction) were inspected using propensity score matching for each subgroup.
All statistical analyses were performed using the R statistical software (R version 4.1.0; The R Foundation for Statistical Computing; Vienna, Austria). Statistical significance was set at p-values of < 0.05.
Results
Overall, 2,887 patients underwent GKRS for metastatic brain tumors between March 2008 and December 2022; 2,002 patients were treated before Icon™ was installed in May 2018 and 885 were treated afterward. Lesions with mid-to-large (volume: >4 mL) brain metastases treated using s-GKRS or f-GKRS were identified. This study included 129 patients with 136 lesions and 70 patients with 78 lesions who underwent s-GKRS and f-GKRS, respectively.
The baseline characteristics of the s-GKRS and f-GKRS groups are summarized in Table 1. The median volumes of the treated target were 9.6 (range: 4.1–47.1) mL and 7.4 (4.0–55.8) mL (p < 0.001), and the median maximum diameters were 30 (range: 10–54) mm and 28 (19–54) mm (p = 0.0012) in s-GKRS and f-GKRS, respectively. There were 63 (46.3%) and 21 (26.9%) large metastases in the s-GKRS and f-GKRS groups, respectively (p = 0.006). The median follow-up times after GKRS were 7.0 (range: 1.0–110.0) months and 7.0 (1.0–42.0) months in the s-GKRS and f-GKRS groups, respectively (p = 0.254).
In the s-GKRS group, 81 (59.6%) and 55 (40.4%) lesions underwent two- and three-staged GKRS, respectively. The median prescription doses of each fraction were 13.0 (10.0–14.0) Gy and 14.0 (13.0–15.0) Gy in two-staged GKRS and were 10.0 (8.0–10.0) Gy, 10.0 (8.0–10.0) Gy, and 10.0 (8.0–12.0) Gy for each fraction in three-staged GKRS, respectively. The target volumes of each fraction were 8.3 (4.1–26.0) mL and 6.9 (1.2–23.3) mL in two-staged GKRS and 10.9 (4.8–47.1) mL, 8.7 (1.6–52.7) mL, and 6.5 (0.9–54.5) mL in three-staged GKRS, respectively. In the f-GKRS group, 51 (65.4%) lesions received more than five fractions. The median target volume was 7.4 (4.0–55.8) mL. The median prescription dose was 35.0 (27.0–45.0) Gy, and the median BED10 was 51.6 (39.0–59.5) Gy. The treatment parameters of the 214 lesions are summarized in Table 2.
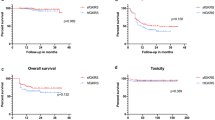
The 6-month and 1-year LC rates were 83.3% (95% confidence interval [CI]: 74.3–89.4%) and 75.2% (95% CI: 64.1–83.3%) in the s-GKRS group, and 82.4% (95% CI: 68.6–90.6%) and 72.0% (95% CI: 56.1–83.0%) in the f-GKRS group, respectively (p = 0.241) (Fig. 1a). The 1-year OS rate and median survival time (MST) in the s-GKRS and f-GKRS groups were 68.7% (95% CI: 58.5–76.9%) and 25 months, and 77.2% (95% CI: 63.4–86.3%) and 36 months, respectively (p = 0.375) (Fig. 1b). The overall incidence rates of adverse events were 2.9% and 7.7% in the s-GKRS and f-GKRS groups, respectively (p = 0.175). None of the patients in either treatment group experienced adverse events associated with CTCAE grade5 toxicity. Three lesions were diagnosed with radiation necrosis (2.2%) and one with hemorrhage in the s-GKRS group. One of the radiation necroses was defined as CTCAE grade4 toxicity. Five lesions (6.4%) were diagnosed with radiation necrosis and one with hemorrhage in the f-GKRS group. There was no lesion defined as CTCAE grade 4 in this group.
