Abstract
Purpose
Fractionated stereotactic radiotherapy (FSRT) using gamma knife is useful for brain metastases. However, several uncertainties derived from fractionation pose issues for maintaining high-level accuracy. This study analyzed interfractional tumor change by performing radiological reassessment at the midterm of FSRT with ≥ 10 fractions, and the significance of replanning was evaluated.
Methods
Data of FSRT using gamma knife with ≥ 10 fractions were retrospectively collected. Interfractional volume changes in MRI at the midterm of the irradiation period were analyzed. Radiological changes after FSRT and final outcomes were also investigated.
Results
Overall, 114 lesions in 74 treatments from 66 patients were included, with previously irradiated lesions accounting for 46%. The median interval between planning and the interfractional MRI was 7 days. The interfractional change rates of tumor volume ranged from − 48 to + 72%. Significant interfractional enlargement was observed in 16 lesions (14%); evident regression was confirmed in 17 lesions (15%). Predictive factors for interfractional enlargement were small tumor and cystic lesion; high biologically effective dose was associated with regression. After FSRT, most lesions regressed within 6 months despite interfractional change type. The incidences of tumor control and radiation necrosis indicated no differences between interfractionally-regressed lesions and others.
Conclusion
This is the first study to evaluate interfractional tumor change in FSRT using gamma knife with ≥ 10 fractions, indicating significant volume changes in 29% of the lesions. These preliminary results suggest that interfractional reassessment of a treatment plan in FSRT with irradiation periods exceeding a week is necessary for more adaptive treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain metastases are the most common intracranial tumors and often affect the patients’ quality of life by causing neurological deterioration [1]. Stereotactic radiosurgery (SRS) has been effectively utilized in the treatment of brain metastases, since it offers less toxicity compared to whole-brain radiotherapy [2,3,4]. Nevertheless, large and/or recurrent brain metastases are still difficult to control using single-fraction SRS due to the risk of radiation injury. Furthermore, unsuitable candidates for surgical resection are not rare among cancer patients, owing to unfavorable conditions or concerns about the delay in systemic therapy. For such cases, fractionated SRS is considered effective because it possesses both the benefits from high-dose irradiation and the maximally reduced risk of damage to normal brain [5,6,7,8,9,10]. As many cancer patients can survive longer than ever before, thanks to the evolution of systemic therapies such as targeted therapies and immunotherapies, more brain metastases have been detected, and long-term treatment efficacy and safety are urgently required.
Gamma knife is a pioneering device for stereotactic radiosurgery, characterized by highly precise irradiation with rigid head-frame fixation, and steep dose gradient, resulting in elevated central dose [11]. The advent of the latest model of Gamma Knife Icon has allowed head fixation not only by frame but also by device-specific thermoplastic mask, enabling consecutive daily irradiation. While the effectiveness of two or three staged gamma knife radiosurgery for brain metastases has been already demonstrated [12, 13], little is known about fractionated stereotactic radiotherapy (FSRT) using gamma knife at present. Since 2018, we have performed FSRT using gamma knife for brain metastases. This was primarily aimed at minimizing the risk of radiation injury. Although it has been mostly performed with five fractions, procedures with more than five fractions have been also applied for specific cases in pursuit of a better outcome.
When performing fractionated radiotherapy, two types of uncertainties in terms of accuracy can potentially become an issue: intrafractional and interfractional changes of the target. The head immobilization during irradiation affects the former, as was discussed in other studies [14, 15], whereas tumor change throughout the irradiation period corresponds to the latter. This would especially matter when the irradiation period exceeds a week. Since an early response after the initial treatment in the staged radiosurgery is often observed [12], we have performed MRI at the midterm of irradiation periods that are of a week or longer and have consequently modified the treatment plan according to target appearance. Therefore, we launched this study, which investigated the interfractional volumetric change and its predictive factors among FSRT using gamma knife with ≥ 10 fractions. Additionally, the effect of replanning at the midterm of irradiation periods was evaluated by the post-treatment clinical course.
