Abstract
Purpose
Stereotactic radiosurgery (SRS) is typically considered for patients who cannot undergo surgical resection for large (> 10 cm3) brain metastases (BMs). Staged SRS requires adaptive planning during each stage of the irradiation period for improved tumor control and reduced radiation damage. However, there has been no study on the tumor reduction rates of this method. We evaluated the outcomes of two-stage SRS across multiple primary cancer types.
Methods
We analyzed 178 patients with 182 large BMs initially treated with two-stage SRS. The primary cancers included breast (BC), non-small cell lung (NSCLC), and gastrointestinal tract cancers (GIC). We analyzed the overall survival (OS), neurological death, systemic death (SD), tumor progression (TP), tumor recurrence (TR), radiation necrosis (RN), and the tumor reduction rate during both stages.
Results
The median survival time after the first Gamma Knife surgery (GKS) procedure was 6.6 months. Compared with patients with BC and NSCLC, patients with GIC had shorter OS and a higher incidence of SD. Compared with patients with NSCLC and GIC, patients with BC had significantly higher tumor reduction rates in both sessions. TP rates were similar among primary cancer types. There was no association of the tumor reduction rate with tumor control. The overall cumulative incidence of RN was 4.2%; further, the RN rates were similar among primary cancer types.
Conclusions
Two-stage SRS should be considered for BC and NSCLC if surgical resection is not indicated. For BMs from GIC, staged SRS should be carefully considered and adapted to each unique case given its lower tumor reduction rate and shorter OS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately 20–40% of patients with cancer develop brain metastases (BMs) [1]. Local control of BMs has become a critical issue since the evolution of chemotherapy. Further, molecular targeting drugs, immune checkpoint inhibitors, and radiotherapy have improved the life expectancy of patients with cancer. Large BMs (> 10 cm3) can typically be treated through surgery and/or whole-brain radiation therapy (WBRT) [2,3,4,5,6,7]. However, patient selection for operative intervention is limited by age, Karnofsky performance status (KPS), tumor location, extracranial disease status, and patient preference [8]. Tumor size is negatively correlated with the tumor control rate [9] and positively correlated with the neurotoxicity risk. Larger BMs should have a decreased prescribed dose; however, low prescribed doses (< 15 Gy) are associated with poor outcomes [10]. Tumor control and radiation damage are challenging in patients with large BMs who cannot undergo craniotomy or WBRT. In these cases, stereotactic radiosurgery (SRS) is an effective option [5, 11,12,13,14,15]. Moreover, SRS by Gamma Knife limits the indication for treatment depending on the metastatic tumor volume [10, 16, 17].
Recently, staged SRS has been developed to deliver a sufficient prescribed radiation dose while reducing the neurotoxicity risk; moreover, previous studies have reported its effectiveness [18,19,20,21,22,23,24,25]. No differences in the outcome have been reported for three-stage and two-stage SRS [24]. In staged SRS, the tumor volume is calculated to allow adaptive planning at the time of second fractions. This leads to reduced prescription volume and neurotoxicity.
To the best of our knowledge, there have been no reports on the tumor reduction rates achieved with staged SRS according to the primary cancer type. We aimed to clarify the role of staged SRS in cancer treatment with regard to the tumor volume reduction rate and tumor control and compared its effectiveness according to primary cancer type to allow the suggestion of appropriate treatment strategies.
Methods
Patient population
The institutional review board of Chiba Cerebral and Cardiovascular Center IRB (IRB number: #456) approved this retrospective study. The main inclusion criteria were as follows: (1) newly diagnosed BMs, (2) tumor volume > 10 cm3, (3) BC, non-small cell lung cancer (NSCLC), or GIC as the primary cancer type. We enrolled 178 patients with 182 lesions treated with two-stage SRS between April 2008 and March 2019 at Chiba Cerebral and Cardiovascular Center and Tsukiji Neurological Clinic. In all patients, a Leksell G frame (Elekta Instrument, Stockholm, Sweden) was secured with screw pins under local anesthesia with adequate sedation, as appropriate. For each dose planning, we obtained gadolinium (Gd)-enhanced T1-weighted magnetic resonance (MR) images. We determined the prescription dose and treatment interval based on a previous report [24]. Neither the Extend system with Perfexion nor the mask system with ICON was used in this series. We did not set margins for the gross, clinical, or planning target volumes. All patients underwent staged SRS alone and not in combination with concurrent WBRT.
