Abstract
The effect of different drying techniques on the phytochemical content and antioxidant activity of Kappaphycus alvarezii (‘crocodile’ morphotype) were investigated. Phytochemical (total phenolic, flavonoid, anthocyanin and carotenoid content) and antioxidant activity of the seaweed were determined. The seaweed was dried using seven types of drying techniques; oven drying (temperature of 40 °C), oven drying (temperature of 80 °C), sun drying, hang drying, sauna drying, shade drying and freeze drying. There were significant differences in the phytochemical content and antioxidant activity between the dried seaweed samples. The total phenolic content of the dried samples were in the range of 26.11 to 53.33 mg gallic acid equivalent (GAE) per 100 g, total flavonoid content from 9.83 to 25.67 mg catechin equivalent (CE) per 100 g, total anthocyanin content from 0.05 to 0.11 mg cyanidin-3-glucoside equivalent (C-3-GE) per gramme and total carotenoid content from 0.03 to 0.24 mg β-carotene equivalent (BCE) per gram, respectively. The oven-dried (40 and 80 °C) and shade-dried samples contained the highest values of phytochemical content as compared to other dried samples tested. Antioxidant activity were determined using 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2-azino-bis-(3-ethyl-benzothiazoline-6-sulphonic acid) (ABTS) and ferric reducing/antioxidant power (FRAP) assays. The oven-dried (40 and 80 °C) and shade-dried samples also displayed stronger scavenging activity and reducing ability as compared to other dried samples tested. The lower values of phytochemical content and weak antioxidant activity were detected in sun-dried and sauna-dried samples. This finding suggested that different drying techniques greatly influence the occurrence of phytochemical content and antioxidant activity in seaweeds.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Seaweeds have been used since the ancient times as food, fodder, fertilizer and as a source of medicinal drugs, and today, they are used as a raw material for industrial production of agar, algin and carrageenan (Mishra et al. 1993). In food manufacturing, seaweeds have been developed as raw or semi-processed food products (Mabeau and Fleurence 1995; Ismail and Tan 2002). They have received significant attention for their potential as a source of natural antioxidants. Seaweed extracts have been demonstrated to display strong antioxidant properties (Kuda et al. 2005; Yuan and Walsh 2006; Martins et al. 2013), protective effects against liver injury caused by carbon tetrachloride (Wong et al. 2000), antiproliferative activity towards HeLa cells (Yuan and Walsh 2006) and other cancer cell lines (Murphy et al. 2014), antimicrobial activity (Valdebenito et al. 1982) and antiviral properties (Chatterji et al. 2004).
Kappaphycus alvarezii, an economically important red tropical seaweed, which is highly demanded for its cell wall polysaccharide, is the most important source of kappa carrageenan (Kumar et al. 2008). It is easily accessible, in huge amounts, for food and pharmaceutical applications. It is cultured in Indonesia, Malaysia, Philippines, Vietnam and several other tropical countries for domestic industries and export (Hurtado et al. 2014; Msuya et al. 2014). In Malaysia, the cultivation of K. alvarezii was conducted in Semporna (eastern coast of Malaysia), where the total production is about 1800 t year−1 (dry weight) (Prud’Homme van Reine and Trono 2001). This study deals with the phytochemical content and antioxidant activity of K. alvarezii (crocodile morphotype—Tan et al. 2013).
Seaweeds collected from the sea are usually dried before being used in any nutritional studies or industrial processing. Drying decreases the water activity which eventually retards microbial growth, helps conserve the desirable qualities and reduces the storage volume (Gupta et al. 2011). According to FAO (1976), seaweeds that are dried properly can be stored for a number of years without appreciable loss of their gel content. The drying technique could be a factor that affects the phytochemical content of seaweed samples. According to Gupta et al. (2011), drying the seaweeds at a lower temperature (less than 40 °C) resulted in 49 and 51 % reduction in the total phenol and total flavonoid content, but the reduction seemed to decline as the drying temperature increased to more than 41 °C.
Sun drying (Carrillo et al. 1992), oven drying (Hamdy and Dawes 1988) and freeze drying (Mabeau et al. 1992) are the three common drying techniques employed in seaweed studies. Thus, this study was designed to employ more drying techniques (oven drying with different temperatures, sun drying, hang drying, sauna drying, shade drying and freeze drying) and to determine the effect of these techniques on the phytochemical content and antioxidant activity of seaweed samples.
