Abstract
Seaweeds are a source of antioxidants and pigments, which can be used as functional ingredients for food, cosmetic, pharmaceutical, and industrial applications. As fresh seaweed has a large amount of water, it usually goes through a drying process in order to be commercialized, which facilitates transportation and storage and also increases shelf time. However, the drying procedures can change the chemical composition and antioxidant properties of seaweeds. Thus, the aim of this study was to evaluate the antioxidant properties and pigment and protein content of four Brazilian macroalgae (Gracilariopsis tenuifrons, Pterocladiella capillacea, Sargassum stenophyllum, and Ulva fasciata) suitable for use as functional bioproducts after processing with three different drying procedures (freeze-drying, oven-drying and silica-drying), using a fresh frozen treatment as control. Among the studied species, S. stenophyllum presented the highest antioxidant activity. For this species, freeze-drying was the procedure with the highest activity in the Folin-Ciocalteu and metal chelating assays, while for the ABTS, DPPH, and FRAP assays, no significant differences were observed between freeze-drying and silica-drying. Freeze-drying also showed low reduction of photosynthetic pigments in G. tenuifrons, P. capillacea, and U. fasciata and was the method that best-preserved protein content in the four species. In general, the antioxidant potential and pigment and protein content for the studied algae decreased in the following order: fresh frozen (control) > freeze-dried > silica-dried > oven-dried. Freeze-drying was the procedure that presented the lowest alteration in functional properties, as the low temperature prevents the degradation of heat-sensitive compounds. In addition, freeze-drying is a vacuum process and the absence of oxygen prevents oxidation reactions of functional ingredients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Marine algae or seaweeds are traditionally used for human and animal consumption in some parts of the world, such as Chile, China, and Ireland, and have been exploited as functional foods due to their nutritional content and bioactive potential (Wells et al. 2017). Functional foods are those that, in addition to nutritional properties, contain bioactive compounds that may have additional health properties. Seaweeds are also a source of phycocolloids, pigments, and antioxidants, which can be used as functional ingredients (Fleurence 2016). Functional ingredients are compounds that may have health benefits and can be marketed as supplements, food additives, or ingredients included in functional foods (Kruger and Mann 2003).
Seaweeds are usually sold in dry form (Barsanti and Gualtieri 2014). In that case, previous pre-processing is necessary because fresh biomass of seaweeds have a large amount of water, usually 70–90% (McDermid and Stuercke 2003), which increases the weight and volume of the material. The retail of fresh raw material is less frequent as it may deteriorate rapidly after harvesting and become a hardship to transport and store. The most common drying processes used for seaweeds are sun-drying, oven-drying and freeze-drying (Ling et al. 2015). The processing characteristics of each method can change the chemical composition and antioxidant properties of seaweed intended for use as functional bioproducts for food and feed, cosmetic, pharmaceutical and industrial applications (Jiménez-Escrig et al. 2001; Le Lann et al. 2008; Gupta et al. 2011). Although there are few studies describing the effects of the drying processes on seaweed extracts (Rioux et al. 2017), interest in this subject has increased in recent years (Chenlo et al. 2018; Stévant et al. 2018; Badmus et al. 2019; Sappati et al. 2019; Silva et al. 2019; Uribe et al. 2019).
The main antioxidants found in seaweeds are phenolic compounds and photosynthetic pigments (chlorophylls, carotenoids and phycobiliproteins), which are also known for their biological activities (anti-cancer, anti-inflammatory, anti-obesity and anti-angiogenic) (Pangestuti and Kim 2011). However, both phenolic compounds and pigments are susceptible to oxidation, especially at high temperature (Humphrey 2004; Moreira et al. 2016). In addition, seaweeds are considered an alternative source of proteins, carbohydrates, fatty acids and minerals, which can undergo degradation under elevated temperature and loss of water.
Some macroalgal potential species for use as functional ingredients are Gracilariopsis tenuifrons (C.J. Bird & E.C. Oliveira) Fredericq & Hommersand, Pterocladiella capillacea (S.G. Gmelin) Santelices & Hommersand (red seaweeds), Sargassum stenophyllum Martius (brown seaweed), and Ulva fasciata Delile (green seaweed). Gracilariopsis tenuifrons and P. capillacea are exploited for the production of good quality agar (Brito and Silva 2005; Cardoso et al. 2014), whereas species of Sargassum and Ulva (sea lettuce) are traditionally used in food products (McDermid and Stuercke 2003; Rioux et al. 2017). Furthermore, Sargassum is used as raw material for alginate production and is recognized as a source of phenolic compounds with several biological activities (Cardoso et al. 2014; Fernando et al. 2016).
The aim of this study was to assess the alteration degree of the antioxidant properties and chemical composition of four Brazilian macroalgae (G. tenuifrons, P. capillacea, S. stenophyllum and U. fasciata) suitable for use as functional bioproducts after processing with three different drying procedures, with fresh frozen treatment as control.
Materials and methods
Biological material and processing of samples
Samples of G. tenuifrons (BG0039) came from the Germplasm Bank of the University of São Paulo (Costa et al. 2012). Samples of P. capillacea (Cibratel II Beach; 24°13′31″S and 46°51′7″W), S. stenophyllum, and U. fasciata (both from Cigarras Beach; 24°76′1″S and 45°41′7″W) were collected from the field, transported to the laboratory and morphologically identified by Dr. Fungyi Chow. Then, each species was cultivated for 8 days in sterilized seawater (32 psu) with 50% von Stosch enrichment solution (Ursi and Plastino 2001 modified from Edwards 1970), in a proportion of 3 g of fresh weight (FW) per 1 L of culture medium. The culture was maintained at 25 ± 1 °C, 14 h photoperiod, 60 ± 5 μmol of photons m−2 s−1 and aeration in alternating periods of 30 min. On the seventh day, the culture medium was replaced in order to avoid nutrient limitation. On the eighth day, seaweeds were washed with distilled water and dried with absorbent paper.