Definition of clinical outcomes
Overall survival (OS) was defined as the interval between the first SRS procedure and death. Tumor progression was defined as a 20% increase in the maximum diameter of the Gd-enhanced lesion since the first SRS [24, 26]. Neurologic death (ND) was defined as death by intracranial disease progression, including tumor recurrence, as well as leptomeningeal and cerebral dissemination. Systemic death was defined as death due to primary lesion progression. Tumor recurrence (TR) and radiation necrosis (RN) were determined using various imaging findings on MR imaging, single-photon emission computed tomography, and methionine positron emission tomography as previously described, as well as the clinical course [27,28,29,30]. The reduction rate was calculated based on the difference in the tumor volume measured by Gamma Plan™ at the first and second SRS. We assessed changes in the tumor burden based on the revised RECIST guideline [31] using final MR imaging and were classified as Partial Response (PR), Progression Disease (PD), or Stable Disease (SD).
Statistical analysis
We obtained patient characteristics regarding sex, age at diagnosis, primary cancer, KPS score, tumor volume, prescribed dose, duration between first and second SRS. Moreover, we used presented continuous variables as median values and categorical factors as percentages. We estimated OS using the Kaplan–Meier method. Tumor control, including TP and TR, was calculated using Gray analysis with competing risk being considered. We considered death as a competing risk for TP, death or RN as a competing risk for TR, and death or TR as a competing risk for RN. The reduction rate was analyzed using a one-way analysis of variance. All statistical analyses were performed using EZR version 1.40 (Saitama Medical Center, Jichii Medical university). A p-value < 0.05 was considered statistically significant.
Results
Patient characteristics
Table 1 shows the clinical characteristics of the patients, which were compared among the primary cancer types. Compared to patients with BC and NSCLC, patients with GIC had significantly worse KPS scores (p = 0.001), more frequent neurological symptoms (p = 0.022), and worse modified recursive partitioning analysis classes [32] (p = 0.0001). There were no significant differences in the age; extracranial disease status, including systemic disease status and extracranial metastases; tumor volume; prescribed dose; and duration from first to second Gamma Knife surgery (GKS) procedure among the three primary cancer types. The period from the date of primary cancer diagnosis to delivery of the first SRS fraction was 36.8 ± 54.2 months.
Clinical outcomes
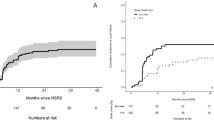
Table 2 shows the comparison of the clinical outcomes among the primary cancer types. The median follow-up duration after the first GKS procedure for the 178 cases was 5.4 months (range 2.5–12.4 months). The median survival time (MST) after the first GKS procedure was 6.6 months. The MST from the first GKS in the patients aged above 65 years was significantly shorter than that in patients aged under 65 years old (p < 0.01). The OS from the first GKS differed among the primary cancer types (Fig. 1a, p = 0.002). Patients with GIC showed a significantly higher systemic death rate compared with those with BC and NSCLC (p = 0.002). A total of 141 patients (79.8%) died before the final data analysis; among them, 20 had experienced ND, including 8 with BC, 3 with GIC, and 9 with lung cancer. The overall cumulative incidence of ND was 11.2% in all patients. The ND causes were determined as carcinomatous meningitis in 10 (50.0%) cases, recurrence of the GKS-treated lesion in 8 (40.0%) cases, and progression of the untreated lesion in 2 (10.0%) cases. We confirmed the response of 122 lesions (67.0%) on final MR imaging. Among 31 cases of BMs from BC, 17(54.8%), 6 (19.4%), and 8 (25.8%) were classified as PR, SD, and PD, respectively. Among 62 lesions with BMs from NSCLC, 32 (51.6%), 16 (25.8%), and 14 (22.6%) were classified as PR, SD, and PD, respectively. Among 29 lesions with BMs from GI tract cancers, 10 (34.5%), 9 (31.0%), and 10 (34.5%) were classified as PR, SD, and PD, respectively. Moreover, there were 60 (33.0%) lesions which we could not obtain information since the image at the second fraction was the last follow-up image. The overall cumulative incidence of PD was 26.2% and the PD rates were similar among the primary cancer types (p = 0.221).