Methods and materials
Patient selection
This retrospective cohort study included FSRT for brain metastases that were performed using the Leksell Gamma Knife Icon (Elekta Instruments, Stockholm, Sweden) between May 2018 and April 2021 at our institution. The inclusion criteria were as follows: patients who underwent FSRT with ≥ 10 fractions and interfractional MRI at the midterm of the irradiation period. In general, FSRT with ≥ 10 fractions was applied for patients with the following tumors and/or characteristics: large, located at highly eloquent areas, previously irradiated, with relatively ambiguous border requiring wide margin, long survival estimated, and with multiple lesions (≥ 15) and extremely long treatment time estimated in the case of single-fraction. Patients who underwent FSRT only for resection cavity, whose tumor was smaller than 0.5 mL, or who underwent cyst drainage via the reservoir system during the irradiation period were excluded because it is difficult to evaluate the effect of irradiation in those cases. The study was conducted in accordance with the tenets of the Declaration of Helsinki (revised version in 2013), and written informed consent was obtained from all patients. The study was approved by the relevant institutional review board (IRB number 21–124).
Treatment procedures
Standardized planning MRI was performed within 3 days before the start of treatment. The sequence on which we mainly utilized to contour the tumor was axial T1-Cube with gadolinium enhancement at a 1.2 mm slice thickness and 0.6 mm interslice spacing (GE Healthcare). All the treatment plans were meticulously created by the same senior physician (AA). The gross tumor volume (GTV) was defined as a contrast-enhancing lesion. The planning target volume (PTV) was typically determined by adding a 0.5 to 2 mm margin to the GTV. The prescribed doses mostly ranged from 40 to 60 Gy in terms of the biologically effective dose, which depended on the history of irradiation, covering 99% and more of the PTV. The interfractional MRI was obtained at the midterm of the irradiation period, using the same protocol as the planning MRI, and the treatment plans were modified, as necessary. Irradiation was performed on consecutive days except for weekends and holidays; thus, it usually took 12 days to reach treatment completion in the case of FSRT with 10 fractions.
Data collection
The following data were retrospectively assessed based on the medical records: age at FSRT, sex, primary disease, location of the lesion, and history of irradiation. Cystic tumor was defined as a lesion with a predominant cystic component. The number of fractions, interval days between planning and interfractional MRI, the prescription dose, and the percentage of the prescription isodose line were correctly identified. Contouring tumor and calculation of volume were performed using the segmentation tool within the planning software, Leksell GammaPlan, by two gamma knife practitioners (AA and MK). The tumor volumes obtained at the planning MRI and interfractional MRI were defined as V1 (consistent with GTV) and V2, respectively. The primary endpoint of this study was the interfractional tumor change rates between V1 and V2, which were calculated by the formula (V2 − V1)/V1. The factors associated with interfractional change exceeding ± 20% were also assessed. After the treatment, patients were generally observed every 1 to 3 months at the referred hospitals or at our institution. The secondary endpoint was the post-treatment changes among the lesions, of which the follow-up data were obtained. This was evaluated in two separate ways. First, the early radiological change of the tumor before 6 months from FSRT was measured (V3), and the change rates were calculated as follows; (V3 − V1)/V1. Second, local tumor control and radiation necrosis were investigated for the lesions with the post-treatment observation period of ≥ 6 months. They were practically diagnosed by reference to several clinical findings such as T1/T2 mismatch on MRI, arterial spin labeling MR perfusion imaging, positron emission tomography, histopathology in the resected cases, and clinical course.
Statistical analysis
First, the patients’ baseline characteristics and treatment parameters were summarized. Considering the possibility that multiple lesions or multiple treatments in the same patient may affect the results, a single index lesion in each patient was analyzed separately. An index lesion was the tumor that had the largest volume in the first treatment in each patient and had no history of previous treatment. Retreated lesions, which indicated progression after any irradiation, were also analyzed. Second, factors potentially affecting interfractional enlargement of ≥ 20% and regression of ≥ 20% were evaluated using bivariate and multivariate logistic regression analyses. Variables entered into the multivariate analysis were determined using the forward and backward stepwise procedure, with a cutoff p value of 0.2. Third, the volume change rates earlier than 6 months after FSRT among the significant interfractional change types were compared using the Mann–Whitney U-test. Finally, the incidences of tumor control and radiation necrosis 6 months after FSRT or later were calculated as a crude rate. They were also analyzed according to the history of interfractional regression and other variables using a two-sided Fisher’s exact test. Statistical significance was set at p < 0.05. All analyses were performed using JMP Pro 16 software (SAS Institute, Cary, NC, USA).