Subsequently, fresh samples of each species were divided, for each treatment, into five subsamples (n = 5) of 200 mg each and five subsamples (n = 5) of 70 mg each. Five 200 mg subsamples and five 70 mg subsamples were subjected to either control treatment (fresh frozen), or one of the drying methods: freeze-drying, oven-drying or silica-drying. Control samples were placed in microtubes, frozen in liquid nitrogen and stored at − 80 °C until analysis. For freeze-drying, the samples were placed in microtubes with a hole in the lid and frozen in liquid nitrogen at atmospheric pressure. Freeze-drying occurred at − 55 °C and 0.021 mbar for 72 h (Christ ALPHA 2–4 LDplus, Germany). For oven-drying, the samples were arranged on pieces of aluminum foil, which were placed in an oven at 40 °C for 48 h. For silica-drying, the samples were placed in centrifuge tubes with silica gel and stored at room temperature (22–25 °C) for a week. After drying, the samples were weighed to determine moisture content.
Samples that initially had 200 mg were used for antioxidant assays, and 70 mg samples were used to determine pigment and protein content. For comparison, the samples were standardized by fresh mass.
Antioxidant potential
Samples of 200 mg FW submitted to each treatment (dried or frozen samples) were ground into a microtube with pistil and liquid nitrogen. The resulting powder was extracted in 1 mL of methanol for 3 h at room temperature and centrifuged for 15 min at 21,255×g and 25 °C. The supernatant (crude extract) was tested in four concentrations (6, 8, 10, and 12 mg mL−1) for each antioxidant assay, without any solvent evaporation. Additionally, crude extract concentration of 4 mg mL−1 for S. stenophyllum was tested. Five different antioxidant potential assays were conducted in 96-well microplates with a final volume of 300 μL, and the absorbance was measured with a UV-Vis spectrophotometer (Epoch 2-BioTek, USA). The 2,2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radical scavenging assay followed the method of Torres et al. (2017) at a wavelength of 734 nm. The 2,2′-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay was performed according to Pires et al. (2017a) and Santos et al. (2019) at 517 nm. The reducing power of the Folin-Ciocalteu reagent was performed according to Pires et al. (2017b) at 760 nm. The ferric reducing antioxidant power (FRAP) assay was performed according to Urrea-Victoria et al. (2016) at 595 nm. The metal chelating activity was performed according to Harb et al. (2016) and at 562 nm.
In the literature antioxidant activity is expressed in different equivalents of standards, such as gallic acid, butylated hydroxyanisole (BHA), quercetin, and Trolox. Therefore, different standard curves were performed, and the respective conversion factor calculated based on gallic acid as control standard (Table S1). The results of antioxidant activity were expressed as percentage of antioxidant activity and gallic acid equivalent (GAE; mg GAE g−1).
The percentage of antioxidant activity (% AOX) for the ABTS radical scavenging, DPPH radical scavenging, and metal chelating assays was determined as
where AbsNC = negative control absorbance; AbsS = sample absorbance.
The percentage of antioxidant activity by the FRAP and Folin-Ciocalteu assays was determined considering as 100% of the antioxidant activity the absorbance value obtained in the maximum concentration of the gallic acid curve. When possible, the EC50 (half-maximum effective concentration) was determined from the percentage values of the all concentrations tested and evaluated with the software GraphPad Prism 6.01 (GraphPad Software, Inc.), using a sigmoidal adjustment model (Chen et al. 2013).
Pigment content and total soluble protein
Extraction of chlorophylls and phycobiliproteins was performed according to Kursar et al. (1983), adapted by Harb et al. (2018). Samples of 70 mg FW submitted to each treatment were ground into a microtube with pistil and liquid nitrogen. The resulting powder was extracted in 1 mL of cold 50 mM sodium phosphate buffer at pH 5.5 and centrifuged at 21,255×g and 4 °C for 15 min. The supernatant (buffer extract) was used for analysis of phycobiliproteins in G. tenuifrons and P. capillacea and for measuring the level of total soluble proteins for all species. The precipitate obtained from the buffer extraction was resuspended in 1.5 mL of methanol and dark-extracted for 3 h at 4 °C. Then, the samples were centrifuged for 15 min at 14,000 rpm and 4 °C, and the supernatant was used for the analysis of chlorophylls. The absorbance was measured by using a UV-Vis spectrophotometer (Epoch 2-BioTek, USA). Pigment content was calculated according to the equations in Table 1.
The quantification of total soluble proteins was performed according to Bradford (1976) with 20% Bio-Rad protein assay solution, at wavelength of 595 nm. Bovine serum albumin (BSA) standard curve was performed at concentrations of 0, 2, 4, 8, and 16 μg mL−1 (y = 0.0508x + 0.0297; R2 = 0.99). The results were expressed in μg g−1.
Data analysis
Statistical analysis was performed using Statistica 10.0 (StatSoft, Inc.). Before the statistical analysis, the data as percentages were transformed into .\( arcsen\sqrt{\times /100} \). The data were tested for normality (Kolmogorov-Smirnov) and homoscedasticity (Bartlett’s test) and subsequent one-way or two-way ANOVA (P < 0.05). A post hoc Newman-Keuls test (P < 0.05) was used to determine significant differences between the treatments. Tables summarizing ANOVA results are given as supplementary material (Tables S2–S5).
Principal component analysis (PCA) was used to assess the correlation between the three drying methods and the control for antioxidant potential, chlorophyll content and protein levels of each studied species. This analysis was performed using the software Past 3.25. Before PCA, the data were standardized by Z-score.
Results
Moisture content was different between species, ranging from 50.02 ± 2.26 to 91.76 ± 0.48% (Table 2). The highest moisture content was found in the silica-dried samples of G. tenuifrons, and the dried samples of P. capillacea showed the lowest moisture content.