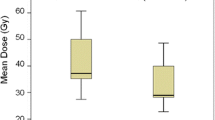
a Kaplan–Meier curves showing overall survival of the patients with different primary cancer types. The median survival time of all the patients was 6.6 months (95% CI 5.3–9.3). The median survival time of the patients with BC, NSCLC, and GIC was 8.9 months (95% CI 5.4–15.0), 9.3 months (95% CI 4.5–11.1), and 5.2 months (95% CI 3.5–5.8), respectively. The overall 6- and 12-month OS rates after the first SRS in all the patients, patients with BC; NSCLC; and GIC were 52% and 33%; 57.1% and 42.9%; 59.3% and 36.8%; and 33.4% and 15.9%; respectively. b The reduction rate was analyzed by one-way analysis of variance. The median tumor volume reduction rate was 46.1%, 18.2%, and 26.6% in patients with BC, GIC, and NSCLC, respectively. BMs from BC showed significantly higher reduction rates than those from GIC (p = 0.001) and NSCLC (p = 0.007)
Tumor control
Thirty-three patients (18.5%) presented with TP; among them, 8, 10, and 15, had BC, GIC, and lung cancer, respectively. Moreover, among the aforementioned patients, 19, 11, 2, and 1 underwent repeated SRS, observation, surgical removal, and WBRT, respectively. The cumulative incidence of TP was 6.8% in all the patients, 0.0% in patients with BC, 14.3% in patients with GIC, and 6.0% in patients with NSCLC at 6 months after the first GKS procedure. The TP rate was not significantly different among the three primary cancer types. There were no cases with mixed/undetermined lesions. Twenty-two patients (12.4%) were associated with TR; among them, 7, 6, and 9 were patients with BC, GIC, and lung cancer, respectively. The cumulative incidence of TR was 2.5% in all the patients at 6 months after the first GKS procedure. The TR rate did not differ significantly among the primary cancer types (Table 2).
The cumulative RN incidence in all the patients was 4.2%. The RN rate was not significantly different among the three primary cancers. RN occurred in 11 patients (6.2%); among them, there were 1, 4, and 6 patients with BC, GIC, and lung cancer, respectively. Further, among the 11 patients with RN, 3, 5, and 3 patients demonstrated Common Terminology Criteria for Adverse Events grade 3 toxicity, grade 2 toxicity, and grade 1 toxicity, respectively. There was no relationship between the reduction rate and the RN rate. Only 7 patients presented with a tumor volume of > 30 cc; among them, there were 1, 3, and 3 patients with BC, GIC, and NSCLC, respectively. All the cases were associated with systemic death. There were no cases with TP and RN. Among the patients with a tumor volume > 20 cc, 4.6% and 13.0% presented with RN and TP at 1 year. In the patients with a tumor volume > 20 cc, 2.8% and 5.7% presented with RN and TP at 1 year. The p values for RN and TP were 0.958 and 0.282, respectively.
Tumor volume reduction during the treatment protocol
The median tumor volume reduction rate after the second GKS procedure was 26.6% (interquartile range [IQR] 7.4–44.8%) in all the patients, 46.1% (IQR 14.1–68.8%) in patients with BC, 18.2% (IQR 3.4–37.0%) in patients with GIC, and 26.6% (IQR 7.2–38.3%) in patients with NSCLC. The tumor volume reduction rate differed significantly among the primary cancer types (p = 0.002) (Fig. 1b). Compared with patients with NSCLC and GIC, the patients with BC had a significantly higher tumor reduction rate. Forty lesions showed volume decreases of < 5% after the second GKS procedure. The primary pathologies of these 40 low-responders were BC in 5 (13.5%), GIC in 12 (24.5%), and NSCLC in 23 (24.0%) with a significant among-group difference (p = 0.011). Among the low-responders, we observed TP in 7 lesions, TR in 4 lesions, and RN in 3 lesions. There was ND due to carcinomatous meningitis and recurrence of the GKS-treated lesion in 1 and 2 patients, respectively.
Prognostic factors for clinical outcomes
Table 3 shows the proportion hazards model for OS. A low KPS score (< 60), active extracranial disease status, GIC, and large tumor volume (> 20 cm3) were identified as unfavorable prognostic factors that independently predicted the OS rates. Table 4 shows the proportion hazards model for TR. The tumor reduction rate was not a prognostic factor for TR. We found that older age (> 65-years old) was a favorable prognostic factor that independently predicted the TR rate. The cumulative incidence of TR at 12 months after the first GKS procedure was 5.4% and 10.1% in patients aged above and below 65 years, respectively (p < 0.01).
Therapeutic factors, including the duration between the first and second GKS or the prescribed dose, were not identified as prognostic factors that could predict either TP or TR.