(a) Local control: s-GKRS vs. f-GKRS in the main cohort of 214 lesions. The 6-month and 1-year local control rates were 83.3% (95% CI: 74.3–89.4%) and 75.2% (95% CI: 64.1–83.3%), respectively for s-GKRS. The 6-month and 1-year local control rates were 82.4% (95% CI: 68.6–90.6%) and 72.0% (95% CI: 56.1–83.0%), respectively, for f-GKRS. (b) Overall survival: s-GKRS vs. f-GKRS in the main cohort of 199 patients. The 1-year overall survival rate and median survival time in the s-GKRS and f-GKRS groups were 68.7% (95% CI: 58.5–76.9%) at 25 months and 77.2% (95% CI: 63.4–86.3%) at 36 months, respectively (p = 0.375). (c) Cumulative incidence of local control failure: s-GKRS vs. f-GKRS in 124 case-matched lesions. No difference was observed in the 6-month and 1-year cumulative incidences of local control failure between the s-GKRS and f-GKRS groups (15.6% vs. 15.9% at 6 months and 25.6% vs. 25.6% at 1 year; p = 0.617). (d) Overall survival: s-GKRS vs. f-GKRS in 117 case-matched patients. The post-GKRS median survival time was shorter in the s-GKRS group (17 months) than in the f-GKRS group (36 months), without significance (p = 0.202), and the 1-year overall survival rate was 62.6% (95% CI: 45.4–75.7%) and 73.9% (95% CI: 58.8–84.3%) in the s-GKRS and f-GKRS groups, respectively. (e) Cumulative incidence of local control failure: s-GKRS vs. f-GKRS (≤5 fr.) in 46 case-matched lesions. No difference was observed in the 6-month and 1-year cumulative incidences of local control failure between the s-GKRS and f-GKRS (≤5 fr.) groups (10.1% vs. 5.7% at 6 months and 19.1% vs. 24.4% at 1 year; p = 0.500). (f) Cumulative incidence of local control failure: s-GKRS vs. f-GKRS (>5 fr.) in 84 case-matched lesions. No difference was observed in the 6-month and 1-year cumulative incidences of local control failure between the s-GKRS and f-GKRS (>5 fr.) groups (11.4% vs. 18.4% at 6 months and 31.7% vs. 22.8% at 1 year; p = 0.878). f-GKRS, fractionated gamma knife radiosurgery; s-GKRS, staged gamma knife radiosurgery; CI, confidence interval; fr., fraction
Propensity score matching selected 124 lesions (62 lesions in each group). The 124 case-matched lesions are summarized in Table 1, and the treatment parameters of the 124 case-matched lesions are summarized in Table 2. No differences were observed in the 6-month and 1-year cumulative incidences of LC failure between the s-GKRS and f-GKRS matched groups (15.6% vs. 15.9% at 6 months, and 25.6% vs. 25.6% at 1 year; p = 0.617) (Fig. 1c). The post-GKRS MST was shorter in the s-GKRS matched group than in the f-GKRS matched group (17 months vs. 36 months) without significance (p = 0.202), and the 1-year OS rates were 62.6% (95% CI: 45.4–75.7%) and 73.9% (95% CI: 58.8–84.2%) in the s-GKRS and f-GKRS matched groups, respectively (Fig. 1d). Adverse events were fewer in the s-GKRS matched group (1.6%) than in the f-GKRS matched group (9.7%), without significance (p = 0.114). No differences were observed in the 6-month and 1-year cumulative incidences of LC failure between s-GKRS and f-GKRS (≤5fraction) matched groups (10.1% vs. 5.7% at 6 months and 19.1% vs. 24.4% at 1 year; p = 0.500) (Fig. 1e), and s-GKRS vs. f-GKRS (>5fraction) matched groups (11.4% vs. 18.4% at 6 months and 31.7% vs. 22.8% at 1 year; p = 0.878) (Fig. 1f). The details of the subgroup analyses are described in Supplemental Digital Content 2.
