Abstract
Hypofractionated stereotactic radiosurgery has become an alternative for metastatic brain tumors (METs). We aimed to analyze the efficacy and safety of frameless hypofractionated Gamma Knife radiosurgery (hfGKRS) in the management of unresected, large METs. All patients who were managed with hfGKRS for unresected, large METs (> 4 cm3) between June 2017 and June 2020 at a single center were reviewed in this retrospective study. Local control (LC), progression-free survival (PFS), overall survival (OS), and toxicities were investigated. A total of 58 patients and 76 METs with regular follow-up were analyzed. LC rate was 98.5% at six months, 96.0% at one year, and 90.6% at 2 years during a median follow-up of 12 months (range, 2–37). The log-rank test indicated no difference in the distribution of LC for any clinical or treatment variable. PFS was 86.7% at 6 months, 66.6% at 1 year, and 58.5% at 2 years. OS was 81% at 6 months, 63.6% at one year, and 50.7% at 2 years. On the log-rank test, clinical parameters such as control status of primary cancer, presence of extracranial metastases, RTOG-RPA class, GPA group, and ds-GPA group were significantly associated with PFS and OS. Patients presented with grade 1 (19.0%), grade 2 (3.5%) and grade 3 (5.2%) side effects. Radiation necrosis was not observed in any patients. Our current results suggest that frameless hfGKRS for unresected, large METs is a rational alternative in selected patients with promising results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metastatic brain tumors (METs) are observed in approximately one-third of cancer patients, with increasing incidence due to improvements in systemic therapies that provide longer survival [1]. The management of METs is multifaceted and includes surgery, systemic treatments, whole-brain radiotherapy (WBRT), and stereotactic radiotherapy (SRT) [2]. The application of stereotactic radiosurgery (SRS), a form of SRT, alone for METs is increasing due to wider availability and heightened experience in the use of SRS, similar survival in subjects with a limited number of METs, and increased risk of impaired neurocognition and quality of life with WBRT [3].
Although single-fraction is efficient at controlling the lesion and saving healthy tissue in small tumors, larger METs (≥ 2–3 cm in maximum diameter or ≥ 4 cm3 in volume) or METs close to dose-limiting organs necessitated lower doses, and these lower doses led to moderate rates of durable local control (LC) [4, 5]. With the development of frameless non-invasive fixation, on-board imaging, and patient motion monitoring systems providing replicable, accurate positions that can be utilized for many fractions, hypofractionated SRS (hfSRS) using 2 to 5 fractions has been implemented for the treatment of METs. Hypofractionation uses the biologic benefits of fractionation and improves the therapeutic ratio between the probabilities of tumor control and normal tissue complications [6, 7]. The literature data theorize the potential of this method [8,9,10,11,12,13,14,15,16,17,18,19,20] in terms of efficiency and safety as opposed to single-fraction SRS (sfSRS) [9, 12, 18, 20,21,22,23,24].
This single-center, retrospective study aimed to describe the efficacy and safety of frameless hypofractionated Gamma Knife Radiosurgery (hfGKRS) in the treatment of unresected, large METs, and to analyze LC, overall survival (OS), intracranial progression-free survival (PFS), and toxicity.
Methods
Ethics statement
This retrospective study with prospectively managed data was authorized by the Institutional Review Board of Koç University (2020.190.IRB1.058) and was performed based on the principles of the Declaration of Helsinki. Informed consent was taken from all participants.
Eligibility and demographic data
This retrospective, single-center study included 58 consecutive patients with 76 large METs who underwent frameless hfGKRS between June 2017 and June 2020. The study inclusion criteria were (a) age ≥ 18 years, (b) a histologically verified systemic malignancy, and (c) MRI-confirmed large METs (> 4 cm3). Patients with a previous history of SRS for local failure, prior surgical resection of the targeted lesion, and inadequate follow-up information (< 2 months) were excluded. All data were retrieved from the patient charts and neuroimaging databases. All cytotoxic, hormone, cytokine (interleukins, interferons), and targeted systemic therapies were collected for each patient. Concurrent systemic therapy was defined as an agent administered on the same day as hfGKRS or within five biological half-lives of the date of hfGKRS [25]. Table 1 tabulates the baseline patient and clinical features.
Radiosurgical technique
hfGKRS was conducted by Leksell Gamma Knife® Icon™ (Elekta Instrument AB, Stockholm, Sweden). A pre-treatment warmed thermoplastic mask was molded over the patient's face, and a reference cone-beam CT (CBCT) scan is obtained. Then pre-treatment 1-mm, thin-slice, volumetric, axial CT, contrast-enhanced T1 images and T2-weighted MRI images were obtained and exported to Leksell Gamma Plan® (version 11.0.3 or version 11.1.1 Elekta Instrument, Stockholm, Sweden). The reference CBCT is registered with the planning MRI using the registration algorithm of the Gamma Plan. Target delineation was performed using T1-weighted MRI, and the gross tumor volume was equal to the clinical target volume and planning target volume. If other metastases were identified in the imaging studies, they were treated concurrently. Shots with collimator sizes 4, 8, and 16 mm were placed inside the target and manipulated to simultaneously achieve high target coverage, high selectivity, gradient index < 3, and reasonable treatment times using the best-fit isodose method until a clinically acceptable plan was formulated by a neurosurgeon, medical physicist, and radiation oncologist in collaboration. A second CBCT was co-registered with the reference CBCT. The new adapted 3D distribution and dose-volume histograms are again reviewed, and if satisfactory, the treatment is delivered. Before every fraction, CBCT was acquired to confirm the exact position of the skull. Automated co-registration was done to define the daily variation in translation and rotation. The program automatically adapts the shot positions to the daily position and recalculates the dose distribution.
As there are no definite guidelines to date with regards to the optimal dose to be used in hfGKRS for METs, the objective was to obtain a biological equivalent dose (BED) > 50Gy using an α/β ratio of 10, when possible [26]. However, additional parameters, such as MET size, histology, the presence of edema, overall metastatic disease in- and outside the central nervous system, concurrent clinical status at the time of treatment (Karnofsky performance status (KPS)/recursive partitioning analysis (RPA)), organ at risk (OAR) proximity, and added biological dose estimates from previous WBRT, were also factored into the dose selection process. Fractionation mostly depended on target volume. Five-fraction treatment was delivered to brain metastases ≥ 10 cm3 and three-fraction treatment to brain metastases > 4 cm3 and < 10 cm3. However, the aforementioned dosing parameters were also taken into account when deemed. The fractionation and dose regimens used in the study were 3 × 7 Gy, 3 × 8 Gy, 3 × 9 Gy, 5 × 5 Gy, and 5 × 6 Gy. METs were irradiated daily.
The OARs were contoured, and the planning objective was to minimize the dose to normal brain tissue as much as possible. Dose constraints to OARs for three fractions and five fractions were as follows: (a) optic pathway: 15.3 Gy to < 0.2 cm3, with a maximum point dose of 17.4 Gy in three fractions, and 23.0 Gy to < 0.2 cm3, with a maximum point dose of 25.0 Gy in five fractions; (b) cochlea: a maximum point dose of 17.1 Gy in three fractions or 25.0 Gy in five fractions; and (c) brainstem: 18.6 Gy to < 0.5 cm3, with a maximum point dose of 23.1 Gy in three fractions, and 23.0 Gy to < 0.5 cm3, with a maximum point dose of 31.0 Gy in five fractions [27]. The entire brain volume was contoured on the treatment planning MRI and mean whole brain dose (MWBD) was calculated. For each plan, all enhancing tumors were outlined and total volume of tumors within that single radiosurgery session was recorded. No other specific dose-volume constraints were applied to other structures.