Discussion
In this study, we assessed two-stage SRS as a strategy for treating large (> 10 cm3) BMs by comparing its clinical outcomes among the different primary cancers. We compared the short-term tumor volume reduction rate and the long-term tumor control, as well as the relationship between tumor volume reduction and tumor control. We found that patients with GIC had a shorter OS than that in patients with BC and NSCLC. BMs from BC showed a higher tumor volume reduction rate than those from GIC. There was no correlation between the tumor reduction rate and tumor control. Additionally, patients under the age of 65 years showed a higher incidence of local recurrence than patients older than 65 years. A study by Serizawa et al. enrolled 1194 patients who had 2–10 BMs treated by SRS. They reported 4 clinical factors that affected local tumor progression based on the Fine-Gray proportional hazards model [26]. These poor prognostic factors were as follows: (1) patients under the age of 65 years, (2) patients with neurological symptoms, (3) patients with larger tumor volumes, and (4) a low prescription dose (< 22 Gy) [26]. In our study, we introduced a competing analysis method for evaluating local recurrence. We accounted for death as a competing risk for TR. The OS of the elderly was significantly shorter than that of younger patients, which might have contributed to the higher local recurrence among younger compared to that among older patients. The period from the date of primary cancer diagnosis to delivery of the first SRS fraction was 42.0 ± 64.5 months and 28.3 ± 29.1 months (p = 0.10) in patients aged above and below 65 years.
Consideration of the primary cancers affecting OS and tumor control
The MST was longest in patients with BC (7.0 months) and shortest (5.3 months) in patients with GIC. This is consistent with a previous study by Nieder et al. who conducted a diagnosis-specific graded prognostic assessment study of 412 patients with BMs. They found that many patients were treated with surgical resection or SRS and that the median OS was 3.6 months from the first treatment day [33]. The primary tumor type was associated with survival. Patients with BC had the most favorable MST (9.0 months) while those with GIC had the least favorable MST (5.3 months). Further, we found that the primary cancer type did not affect tumor control. Specifically, BMs from GIC with a short OS could be controlled to a similar extent as those from BC with a long OS. Even with control of intracranial metastases, patients with BMs from GIC have a short OS, which suggests that the survival prognosis is likely dependent on the progression of extracranial lesions in these patients.
Consideration of the tumor reduction rate according to the primary cancer type and the relationship between the tumor reduction rate and tumor control
Small cell lung cancer, lymphoma, and germ cell tumors are regarded as highly radiosensitive tumors; contrastingly, malignant melanoma, renal cell carcinoma, and sarcoma are regarded as radioresistant tumors [34, 35]. NSCLC, BC, and GIC are regarded as intermediate radiosensitive tumors. This study involved targeting these intermediate radiosensitive tumors. Differences in the reduction rate might have occurred due to differences in the radiosensitivity of the primary cancers and vascular effects. Ahmed et al. assessed radiosensitivity in patients with lung metastases [36]. They reported that lung metastases from BC showed higher radiosensitivity than those from GIC. It is unlikely that BMs and lung metastases have identical radiosensitivity; however, it is possible that BMs from BC have a higher radiosensitivity than BMs from GIC. Kocher et al. assessed and reported the vascular effects of radiosurgery with single fraction based on computer simulation [37]. We hypothesized that the vascular effect could differ among the primary cancer types. To the best of our knowledge, there has been no previous study comparing the tumor reduction rate and tumor control between different tumor groups with moderately radiosensitive tumors. Higuchi et al. reported the efficacy and safety of staged SRS for large BM treatment [18]. Their participants included 43 patients with large BMs (> 10 cm3) treated using three-stage SRS without WBRT. The peripheral dose was 10 Gy and the interval between fractions was 2 weeks. They found that the mean tumor volume decreased by 18.8% after the second fraction. However, they did not report differences in the tumor volume reduction rate among the primary cancer types. In our study, the mean tumor volume decreased by 26.6% after the second fraction. We speculate that these differences in the reported tumor volume reduction rate could have resulted from differences in the treatment interval, prescribed dose, and patient characteristics. In our study, large BMs from BC showed significantly greater volume reduction than those from GIC and NSCLC.
A previous study reported a correlation between the tumor volume reduction rate and tumor control [20]. This previous study had longer treatment intervals and concomitantly longer reduction rate evaluation intervals. The treatment interval affects the tumor reduction rate, which could have contributed to the differences in our study and the aforementioned previous study. Contrastingly, some cancers show high radiosensitivity but poor tumor control, including SCLC [34, 35] Adenocarcinoma is known to have a slow radiation response [38]. Therefore, there is possibly no relationship between the reduction rate and tumor control. Staged SRS was developed to reduce the radiation prescription volume and toxicity by re-planning the irradiation range according to short-term tumor shrinkage for large BMs [18]. However, studies on the reduction rate and tumor control with staged SRS remain scares and future studies should assess more cases. In this study, as in other similar studies, we discussed the relationship between tumor control and OS. In contrast to our findings, a previous study reported that tumor reduction contributes to improved KPS scores [20]. Since improved KPS scores could expand treatment options, future studies should consider the relationship between tumor reduction, tumor control, and OS.