Antioxidant potential
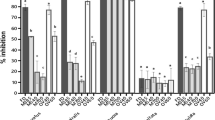
For G. tenuifrons, the antioxidant potential varied among the different crude extract concentrations (from 6 to 12 mg mL−1) for almost all antioxidant assays and drying methods (Fig. 1). Improving antioxidant action under increasing extract concentration was evidenced by the ABTS, DPPH, Folin-Ciocalteu, and FRAP assays (Fig. 1a–d, respectively). For the ABTS assay, extracts of control frozen samples (28.55 ± 1.95%) and freeze-dried samples (25.15 ± 2.04%) of G. tenuifrons achieved the highest ABTS radical scavenging activity at 12 mg mL−1 extract concentration when compared with extract from oven and silica-drying treatments (Fig. 1a). Observing the results of DPPH activity, all tested treatments and concentrations for crude extracts of G. tenuifrons showed low inhibition of DPPH radical (< 15%), and several treatments had no antioxidant activity (Fig. 1b). For the Folin-Ciocalteu assay (Fig. 1c), the highest antioxidant activity was found in control frozen samples (100.00 ± 6.11%) and the lowest one in oven-drying samples, with a reduction of 58% in antioxidant activity. The FRAP assay did not show significant differences in antioxidant action between extracts from control frozen samples and their respective extract concentration of freeze-drying and silica-drying treatments, although there was a reduction in antioxidant activity in oven-dried samples (Fig. 1d). For the metal chelating assay (Fig. 1e), no significant differences were observed between the control frozen, freeze-dried, and oven-dried samples, as well as between the concentrations. Silica-dried samples showed a reduction in antioxidant activity compared to control frozen samples at 6, 8, and 10 mg mL−1.
Percentage of antioxidant activity (mean ± SD; n = 5) of crude methanolic extracts of Gracilariopsis tenuifrons at concentrations of 6, 8, 10, and 12 mg mL−1 and under different drying methods a ABTS radical scavenging assay. b DPPH radical scavenging assay. c Reducing power of the Folin-Ciocalteu reagent assay. d ferric reducing antioxidant power (FRAP) assay. e Metal chelating assay. Different letters represent significant differences (p < 0.05)
For P. capillacea improving antioxidant action under increasing extract concentration was observed in the ABTS, DPPH, Folin-Ciocalteu, and FRAP assays (Fig. 2a–d, respectively). For the ABTS assay, frozen (95.22 ± 2.44%) and freeze-dried (78.84 ± 8.40%) crude extracts of P. capillacea showed the highest activity (Fig. 2a). The ABTS radical scavenging activity was reduced by about 60% in oven-dried and silica-dried samples. The highest inhibition of DPPH radical was found in control frozen samples (47.55 ± 7.33%), and the drying treatments showed a significant reduction (over 30%) in antioxidant activity (Fig. 2b). For the Folin-Ciocalteu assay, the highest activity was found in the crude extracts from control frozen samples (100.00 ± 14.77%) when compared to freeze-dried, oven-dried, and silica-dried samples (Fig. 2c). For the FRAP assay (Fig. 2d), control frozen samples showed higher antioxidant activity, followed by freeze-dried (63.39 ± 14.46%), silica-dried (42.34 ± 8.12%), and oven-dried samples (22.49 ± 2.17%) (Fig. 2d). For metal chelating activity (Fig. 2e), no significant differences were observed between treatments and concentrations.
Percentage of antioxidant activity (mean ± SD; n = 5) of crude methanolic extracts of Pterocladiella capillacea at concentrations of 6, 8, 10, and 12 mg mL−1 and under different drying methods. a ABTS radical scavenging assay. b DPPH radical scavenging assay. c Reducing power of the Folin-Ciocalteu reagent assay. d Ferric reducing antioxidant power (FRAP) assay. e Metal chelating assay. Different letters represent significant differences (p < 0.05)
The antioxidant potential of S. stenophyllum in the DPPH, Folin-Ciocalteu, and FRAP assays showed improving antioxidant action under increasing extract concentration (Fig. 3b–d, respectively). In the ABTS radical scavenging assay, crude extracts from frozen samples of S. stenophyllum presented an activity of 100% in the lowest concentration tested (4 mg mL−1; Fig. 3a). Oven-dried samples showed a reduction of approximately 12% in the ABTS radical scavenging activity compared to control frozen samples at 4 mg mL−1 (Fig. 3a). In the inhibition of DPPH (Fig. 3b), control frozen and freeze-dried samples exhibited the highest activity (100.00 ± 5.10% and 90.17 ± 8.71%, respectively). The oven-dried samples showed no activity in the inhibition of DPPH radical at any of the tested concentrations (Fig. 3b). For the Folin-Ciocalteu assay (Fig. 3c), crude extracts from control frozen samples showed the highest activity (100.00 ± 16.74%), and the lowest activity was found in the oven-dried samples with a reduction of 85% in antioxidant potential compared to frozen samples (Fig. 3c). In the FRAP assay (Fig. 3d), there was no significant differences between the antioxidant activity of control frozen (100.00 ± 25.95%), freeze-dried (79.37 ± 11.83%), and silica-dried (82.17 ± 11.97%) samples and the lower activity was found in the oven-dried samples (18.50 ± 1.69%) (Fig. 3d). The highest metal chelating activity was found in the frozen samples (37.59 ± 7.92% at 12 mg mL−1; Fig. 3e), followed by the freeze-dried samples (17.80 ± 4.26% at 12 mg mL−1). The oven-dried and silica-dried samples showed metal chelating activity lower than 10% at all concentrations (Fig. 3e).