Discussion
The existing literature in the past decade has established the efficacy of s-GKRS for large-sized brain metastases [6,7,8, 16,17,18,19,20,21,22,23]. The present study’s outcomes were as follows: 6-month LC, 83.3%; 1-year LC, 75.2%; MST, 25.0 months; and 1-year OS, 68.7%. According to previous reports, the 6-month and 1-year LC rates were 85.0–100% and 61.0–92.0%, and the MST and 1-year OS rates were 7.0–24.7 months and 35.2–60.0%, respectively. The latest report of s-GKRS in 2022 showed the most favorable outcome in regard to LC, but the study included small lesions and the maximum range of tumor volume was smaller than previously reported [23]. According to these factors, our study’s outcomes were comparable with those of previous reports (Table 3).
Delivery of high doses of radiation by HFRT minimizes adverse events by maintaining LC, and LINAC-based equipment delivering HFRT for large brain metastases has been discussed in several publications. The conventional LINAC series focused on lesions sized > 4 mL or > 2 cm in diameter, reporting a 1-year OS of 56.0–69.0% and 1-year LC of 61.0–100% [24,25,26,27]. In comparison, the CyberKnife series in the literature reported a 1-year OS and 1-year LC of 13.0–69.4% and 63.0–92.4%, respectively [2, 28,29,30]. A systematic review of 1049 metastases sized > 2 cm in diameter collected from 15 series concluded that HFRT showed better LC when administered safely than single-fraction GKRS [31]. Conversely, the biggest disadvantage of HFRT using LINAC is that the gradient index is inferior to that of GKRS and 10 times higher in extracranial exposure to radiation doses. It should be noted that when comparing extracranial doses of various treatment equipment, Leksell Gamma Knife® Perfexion™, the predecessor of Icon™, was superior in terms of radiation protection [32]. In particular, one report compared the treatment plans with gamma knife and other LINAC-based devices for large brain metastases. Gamma knife demonstrated that the sharpest dose fell off in normal brain tissue despite having the highest dose within the tumor [33]. After introducing Leksell Gamma Knife® Icon™ in 2015, the fractionation ability was introduced for gamma knifes. Icon™ was designed with optional thermoplastic mask fixation. To maintain its accuracy as high as those of its predecessors, a high-definition motion management system, which is an infrared stereoscopic camera that detects a nose marker’s displacement, was installed in the equipment settings. These new features allowed the fractionation of GKRS with mask, which is considered safer and less invasive than conventional single-fraction GKRS with frame fixation. One study reported that f-GKRS distributed a higher dose to the tumor and a lower dose to the normal brain tissue than s-GKRS by comparing the BED of their treatment plans [34]. According to their report, theoretically, f-GKRS should be more effective and safer for treating large brain metastases. Additionally, Grimm et al. compared frame fixation and mask fixation GKRS, and showed that mask fixation using GKRS was safer regarding radiation necrosis [35]. One study analyzed patients who underwent frame fixation or mask fixation GKRS using a questionnaire and reported that patients were more comfortable and less likely to experience pain with mask fixation [36]. Another study compared frame fixation and mask fixation single-fraction GKRS, and reported similar outcomes between the two groups, although the mean pain scale score was higher in patients with frame fixation. Patients also experienced disadvantages of mask fixation, such as longer treatment time and higher extracranial doses due to the cone-beam computed tomography [37]. Furthermore, mask fixation has been reported to show a significantly higher degree of error variability, even though the motion error was < 1 mm in the translational direction and 1° in the rotational direction for both fixations [38]. Hence, this fact had to be considered when treating small lesions and lesions near critical structures. Conversely, this could be a negligible factor when treating large brain metastases. Only a few studies have reported the outcomes of f-GKRS. This was validated in another study that reported a 1-year LC of 84.8% with a low incidence of symptomatic adverse events, although target volumes > 4.5 mL were a significant predictor of symptomatic adverse events [39]. Kim et al. first reported f-GKRS for large brain metastases. The treatment was performed using Leksell Gamma Knife® Perfexion™, with frame fixation of 2–4 fractions on consecutive days [40]. In their study, the BED10, median OS, and 1-year OS were 24.2–60.0 (median: 43.2) Gy, 16.2 months, and 66.7%, respectively. However, their study’s greatest challenge was the treatment protocol involving attaching a painful frame for every fraction during treatment. Two preliminary studies have been published on frameless f-GKRS for large metastatic brain tumors. Moreover, a prescription dose of 21–40 Gy, when administered for 3–5 consecutive days, showed median BED10 of 51.3 (range: 35.7–72.0) Gy, median OS of 12.0 months, and 1-year OS of 93.3% [11]. In a study with three or five fractions at 5–9 Gy per fraction, BED10 was 35.7–51.3 Gy, LC was 98.5% at 6 months and 96.0% at 1 year, and median OS and 1-year OS were 23.2 months and 63.6%, respectively (Table 4) [10]. In the present circumstances, further investigation is required in the field of frameless f-GKRS for large metastatic brain tumors.