Results
Patient characteristics
A total of 114 lesions in 74 treatments from 66 patients were included in this study. Among them, eight treatments were secondary or third treatments for the same patients, and 46% (52 lesions) were irradiated repeatedly for progression after stereotactic radiosurgery/radiotherapy (38 lesions), whole-brain radiotherapy (12 lesions), and both (2 lesions). As a result, index lesions in each patient accounted for 36% (41 lesions) of all the lesions. The baseline characteristics and treatment parameters of overall, index, and retreated lesions are summarized in Table 1. Fifty percent (57 lesions) of the overall lesions originated from non-small cell lung cancer (NSCLC), followed by breast cancer (24%). Eighty-three lesions (73%) were treated with 10 fractions, and the remaining ones were treated with > 10 fractions. Cystic tumors accounted for 18% (20 lesions). The median interval between the planning MRI and the interfractional MRI was 7 days (range 6–15 days).
Interfractional change of the tumor volume
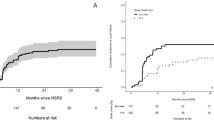
For the entire cohort, the interfractional change of the tumor volumes ranged from − 48% to + 72% (interquartile range − 11% to + 10%) at the midterm of the irradiation period (Fig. 1A). Sixteen lesions (14%) experienced ≥ 20% enlargement of the initial volume, whereas 17 lesions (15%) had reduced volumes of ≥ 20%. The volume of other lesions remained within ± 20%. Concerning index lesions in each patient, significant enlargement and regression were observed in four lesions (10%) and nine lesions (22%), while they were recognized in eight lesions (15%) and five lesions (10%) among the retreated lesions (Fig. 1B, C). Among the overall lesions, the factors associated with interfractional enlargements of ≥ 20% by multivariate analysis were lower volume (continuous variable; odds ratio [OR] per 1 mL increase 0.73, 95% confidence interval [CI] 0.57–0.94, p = 0.016) and cystic lesion (vs. non-cystic lesion; OR 20.07, 95% CI 4.84–83.16, p < 0.001) (Table 2). In the subgroup of retreated lesions, cystic lesion was also associated with significant enlargement (OR 337.43, 95% CI 8.50–13,395.2). Regarding interfractional regressions of ≥ 20%, the only significantly associated factor among the overall lesions was biologically effective dose (α/β = 10) of the prescription dose (BED10) (continuous variable; OR per 1 Gy increase 1.21, 95% CI 1.06–1.37, p = 0.004), and the result was similar in the subgroup analysis (Table 3). An illustrative case of interfractional regression is presented in Fig. 2. As a result of interfractional MRI, a total of 63 (85%) of the 74 treatment plans were eventually modified at the midterm of the irradiation period, corresponding to any target change.
A case of large brain metastasis that originated from non-small cell lung carcinoma. A and B Axial and coronal T1-weighted contrast-enhanced MRI at the fractionated radiotherapy using gamma knife planning, respectively. The tumor volume was 16 mL and was treated with a prescription dose of 45 Gy in 15 fractions with 75% isodose coverage. The yellow line represents the 45-Gy line, covering the whole target. C and D The interfractional MRI obtained after eight fractions at the interval of 11 days from the planning MRI remarkably indicated tumor regression. Axial and coronal T1-weighted contrast-enhanced MRI performed under the same protocol. E and F The irradiated field was adjusted to tighten according to the result of interfractional MRI. Considering the evident regression of the tumor after eight fractions, no margin was set at replanning to avoid excessive irradiation to normal brain tissues around the lesion. G and H Follow-up MRI at 3 months after radiotherapy indicated successful tumor control and no radiation necrosis thus far.