Follow-up and outcome measures
Patients underwent a regular clinical neurological examination and diagnostic imaging of the brain 2 months after the initial hfGKRS procedure and then approximately every 2–4 months thereafter, according to the imaging and clinical characteristics. All pre-treatment and post-treatment radiological examinations were evaluated by a team comprised of a radiation oncologist, neurosurgeon, and neuroradiologist. Response assessment of target lesions was performed according to the Response Assessment in Neuro-Oncology Brain Metastases (RANO-BM) group as complete response (tumor disappearance), partial response (≥ 30% decrease in longest diameter), progressive disease (≥ 20% increase in the longest diameter), and stable otherwise [28]. A complete response, partial response, and stable disease were indicative of LC. The LC was assessed throughout the study until local failure or death. Intracranial PFS was defined as the time interval until distant intracranial recurrence or local failure or death, and overall survival (OS) was defined as the interval between hfGKRS and date of last follow-up or death. All patients were classified using the RPA classification system of the Radiation Therapy Oncology Group (RTOG) [29], Graded Prognostic Assessment (GPA) [30], and diagnosis-specific graded prognostic assessment (ds-GPA) score [31]. The updated Graded Prognostic Assessment for lung cancer using molecular markers (Lung-molGPA) [32] and Graded Prognostic Assessment for melanoma using molecular markers (Melanoma-molGPA) [33] were also calculated.
Radiation necrosis (RN) was determined regarding the radiological criteria proposed by Minniti et al. [34]. In addition to standard radiological modalities such as dynamic susceptibility-weighted contrast-enhanced (DSC) perfusion MRI, fludeoxyglucose F18 (FDG) positron emission tomography (PET) and MR-spectroscopy (MR-SPECT), Elements Contrast Clearance Analysis (BrainLAB AG, Munich, Germany) was used to calculate color-coded Treatment Response Assessment Maps (TRAMs) by subtracting delayed T1-CE images from early T1-CE images. Following injection, the contrast agent spreads rapidly throughout the circulation system and is removed by the kidneys within a few hours. Therefore, vessels typically exhibit a rapid increase in signal intensity, followed by a relatively rapid clearance. Subtraction of signal intensities in voxels, including vessels, is thus negative (depicted in blue). Pathologies with dense vasculature also typically exhibit a rapid increase in signal intensity followed by a relatively rapid clearance, although the peak intensity is delayed due to blood-brain barrier disruption. The subtraction is negative (blue) as well. Areas with damaged vasculature typically exhibit slow accumulation of contrast, and thus the subtraction is positive (depicted in red). In summary, areas of contrast accumulation (low vascular activity) were illustrated in red, and areas of contrast clearance (high vascular activity) were illustrated in blue. A subjective diagnosis of “persistent tumoral lesion” was based on the presence of blue areas, and a diagnosis of “radiation effect” was made in case of red areas [35]. Toxicity was ranked with the Common Terminology Criteria for Adverse Events version 5.
Statistical analysis
All analyses were conducted utilizing SPSS 21.0 (Statistical Package for Social Sciences, SPSS Inc., Chicago, IL, USA). Descriptive statistics were used to define the study cohort. LC, intracranial PFS, and RN analyses were performed using a per-tumor basis. Evaluation of OS was conducted on a per-patient basis. Kaplan-Meier survival plots were applied to estimate LC, intracranial PFS, and OS distributions. Log-Rank test was used to assess predictive factors on survival outcomes, which included age (≥ 65 years vs. < 65 years), gender, primary cancer type, tumor location (eloquent vs. non-eloquent), MET volume (RTOG group A 4–14 cm3 vs. RTOG group B > 14 cm3), total METs volume (≥ 15 cm3 vs. < 15 cm3), number of irradiated METs (multiple vs. solitary), status of primary cancer, extracranial metastases, pre-treatment KPS score (≥ 70 vs. < 70), RTOG-RPA class (I, II, III), GPA scores (0–1, 1.5–2.5, 3, 3.5–4) DS-GPA score (0–1, 1.5–2, 2.5–3, 3.5–4), prior conventional systemic agents, prior WBRT, and prior craniotomy for other than the targeted lesion. Prognostic factors that were found significant (p ≤ 0.2) were included in the multivariate analysis using a Cox proportional hazards regression model. All tests were two-sided, and p < 0.05 was regarded as statistically significant.
Results
General characteristics
Fifty-eight subjects with a total number of 131 METs were reviewed, and 76 unresected, large METs were included in the analysis. The median age of patients was 59.5 years (range, 32-83). The most common primary diagnosis was non-small cell lung carcinoma (NSCLC) (n = 24), and the most common location was cerebellum (n = 25). Twenty-seven patients (46.6%) had a single tumor, and the median number of METs and large METs treated per patient was 2 (range, 1–10) and 1 (range, 1–3), respectively. Sixty-six lesions were in the RTOG Group A (median = 5.55 cm3), and ten lesions were in Group B (median = 16.5 cm3). Those ten lesions were not resected due to eloquent area localization (n = 4), low KPS (n = 3), and patient preference (n = 3). Twenty patients (34.5%) had previously undergone cranial surgery for METs other than the targeted lesions. Nine patients (15.5%) previously received WBRT. The median duration between diagnosis of metastasis and hfGKRS was 2.5 weeks (range, 0–316). hfGKRS was delivered in 3 daily fractions in 54 lesions (median volume = 5.2 cm3) or 5 daily fractions in 22 lesions (median volume = 13.3 cm3). The median target volume was 6.15 cm3 (range, 4-22.2). The median total dose to the margin, dose per fraction and the isodose line was 27 Gy (range, 21–30), 9 Gy (range, 5–9), and 45% (range, 40–60), respectively. The median MWBD per treatment was 3.4 Gy (range 1.6–4.9 Gy). A summary of the baseline features of this study is shown in Table 1.
Systemic treatments
Among all patients, 33 patients (56.9%) were treated with chemotherapy or hormone therapy at the time of hfGKRS; however, none of these were concurrent. Among these 33 patients, 19 patients (57.6%) had received cytotoxic therapies, and 14 patients (42.4%) received hormone therapies. Only two patients (3.5%) with non-small cell lung cancer were on concurrent targeted therapy with epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs).
Local control and progression
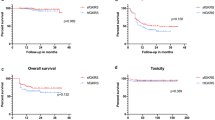
The median clinical and radiologic follow-up was 12 months (range, 2–37 months). The local responses were regarded as complete response in 15 METs (19.7%), partial response in 54 METS (71.1%), and stable disease in four METs (5.3%). The post-hfGKRS median METs volume was 1.3 cm3 (range, 0-11.3). The median volume percent changes in responders and stable disease were 80% (range, 65–100) and 27.5% (range, 15–35%), respectively. An illustrative case is shown in Fig. 1. Progressive disease was observed in three METs (3.9%), with a median volume percent change of − 25%. The LC rate was 98.5% at 6 months, 96.0% at one year, and 90.6% at 2 years (Fig. 2). The log-rank test indicated no difference in the distribution of LC for any clinical or treatment variable. New METs were detected in 16 subjects and were managed with repeat GKRS in 15 subjects after a median period of 10 months (range, 2–14) and WBRT in one subject after 5 months. The mean local/distant intracranial PFS was 25.6 months (95%CI 21.0–30.1). Intracranial PFS was 86.7% at 6 months, 66.6% at 1 year, and 58.5% at 2 years (Fig. 2). The log-rank test indicated that control status of primary cancer (p = 0.001), presence of extracranial metastases (p < 0.0001), RTOG-RPA class (p < 0.0001), GPA group (p = 0.013), and ds-GPA group (p = 0.002) were predictive factors of intracranial PFS. However, only RTOG-RPA class (p = 0.022) remained significant in multivariate analysis.