The advantage of f-GKRS is that it increases the total radiation dose delivered to lesions, while allowing reduction of the radiation dose delivered to normal brain tissue. This should be a more effective and safer option for managing large brain metastases. The present study analyzed the differences in LC between s-GKRS and f-GKRS for mid-to-large brain metastases. Propensity score matching was performed to reduce biases and heterogeneous factors; f-GKRS was as effective as s-GKRS for mid-to-large brain metastases. OS was longer in the f-GKRS group than in the s-GKRS group, with no statistical significance. Additionally, f-GKRS should be considered a substitute for patients ineligible to undergo the entire s-GKRS treatment. The shorter treatment period of f-GKRS can be an added advantage for patients with large brain metastases. If the treatment of the brain lesion is accomplished earlier, the patient could swiftly move on to the succeeding treatment for the primary lesion.
Limitations
A limitation of this study is that the confounding factors of concurrent systemic therapy could not be eliminated. Since this was a retrospective study investigating two different treatment approaches performed in different eras, the paradigm shift of systemic therapy between both eras [41] was not considered. Targeted therapy has shown drastic advances in variety and effectiveness in recent years, potentially influencing outcomes. In the future, a well-designed multi-institutional prospective cohort study is needed to investigate accurate OS and LC. An additional limitation is that, compared to previous studies, the present study included cases treated by a relatively higher number of fractions. Conventionally, hypofraction is defined as five or fewer fractions, and previous publications concerning Leksell Gamma Knife® Icon™ are as such. However, more than half of our cases were fractionated into more than five categories, which does not meet the definition of hypofraction. This difference should be carefully considered when interpreting study data. The aim of increasing the number of fractions was to minimize the risk of radiation injury, and the results of propensity score matched analysis of s-GKRS vs. f-GKRS (> 5 fraction) demonstrated the possibility of the effectiveness of more than five fraction GKRS. However, little is known about f-GKRS performed with more than five fractions. Accordingly, the choice of fractionation number should be carefully investigated in the future, and further accumulation of cases is required.
Conclusion
To the best of our knowledge, this study is the first to compare f-GKRS and s-GKRS for relatively large brain metastases. Our report showed that f-GKRS is as effective as s-GKRS, and this strategy should be considered an alternative management strategy for patients unsuitable for frame fixation.
Data Availability
The datasets generated and/or analyzed during the current study are available by contacting the corresponding author (R.N., rnrn46_8447@yahoo.co.jp).