Radiological change of the tumor in the early phase after FSRT
There were 74 lesions for which the tumor volumes within 6 months after FSRT (median 3 months, range 1.5–6 months) were obtained. Compared with the initial volume (V1), 57 lesions (77%) indicated any regression, and the median change rates were − 50% (range − 98% to 90%, interquartile range − 80% to − 5%). One patient who experienced a 90% expansion of the lesion eventually underwent cyst drainage and secondary FSRT 5 months after the initial treatment. These post-treatment change rates were not statistically different among the following groups: the lesions with interfractional enlargement of ≥ 20% vs. interfractional change within ± 20% (p = 0.571), interfractional enlargement of ≥ 20% vs. regression of ≥ 20% (p = 0.463), and within ± 20% vs. regression of ≥ 20% (p = 0.181).
Tumor control and radiation necrosis at the final follow-up
There were 64 lesions that had follow-up periods of ≥ 6 months (median 15 months, range 6–32). Among them, 39 tumors (61%) were under control at the final follow-up. Tumor control rates were not statistically different between the group that had experienced any regression at the interfractional evaluation and the remaining group that had not (p = 0.797). Other factors, including retreated lesion (p = 0.795), cystic lesion (p = 1.000), breast cancer (p = 0.074), larger lesion (p = 0.251), and higher BED10 (p = 0.139), were not associated with tumor control, although NSCLC was the predictive factor of higher tumor control (OR 5.41, p = 0.002). Conversely, eight lesions (12.5%) had experienced radiation necrosis until the last follow-up. One of these lesions was resected and histopathologically confirmed without evidence of recurrence. There was no statistical difference in the incidence of radiation necrosis according to the history of interfractional regression (p = 0.282), retreated lesion (p = 0.245), cystic lesion (p = 0.582), breast cancer (p = 0.668), NSCLC (p = 1.000), larger lesion (p = 0.576), and higher BED10 (p = 0.456).
Discussion
This is the first study that retrospectively demonstrated interfractional volumetric change of tumor during FSRT using gamma knife with ≥ 10 fractions for brain metastases. The significant increase and decrease of interfractional volume were recognized in 14% and 15% of the overall lesions, respectively. Cystic tumors and smaller tumors tended to be enlarged, while lesions treated with higher BED10 were likely to shrink even during the irradiation period. Some literatures have focused on interfractional target change during linear accelerator (LINAC)-based stereotactic radiotherapy for brain metastases [16,17,18,19]. According to these, interfractional target enlargement was recognized in 11–19% of lesions, and interfractional regression was observed in 14–22% of lesions treated by stereotactic radiotherapy with 3 to 13 fractions, within the median interval of 6–9 days. The predictive factors of tumor change were reported to be adenocarcinoma (vs. other pathological types), steroid administration before radiotherapy (vs. after radiotherapy) [18], and high speed of enlargement before treatment [19]. With the advent of new technologies, gamma knife procedure is beginning to play an important role in recent years, not only for small, well-demarcated brain metastases, but also relatively large or intractable ones through fractionation. Gamma knife radiosurgery is basically characterized by both a sharper dose falloff outside the tumor and a rapid dose increase inside the target, as the isodose line of 50–60% of a maximum dose has been usually prescribed on the tumor edge in the case of single-fraction. Higher dose to the tumor core and minimized irradiation to normal brain tissue around the target have brought about good outcomes. Considering these facts, the interfractional volume change must be a more compelling matter in radiotherapy using gamma knife than in LINAC-based radiotherapy, which has a rather homogenous dose-coverage. Therefore, it is mandatory to recognize the possible uncertainty regarding accuracy in order to exploit the advantage of gamma knife treatments. In fact, Lee et al., who applied fractionated gamma knife radiosurgery in 3–5 fractions for large brain metastases, reported that 18 of 40 lesions (45%) showed decreased tumor volume after only one or two fractions [20]. Although our study differs from theirs in terms of the number of fractions and dose per fraction, and consequently, the frequency and pattern of tumor change, both results imply that adaptive replanning should be performed in fractionated gamma knife radiosurgery or radiotherapy.