Materials and methods
Plant materials and sample preparation
Samples of Kappaphycus alvarezii were collected from Pulau Salakan, Semporna, Sabah, Malaysia, between May and June 2013. Fresh seaweeds were thoroughly washed with distilled water, and their holdfasts and visible epiphytes were removed. The cleaned seaweeds were divided into seven groups. The first group was dried in a ventilated oven for 24 h with a lower temperature of 40 °C [the oven-dried (40 °C) group], and the second group was dried in a ventilated oven for 24 h at a higher temperature of 80 °C [the oven-dried (80 °C) group]. The third group was dried under direct sunlight for 3–4 days (the sun-dried group); the fourth group was dried by hanging them under the sunlight until they changed to purplish colour and letting them dry under the sunlight for 3–4 days (the hang-dried group); the fifth group was dried by putting them inside a clear plastic bag and placing them under the sunlight for a half day until they changed to whitish colour, before removing them from the clear plastic bag and letting them dry under the sunlight for 3–4 days (the sauna-dried group); the sixth group was dried by placing them in a shaded place for 2 days and then drying them in a ventilated oven at 70 °C for 4 h (the shade-dried group) (Kumar et al. 2008); and the last group was frozen in a −80 °C freezer for 24 h and then dried in a freeze drier (Labconco, USA) for 2 days (the freeze-dried group). Each of the samples was dried until the moisture content was less than 10 %. All samples were ground into fine powder using a dry grinder. The ground samples were sieved to get uniform size, then kept in an air-tight container and stored in a freezer (−20 °C) until further analysis.
Extraction
Samples (1 g) were extracted for 2 h with 80 % methanol with a ratio of 1:10 at room temperature on an orbital shaker set at 200 rpm (Velioglu et al. 1998). The mixture was then centrifuged at 1400 rpm for 20 min, and the supernatant was decanted into a 15 mL vial. The pellet was re-extracted under identical conditions. The supernatants were combined and used for measurement of total antioxidant activity, total phenolic, total flavonoid, total anthocyanin and total carotenoid content.
DPPH free radical scavenging assay
The scavenging activity of the extract was measured using 2,2-diphenyl-1-picrylhydrazyl (DPPH) (Sigma Aldrich, USA) assay as described by Mensor et al. (2001) with some modifications. An aliquot (2.5 mL) of extract was mixed with 1 mL of 0.3 mM of DPPH in absolute methanol. The mixture was shaken vigorously and kept in the dark for 30 min at room temperature. The absorbance of the mixture was measured at 518 nm, and the free-radical scavenging activity was calculated as follows:
The scavenging percentage of the extract was plotted, and the final result was interpreted as the 50 % inhibition (IC50) value (the concentration of substrate that causes 50 % loss of DPPH activity).
FRAP (Ferric reducing/antioxidant power) assay
The assay was conducted according to Benzie and Strain (1996) with some modifications. The working reagent was produced by mixing 300 mM acetate buffer (pH 3.6); 10 mM 2,4,6-tripyridyl-s-triazine (TPTZ) (Sigma Aldrich, USA) solution and 20 mM FeCl3.6H2O in the ratio of 10:1:1, freshly prepared and warmed to 37 °C in the water bath. The reaction was carried out in microtiter plate. The blank reading was measured by dispending 200 μL of this working solution to each well of the microtiter plate and read at 593 nm. Then, the sample reading was measured by adding 20 μL of the extract into the 200 μL of the working solution to initiate the reaction, and the absorbance was measured at 593 nm after exactly 4 min. Standards of known Fe (II) concentrations were run using several concentrations between 0 and 1000 μg mL−1. A standard curve was then plotted. The result was expressed as the concentration of the antioxidant having a ferric reducing ability (mM ferric reduction to ferrous in 1 g of dried sample).
ABTS decolorization assay
2,2-azino-bis-(3-ethyl-benzothiazoline-6-sulphonic acid) (Sigma Aldrich, USA) assay was carried out according to Re et al. (1999) with some modifications. Working ABTS solution (7 mM) and potassium persulphate (2.45 mM) was mixed, and the mixture was allowed to stand overnight to generate an ABTS free radical cation solution. The mixture was diluted with 80 % methanol in order to obtain absorbance of 0.7 ± 0.2 units at 734 nm. An aliquot (200 μL) of extract was mixed with 2 mL of ABTS solution, and the mixture was shaken for 45 s before measured at 734 nm. Ascorbic acid (Sigma Aldrich), with the concentration of 0 to 100 mg mL−1, was used as a standard and calibration. The result was expressed as milligrammes ascorbic acid equivalents antioxidant capacity (AEAC) in 1 g of dried sample.