References
Serizawa T, Higuchi Y, Nagano O (2013) Stereotactic radiosurgery for brain metastases. Neurosurg Clin N Am 24:597–603. https://doi.org/10.1016/j.nec.2013.05.007
Chon H, Yoon K, Lee D, Kwon DH, Cho YH (2019) Single-fraction versus hypofractionated stereotactic radiosurgery for medium-sized brain metastases of 2.5 to 3 cm. J Neurooncol 145:49–56. https://doi.org/10.1007/s11060-019-03265-1
Zimmerman AL, Murphy ES, Suh JH et al (2016) Treatment of large brain metastases with stereotactic radiosurgery. Technol Cancer Res Treat 15:186–195. https://doi.org/10.1177/1533034614568097
Han JH, Kim DG, Chung HT, Paek SH, Park CK, Jung HW (2012) Radiosurgery for large brain metastases. Int J Radiat Oncol Biol Phys 83:113–120. https://doi.org/10.1016/j.ijrobp.2011.06.1965
Yang HC, Kano H, Lunsford LD, Niranjan A, Flickinger JC, Kondziolka D (2011) What factors predict the response of larger brain metastases to radiosurgery. Neurosurgery 68:682–690 discussion 690. https://doi.org/10.1227/NEU.0b013e318207a58b
Higuchi Y, Serizawa T, Nagano O et al (2009) Three-staged stereotactic radiotherapy without whole brain irradiation for large metastatic brain tumors. Int J Radiat Oncol Biol Phys 74:1543–1548. https://doi.org/10.1016/j.ijrobp.2008.10.035
Serizawa T, Higuchi Y, Yamamoto M et al (2018) Comparison of treatment results between 3- and 2-stage Gamma Knife radiosurgery for large brain metastases: a retrospective multi-institutional study. J Neurosurg 131:227–237. https://doi.org/10.3171/2018.4.JNS172596
Yomo S, Oda K, Oguchi K (2020) Single- versus 2-session gamma knife surgery for symptomatic midsize brain metastases: a propensity score-matched analysis. J Neurosurg 133:1646–1654. https://doi.org/10.3171/2019.7.JNS191193
Lehrer EJ, Peterson JL, Zaorsky NG et al (2019) Single versus multifraction stereotactic radiosurgery for large brain metastases: an international meta-analysis of 24 trials. Int J Radiat Oncol Biol Phys 103:618–630. https://doi.org/10.1016/j.ijrobp.2018.10.038
Samanci Y, Sisman U, Altintas A et al (2021) Hypofractionated frameless gamma knife radiosurgery for large metastatic brain tumors. Clin Exp Metastasis 38:31–46. https://doi.org/10.1007/s10585-020-10068-6
Park HR, Park KW, Lee JM et al (2019) Frameless fractionated gamma knife radiosurgery with ICON™ for large metastatic brain tumors. J Korean Med Sci 34:e57. https://doi.org/10.3346/jkms.2019.34.e57
Noda R, Akabane A, Kawashima M, Oshima A, Tsunoda S, Segawa M, Inoue T (2022) Fractionated gamma knife radiosurgery after cyst aspiration for large cystic brain metastases: case series and literature review. Neurosurg Rev 45:3457–3465. https://doi.org/10.1007/s10143-022-01835-y
Kawashima M, Akabane A, Noda R, Segawa M, Tsunoda S, Inoue T (2022) Interfractional change of tumor volume during fractionated stereotactic radiotherapy using gamma knife for brain metastases. J Neurooncol 159:409–416. https://doi.org/10.1007/s11060-022-04075-8
Masucci GL (2018) Hypofractionated radiation therapy for large brain metastases. Front Oncol 8:379. https://doi.org/10.3389/fonc.2018.00379
van Leeuwen CM, Oei AL, Crezee J, Bel A, Franken NAP, Stalpers LJA, Kok HP (2018) The alfa and beta of tumours: a review of parameters of the linear-quadratic model, derived from clinical radiotherapy studies. Radiat Oncol 13:96. https://doi.org/10.1186/s13014-018-1040-z
Yomo S, Hayashi M, Nicholson C (2012) A prospective pilot study of two-session gamma knife surgery for large metastatic brain tumors. J Neurooncol 109:159–165. https://doi.org/10.1007/s11060-012-0882-8
Yomo S, Hayashi M (2014) A minimally invasive treatment option for large metastatic brain tumors: long-term results of two-session gamma knife stereotactic radiosurgery. Radiat Oncol 9:132. https://doi.org/10.1186/1748-717X-9-132