The findings of the present study suggest the following issues. First, judging by the result that almost 30% of all lesions indicated significant volumetric fluctuations, interfractional MRI is indispensable for FSRT with ≥ 10 fractions or an irradiation period of a week or longer. Naturally, the shortage of dose-coverage due to tumor enlargement during the irradiation period would deteriorate tumor control, and an overdose for normal brain tissue around the tumor owing to tumor shrinkage could induce radiation necrosis more frequently. In contrast, the incidences of tumor control and radiation necrosis did not significantly differ between the groups of interfractionally-regressed lesions and that of the remaining lesions in this study, possibly demonstrating that replanning was effective to prevent such worse courses. Second, interfractional evaluation of MRI enabled us to make more subtle adjustments by confirming the initial response to irradiation and to modify the plan accordingly. If tumor volume decreased at the midterm of irradiation, we could tighten the irradiated field by reducing the margin because the tumor is estimated to become even smaller at the completion of treatment. On the other hand, a more adequate margin would be needed in case of tumor enlargement at the interfractional MRI, in order to keep an optimal coverage on the whole tumor. It would also be effective to lower the prescribed percentage isodose line to raise the central dose for the tumor core. Even if interfractional change is volumetrically slight, these fine modifications to the original plan, made by confirming the extremely early response of the tumor, could be useful for more adaptive treatment.
There are several limitations in this study. First, middle- to long-term outcomes of FSRT with an interfractional MRI have not been thoroughly revealed yet, due to the limited number of lesions that had a follow-up period of ≥ 6 months. The effect of interfractional MRI and replanning on tumor control and radiation necrosis should be considered in future studies with a larger number of cases. Second, the possibility of target displacement without volumetric change has not been analyzed. Displacement is common in tumors with evident brain edema and those under steroids administration. Most displacements are accompanied by an increase or decrease in tumor volume to some extent, and this is the reason that we performed replanning for most treatments without significant volumetric change. Third, the effect of concurrent systemic therapy is likely not negligible. Ongoing tyrosine-kinase inhibitors and anti-VEGF inhibitors could potentially affect the way the tumor is depicted with contrast enhancement. Despite these limitations, the present study successfully indicated the significant changes in tumor volumes during the irradiation period and the importance of treatment replanning.
Conclusion
From our initial experience of FSRT using gamma knife with ≥ 10 fractions, the radiological assessment revealed that 29% of the irradiated brain metastases became enlarged or regressed as early as the midterm of the irradiation period. Interfractional evaluation and replanning are requirements for highly accurate FSRT with an irradiation period of a week or longer, possibly contributing to better outcomes. Further investigation is needed to confirm the outcome of this approach.
Data availability
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
References
Soffietti R, Abacioglu U, Baumert B et al (2017) Diagnosis and treatment of brain metastases from solid tumors: guidelines from the European Association of Neuro-Oncology (EANO). Neuro Oncol 19:162–174. https://doi.org/10.1093/neuonc/now241
Yamamoto M, Serizawa T, Shuto T et al (2014) Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol 15:387–395. https://doi.org/10.1016/s1470-2045(14)70061-0
Brown PD, Jaeckle K, Ballman KV et al (2016) Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA 316:401–409. https://doi.org/10.1001/jama.2016.9839
Kann BH, Park HS, Johnson SB, Chiang VL, Yu JB (2017) Radiosurgery for brain metastases: changing practice patterns and disparities in the United States. J Natl Compr Canc Netw 15:1494–1502. https://doi.org/10.6004/jnccn.2017.7003
Minniti G, Scaringi C, Paolini S et al (2016) Single-fraction versus multifraction (3 × 9 Gy) stereotactic radiosurgery for large (>2 cm) brain metastases: a comparative analysis of local control and risk of radiation-induced brain necrosis. Int J Radiat Oncol Biol Phys 95:1142–1148. https://doi.org/10.1016/j.ijrobp.2016.03.013