An illustrative case treated with hypofractionated Gamma Knife radiosurgery. a A 46-year-old male patient was simultaneously diagnosed with a metastatic lesion in the head of the caudate nucleus and non-small cell lung carcinoma. The metastatic lesion was irradiated with a marginal dose of 24 Gy in 3 fractions (targeted to 45% isodose line) (The treatment plan is given on the right). b 4 months and c 10 months after radiosurgery, the lesion had shrunk dramatically. d A final follow-up imaging scan, obtained 16 months after radiosurgery, showed that the metastatic lesion had nearly disappeared. The patient was still alive at the analysis.
Overall survival
In the last follow-up, 33 subjects (56.9%) were alive, and 25 subjects (43.1%) were dead. Twenty deaths (80.0%) were due to primary cancer progression, while five deaths (20.0%) were due to progressive brain disease. Leptomeningeal metastasis (LM) was observed in one patient (1.7%) with a breast carcinoma diagnosis who had a surgical resection for a cerebellar MET. The distribution of RTOG RPA, GPA, and ds-GPA are presented in Table 1. The mean OS was 23.2 months (95%CI 19.2–27.2). OS was 81% at 6 months, 63.6% at one year, and 50.7% at 2 years (Fig. 2). Furthermore, the median survival after the diagnosis of METs was 14 months (range, 2-94). On log-rank test, clinical parameters such as control status of primary cancer (p = 0.008), presence of extracranial metastases (p < 0.0001), RTOG-RPA class (p < 0.0001), GPA group (p = 0.001), ds-GPA group (p < 0.0001) and Lung-molGPA (p < 0.0001) were significantly associated with OS (Table 2). However, only presence of extracranial metastases (p = 0.041) remained significant in multivariate analysis.
Toxicity
hfGKRS was well tolerated. Grade 1 toxicities (headache, dizziness, and somnolence) occurred in 11 patients (19.0%), grade 2 toxicities (headache) occurred in two patients (3.5%), and grade 3 toxicities (edema cerebral) occurred in three patients (5.2%). The median time to onset of edema was 1 month. All patients with edema were managed with short-term steroid treatment. No RN was observed in this study.
Discussion
Although hfSRS with linear accelerator-based radiosurgery is well-defined, the present study demonstrated the preliminary frameless hfGKRS experience in 58 patients with 76 unresected, large METs who were treated with Gamma Knife ICONTM as initial treatment at a single institution, showing an LC rate of 98.5% at 6 months and 96.0% at one year, and grade 3 toxicity rates of 5.2%. This study has the highest number of MET patients treated with Leksell Gamma Knife® Icon™. Our results are compatible with the previously reported results of hfSRS as an upfront treatment for METs. This study showed that frameless hfGKRS for METs is well-tolerated, with satisfactory efficiency and safety.
Whole-brain radiotherapy and single-fraction radiosurgery
The management of METs is complicated and affected by several tumor-related and patient-related factors. Although surgical resection succeeded by adjuvant irradiation may be the most favored treatment for METs, radiation may become the main modality in treating the patients who were not surgical candidates [36, 37]. WBRT was deemed as the standard treatment for treating patients with METs when surgery is not applicable or not demanded [38]; however, both moderate LC and worries about toxicities associated with WBRT have caused considerable interest in managing METs with focal irradiation [3, 39]. sfSRS, which is a subgroup of SRT approach, has been preferred to WBRT for several years with excellent LC in selected patients with METs [40]. While single-fraction treatment of small tumors was both efficient at tumor control and saving healthy tissue [41], the capacity to safely deliver a satisfactory dose with optimal results in a single fraction to larger lesions was restricted to tumor diameters above 2–3 cm, as determined in the Radiation Therapy Oncology Group (RTOG) trial 90-05 [4]. In that study, it was concluded that as tumors increased in diameter, the dose must be reduced to consider the risk of severe toxicity, and dose limits of 24 Gy, 18 Gy, and 15 Gy were established for lesions < 2.0, 2.0–3.0, and 3.1–4.0 cm in maximum dimension. Contradictorily, as tumor diameter increases, tumor volume and the number of tumor cells increase to a great extent; thus, the reduced dose affects tumor control adversely. Indeed, the 1-year LC rates for tumors > 3 cm utilizing the RTOG-recommended 15 Gy dose were 37%–62% [4, 42]. A study by Vogelbaum et al. [43] determined that the dose limit demanded by increasing size caused a 1-year LC rate of 85% in METs managed with 24 Gy, compared with 49% in METs treated with 18 Gy and 45% in METs treated with 15 Gy. However, we did not find such a relationship between LC and the delivered dose. To overcome this, one would fancy administering higher doses to the larger volumes to kill comparable proportions of tumor cells. However, high-dose sfSRS in subjects with small or large METs or patients who had local failure following a previous SRS yield a higher risk of RN [4, 18, 20, 24, 42,43,44,45,46], and the relationship between the irradiated brain volume with a high single dose and risk of RN became apparent as parameters such as V12Gy and V10Gy were confirmed to be predicting [47]. Regardless of the sfSRS or hfSRS, it should be kept in mind that there likely is a point at which the number of brain metastases treated with SRS results in a significant dose of radiation to the whole brain. Despite this risk, SRS was accepted to be neurologically safer even when doses approach those seen in a single WBRT. Jairam et al. [48] suggested that a MWBD of 4 Gy can be both used as clinical benchmarks for theoretically safe administration of SRS to the brain. In our study, the median MWBD per treatment was 3.4 Gy (range 1.6–4.9 Gy). Another limitation of sfSRS is METs near OARs like the brainstem, optic pathways, and cochlea. In such cases, traditional sfSRS resulted in higher adverse radiation effects [49]. In a study by Yuan et al. [50], it was shown that a low dose to OARs could be maintained despite SRS with repeat GKRS, and thus the low cumulative doses to the brain and the hippocampi may potentially spare these patients from radiation-induced neurocognitive decline as commonly seen with WBRT.
Fractionation
Several novel strategies have been explored to overwhelm the moderate LC achieved in the treatment of METs and minimize the SRS-related toxicity. Dose fractionation was considered a possible alternative for enhancing the total dose delivered to the lesion and restraining the toxicity to surrounding tissues. Differing from sfSRS, where patients are conventionally immobilized with an invasive frame, non-invasive stereotactic systems with a mask or a re-locatable frame are regularly used for multi-fraction SRS. Retrospective and prospective studies evaluating hfSRS [8,9,10,11,12,13,14,15,16,17,18,19,20] and staged SRS [51,52,53,54,55] have been studied in the treatment of METs. The basis was to increase the prescribed dose and the LC while reducing the radiation-related morbidity by dividing the dose over time and possibly to smaller targets during the second treatment. The treatment of METs with sfSRS, hfSRS, and staged SRS (SSRS) in studies from the last 10 years is shown in Table 3, where particular study features, treatment responses, and toxicities are analyzed. A BED10 comparison is also presented using the linear-quadratic model [56].