Percentage of antioxidant activity (mean ± SD; n = 5) of crude methanolic extracts of Sargassum stenophyllum at concentrations of 4, 6, 8, 10, and 12 mg mL−1 and under different drying methods. a ABTS radical scavenging assay. b DPPH radical scavenging assay. c Reducing power of the Folin-Ciocalteu reagent assay. d Ferric reducing antioxidant power (FRAP) assay. e Metal chelating assay. Different letters represent significant differences (p < 0.05)
The antioxidant potential of crude extract from U. fasciata showed improving antioxidant action under increasing extract concentration in the ABTS, FRAP, and metal chelating assays (Fig. 4a, d, e, respectively). For the inhibition of ABTS radical (Fig. 4a), the crude extracts from freeze-dried samples showed the highest antioxidant potential (52.39 ± 6.78%), whereas silica-dried (11.33 ± 2.87%) samples showed the lowest potential compared to control frozen. Activity of DPPH radical scavenger was low in all treatments and concentrations (< 20%) (Fig. 4b). In the Folin-Ciocalteu assay, no significant differences were observed between control frozen and freeze-dried samples (Fig. 4c). The antioxidant potential, measured by the Folin-Ciocalteu assay, of silica-dried samples was reduced by approximately 34% compared to control frozen sample. In the FRAP assay, freeze-dried and oven-dried samples exhibited antioxidant activity respectively three times and twice as great as frozen samples (Fig. 4d). No significant differences were observed between the frozen and silica-dried samples in the FRAP assay. The metal chelating activity was higher in crude extract from control frozen samples (57.00 ± 5.43%; Fig. 4e). No significant differences were observed between freeze-dried, oven-dried, and silica-dried samples for this last assay, which showed a reduction of approximately 40% in antioxidant activity compared to control frozen samples (Fig. 4e).
Percentage of antioxidant activity (mean ± SD; n = 5) of crude methanolic extracts of Ulva fasciata at concentrations of 4, 6, 8, 10, and 12 mg mL−1 and under different drying methods. a ABTS radical scavenging assay. b DPPH radical scavenging assay. c Reducing power of the Folin-Ciocalteu reagent assay. d Ferric reducing antioxidant power (FRAP) assay. e Metal chelating assay. Different letters represent significant differences (p < 0.05)
The EC50 value could not be calculated for most assays and conservation methods because the percentage of antioxidant activity was lower than 50% (Table 3), so the EC50 values of the extracts are outside the concentration range tested. For G. tenuifrons, it was possible to calculate the EC50 for the Folin-Ciocalteu and FRAP assays. For the Folin-Ciocalteu, the lowest value of EC50 was found in control frozen samples (4.87 mg mL−1) and the highest value in oven-dried and silica-dried samples (> 12.00 mg mL−1). For the FRAP assay, the silica-dried samples had the lowest value (5.03 mg mL−1) and the oven-dried samples had the highest value of EC50 (> 12.00 mg mL−1). For P. capillacea, the EC50 was calculated for the ABTS and FRAP assays, and in both of them, the lowest value was found in the frozen samples (6.11 mg mL−1 and 5.57 mg mL−1, respectively). For S. stenophyllum, it was possible to calculate the EC50 for DPPH, Folin-Ciocalteu, and FRAP assays. In these three tests, the lowest values were found in the frozen samples (4.23, 3.60, and 5.33 mg mL−1, respectively) and the highest values in oven-dried samples (> 12.00 mg mL−1). For U. fasciata, it was possible to calculate the EC50 for the ABTS and metal-chelating assays. The freeze-dried samples had the lowest value for the ABTS assay (10.69 mg mL−1) and the frozen samples for the metal-chelating assay (10.12 mg mL−1).
To compare the antioxidant potential within samples from different drying procedures among the four species studied, the results were expressed as gallic acid equivalents (Fig. 5). Based on the comparison of antioxidant activity between concentrations, the highest concentration (12 mg mL−1) was selected to determine the standard equivalent. Except for the ABTS assay in S. stenophyllum, as there was activity saturation, the analyses were led with 4 mg mL−1 extract concentration. For the ABTS, DPPH, Folin-Ciocalteu, and FRAP assays, the frozen, freeze-dried, and silica-dried samples of S. stenophyllum presented the highest antioxidant potential among all species and treatments (Fig. 5a–d, respectively). For the metal-chelating assay, the frozen samples of S. stenophyllum and U. fasciata presented the highest antioxidant potential.
Antioxidant activity in gallic acid equivalents (mean ± SD; n = 5) of methanolic crude extracts of G. tenuifrons, P. capillacea, S. stenophyllum, and U. fasciata at concentrations of 4 mg mL−1 (only for ABTS assay in S. stenophyllum) and 12 mg mL−1 under different drying methods. a ABTS radical scavenging assay. b DPPH radical scavenging assay. c Reducing power of the Folin-Ciocalteu reagent assay. d Ferric reducing antioxidant power (FRAP) assay. e Metal chelating assay. Different letters represent significant differences (p < 0.05)
Pigment content and total soluble proteins
Chlorophyll a concentration in G. tenuifrons (Fig. 6a) was higher in control frozen samples 329.45 ± 28.53 μg g−1) and lower in oven-dried samples (127.52 ± 28.56 μg g−1). In P. capillacea (Fig. 6b), no significant differences were observed between control frozen, freeze-dried, and oven-dried samples, while silica-dried samples showed the lowest value (466.65 ± 3.73 μg g−1). The concentration of chlorophyll a and chlorophyll c in control frozen and silica-dried samples of S. stenophyllum (Fig. 6c) was higher than the one found in freeze-dried and oven-dried samples. No significant differences were observed in the concentration of chlorophyll a and chlorophyll b in frozen, freeze-dried, and silica-dried U. fasciata (Fig. 6d). However, oven-dried samples showed lower concentrations of chlorophyll a and chlorophyll b (352.87 ± 26.63 μg g−1 and 370.66 ± 30.37 μg g−1, respectively) than the control frozen sample (428.88 ± 44.56 μg g−1 and 478.47 ± 81.72 μg g−1, respectively).