Lehrer EJ, Peterson JL, Zaorsky NG et al (2019) Single versus multifraction stereotactic radiosurgery for large brain metastases: an international meta-analysis of 24 trials. Int J Radiat Oncol Biol Phys 103:618–630. https://doi.org/10.1016/j.ijrobp.2018.10.038
Ogura K, Mizowaki T, Ogura M et al (2012) Outcomes of hypofractionated stereotactic radiotherapy for metastatic brain tumors with high risk factors. J Neurooncol 109:425–432. https://doi.org/10.1007/s11060-012-0912-6
Redmond KJ, Gui C, Benedict S et al (2021) Tumor control probability of radiosurgery and fractionated stereotactic radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys 110:53–67. https://doi.org/10.1016/j.ijrobp.2020.10.034
Milano MT, Grimm J, Niemierko A et al (2021) Single- and multifraction stereotactic radiosurgery dose/volume tolerances of the brain. Int J Radiat Oncol Biol Phys 110:68–86. https://doi.org/10.1016/j.ijrobp.2020.08.013
Gondi V, Bauman G, Bradfield L et al (2022) Radiation therapy for brain metastases: an ASTRO clinical practice guideline. Pract Radiat Oncol. https://doi.org/10.1016/j.prro.2022.02.003
Lindquist C (1989) Gamma knife surgery for recurrent solitary metastasis of a cerebral hypernephroma: case report. Neurosurgery 25:802–804. https://doi.org/10.1097/00006123-198911000-00018
Higuchi Y, Serizawa T, Nagano O et al (2009) Three-staged stereotactic radiotherapy without whole brain irradiation for large metastatic brain tumors. Int J Radiat Oncol Biol Phys 74:1543–1548. https://doi.org/10.1016/j.ijrobp.2008.10.035
Serizawa T, Higuchi Y, Yamamoto M et al (2018) Comparison of treatment results between 3- and 2-stage Gamma Knife radiosurgery for large brain metastases: a retrospective multi-institutional study. J Neurosurg 131:227–237. https://doi.org/10.3171/2018.4.JNS172596
Schasfoort J, Ruschin M, Sahgal A et al (2021) Quantifying the sensitivity of target dose on intra-fraction displacement in intra-cranial stereotactic radiosurgery. Pract Radiat Oncol. https://doi.org/10.1016/j.prro.2021.11.012
Wright G, Schasfoort J, Harrold N, Hatfield P, Bownes P (2019) Intra-fraction motion gating during frameless Gamma Knife. J Radiosurg SBRT 6:67–76
Uto M, Ogura K, Katagiri T, Takehana K, Mizowaki T (2021) Interfractional target changes in brain metastases during 13-fraction stereotactic radiotherapy. Radiat Oncol 16:140. https://doi.org/10.1186/s13014-021-01869-4
Ohtakara K, Hoshi H (2014) Target volume geometric change and/or deviation from the cranium during fractionated stereotactic radiotherapy for brain metastases: potential pitfalls in image guidance based on bony anatomy alignment. J Med Imaging Radiat Oncol 58:729–736. https://doi.org/10.1111/1754-9485.12194
Kubo K, Kenjo M, Doi Y et al (2019) MRI appearance change during stereotactic radiotherapy for large brain metastases and importance of treatment plan modification during treatment period. Jpn J Radiol 37:850–859. https://doi.org/10.1007/s11604-019-00886-4
Hessen E, Nijkamp J, Damen P et al (2020) Predicting and implications of target volume changes of brain metastases during fractionated stereotactic radiosurgery. Radiother Oncol 142:175–179. https://doi.org/10.1016/j.radonc.2019.07.011
Lee MH, Kim KH, Cho KY et al (2019) Volumetric changes of intracranial metastases during the course of fractionated stereotactic radiosurgery and significance of adaptive planning. J Neurosurg. https://doi.org/10.3171/2019.3.JNS183130
Funding
The authors declare that no funds, grants, or other support was received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
MK and AA contributed to the study conception and design. Material preparation, data collection, and analysis were performed by MK, RN, and AA. The first draft of the manuscript was written by MK, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was conducted in accordance with the tenets of the Declaration of Helsinki. Approval was given by the relevant IRB (IRB number 21-24).
Consent to participate
Written informed consent was obtained from all patients included in this study.
Consent to publish
Informed consent was obtained from all patients whose images were included in publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kawashima, M., Akabane, A., Noda, R. et al. Interfractional change of tumor volume during fractionated stereotactic radiotherapy using gamma knife for brain metastases. J Neurooncol 159, 409–416 (2022). https://doi.org/10.1007/s11060-022-04075-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-022-04075-8