Hasegawa T, Kato T, Yamamoto T, Iizuka H, Nishikawa T, Ito H, Kato N (2017) Multisession gamma knife surgery for large brain metastases. J Neurooncol 131:517–524. https://doi.org/10.1007/s11060-016-2317-4
Angelov L, Mohammadi AM, Bennett EE et al (2018) Impact of 2-staged stereotactic radiosurgery for treatment of brain metastases ≥ 2 cm. J Neurosurg 129:366–382. https://doi.org/10.3171/2017.3.JNS162532
Dohm A, McTyre ER, Okoukoni C et al (2018) Staged stereotactic radiosurgery for large brain metastases: local control and clinical outcomes of a one-two punch technique. Neurosurgery 83:114–121. https://doi.org/10.1093/neuros/nyx355
Yamamoto M, Higuchi Y, Serizawa T et al (2018) Three-stage gamma knife treatment for metastatic brain tumors larger than 10 cm3: a 2-institute study including re-analyses of earlier results using competing risk analysis. J Neurosurg 129:77–85. https://doi.org/10.3171/2018.7.GKS181392
Ito D, Aoyagi K, Nagano O, Serizawa T, Iwadate Y, Higuchi Y (2020) Comparison of two-stage gamma knife radiosurgery outcomes for large brain metastases among primary cancers. J Neurooncol 147:237–246. https://doi.org/10.1007/s11060-020-03421-y
Cho A, Medvedeva K, Kranawetter B et al (2022) How to dose-stage large or high-risk brain metastases: an alternative two-fraction radiosurgical treatment approach. J Neurosurg 137:1666–1675. https://doi.org/10.3171/2022.2.JNS212440
Marcrom SR, McDonald AM, Thompson JW et al (2017) Fractionated stereotactic radiation therapy for intact brain metastases. Adv Radiat Oncol 2:564–571. https://doi.org/10.1016/j.adro.2017.07.006
Minniti G, Scaringi C, Paolini S et al (2016) Single-fraction versus multifraction (3 × 9 gy) stereotactic radiosurgery for large (> 2 cm) brain metastases: a comparative analysis of local control and risk of radiation-induced brain necrosis. Int J Radiat Oncol Biol Phys 95:1142–1148. https://doi.org/10.1016/j.ijrobp.2016.03.013
Navarria P, Pessina F, Cozzi L et al (2016) Hypo-fractionated stereotactic radiotherapy alone using volumetric modulated arc therapy for patients with single, large brain metastases unsuitable for surgical resection. Radiat Oncol 11:76. https://doi.org/10.1186/s13014-016-0653-3
Feuvret L, Vinchon S, Martin V et al (2014) Stereotactic radiotherapy for large solitary brain metastases. Cancer Radiother 18:97–106. https://doi.org/10.1016/j.canrad.2013.12.003
Jeong WJ, Park JH, Lee EJ, Kim JH, Kim CJ, Cho YH (2015) Efficacy and safety of fractionated stereotactic radiosurgery for large brain metastases. J Korean Neurosurg Soc 58:217–224. https://doi.org/10.3340/jkns.2015.58.3.217
Wegner RE, Leeman JE, Kabolizadeh P, Rwigema JC, Mintz AH, Burton SA, Heron DE (2015) Fractionated stereotactic radiosurgery for large brain metastases. Am J Clin Oncol 38:135–139. https://doi.org/10.1097/COC.0b013e31828aadac
Murai T, Ogino H, Manabe Y et al (2014) Fractionated stereotactic radiotherapy using CyberKnife for the treatment of large brain metastases: a dose escalation study. Clin Oncol (R Coll Radiol) 26:151–158. https://doi.org/10.1016/j.clon.2013.11.027
Lee EJ, Choi KS, Park ES, Cho YH (2021) Single- and hypofractionated stereotactic radiosurgery for large (> 2 cm) brain metastases: a systematic review. J Neurooncol 154:25–34. https://doi.org/10.1007/s11060-021-03805-8
Lindquist C, Paddick I (2007) The Leksell gamma knife perfexion and comparisons with its predecessors. Neurosurgery 61:130–140 discussion 140–141. https://doi.org/10.1227/01.neu.0000316276.20586.dd
Cao H, Xiao Z, Zhang Y et al (2019) Dosimetric comparisons of different hypofractionated stereotactic radiotherapy techniques in treating intracranial tumors > 3 cm in longest diameter. J Neurosurg 132:1024–1032. https://doi.org/10.3171/2018.12.JNS181578
Cui T, Weiner J, Danish S et al (2022) Evaluation of biological effective dose in gamma knife staged stereotactic radiosurgery for large brain metastases. Front Oncol 12:892139. https://doi.org/10.3389/fonc.2022.892139