Hypofractionated stereotactic radiosurgery
Wiggenraad et al. [20] first described the use of hfSRS for METs in 2012. Since then, various single-institution series have published hfSRS results, indicating trends toward better or equal LC rates with fewer RN despite the larger irradiated volumes (Table 3). In the meta-analysis of 24 studies, Lehrer et al. [57] compared LC rates of sfSRS and hfSRS in the upfront and postoperative settings. The study included a total of 1094 subjects with 1157 large METs in the upfront group. In the upfront hfSRS group, the median OS during a median follow-up of 10 months was 11.8 months, and the most common hfSRS dose was 27 Gy in 3 fractions (range, 24–35 Gy in 2–5 fractions). BEDs ranged from 43.2 to 76.2 Gy in the upfront hfSRS studies. The mean OS was 23.2 months during a median follow-up of 12 months, and, similarly, the median total dose to the margin was 27 Gy, with a median dose per fraction of 9 Gy in our study. The most commonly used SRS platform was Gamma Knife (Elekta AB, Stockholm, Sweden), which was used in 10 of 24 studies. The data in the study by Lehrer et al. [57] were segmented into groups based on RTOG 90-05 definitions as Group A (4–14 cm3 or about 2–3 cm in diameter) and Group B (> 14 cm3 or about > 3 cm in diameter). The median tumor volume was 15.7 cm3 (range, 5.9–29.4 cm3) for upfront hfSRS, with a median tumor volume for Group A of 10.5 cm3 (range, 5.9–12.6 cm3) and Group B of 17.6 cm3 (range, 15.6–29.4 cm3). The median tumor volume in our study was 6.15 cm3 (range, 4–22.2), with a median tumor volume for RTOG 90-05 Group A of 5.5 cm3 (range, 4–3.6) and Group B of 16.5 cm3 (range, 14.4–22.2). LC rates at 1-year ranged from 59 to 100% in the meta-analysis. They found that hfSRS potentially afforded a 20% relative increase in 1-year LC in METs measuring 4 to 14cm3 and/or 2 to 3 cm in diameter treated with upfront intent. However, they did not observe an LC benefit in larger METs measuring > 14 cm3 and/or > 3 cm in diameter. In our study, the LC rate at one year was 96.0%. Interestingly, LC was better in our study in RTOG Group B tumors than Group A tumors, as all three local failures in the study were observed in Group A. All three METs were of skin origin (malign melanoma), and it is already known that there is a significant relationship between local failure and tumor histology and that melanomas have the highest relative risk for local failure after SRS [58]. As the suitable dose selection and fractionation for large brain metastases remain controversial, personal experiences along with tumor characteristics, concurrent clinical status, and current or previous treatment history affect the dosing and fractionation schemes. In this study, the prescription dose was decided based on a linear-quadratic model that assumed an α/β ratio of 10 Gy for malignant tumors. A larger tumor volume and lower total marginal dose are suggested as potential candidates for local control failure; however, no factors were found to be significant in the univariate analyses in this study. While the dose delivered via hfGKRS could theoretically be increased, prescription doses are currently constrained by the volume of the tumor being treated to avoid RN. It is necessary to clarify the optimum dose, interval, and number of fractionations to treat large brain metastases in the future.
Radiation necrosis
RN is the most significant late toxicity described following SRS. Its importance is increasing in patients with METs, as they started living longer, thus having an increased risk of exhibiting RN after SRS. Regarding the radiobiological principles of reduced healthy tissue toxicity with increased fractionation, hfSRS can decrease RN incidence. hfSRS regimens allow for a higher BED administration due to interludes between fractions, which is not achievable with sfSRS. Minniti et al. [12] analyzed 289 subjects with METs > 2.0 cm who underwent upfront SRS and found that the radiological changes indicative of RN were significantly higher in patients who received a median dose of 18 Gy with sfSRS (27.7%) as compared with those who received a total dose of 27 Gy in 3 fractions (14.4%) (p = 0.04). In their meta-analysis, Lehrer et al. [57] also analyzed the RN incidence stratified by the fractionation scheme. They found a significantly higher random effects estimate for the incidence of RN in the upfront sfSRS group (18.2%) compared to the upfront hfSRS group (7.1%) (p = 0.02). Prabhu et al. [21] reported a study where patients managed with upfront sfSRS with a median tumor volume of 5.9 cm3 (RTOG 90-05 Group A) received a single fraction of 18 Gy. These patients encountered an RN rate of 17.2%. In contrast, Navarria et al. [13] analyzed 102 patients who underwent hfSRS for a median maximum diameter of BM of 2.9 cm (RTOG 90-05 Group A) and found an RN incidence of 5.9%. Han et al. [23] reported an RN incidence of 38.8% (18.8% unacceptable toxicity levels) in 80 patients who were managed with upfront sfSRS for a median tumor volume of 22.4 cm3 (RTOG 90-05 Group B) and received a median single dose of 13.8 Gy. In contrast, Kim et al. [11] published an RN incidence of 2.7% in 36 patients with a mean gross tumor volume of 18.3 cm3 and received a median dose of 8 Gy with three fractions for three consecutive days. The meta-analysis by Lehrer et al. [57] reported that the random effects estimate for RN incidence stratified by increasing tumor size were not statistically significant between upfront SRS Group A (11.3%) and Group B (7.6%) (p = 0.49). However, they found that the random effects estimate for RN was significantly (p = 0.003) higher in Group A METs treated with upfront sfSRS than Group A METs treated with upfront hfSRS (23.1% vs. 7.3%). In our study, comparable to the recently published studies by Park et al. [8] and Chon et al. [9], RN was not observed in any patients.
Staged stereotactic radiosurgery
sSRS was suggested to be the major competing technique for hfSRS. This method is based on providing an increased overall dose in two or three individual sessions and is established on the assumption that volume reduction following the initial SRS may enhance the therapeutic ratio by reducing the volume of the second stage of the procedure. Dissimilar from the daily hfSRS, sSRS intends to decrease toxicity with a more extended delay between fractions, benefiting from the interval volume reduction that is usually seen between stages so that less brain volume is treated in the following stages. Angelov et al. [52] claimed that the high doses in every session might improve the killing of tumor cells by endothelial cell apoptosis, microvascular dysfunction, and the disruption of tumor perfusion that occurs at a threshold of 10 Gy and is typically not obtained with hfSRS, where fraction doses are generally 6–9 Gy. They also stated that the repair half-time for late radiation effects could be as long as 76 h; therefore, they preferred a 30-day interlude between stages to provide ten half-lives for repair and lessen RN risk [59]. Although successful results were reported, there is a volume restriction for a MET that can effectively be managed with this technique. It is vague what the upper limit of the volume is that can be managed with sSRS, as fractional doses would likely require to be decreased for larger lesions, and it is indefinite whether this would unfavorably affect LC. In fact, the LC rates published up-to-date range between 61 and 86.7% with being poor compared to hfSRS rates (Table 3). Besides, sSRS is proposed to safely deliver up to 73 Gy of BED10 to the tumor itself by augmenting the dose usually delivered to the METs and optimizing LC; the rate of RN in these studies, even with recently published ones, are approximately 10%. A BED10 value ≥ 50Gy has been recommended for hfSRS regimens, and a 1-year LC of 96.0% and a RN rate of 0% were achieved in our study with a BED10 value of 51.3 [60].
Leptomeningeal metastasis
LM is often associated with poor OS, typically measured in weeks to months, which makes reducing the rate of LM a potentially important goal [61]. In our cohort, one patient who had undergone surgical resection for a cerebellar MET had a LM and died after 5 months. It seems important to use a treatment strategy that effectively controls METs while minimizing the risk of iatrogenically introduced LM. One such treatment strategy is the utilization of upfront SRS, which has been reported to reduce the risk of LM in patients requiring surgical resection of a MET [62].