Consideration of treatment strategy for patients with large BMs by SRS
The tumor control rate at 1 year after treatment with other modalities, i.e., LINIAC, Cyber knife and staged Gamma Knife, were reported to be 81% [39], 87% [40], and 75.9–79.3% [18, 24], respectively. We found that the tumor control rate at 1 year after the first procedure was 88.5%. Comparisons with other findings are limited due to differences, including focusing on smaller tumor volume and differences in the percentage of the primary cancer types. The incidence of carcinomatous meningitis in our study was similar to that in a previous report [24]. Making a simple comparison is difficult; however, our results provide considerable evidence that staged SRS might be a treatment option for some cases. It is recommended that BMs with a diameter > 3 cm should be surgically excised and/or treated with WBRT or SRS [6, 7]. Surgery should be performed for any primary cancer type if the patient is young, has a tumor in a non-eloquent area, shows a good KPS score, and has no active extracranial lesions. However, in some cases, choosing surgery might be difficult due to the patient’s age, tumor localization in an eloquent area, poor general condition of the patient, presence of active extracranial lesions, and patient preference. In these cases, particularly for patients with large BMs from BC and NSCLC, staged SRS might be a treatment option that allows for short-term tumor reduction and KPS score improvement. Contrastingly, in patients with large BMs arising from GIC, it is difficult to expect significant tumor reduction; further, the progression of extracranial lesions could cause death within a relatively short period. In these cases, the adaptation of staged SRS should follow careful consideration.
Limitations and prospects
This study has several limitations. All our patients were treated with two-stage SRS as the initial therapy for large BMs. Despite advances in oncologic therapies, we could not analyze the effect of gene mutations or novel oncologic therapies, including molecular targeting drugs, since we could not obtain the relevant information. However, Yomo et al. reported that treatment with TKI affected survival; however, it did not affect tumor control. Therefore, we suggested that the effect of TKI on local control might be minimal [41].
There remains controversy regarding the maximum volume treatable by SRS, optimal interfraction interval, and optimal prescribed dose. We focused on patients with a tumor volume of less than 30 cc; therefore, we can tentatively consider that the therapeutic maximum volume for two-staged SRS is 30 cc. There is a need for further prospective studies that analyze more information on gene mutations and detailed oncologic therapies.
Conclusion
To the best of our knowledge, this is the first study to report the tumor reduction rate among primary cancer types after two-stage SRS using a large sample size. In this study, we found that the tumor reduction rate during both sessions was not a prognostic factor for tumor control. In patients with large BMs from BC, staged SRS could reduce the tumor size. In patients with large BMs from NSCLC, staged SRS could improve tumor control compared to single fraction, which is similar to BC. Therefore, clinical care in this regard could consider staged SRS. Careful consideration should be placed when adapting two-stage SRS for patients with large BMs from GIC since these patients show a lower tumor reduction rate and shorter OS.
References
Mehta MP, Tsao MN, Whelan TJ, Morris DE, Hayman JA, Flickinger JC, Mills M, Rogers CL, Souhami L (2005) The American Society for Therapeutic Radiology and Oncology (ASTRO) evidence-based review of the role of radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys 63:37–46. https://doi.org/10.1016/j.ijrobp.2005.05.023