Grimm MA, Köppen U, Stieler F et al (2020) Prospective assessment of mask versus frame fixation during gamma knife treatment for brain metastases. Radiother Oncol 147:195–199. https://doi.org/10.1016/j.radonc.2020.05.011
Pavlica M, Dawley T, Goenka A, Schulder M (2021) Frame-based and mask-based stereotactic radiosurgery: the patient experience, compared. Stereotact Funct Neurosurg 99:241–249. https://doi.org/10.1159/000511587
Régis J, Merly L, Balossier A et al (2022) Mask-based versus frame-based gamma knife ICON radiosurgery in brain metastases: a prospective randomized trial. Stereotact Funct Neurosurg 100:86–94. https://doi.org/10.1159/000519280
Carminucci A, Nie K, Weiner J, Hargreaves E, Danish SF (2018) Assessment of motion error for frame-based and noninvasive mask-based fixation using the Leksell gamma knife icon radiosurgery system. J Neurosurg 129:133–139. https://doi.org/10.3171/2018.7.GKS181516
Yan M, Holden L, Wang M et al (2022) Gamma knife icon based hypofractionated stereotactic radiosurgery (GKI-HSRS) for brain metastases: impact of dose and volume. J Neurooncol 159:705–712. https://doi.org/10.1007/s11060-022-04115-3
Kim JW, Park HR, Lee JM et al (2016) Fractionated stereotactic gamma knife radiosurgery for large brain metastases: a retrospective, single center study. PLoS ONE 11:e0163304. https://doi.org/10.1371/journal.pone.0163304
Khan M, Spicer J (2019) The evolving landscape of cancer therapeutics. Handb Exp Pharmacol 260:43–79. https://doi.org/10.1007/164_2019_312
Funding
The authors declare that no funds, grants or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, and statistical analysis, figure preparation were performed by Ryuichi Noda. The first draft of the manuscript was written by Ryuichi Noda and all authors commented on previous versions of the manuscript. Statistical analysis was reviewed by Mariko Kawashima and Atsuya Akabane. Data collection was performed by Ryuichi Noda, Mariko Kawashima, Atsuya Akabane. The study was directed by Tomohiro Inoue and Atsuya Akabane. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki (revised version, 2013). The study protocol was approved by the Institutional Review Board of NTT Medical Center, Tokyo (approval number: 21‒71).
Consent to participate
Written informed consent was obtained from all patients.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Digital Content 1
: Fig. a Schematic of staged gamma knife radiosurgery treatment protocol. The prescription dose of each fraction was set to 11.8–14.2 Gy for the two-staged GKRS and 9.0–11.0 Gy for the three-staged GKRS. The treatment intervals were completed within 6 weeks, with ≥ 12 days between each fraction. Fig. b Schematic of fractionated gamma knife radiosurgery treatment protocol. The prescription dose of each fraction for more than five-fraction cases were set to 3.0–5.0 Gy, and for five or fewer fraction cases were set to 6.0–9.5 Gy. Interfractional evaluation by MRI was performed when the treatment continued for more than five fractions (> 1 week), and the treatment plan was modified if necessary, as reported previously. GKRS, gamma knife radiosurgery; MRI, magnetic resonance images
Supplemental Digital content 2:
Table 1. Clinical characteristics of the 163 lesions of s-GKRS vs. f-GKRS (≤5 fr.) and the case-matched 46 lesions. Table 2. Clinical characteristics of the 187 lesions of s-GKRS vs. f-GKRS (>5 fr.) and the case-matched 84 lesions. Table 3. Summary of treatment parameters of f-GKRS (≤5 fr.) lesions and f-GKRS (>5 fr.) lesions
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Noda, R., Kawashima, M., Segawa, M. et al. Fractionated versus staged gamma knife radiosurgery for mid-to-large brain metastases: a propensity score-matched analysis. J Neurooncol 164, 87–96 (2023). https://doi.org/10.1007/s11060-023-04374-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-023-04374-8