The role of systemic treatments
The future of MET management is signified on personalized therapeutics targeted to specific tumor molecular pathways, such as those involved in the blood-brain barrier transgression, cell-cell adhesion, and angiogenesis. Personalized therapies should, therefore, be preferred based on MET tissue whenever feasible. EGFR-TKIs have succeeded in notable clinical improvements, with promising toxicity profiles, in NSCLC patients harboring EGFR mutations [63]. Two recent studies have demonstrated that combined use of GKRS and EGFR-TKI was correlated with increased OS in patients with METs from NSCLC [64, 65]. Another topic of great interest is whether TKIs exacerbate the toxicity of treatment. Preclinical and clinical data revealed that cancer cells with EGFR mutations are radiosensitive and that EGFR-TKI is considered a radiosensitizer. One study reported a significantly greater 12-months cumulative incidence of RN in patients who had used TKIs within 30 days of SRS (10.9 vs. 6.4%, p = 0.04) [66]. On the other hand, Yomo et al. demonstrated no substantial differences between the SRS with versus without EGFR-TKI use in terms of either local efficacy or toxicity at the site of stereotactic irradiation [65]. In our study, only two patients were on concurrent EGFR-TKI therapy, and they were still alive during the study with median follow-up periods of 37 months and 30 months. Besides, no toxicity was observed in these patients. However, we cannot make any assumptions on the effect of concurrent TKI therapy in our cohort, as there were only two patients. Careful documentation of systemic agents use before and after GKRS is necessary. Reducing the irradiation dose delivered to tumors in patients treated with novel systemic agents, as an approach to avoiding radiation injury, should perhaps be considered. In our center, MET patients are discussed with medical oncologists before and after GKRS to ensure proper treatment planning.
Perspective
Regarding the increased experience with favorable outcomes, hfSRS is a vital alternative to treat patients with METs. The progressive growth of brain metastases is often associated with advanced primary cancer. Although detailed information on the relationship between MET cells and host immune response is scarce, more and more evidence suggesting that an effective immune response may have great potential in mediating the tumor dynamics. Therefore, a profound understanding of the pathogenesis of METs, tumor dynamics, and immune microenvironment is of great importance in the prognosis. As mentioned earlier, the suitable dose selection and fractionation for large brain metastases remain controversial, and it is necessary to clarify the optimum dose, interval, and number of fractionations for the treatment of large brain metastases. This clarification becomes more important as novel treatment options such as targeted agents and immunotherapy have come into play. Early evidence suggests synergy between SRS and immune system modulation, presumably due to an abscopal response mediated by SRS-induced tumor associated antigen release. Nowadays, there has been increased enthusiasm in exploring the synergy of SRS and immunotherapy in the treatment of METs. There are more than 80 clinical trials in progress on SRS trying to find an answer to dose escalation (NCT03412812), dose reduction for brain metastasis on immunotherapy (NCT04047602), and fractionation on immunotherapy (NCT04427228). A recent systematic review and meta-analysis by Sha et al. [67] including 51 studies on toxicity in combination immune checkpoint inhibitor and radiation therapy revealed comparable grade 3–4 toxicities in immune checkpoint inhibitor with radiation therapy (16.3%; 95% CI 11.1–22.3%) and immune checkpoint inhibitor alone (22.3%; 95% CI 18.1–26.9%) in CNS melanoma metastases, NSCLC, and prostate cancer.
Limitations
The major limitation of the present study was the retrospective nature of the analysis. Another limitation of our study was a short follow-up time due to the poor prognosis for these patients. The dominance of NSCLC histopathology in our study might impact the general applicability of the outcomes. The low rate of RN might be due to the short follow-up period. Besides, results from a single institution might be prone to patient selection bias. Additionally, it should be noted that poor compliance might be observed with mask-based treatment in claustrophobic patients. The findings of the current study are intended to be hypothesis-generating, and additional studies are needed to classify the most proper forms for hfSRS based on tumor and patient features.
Conclusion
The results of the current study with the highest number of unresected, large MET patients treated with hypofractionated Leksell Gamma Knife® Icon™ propose a superior balance of efficiency and toxicity of frameless upfront hfGKRS in the management of METs. Thus, frameless upfront hfGKRS represents a reasonable therapeutic option in selected patients with risks for greater toxicity due to increasing treatment volume and with lesions in close proximity to critical normal structures that limit the use of sfSRS.
References
Soffietti R, Ruda R, Trevisan E (2008) Brain metastases: current management and new developments. Curr Opin Oncol 20(6):676–684. https://doi.org/10.1097/CCO.0b013e32831186fe
Owonikoko TK, Arbiser J, Zelnak A, Shu HK, Shim H, Robin AM, Kalkanis SN, Whitsett TG, Salhia B, Tran NL, Ryken T, Moore MK, Egan KM, Olson JJ (2014) Current approaches to the treatment of metastatic brain tumours. Nat Rev Clin Oncol 11(4):203–222. https://doi.org/10.1038/nrclinonc.2014.25
Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, Arbuckle RB, Swint JM, Shiu AS, Maor MH, Meyers CA (2009) Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 10(11):1037–1044. https://doi.org/10.1016/S1470-2045(09)70263-3
Shaw E, Scott C, Souhami L, Dinapoli R, Kline R, Loeffler J, Farnan N (2000) Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90–05. Int J Radiat Oncol Biol Phys 47(2):291–298. https://doi.org/10.1016/s0360-3016(99)00507-6
Masucci GL (2018) Hypofractionated radiation therapy for large brain metastases. Front Oncol 8:379. https://doi.org/10.3389/fonc.2018.00379
Nahum AE (2015) The radiobiology of hypofractionation. Clin Oncol 27(5):260–269. https://doi.org/10.1016/j.clon.2015.02.001
Sahgal A, Ma L, Chang E, Shiu A, Larson DA, Laperriere N, Yin FF, Tsao M, Menard C, Basran P, Letourneau D, Heydarian M, Beachey D, Shukla V, Cusimano M, Hodaie M, Zadeh G, Bernstein M, Schwartz M (2009) Advances in technology for intracranial stereotactic radiosurgery. Technol Cancer Res Treat 8(4):271–280. https://doi.org/10.1177/153303460900800404