Navarria P, Pessina F, Cozzi L, Ascolese AM, De Rose F, Fogliata A, Franzese C, Franceschini D, Tozzi A, D'Agostino G, Comito T, Iftode C, Maggi G, Reggiori G, Bello L, Scorsetti M (2016) Hypo-fractionated stereotactic radiotherapy alone using volumetric modulated arc therapy for patients with single, large brain metastases unsuitable for surgical resection. Radiat Oncol 11:76. https://doi.org/10.1186/s13014-016-0653-3
Murai T, Ogino H, Manabe Y, Iwabuchi M, Okumura T, Matsushita Y, Tsuji Y, Suzuki H, Shibamoto Y (2014) Fractionated stereotactic radiotherapy using CyberKnife for the treatment of large brain metastases: a dose escalation study. Clin Oncol 26:151–158. https://doi.org/10.1016/j.clon.2013.11.027
Han JH, Kim DG, Chung HT, Paek SH, Park CK, Jung HW (2012) Radiosurgery for large brain metastases. Int J Radiat Oncol Biol Phys 83:113–120. https://doi.org/10.1016/j.ijrobp.2011.06.1965
Arvold ND, Lee EQ, Mehta MP, Margolin K, Alexander BM, Lin NU, Anders CK, Soffietti R, Camidge DR, Vogelbaum MA, Dunn IF, Wen PY (2016) Updates in the management of brain metastases. Neuro-oncology 18:1043–1065. https://doi.org/10.1093/neuonc/now127
Kocher M, Soffietti R, Abacioglu U, Villa S, Fauchon F, Baumert BG, Fariselli L, Tzuk-Shina T, Kortmann RD, Carrie C, Ben Hassel M, Kouri M, Valeinis E, van den Berge D, Collette S, Collette L, Mueller RP (2011) Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J Clin Oncol 29:134–141. https://doi.org/10.1200/jco.2010.30.1655
Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, Markesbery WR, Macdonald JS, Young B (1990) A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 322:494–500. https://doi.org/10.1056/nejm199002223220802
Ewend MG, Elbabaa S, Carey LA (2005) Current treatment paradigms for the management of patients with brain metastases. Neurosurgery 57:S66–77; discussion S61–64. https://doi.org/10.1227/01.neu.0000182739.84734.6e
Serizawa T, Higuchi Y, Nagano O, Sato Y, Yamamoto M, Ono J, Saeki N, Miyakawa A, Hirai T (2012) Analysis of 2000 cases treated with Gamma Knife surgery: validating eligibility criteria for a prospective multi-institutional study of stereotactic radiosurgery alone for treatment of patients with 1–10 brain metastases (JLGK0901) in Japan. J Radiosurg SBRT 2:19–27
Shiau CY, Sneed PK, Shu HK, Lamborn KR, McDermott MW, Chang S, Nowak P, Petti PL, Smith V, Verhey LJ, Ho M, Park E, Wara WM, Gutin PH, Larson DA (1997) Radiosurgery for brain metastases: relationship of dose and pattern of enhancement to local control. Int J Radiat Oncol Biol Phys 37:375–383. https://doi.org/10.1016/s0360-3016(96)00497-x
Linskey ME, Andrews DW, Asher AL, Burri SH, Kondziolka D, Robinson PD, Ammirati M, Cobbs CS, Gaspar LE, Loeffler JS, McDermott M, Mehta MP, Mikkelsen T, Olson JJ, Paleologos NA, Patchell RA, Ryken TC, Kalkanis SN (2010) The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol 96:45–68. https://doi.org/10.1007/s11060-009-0073-4
Ellis TL, Neal MT, Chan MD (2012) The role of surgery, radiosurgery and whole brain radiation therapy in the management of patients with metastatic brain tumors. Int J Surg Oncol 2012:952345. https://doi.org/10.1155/2012/952345
Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, Werner-Wasik M, Demas W, Ryu J, Bahary JP, Souhami L, Rotman M, Mehta MP, Curran WJ Jr (2004) Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 363:1665–1672. https://doi.org/10.1016/s0140-6736(04)16250-8
Bowden G, Kano H, Caparosa E, Park SH, Niranjan A, Flickinger J, Lunsford LD (2015) Gamma Knife radiosurgery for the management of cerebral metastases from non-small cell lung cancer. J Neurosurg 122:766–772. https://doi.org/10.3171/2014.12.jns141111
Devoid HM, McTyre ER, Page BR, Metheny-Barlow L, Ruiz J, Chan MD (2016) Recent advances in radiosurgical management of brain metastases. Front Biosci 8:203–214. https://doi.org/10.2741/s458
Serizawa T, Saeki N, Higuchi Y, Ono J, Iuchi T, Nagano O, Yamaura A (2005) Gamma Knife surgery for brain metastases: indications for and limitations of a local treatment protocol. Acta Neurochir 147:721–726; discussion 726. https://doi.org/10.1007/s00701-005-0540-4