Concentration of photosynthetic pigments and total soluble proteins (TSP) (mean ± SD; n = 5) of samples under different drying methods. aGracilariopsis tenuifrons. bPterocladiella capillacea. cSargassum stenophyllum. dUlva fasciata. Different letters represent significant differences (p < 0.05). Different types of letters represent independent analysis
The highest concentration of phycoerythrin and phycocyanin in G. tenuifrons (Fig. 6a) was found in freeze-dried samples (867.36 ± 115.46 μg g−1 and 91.46 ± 22.11 μg g−1), whereas silica-dried samples showed the lowest concentration (321.47 ± 58.68 μg g−1 and 36.98 ± 5.48 μg g−1). In P. capillacea (Fig. 6b), control frozen samples showed the highest concentration of phycoerythrin and phycocyanin (628.37 ± 34.86 μg g−1 and 58.14 ± 3.51 μg g−1), and oven-dried (166.50 ± 33.93 μg g−1 and 17.75 ± 3.23 μg g−1) and silica-dried samples (129.87 ± 56.98 μg g−1 and 14.96 ± 8.76 μg g−1) showed the lowest concentration.
The total soluble protein concentration (TSP) for G. tenuifrons (Fig. 6a) and P. capillacea (Fig. 6b) followed the same trend of phycobiliprotein concentration for each species. Larger levels of TSP in S. stenophyllum were found in freeze-dried (1543.71 ± 212.64 μg g−1) and in silica-dried samples (1743.49 ± 136.00 μg g−1) (Fig. 6c), and in U. fasciata in the frozen (1119.16 ± 220.31 μg g−1) and freeze-dried samples (1292.93 ± 211.18 μg g−1) (Fig. 6d).
Principal component analysis
The results of the antioxidant potential, pigment content, and total soluble protein (TSP) were analyzed using the principal component analysis (PCA) for each species separately.
For G. tenuifrons (Fig. 7a), component 1 (PC1) explains 47.98% of the data variance and component 2 (PC2) explains 28.77%. It is possible to observe the formation of four groups; each grouping encompasses separate treatments. The freeze-dried and frozen control samples are correlated with high pigments and TSP content. The frozen control group had greater influence of the antioxidant potential and chlorophyll a. The other groups appeared to not have been greatly affected by the eigenvectors.
Principal component analysis (PCA) for antioxidant assays (ABTS, chelating, DPPH, Folin, FRAP), pigment content (Chla chlorophyll a; Chlb chlorophyll b; Chlc chlorophyll c; PC phycocyanin; PE phycoerythrin) and total soluble proteins (TSP) for each species drying treatment. aGracilariopsis tenuifrons. bPterocladiella capillacea. cSargassum stenophyllum. dUlva fasciata
For P. capillacea (Fig. 7b), PC1 explains 74.41% of the data variance and PC2 13.26%. As observed for G. tenuifrons, four groups were formed and freeze-dried and frozen control samples had greater influence of Folin, DPPH, pigments, and TSP eigenvectors. The FRAP and ABTS had greater influence on the freeze-dried samples, while the chlorophyll had the greatest influence on the frozen control and oven-dried samples.
For S. stenophyllum (Fig. 7c), PC1 explains 61.76% of the data variance and PC2 explains 21.00%, with one group including the oven-dried samples and another group with the other treatments. For U. fasciata (Fig. 7d), PC1 explains 42.82% of the data variance and PC2 31.14%. The frozen control and freeze-dried samples were positively correlated with antioxidant potential, pigment content, and TSP compared to oven-dried and silica-dried samples.
In general, for the four species, the frozen and freeze-dried samples had the highest influence on antioxidant potential, pigment content, and TSP. In S. stenophyllum, silica-dried samples also showed preservation of the stability of pigments and TSP.
Discussion
Drying processes may alter the functional properties of seaweed extracts, and the magnitude of these changes may vary between different processes. For application of seaweed as a functional ingredient, it is important to evaluate the changes and the loss of properties of interest that each drying process can cause, as well as the feasibility of each one of them.
To analyze antioxidant properties, five assays were used, which evaluate different mechanisms of action and present reaction systems with different conditions (reactive species, pH, solvent and temperature). These factors may affect the reactivity of the substances present in the samples, so it is recommended to use different antioxidant assays in parallel (Frankel and Meyer 2000).
The ABTS, FRAP, and Folin-Ciocalteu assays are based on the ability of the sample to transfer electrons (Huang et al. 2005). The Folin-Ciocalteu assay is widely used to quantify the content of total phenolic compounds, but it measures the reducing capacity of the sample as the reaction is not specific. The DPPH assay is mainly based on electron transfer but also involves hydrogen atom transfer reactions. The metal-chelating assay is based on the ability of the sample to chelate Fe2+. Transition metals, like iron, can catalyze free radical formation reactions. Thus, chelating agents decrease the pro-oxidant effect of metal ions and increase the activation energy of free radical formation reactions (Gordon 1990).
Sargassum stenophyllum showed the highest antioxidant potential compared to other species. Frozen, freeze-dried, and silica-dried samples of this species showed higher antioxidant potential for the ABTS, DPPH, Folin-Ciocalteu, and FRAP assays. These results agree with those of Silva et al. (2019), who found a higher activity in the Folin-Ciocalteu (11.0 ± 0.28 mg GAE g−1) and ABTS assays (IC50 = 0.06 ± 0.01 mg mL−1 extract) in Fucus vesiculosus L. (brown seaweed) than in Gracilaria sp. (red seaweed) and Ulva rigida (green seaweed). In general, brown seaweeds present higher antioxidant activity than red and green seaweeds due to their high content of phenolic compounds (Zubia et al. 2007; Holdt and Kraan 2011).