Park HR, Park KW, Lee JM, Kim JH, Jeong SS, Kim JW, Chung HT, Kim DG, Paek SH (2019) Frameless fractionated gamma knife radiosurgery with ICON for large metastatic brain tumors. J Korean Med Sci 34(8):e57. https://doi.org/10.3346/jkms.2019.34.e57
Chon H, Yoon K, Lee D, Kwon DH, Cho YH (2019) Single-fraction versus hypofractionated stereotactic radiosurgery for medium-sized brain metastases of 2.5 to 3 cm. J Neuro-Oncol 145(1):49–56. https://doi.org/10.1007/s11060-019-03265-1
Marcrom SR, McDonald AM, Thompson JW, Popple RA, Riley KO, Markert JM, Willey CD, Bredel M, Fiveash JB (2017) Fractionated stereotactic radiation therapy for intact brain metastases. Adv Radiat Oncol 2(4):564–571. https://doi.org/10.1016/j.adro.2017.07.006
Kim JW, Park HR, Lee JM, Kim JW, Chung HT, Kim DG, Jung HW, Paek SH (2016) Fractionated stereotactic gamma knife radiosurgery for large brain metastases: a retrospective, single center study. PloS One 11(9):e0163304. https://doi.org/10.1371/journal.pone.0163304
Minniti G, Scaringi C, Paolini S, Lanzetta G, Romano A, Cicone F, Osti M, Enrici RM, Esposito V (2016) Single-fraction versus multifraction (3 x 9 Gy) stereotactic radiosurgery for large (>2 cm) brain metastases: a comparative analysis of local control and risk of radiation-induced brain necrosis. Int J Radiat Oncol Biol Phys 95(4):1142–1148. https://doi.org/10.1016/j.ijrobp.2016.03.013
Navarria P, Pessina F, Cozzi L, Ascolese AM, De Rose F, Fogliata A, Franzese C, Franceschini D, Tozzi A, D’Agostino G, Comito T, Iftode C, Maggi G, Reggiori G, Bello L, Scorsetti M (2016) Hypo-fractionated stereotactic radiotherapy alone using volumetric modulated arc therapy for patients with single, large brain metastases unsuitable for surgical resection. Radiat Oncol 11:76. https://doi.org/10.1186/s13014-016-0653-3
Jeong WJ, Park JH, Lee EJ, Kim JH, Kim CJ, Cho YH (2015) Efficacy and safety of fractionated stereotactic radiosurgery for large brain metastases. J Korean Neurosurg Soc 58(3):217–224. https://doi.org/10.3340/jkns.2015.58.3.217
Wegner RE, Leeman JE, Kabolizadeh P, Rwigema JC, Mintz AH, Burton SA, Heron DE (2015) Fractionated stereotactic radiosurgery for large brain metastases. Am J Clin Oncol 38(2):135–139. https://doi.org/10.1097/COC.0b013e31828aadac
Minniti G, D’Angelillo RM, Scaringi C, Trodella LE, Clarke E, Matteucci P, Osti MF, Ramella S, Enrici RM, Trodella L (2014) Fractionated stereotactic radiosurgery for patients with brain metastases. J Neuro-Oncol 117(2):295–301. https://doi.org/10.1007/s11060-014-1388-3
Inoue HK, Sato H, Seto K, Torikai K, Suzuki Y, Saitoh J, Noda SE, Nakano T (2014) Five-fraction CyberKnife radiotherapy for large brain metastases in critical areas: impact on the surrounding brain volumes circumscribed with a single dose equivalent of 14 Gy (V14) to avoid radiation necrosis. J Radiat Res 55(2):334–342. https://doi.org/10.1093/jrr/rrt127
Feuvret L, Vinchon S, Martin V, Lamproglou I, Halley A, Calugaru V, Chea M, Valery CA, Simon JM, Mazeron JJ (2014) Stereotactic radiotherapy for large solitary brain metastases. Cancer Radiother: J de la Societe Francaise de Radiotherapie Oncologique 18(2):97–106. https://doi.org/10.1016/j.canrad.2013.12.003
Murai T, Ogino H, Manabe Y, Iwabuchi M, Okumura T, Matsushita Y, Tsuji Y, Suzuki H, Shibamoto Y (2014) Fractionated stereotactic radiotherapy using CyberKnife for the treatment of large brain metastases: a dose escalation study. Clin Oncol 26(3):151–158. https://doi.org/10.1016/j.clon.2013.11.027
Wiggenraad R, Verbeek-de Kanter A, Mast M, Molenaar R, Kal HB, Lycklama a Nijeholt G, Vecht C, Struikmans H (2012) Local progression and pseudo progression after single fraction or fractionated stereotactic radiotherapy for large brain metastases. A single centre study. Strahlentherapie und Onkologie: Organ der Deutschen Rontgengesellschaft 188(8):696–701. https://doi.org/10.1007/s00066-012-0122-3
Prabhu RS, Press RH, Patel KR, Boselli DM, Symanowski JT, Lankford SP, McCammon RJ, Moeller BJ, Heinzerling JH, Fasola CE, Asher AL, Sumrall AL, Buchwald ZS, Curran WJ Jr, Shu HG, Crocker I, Burri SH (2017) Single-fraction stereotactic radiosurgery (SRS) alone versus surgical resection and SRS for large brain metastases: a multi-institutional analysis. Int J Radiat Oncol Biol Phys 99(2):459–467. https://doi.org/10.1016/j.ijrobp.2017.04.006
Zimmerman AL, Murphy ES, Suh JH, Vogelbaum MA, Barnett GH, Angelov L, Ahluwalia M, Reddy CA, Chao ST (2016) Treatment of Large Brain Metastases With Stereotactic Radiosurgery. Technology in cancer research & treatment 15(1):186–195. https://doi.org/10.1177/1533034614568097
Han JH, Kim DG, Chung HT, Paek SH, Park CK, Jung HW (2012) Radiosurgery for large brain metastases. Int J Radiat Oncol Biol Phys 83(1):113–120. https://doi.org/10.1016/j.ijrobp.2011.06.1965
Yang HC, Kano H, Lunsford LD, Niranjan A, Flickinger JC, Kondziolka D (2011) What factors predict the response of larger brain metastases to radiosurgery? Neurosurgery 68 (3):682-690; discussion 690. https://doi.org/https://doi.org/10.1227/NEU.0b013e318207a58b
Kim JM, Miller JA, Kotecha R, Xiao R, Juloori A, Ward MC, Ahluwalia MS, Mohammadi AM, Peereboom DM, Murphy ES, Suh JH, Barnett GH, Vogelbaum MA, Angelov L, Stevens GH, Chao ST (2017) The risk of radiation necrosis following stereotactic radiosurgery with concurrent systemic therapies. J Neuro-Oncol 133(2):357–368. https://doi.org/10.1007/s11060-017-2442-8
Fokas E, Henzel M, Surber G, Kleinert G, Hamm K, Engenhart-Cabillic R (2012) Stereotactic radiosurgery and fractionated stereotactic radiotherapy: comparison of efficacy and toxicity in 260 patients with brain metastases. J Neuro-Oncol 109(1):91–98. https://doi.org/10.1007/s11060-012-0868-6
Benedict SH, Yenice KM, Followill D, Galvin JM, Hinson W, Kavanagh B, Keall P, Lovelock M, Meeks S, Papiez L, Purdie T, Sadagopan R, Schell MC, Salter B, Schlesinger DJ, Shiu AS, Solberg T, Song DY, Stieber V, Timmerman R, Tome WA, Verellen D, Wang L, Yin FF (2010) Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys 37(8):4078–4101. https://doi.org/10.1118/1.3438081
Lin NU, Lee EQ, Aoyama H, Barani IJ, Barboriak DP, Baumert BG, Bendszus M, Brown PD, Camidge DR, Chang SM, Dancey J, de Vries EG, Gaspar LE, Harris GJ, Hodi FS, Kalkanis SN, Linskey ME, Macdonald DR, Margolin K, Mehta MP, Schiff D, Soffietti R, Suh JH, van den Bent MJ, Vogelbaum MA, Wen PY (2015) Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol 16(6):e270-278. https://doi.org/10.1016/s1470-2045(15)70057-4
Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, McKenna WG, Byhardt R (1997) Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 37(4):745–751. https://doi.org/10.1016/s0360-3016(96)00619-0
Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W (2008) A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys 70(2):510–514. https://doi.org/10.1016/j.ijrobp.2007.06.074
Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, Sneed PK, Chao ST, Weil RJ, Suh J, Bhatt A, Jensen AW, Brown PD, Shih HA, Kirkpatrick J, Gaspar LE, Fiveash JB, Chiang V, Knisely JP, Sperduto CM, Lin N, Mehta M (2012) Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol: Off J Am Soc Clin Oncol 30(4):419–425. https://doi.org/10.1200/JCO.2011.38.0527
Sperduto PW, Yang TJ, Beal K, Pan H, Brown PD, Bangdiwala A, Shanley R, Yeh N, Gaspar LE, Braunstein S, Sneed P, Boyle J, Kirkpatrick JP, Mak KS, Shih HA, Engelman A, Roberge D, Arvold ND, Alexander B, Awad MM, Contessa J, Chiang V, Hardie J, Ma D, Lou E, Sperduto W, Mehta MP (2017) Estimating survival in patients with lung cancer and brain metastases: an update of the graded prognostic assessment for lung cancer using molecular markers (Lung-molGPA). JAMA Oncol 3(6):827–831. https://doi.org/10.1001/jamaoncol.2016.3834
Sperduto PW, Jiang W, Brown PD, Braunstein S, Sneed P, Wattson DA, Shih HA, Bangdiwala A, Shanley R, Lockney NA, Beal K, Lou E, Amatruda T, Sperduto WA, Kirkpatrick JP, Yeh N, Gaspar LE, Molitoris JK, Masucci L, Roberge D, Yu J, Chiang V, Mehta M (2017) Estimating Survival in Melanoma Patients With Brain Metastases: An Update of the Graded Prognostic Assessment for Melanoma Using Molecular Markers (Melanoma-molGPA). International journal of radiation oncology, biology, physics 99(4):812–816. https://doi.org/10.1016/j.ijrobp.2017.06.2454
Minniti G, Clarke E, Lanzetta G, Osti MF, Trasimeni G, Bozzao A, Romano A, Enrici RM (2011) Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiation Oncol 6:48. https://doi.org/10.1186/1748-717X-6-48
Zach L, Guez D, Last D, Daniels D, Grober Y, Nissim O, Hoffmann C, Nass D, Talianski A, Spiegelmann R, Tsarfaty G, Salomon S, Hadani M, Kanner A, Blumenthal DT, Bukstein F, Yalon M, Zauberman J, Roth J, Shoshan Y, Fridman E, Wygoda M, Limon D, Tzuk T, Cohen ZR, Mardor Y (2015) Delayed contrast extravasation MRI: a new paradigm in neuro-oncology. Neuro-Oncol 17(3):457–465. https://doi.org/10.1093/neuonc/nou230
Luther N, Kondziolka D, Kano H, Mousavi SH, Engh JA, Niranjan A, Flickinger JC, Lunsford LD (2013) Predicting tumor control after resection bed radiosurgery of brain metastases. Neurosurgery 73 (6):1001-1006; discussion 1006. https://doi.org/https://doi.org/10.1227/NEU.0000000000000148
Kalkanis SN, Kondziolka D, Gaspar LE, Burri SH, Asher AL, Cobbs CS, Ammirati M, Robinson PD, Andrews DW, Loeffler JS, McDermott M, Mehta MP, Mikkelsen T, Olson JJ, Paleologos NA, Patchell RA, Ryken TC, Linskey ME (2010) The role of surgical resection in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neuro-Oncol 96(1):33–43. https://doi.org/10.1007/s11060-009-0061-8
Lin X, DeAngelis LM (2015) Treatment of brain metastases. J Clin Oncol: Off J Am Soc Clin Oncol 33(30):3475–3484. https://doi.org/10.1200/JCO.2015.60.9503
Brown PD, Jaeckle K, Ballman KV, Farace E, Cerhan JH, Anderson SK, Carrero XW, Barker FG 2nd, Deming R, Burri SH, Menard C, Chung C, Stieber VW, Pollock BE, Galanis E, Buckner JC, Asher AL (2016) Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. Jama 316(4):401–409. https://doi.org/10.1001/jama.2016.9839
Kocher M, Soffietti R, Abacioglu U, Villa S, Fauchon F, Baumert BG, Fariselli L, Tzuk-Shina T, Kortmann RD, Carrie C, Ben Hassel M, Kouri M, Valeinis E, van den Berge D, Collette S, Collette L, Mueller RP (2011) Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J Clin Oncol: Off J Am Soc Clin Oncol 29(2):134–141. https://doi.org/10.1200/JCO.2010.30.1655
Mohammadi AM, Schroeder JL, Angelov L, Chao ST, Murphy ES, Yu JS, Neyman G, Jia X, Suh JH, Barnett GH, Vogelbaum MA (2017) Impact of the radiosurgery prescription dose on the local control of small (2 cm or smaller) brain metastases. J Neurosurg 126(3):735–743. https://doi.org/10.3171/2016.3.JNS153014
Molenaar R, Wiggenraad R, Verbeek-de Kanter A, Walchenbach R, Vecht C (2009) Relationship between volume, dose and local control in stereotactic radiosurgery of brain metastasis. Br J Neurosurg 23(2):170–178. https://doi.org/10.1080/02688690902755613
Vogelbaum MA, Angelov L, Lee SY, Li L, Barnett GH, Suh JH (2006) Local control of brain metastases by stereotactic radiosurgery in relation to dose to the tumor margin. J Neurosurg 104(6):907–912. https://doi.org/10.3171/jns.2006.104.6.907
Kohutek ZA, Yamada Y, Chan TA, Brennan CW, Tabar V, Gutin PH, Yang TJ, Rosenblum MK, Ballangrud A, Young RJ, Zhang Z, Beal K (2015) Long-term risk of radionecrosis and imaging changes after stereotactic radiosurgery for brain metastases. J Neuro-Oncol 125(1):149–156. https://doi.org/10.1007/s11060-015-1881-3
Sneed PK, Mendez J, Vemer-van den Hoek JG, Seymour ZA, Ma L, Molinaro AM, Fogh SE, Nakamura JL, McDermott MW (2015) Adverse radiation effect after stereotactic radiosurgery for brain metastases: incidence, time course, and risk factors. J Neurosurg 123(2):373–386. https://doi.org/10.3171/2014.10.JNS141610
Varlotto JM, Flickinger JC, Niranjan A, Bhatnagar AK, Kondziolka D, Lunsford LD (2003) Analysis of tumor control and toxicity in patients who have survived at least one year after radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys 57(2):452–464. https://doi.org/10.1016/s0360-3016(03)00568-6
Blonigen BJ, Steinmetz RD, Levin L, Lamba MA, Warnick RE, Breneman JC (2010) Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 77(4):996–1001. https://doi.org/10.1016/j.ijrobp.2009.06.006
Jairam V, Chiang VL, Bond J, Yu JB (2015) Equivalent whole brain dose for patients undergoing gamma knife for multiple lesions. J Radiosurg SBRT 3(3):187–191
Vachhrajani S, Fawaz C, Mathieu D, Menard C, Cusimano MD, Gentili F, Hodaie M, Kenny B, Kulkarni AV, Laperriere N, Schwartz M, Tsao M, Bernstein M (2008) Complications of gamma knife surgery: an early report from 2 Canadian centers. J Neurosurg 109(Suppl):2–7. https://doi.org/10.3171/JNS/2008/109/12/S2
Yuan J, Lee R, Dusenbery KE, Lee CK, Mathew DC, Sperduto PW, Watanabe Y (2018) Cumulative doses to brain and other critical structures after multisession gamma knife stereotactic radiosurgery for treatment of multiple metastatic tumors. Front Oncol 8:65. https://doi.org/10.3389/fonc.2018.00065