Mori Y, Kondziolka D, Flickinger JC, Kirkwood JM, Agarwala S, Lunsford LD (1998) Stereotactic radiosurgery for cerebral metastatic melanoma: factors affecting local disease control and survival. Int J Radiat Oncol Biol Phys 42:581–589. https://doi.org/10.1016/s0360-3016(98)00272-7
Higuchi Y, Serizawa T, Nagano O, Matsuda S, Ono J, Sato M, Iwadate Y, Saeki N (2009) Three-staged stereotactic radiotherapy without whole brain irradiation for large metastatic brain tumors. Int J Radiat Oncol Biol Phys 74:1543–1548. https://doi.org/10.1016/j.ijrobp.2008.10.035
Yomo S, Hayashi M, Nicholson C (2012) A prospective pilot study of two-session Gamma Knife surgery for large metastatic brain tumors. J Neurooncol 109:159–165. https://doi.org/10.1007/s11060-012-0882-8
Yomo S, Hayashi M (2014) A minimally invasive treatment option for large metastatic brain tumors: long-term results of two-session Gamma Knife stereotactic radiosurgery. Radiation Oncology 9:132. https://doi.org/10.1186/1748-717x-9-132
Hasegawa T, Kato T, Yamamoto T, Iizuka H, Nishikawa T, Ito H, Kato N (2017) Multisession Gamma Knife surgery for large brain metastases. J Neurooncol 131:517–524. https://doi.org/10.1007/s11060-016-2317-4
Angelov L, Mohammadi AM, Bennett EE, Abbassy M, Elson P, Chao ST, Montgomery JS, Habboub G, Vogelbaum MA, Suh JH, Murphy ES, Ahluwalia MS, Nagel SJ, Barnett GH (2018) Impact of 2-staged stereotactic radiosurgery for treatment of brain metastases ≥ 2 cm. J Neurosurg 129:366–382. https://doi.org/10.3171/2017.3.jns162532
Dohm AE, Hughes R, Wheless W, Lecompte M, Lanier C, Ruiz J, Watabe K, Xing F, Su J, Cramer C, Laxton A, Tatter S, Chan MD (2018) Surgical resection and postoperative radiosurgery versus staged radiosurgery for large brain metastases. J Neurooncol 140:749–756. https://doi.org/10.1007/s11060-018-03008-8
Serizawa T, Higuchi Y, Yamamoto M, Matsunaga S, Nagano O, Sato Y, Aoyagi K, Yomo S, Koiso T, Hasegawa T, Nakazaki K, Moriki A, Kondoh T, Nagatomo Y, Okamoto H, Kohda Y, Kawai H, Shidoh S, Shibazaki T, Onoue S, Kenai H, Inoue A, Mori H (2018) Comparison of treatment results between 3- and 2-stage Gamma Knife radiosurgery for large brain metastases: a retrospective multi-institutional study. J Neurosurg 131:227–237. https://doi.org/10.3171/2018.4.jns172596
Yamamoto M, Higuchi Y, Serizawa T, Kawabe T, Nagano O, Sato Y, Koiso T, Watanabe S, Aiyama H, Kasuya H (2018) Three-stage Gamma Knife treatment for metastatic brain tumors larger than 10 cm3: a 2-institute study including re-analyses of earlier results using competing risk analysis. J Neurosurg 129:77–85. https://doi.org/10.3171/2018.7.gks181392
Serizawa T, Yamamoto M, Higuchi Y, Sato Y, Shuto T, Akabane A, Jokura H, Yomo S, Nagano O, Kawagishi J, Yamanaka K (2019) Local tumor progression treated with Gamma Knife radiosurgery: differences between patients with 2–4 versus 5–10 brain metastases based on an update of a multi-institutional prospective observational study (JLGK0901). J Neurosurg. https://doi.org/10.3171/2019.1.jns183085
Chernov M, Hayashi M, Izawa M, Ochiai T, Usukura M, Abe K, Ono Y, Muragaki Y, Kubo O, Hori T, Takakura K (2005) Differentiation of the radiation-induced necrosis and tumor recurrence after Gamma Knife radiosurgery for brain metastases: importance of multi-voxel proton MRS. Minim Invasive Neurosurg MIN 48:228–234. https://doi.org/10.1055/s-2005-870952
Kano H, Kondziolka D, Lobato-Polo J, Zorro O, Flickinger JC, Lunsford LD (2010) T1/T2 matching to differentiate tumor growth from radiation effects after stereotactic radiosurgery. Neurosurgery 66:486–491; discussion 491–482. https://doi.org/10.1227/01.neu.0000360391.35749.a5
Minamimoto R, Saginoya T, Kondo C, Tomura N, Ito K, Matsuo Y, Matsunaga S, Shuto T, Akabane A, Miyata Y, Sakai S, Kubota K (2015) Differentiation of brain tumor recurrence from post-radiotherapy necrosis with 11C-methionine PET: visual assessment versus quantitative assessment. PLoS ONE 10:e0132515. https://doi.org/10.1371/journal.pone.0132515