Considering the five antioxidant assays, there were significant differences in antioxidant potential between the algal drying methods in the four studied species. In general, the antioxidant potential of the samples follows this order: frozen (− 80 °C) > freeze-dried > silica-dried > oven-dried (40 °C). Cruces et al. (2016) also observed a reduction in antioxidant potential in oven-dried and silica-dried samples of Lessonia spicata compared to frozen (control) and freeze-dried samples. According to these authors, the long time required for drying the samples in silica can lead to oxidation processes or degradation of phenolic compounds and thus the loss of antioxidant capacity. In the oven-drying process, oxidation of molecules with antioxidant activity can occur because of the action of temperature.
The Folin-Ciocalteu assay showed a significant decrease in the amount of reducing compounds in silica-dried and oven-dried samples compared to the frozen ones. Studies with seaweeds (Jiménez-Escrig et al. 2001; Le Lann et al. 2008; Gupta et al. 2011) and terrestrial plants (Lim and Murtijaya 2007; Mudau and Ngezimana 2014) also showed a decrease in the reducing compounds in oven-dried samples. This reduction could be due to degradation of phenolic compounds at high temperatures, starting at 40 °C (Le Lann et al. 2008; Moreira et al. 2016). Other studies also showed antioxidant potential decrease in macroalgae due to high temperatures. Jiménez-Escrig et al. (2001) found a reduction of the antioxidant potential of oven-dried samples (50 °C) compared to frozen samples of Fucus sp. (brown seaweed) and Porphyra sp. (red algae). In a study by Gupta et al. (2011) samples of Himanthalia elongata dried at 35 and 40 °C showed the lowest antioxidant potential by DPPH assay than samples dried at 25 and 30 °C. Le Lann et al. (2008), in a study with two species of brown algae, observed through the DPPH assay a reduction in antioxidant potential of oven-dried (50 °C for 48 h) compared to frozen (− 20 °C) and freeze-dried (for 72 h) samples.
Decrease of phenolic content and antioxidant activities of samples dried at high temperatures may occur due to the release of phenolic compounds bound to the cell wall, thermal degradation of phenolic compounds by oxidative enzymes, changes in the chemical structure, and binding phenolic compounds with other substances, such as proteins, affecting their extractability (Maillard and Berset 1995; Le Lann et al. 2008; Martín-Cabrejas et al. 2009; Moreira et al. 2016).
In the present study, in general, freeze-drying was the best drying method to preserve the antioxidant potential because the low temperature prevents degradation of heat-sensitive compounds (Le Lann et al. 2008). Besides, freeze-drying is a vacuum process and the absence of oxygen prevents oxidation reactions and degradation of functional ingredients (Murgatroyd 1997). However, in most cases, the assays for freeze-dried samples demonstrated reduction of antioxidant potential compared to the frozen samples, probably because the loss of water can cause the destabilization of bioactive molecules. Furthermore, according to Li et al. (2006) in the absence of water, the cellular components may adhere to each other, making extraction more difficult by different solvents, which can reduce the antioxidant potential of the extracts.
In contrast, for the FRAP and ABTS assays, frozen samples of U. fasciata showed lower antioxidant potential than freeze-dried and oven-dried samples. This might be due to the fact that freeze-drying and oven-drying facilitate the breakage of the cell wall and cell membrane, which eases the grinding of the samples and makes the extraction of antioxidant compounds more effective (Stafford et al. 2005). According to Moreira et al. (2016), the size of the sample particles affects the extraction of phenolic compounds and antioxidant potential. However, particle size has not been measured in the present work. Freeze-drying was also the drying method that best preserved the pigment content and TSP. Works with terrestrial plants (Mahanom et al. 1999; Mudau and Ngezimana 2014; Rubinskienė et al. 2015) and macroalgae (Tello-Ireland et al. 2011) have demonstrated that freeze-drying, when compared to oven-drying, is the best drying method for the preservation of pigments.
High temperatures can promote oxidative reactions that may cause pigments and protein denaturation (Tello-Ireland et al. 2011), which explains the lower levels of these compounds in samples dried in the oven (40 °C) compared to freeze-drying. Silva et al. (2019), analyzing the effects of oven-drying, noted that the use of high temperatures result in the degradation of photosynthetic pigments in Gracilaria sp., F. vesiculosus, and U. rigida samples.
For the use of seaweed as a functional ingredient, it is important that the drying process maintain its antioxidant properties and chemical composition. Sun-drying is the most used process for drying plants and seaweed, as it is cheaper and allows for large-scale production, but some food products should not be dried in the sun because it can cause the degradation of compounds of interest and lower the quality of the final product (Jairaj et al. 2009). The results of the present study suggest that, when possible, it is best to use freeze-drying, because that was the process that caused the least change in the antioxidant potential and pigment content when compared to oven-drying and silica-drying. However, freeze-drying has a high cost and, from a commercial perspective, the choice of drying method should also consider economic factors and depend on the application intended for the seaweed. Among the studied species, S. stenophyllum is the best suitable one as a functional ingredient because it presented the highest antioxidant activity. However, in order to be considered a functional food or ingredient, the nutritional properties and food safety must also be evaluated.
Conclusions
The reactivity results in the five antioxidant assays varied even for the same species and the same drying method, demonstrating that the use of more than one assay is important for a complete analysis of the antioxidant potential of macroalgal extracts. Sargassum stenophyllum presented the highest antioxidant activity and is the best suitable species for use as a functional ingredient, although the nutritional properties and the food safety of this species must still be evaluated.
The choice of drying method can vary considering the application intended for the seaweed, the costs, the logistics for the processing, and the possible loss of properties of interest. The present study demonstrated that, among the analyzed drying processes, freeze-drying was the one that presented overall the lowest alteration of antioxidant potential and pigment content in relation to the control (frozen).