Dohm A, McTyre ER, Okoukoni C, Henson A, Cramer CK, LeCompte MC, Ruiz J, Munley MT, Qasem S, Lo HW, Xing F, Watabe K, Laxton AW, Tatter SB, Chan MD (2018) Staged stereotactic radiosurgery for large brain metastases: local control and clinical outcomes of a one-two punch technique. Neurosurgery 83(1):114–121. https://doi.org/10.1093/neuros/nyx355
Angelov L, Mohammadi AM, Bennett EE, Abbassy M, Elson P, Chao ST, Montgomery JS, Habboub G, Vogelbaum MA, Suh JH, Murphy ES, Ahluwalia MS, Nagel SJ, Barnett GH (2018) Impact of 2-staged stereotactic radiosurgery for treatment of brain metastases >/= 2 cm. J Neurosurg 129(2):366–382. https://doi.org/10.3171/2017.3.JNS162532
Hasegawa T, Kato T, Yamamoto T, Iizuka H, Nishikawa T, Ito H, Kato N (2017) Multisession gamma knife surgery for large brain metastases. J Neuro-Oncol 131(3):517–524. https://doi.org/10.1007/s11060-016-2317-4
Yomo S, Hayashi M (2014) A minimally invasive treatment option for large metastatic brain tumors: long-term results of two-session Gamma Knife stereotactic radiosurgery. Radiat Oncol 9:132. https://doi.org/10.1186/1748-717X-9-132
Yomo S, Hayashi M, Nicholson C (2012) A prospective pilot study of two-session Gamma Knife surgery for large metastatic brain tumors. J Neuro-Oncol 109(1):159–165. https://doi.org/10.1007/s11060-012-0882-8
Park C, Papiez L, Zhang S, Story M, Timmerman RD (2008) Universal survival curve and single fraction equivalent dose: useful tools in understanding potency of ablative radiotherapy. Int J Radiat Oncol Biol Phys 70(3):847–852. https://doi.org/10.1016/j.ijrobp.2007.10.059
Lehrer EJ, Peterson JL, Zaorsky NG, Brown PD, Sahgal A, Chiang VL, Chao ST, Sheehan JP, Trifiletti DM (2019) Single versus multifraction stereotactic radiosurgery for large brain metastases: an international meta-analysis of 24 trials. Int J Radiat Oncol Biology Phys 103(3):618–630. https://doi.org/10.1016/j.ijrobp.2018.10.038
Black PJ, Page BR, Lucas JT Jr, Hughes RT, Laxton AW, Tatter SB, Munley MT, Chan MD (2015) Factors that determine local control with gamma knife radiosurgery: the role of primary histology. J Radiosurg SBRT 3(4):281–286
Bender ET (2012) Brain necrosis after fractionated radiation therapy: is the halftime for repair longer than we thought? Med Phys 39(11):7055–7061. https://doi.org/10.1118/1.4762562
Remick JS, Kowalski E, Khairnar R, Sun K, Morse E, Cherng HR, Poirier Y, Lamichhane N, Becker SJ, Chen S, Patel AN, Kwok Y, Nichols E, Mohindra P, Woodworth GF, Regine WF, Mishra MV (2020) A multi-center analysis of single-fraction versus hypofractionated stereotactic radiosurgery for the treatment of brain metastasis. Radiat Oncol 15(1):128. https://doi.org/10.1186/s13014-020-01522-6
Le Rhun E, Weller M, Brandsma D, Van den Bent M, de Azambuja E, Henriksson R, Boulanger T, Peters S, Watts C, Wick W, Wesseling P, Ruda R, Preusser M, Board EE, clinicalguidelines@esmo.org EGCEa (2017) EANO-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with leptomeningeal metastasis from solid tumours. Ann Oncol: Off J Eur Soc Med Oncol 28 Suppl 4:iv84-iv99. https://doi.org/https://doi.org/10.1093/annonc/mdx221
Patel KR, Burri SH, Asher AL, Crocker IR, Fraser RW, Zhang C, Chen Z, Kandula S, Zhong J, Press RH, Olson JJ, Oyesiku NM, Wait SD, Curran WJ, Shu HK, Prabhu RS (2016) Comparing preoperative with postoperative stereotactic radiosurgery for resectable brain metastases: a multi-institutional analysis. Neurosurgery 79(2):279–285. https://doi.org/10.1227/NEU.0000000000001096
Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, Porta R, Cobo M, Garrido P, Longo F, Moran T, Insa A, De Marinis F, Corre R, Bover I, Illiano A, Dansin E, de Castro J, Milella M, Reguart N, Altavilla G, Jimenez U, Provencio M, Moreno MA, Terrasa J, Munoz-Langa J, Valdivia J, Isla D, Domine M, Molinier O, Mazieres J, Baize N, Garcia-Campelo R, Robinet G, Rodriguez-Abreu D, Lopez-Vivanco G, Gebbia V, Ferrera-Delgado L, Bombaron P, Bernabe R, Bearz A, Artal A, Cortesi E, Rolfo C, Sanchez-Ronco M, Drozdowskyj A, Queralt C, de Aguirre I, Ramirez JL, Sanchez JJ, Molina MA, Taron M, Paz-Ares L, Spanish Lung Cancer Group in Collaboration with Groupe Francais de P-C, Associazione Italiana Oncologia T (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13(3):239–246. https://doi.org/10.1016/S1470-2045(11)70393-X
Lee CC, Hsu SPC, Lin CJ, Wu HM, Chen YW, Luo YH, Chiang CL, Hu YS, Chung WY, Shiau CY, Guo WY, Hung-Chi Pan D, Yang HC (2019) Epidermal growth factor receptor mutations: association with favorable local tumor control following Gamma Knife radiosurgery in patients with non-small cell lung cancer and brain metastases. J Neurosurg. https://doi.org/10.3171/2019.4.JNS19446
Yomo S, Serizawa T, Yamamoto M, Higuchi Y, Sato Y, Shuto T, Akabane A, Jokura H, Kawagishi J, Aoyama H (2019) The impact of EGFR-TKI use on clinical outcomes of lung adenocarcinoma patients with brain metastases after Gamma Knife radiosurgery: a propensity score-matched analysis based on extended JLGK0901 dataset (JLGK0901-EGFR-TKI). J Neuro-Oncol 145(1):151–157. https://doi.org/10.1007/s11060-019-03282-0
Juloori A, Miller JA, Parsai S, Kotecha R, Ahluwalia MS, Mohammadi AM, Murphy ES, Suh JH, Barnett GH, Yu JS, Vogelbaum MA, Rini B, Garcia J, Stevens GH, Angelov L, Chao ST (2019) Overall survival and response to radiation and targeted therapies among patients with renal cell carcinoma brain metastases. J Neurosurg. https://doi.org/10.3171/2018.8.JNS182100
Sha CM, Lehrer EJ, Hwang C, Trifiletti DM, Mackley HB, Drabick JJ, Zaorsky NG (2020) Toxicity in combination immune checkpoint inhibitor and radiation therapy: a systematic review and meta-analysis. Radiother Oncol: J Eur Soc Ther Radiol Oncol 151:141–148. https://doi.org/10.1016/j.radonc.2020.07.035
Funding
None.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design, or analysis and interpretation of data, and drafting the article or revising it critically for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the Ethics Committee of Koç University (2020.190.IRB1.058).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Patients signed informed consent regarding publishing their data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Samanci, Y., Sisman, U., Altintas, A. et al. Hypofractionated frameless gamma knife radiosurgery for large metastatic brain tumors. Clin Exp Metastasis 38, 31–46 (2021). https://doi.org/10.1007/s10585-020-10068-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-020-10068-6