Serizawa T, Saeki N, Higuchi Y, Ono J, Matsuda S, Sato M, Yanagisawa M, Iuchi T, Nagano O, Yamaura A (2005) Diagnostic value of thallium-201 chloride single-photon emission computerized tomography in differentiating tumor recurrence from radiation injury after Gamma Knife surgery for metastatic brain tumors. J Neurosurg 102(Suppl):266–271. https://doi.org/10.3171/jns.2005.102.s_supplement.0266
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Serizawa T, Higuchi Y, Nagano O, Matsuda S, Aoyagi K, Ono J, Saeki N, Iwadate Y, Hirai T, Takemoto S, Shibamoto Y (2017) Robustness of the neurological prognostic score in brain metastasis patients treated with Gamma Knife radiosurgery. J Neurosurg 127:1000–1006. https://doi.org/10.3171/2016.8.jns16528
Nieder C, Andratschke NH, Geinitz H, Grosu AL (2012) Diagnosis-specific graded prognostic assessment score is valid in patients with brain metastases treated in routine clinical practice in two European countries. Med Sci Monit 18:450–455. https://doi.org/10.12659/msm.883213
Meyners T, Heisterkamp C, Kueter JD, Veninga T, Stalpers LJ, Schild SE, Rades D (2010) Prognostic factors for outcomes after whole-brain irradiation of brain metastases from relatively radioresistant tumors: a retrospective analysis. BMC Cancer 10:582. https://doi.org/10.1186/1471-2407-10-582
Brown PD, Brown CA, Pollock BE, Gorman DA, Foote RL (2008) Stereotactic radiosurgery for patients with "radioresistant" brain metastases. Neurosurgery 62(Suppl 2):790–801. https://doi.org/10.1227/01.neu.0000316283.45242.e1
Ahmed KA, Scott JG, Arrington JA, Naghavi AO, Grass GD, Perez BA, Caudell JJ, Berglund AE, Welsh EA, Eschrich SA, Dilling TJ, Torres-Roca JF (2018) Radiosensitivity of lung metastases by primary histology and implications for stereotactic body radiation therapy using the genomically adjusted radiation dose. J Thorac Oncol 13:1121–1127. https://doi.org/10.1016/j.jtho.2018.04.027
Kocher M, Treuer H, Voges J, Hoevels M, Sturm V, Muller RP (2000) Computer simulation of cytotoxic and vascular effects of radiosurgery in solid and necrotic brain metastases. Radiother Oncol 54:149–156. https://doi.org/10.1016/s0167-8140(99)00168-1
Kubo K, Kenjo M, Doi Y, Nakao M, Miura H, Ozawa S, Nagata Y (2019) MRI appearance change during stereotactic radiotherapy for large brain metastases and importance of treatment plan modification during treatment period. Jpn J Radiol 37: 850-859. doi:10.1007/s11604-019-00886-4.
Aoyama H, Shirato H, Onimaru R, Kagei K, Ikeda J, Ishii N, Sawamura Y, Miyasaka K (2003) Hypofractionated stereotactic radiotherapy alone without whole-brain irradiation for patients with solitary and oligo brain metastasis using noninvasive fixation of the skull. Int J Radiat Oncol Biol Phys 56:793–800. https://doi.org/10.1016/s0360-3016(03)00014-2
Jeong WJ, Park JH, Lee EJ, Kim JH, Kim CJ, Cho YH (2015) Efficacy and Safety of Fractionated Stereotactic Radiosurgery for Large Brain Metastases. J Korean Neurosurg Soc 58:217–224. https://doi.org/10.3340/jkns.2015.58.3.217
Yomo S, Serizawa T, Yamamoto M, Higuchi Y, Sato Y, Shuto T, Akabane A, Jokura H, Kawagishi J, Aoyama H (2019) The impact of EGFR-TKI use on clinical outcomes of lung adenocarcinoma patients with brain metastases after Gamma Knife radiosurgery: a propensity score-matched analysis based on extended JLGK0901 dataset (JLGK0901-EGFR-TKI). J Neurooncol 145:151–157. https://doi.org/10.1007/s11060-019-03282-0
Funding
This study received no financial support.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Daisuke Ito, Kyoko Aoyagi, Osamu Nagano, Yoshinori Higuchi, and Toru Serizawa. The first draft of the manuscript was written by Daisuke Ito and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Research involving human participants
The institutional review board of Chiba Cerebral and Cardiovascular Center IRB (IRB Number: #456) approved this retrospective study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ito, D., Aoyagi, K., Nagano, O. et al. Comparison of two-stage Gamma Knife radiosurgery outcomes for large brain metastases among primary cancers. J Neurooncol 147, 237–246 (2020). https://doi.org/10.1007/s11060-020-03421-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-020-03421-y