References
Badmus UO, Taggart MA, Boyd KG (2019) The effect of different drying methods on certain nutritionally important chemical constituents in edible brown seaweeds. J Appl Phycol 31:3883–3897
Barsanti L, Gualtieri P (2014) Algae: anatomy, biochemistry, and biotechnology. CRC Press, Boca Raton, pp 267–308
Beer S, Eshel A (1985) Determining phycoerythrin and phycocyanin concentrations in aqueous crude extracts of red algae. Mar Freshw Res 36:785–792
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brito LL, Silva S (2005) Fenología y ciclo de vida del alga Gracilariopsis tenuifrons (Gracilariaceae) en Sucre, Venezuela. J Trop Biol 53:67–73
Cardoso SM, Carvalho LG, Silva PJ, Rodrigues MS, Pereira OR, Pereira L (2014) Bioproducts from seaweeds: a review with special focus on the Iberian Peninsula. Curr Org Chem 18:896–917
Chen Z, Bertin R, Froldi G (2013) EC50 estimation of antioxidant activity in DPPH assay using several statistical programs. Food Chemistry 138:414–420
Chenlo F, Arufe S, Díaz D, Torres MD, Sineiro J, Moreira R (2018) Air-drying and rehydration characteristics of the brown seaweeds, Ascophylum nodosum and Undaria pinnatifida. J Appl Phycol 30:1259–1270
Costa ES, Plastino EM, Petti R, Oliveira EC, Mariana MC (2012) The Gracilariaceae Germplasm bank of the University of São Paulo, Brazil - a DNA barcoding approach. J Appl Phycol 24:1643–1653
Cruces E, Rojas-Lillo Y, Ramirez-Kushel E, Atala E, López-Alarcón C, Lissi E, Gómez I (2016) Comparison of different techniques for the preservation and extraction of phlorotannins in the kelp Lessonia spicata (Phaeophyceae): assays of DPPH, ORAC-PGR, and ORAC-FL as testing methods. J Appl Phycol 28:573–580
Edwards P (1970) Illustrated guide to the seaweeds and sea grasses in the vicinity of Port Aransas. University of Texas Marine Science Institute, Port Aransas
Fernando IPS, Kim M, Son KT, Jeong Y, Jeon YJ (2016) Antioxidant activity of marine algal polyphenolic compounds: a mechanistic approach. J Med Food 19:1–14
Fleurence J (2016) Seaweeds as food. In: Fleurence J, Levine I (eds) Seaweed in health and disease prevention. Elsevier, Amsterdam, pp 149–167
Frankel EN, Meyer AS (2000) The problems of using one-dimensional methods to evaluate multifunctional food and biological antioxidants. J Sci Food Ag 80:1925–1941
Gordon MH (1990) The mechanism of antioxidant action in vitro. In: Hudson BJF (ed) Food antioxidants. Springer, Dordrecht pp 1-18
Gupta S, Cox S, Abu-Ghannam N (2011) Effect of different drying temperatures on the moisture and phytochemical constituents of edible Irish brown seaweed. LWT Food Sci Technol 44:1266–1272
Harb TB, Torres PB, Pires JS, Santos DYAC, Chow F (2016) Ensaio em microplaca do potencial antioxidante através do sistema quelante de metais para extratos de algas. Universidade de São Paulo, Instituto de Biociências
Harb TB, Nardelli A, Chow F (2018) Physiological responses of Pterocladiella capillacea (Rhodophyta, Gelidiales) under two light intensities. Photosynthetica 56:1093–1106
Holdt SL, Kraan S (2011) Bioactive compounds in seaweed: functional food applications and legislation. J Appl Phycol 23:543–597
Huang D, Ou B, Prior RL (2005) The chemistry behind antioxidant capacity assays. J Agric Food Chem 53:1841–1856
Humphrey A (2004) Chlorophyll as a color and functional ingredient. J Food Sci 69:C422–C425
Jairaj KS, Singh SP, Srikant K (2009) A review of solar dryers developed for grape drying. Sol Energy 83:1698–1712
Jiménez-Escrig A, Jiménez-Jiménez I, Pulido R, Saura-Calixto F (2001) Antioxidant activity of fresh and processed edible seaweeds. J Sci Food Agric 81:530–534
Kruger CL, Mann SW (2003) Safety evaluation of functional ingredients. Food Chem Toxicol 41:793–805
Kursar TA, Van der Meer J, Alberte RS (1983) Light-harvesting system of the red alga Gracilaria tikvahiae. Plant Physiol 73:361–369
Le Lann K, Jégou C, Stiger-Pouvreau V (2008) Effect of different conditioning treatments on total phenolic content and antioxidant activities in two Sargassacean species: comparison of the frondose Sargassum muticum (Yendo) Fensholt and the cylindrical Bifurcaria bifurcata R. Ross. Phycol Res 56:238–245
Li B, Smith B, Hossain M (2006) Extraction of phenolics from citrus peels: I. solvent extraction method. Sep Purif Technol 48:182–188
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: measurement and characterization by UV-VIS. Curr Protocols Food Analyt Chem 1:F4.3.1–F4.3.8
Lim YY, Murtijaya J (2007) Antioxidant properties of Phyllanthus amarus extracts as affected by different drying methods. LWT Food Sci Technol 40:1664–1669
Ling ALM, Yasir S, Matanjun P, Abu Bakar MF (2015) Effect of different drying techniques on the phytochemical content and antioxidant activity of Kappaphycus alvarezii. J Appl Phycol 27:1717–1723
Mahanom H, Azizah A, Dzulkifly M (1999) Effect of different drying methods on concentrations of several phytochemicals in herbal preparation of 8 medicinal plants leaves. Malays J Nutr 5:47–54
Maillard MN, Berset C (1995) Evolution of antioxidant activity during kilning: role of insoluble bound phenolic acids of barley and malt. J Agric Food Chem 43:1789–1793
Martín-Cabrejas MA, Aguilera Y, Pedrosa M, Cuadrado C, Hernández T, Díaz S, Esteban RM (2009) The impact of dehydration process on antinutrients and protein digestibility of some legume flours. Food Chem 114:1063–1068
McDermid KJ, Stuercke B (2003) Nutritional composition of edible Hawaiian seaweeds. J Appl Phycol 15:513–524
Moreira R, Chenlo F, Sineiro J, Arufe S, Sexto S (2016) Drying temperature effect on powder physical properties and aqueous extract characteristics of Fucus vesiculosus. J Appl Phycol 28:2485–2494
Mudau FN, Ngezimana W (2014) Effect of different drying methods on chemical composition and antimicrobial activity of bush tea (Athrixia phylicoides). Int J Agric Biol 16:1560–8530
Murgatroyd K (1997) The freeze drying process. In: Cameron P (ed.) Good Pharmaceutical Freeze-Drying Practice. Interpharm Press, pp. 1–57
Pangestuti R, Kim SK (2011) Biological activities and health benefit effects of natural pigments derived from marine algae. J Funct Foods 3:255–266
Pires J, Torres PB, Santos DYAC, Chow F (2017a) Ensaio em microplaca do potencial antioxidante através do método de sequestro do radical livre DPPH para extratos de algas. Instituto de Biociências, Universidade de São Paulo, São Paulo
Pires J, Torres PB, Santos DYAC, Chow F (2017b) Ensaio em microplaca de substâncias redutoras pelo método do Folin-Ciocalteu para extratos de algas. Instituto de Biociências, Universidade de São Paulo, São Paulo.
Rioux LE, Beaulieu L, Turgeon SL (2017) Seaweeds: a traditional ingredient for new gastronomic sensation. Food Hydrocoll 68:255–265
Ritchie RJ (2008) Universal chlorophyll equations for estimating chlorophylls a, b, c, and d and total chlorophylls in natural assemblages of photosynthetic organisms using acetone, methanol, or ethanol solvents. Photosynthetica 46:115–126
Rubinskienė M, Viškelis P, Dambrauskienė E, Viškelis J, Karklelienė R (2015) Effect of drying methods on the chemical composition and colour of peppermint (Mentha × piperita L .) leaves. Zemdirbyste-Agriculture 102:223–228
Santos JP, Torres PB, Santos DYAC, Motta LB, Chow F, (2019) Seasonal effects on antioxidant and anti-HIV activities of Brazilian seaweeds. J Appl Phycol 31:1333–1341
Sappati PK, Nayak B, VanWalsum PV, Mulrey OT (2019) Combined effects of seasonal variation and drying methods on the physicochemical properties and antioxidant activity of sugar kelp (Saccharina latissima). J Appl Phycol 31:1311–1332
Silva AFR, Abreu H, Silva MAS, Cardoso SM (2019) Effect of oven-drying on the recovery of valuable compounds from Ulva rigida, Gracilaria sp. and Fucus vesiculosus. Mar Drugs 17:90
Stafford GI, Jäger AK, Van Staden J (2005) Effect of storage on the chemical composition and biological activity of several popular South African medicinal plants. J Ethnopharmacol 97:107–115
Stévant P, Indergård E, Ólafsdóttir A, Marfaing H, Larssen WE, Fleurence J, Roleda MY, Rustad T, Slizyte R, Nordtvedt TS (2018) Effects of drying on the nutrient content and physicochemical and sensory characteristics of the edible kelp Saccharina latissima. J Appl Phycol 30:2587–2599
Tello-Ireland C, Lemus-Mondaca R, Vega-Gálvez A, López J, Di Scala K (2011) Influence of hot-air temperature on drying kinetics, functional properties, colour, phycobiliproteins, antioxidant capacity, texture and agar yield of alga Gracilaria chilensis. LWT Food Sci Technol 44:2112–2118
Torres PB, Pires JS, Santos DYAC, Chow F (2017) Ensaio do potencial antioxidante de extratos de algas através do sequestro do ABTS•+ em microplaca. Instituto de Biociências, Universidade de São Paulo
Uribe E, Vega-Gálvez A, García V, Pastén A, López J, Goñi G (2019) Effect of different drying methods on phytochemical content and amino acid and fatty acid profiles of the green seaweed, Ulva spp. J Appl Phycol 31:1967–1979
Urrea-Victoria V, Pires J, Torres PB, Santos DYAC, Chow F (2016) Ensaio antioxidante em microplaca do poder de redução do ferro (FRAP) para extratos de algas. Instituto de Biociências, Universidade de São Paulo
Ursi S, Plastino EM (2001) Crescimento in vitro de linhagens de coloração vermelha e verde clara de Gracilaria birdiae (Gracilariales, Rhodophyta) em dois meios de cultura: análise de diferentes estádios reprodutivos. Braz J Bot 24:587–594
Wells ML, Potin P, Craigie JS, Raven JA, Merchant SS, Helliwell KE, Smith AG, Camire ME, Brawley SH (2017) Algae as nutritional and functional food sources: revisiting our understanding. J Appl Phycol 29:949–982
Zubia M, Robledo D, Freile-Pelegrin Y (2007) Antioxidant activities in tropical marine macroalgae from the Yucatan peninsula, Mexico. J Appl Phycol 19:449–458
Acknowledgments
We are thankful to Rosário Petti and Willian Oliveira for technical support.
Funding
Financial support was provided by FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) (15/02008-4; BIOTA/FAPESP 13/50731-1).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Amorim, A.M., Nardelli, A.E. & Chow, F. Effects of drying processes on antioxidant properties and chemical constituents of four tropical macroalgae suitable as functional bioproducts. J Appl Phycol 32, 1495–1509 (2020). https://doi.org/10.1007/s10811-020-02059-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-020-02059-7