Determination of total phenolic content
Total phenolic (TP) content of the extract was determined according to the Folin-Ciocalteu method (Velioglu et al. 1998) with some modifications. An aliquot (100 μL) of extract was mixed with 0.75 mL of Folin-Ciocalteu reagent (Fisher, UK) (previously diluted 10-fold with distilled water) and allowed to stand at room temperature for 5 min. Then, 0.75 mL of sodium bicarbonate (60 g L−1) solution was added into the mixture. The mixture was incubated at room temperature and kept in the dark for 90 min. After 90 min, the absorbance was measured at 725 nm. A standard curve was plotted using different concentrations of gallic acid (Sigma Aldrich), and the amount of total phenolic was expressed as gallic acid equivalents (GAE) in milligram per 100 g of dried sample (Sabeena and Jacobsen 2013).
Determination of total flavonoid content
Total flavonoid (TF) content of the extract was determined according to Aluminium Chloride Calorimetric Assay (Zhishen et al. 1999). An aliquot (1 mL) of extract was mixed with 4 mL of distilled water. At 0 min, 0.3 mL of 5 % NaNO2 was added to the mixture. After 5 min, 0.6 mL of 10 % AlCl3.6H2O was added, and after 6 min, 2 mL of 1 M NaOH was added. The mixture was mixed, and the absorbance was measured at 510 nm. A standard curve was plotted using different concentration of catechin (Sigma Aldrich), and the amount of total flavonoid was expressed as catechin equivalents (CE) in milligram per 100 g of dried sample.
Determination of total anthocyanin content
Total anthocyanin (TA) content was measured using a spectrophotometric pH differential protocol, as described by Giusti and Wrolstad (2001) with some modifications. Briefly, 0.5 mL of extract was mixed thoroughly with 3.5 mL of 0.025 M potassium chloride buffer; pH 1. The mixture was stirred and allowed to stand at room temperature for 15 min. Then, the absorbance was measured at 515 and 700 nm against distilled water as a blank. The extract was then combined similarly with 3.5 mL of 0.025 M sodium acetate buffer; pH 4.5. The mixture was stirred and allowed to stand at room temperature for 15 min. The mixture was measured at the same wavelengths. The total anthocyanins content was calculated using the following equation:
Where A is absorbance = (A 515–A 700) pH 1.0–(A 515–A 700) pH 4.5; MW is molecular weight for cyanidin-3-glucoside =449.2; DF is the factor of the extract; ɛ is the molar absorbtivity of cyanidin-3-glucoside =26,900; C is the concentration of the buffer in mg mL−1 = 0.025. Result was expressed as milligram of cyanidin-3-glucoside (C-3-GE) equivalents in 1 g of dried sample.
Determination of total carotenoid content
Total carotenoid (TC) content was measured according to Hess et al. (1991) method with some modifications. Briefly, 300 μL of extract was added to 300 μL of distilled water and 600 μL of solvent. Then, the mixture was combined with 1.2 mL of n-hexane and then centrifuged for 5 min at 4 °C. The absorbance was measured at 350 nm using a spectrophotometer. A standard curve was plotted using different concentration of β-carotene (Sigma Aldrich), and the amount of total carotenoid content was expressed as β-carotene equivalents (BC) in milligram per gram of dried sample.
Statistical analysis
All experiments were carried out in three replicates in three independent experiments. The results were presented as mean ± standard deviation (SD) using Prism 5 software. The data were statistically analysed by one-way ANOVA and Duncan post hoc test. The level of statistical significance was set at α = 0.05. Pearson’s correlation analysis was done to correlate the phytochemical content and antioxidant potential in the samples.
Results
Table 1 shows the results of primary screening of antioxidant activity of all dried samples extracts using DPPH, FRAP and ABTS assays. The antioxidant activities of the dried samples determined by FRAP assay were in the range of 1.53 ± 0.14 to 7.81 ± 0.47 mM ferric reduction to ferrous equivalents in 1 g of dried sample and the sequence of antioxidant activity was as follows: oven-dried (40 °C) > oven-dried (80 °C) > shade-dried > hang-dried > freeze-dried > sauna-dried > sun-dried. The antioxidant activities of the dried samples determined by ABTS assay were in the range of 0.20 ± 0.03 to 0.54 ± 0.01 mg Aascorbic acid equivalents antioxidant capacity (AEAC) in 1 g of dried sample, and the sequence of antioxidant activity was as follows: oven-dried (40 °C) > oven-dried (80 °C) > shade-dried > hang-dried > freeze-dried and sun-dried > sauna-dried. The radical scavenging activity of dried samples extracts is shown in Fig. 1 and expressed as percentage reduction of the initial DPPH∙ absorption by the tested compound. The best radical scavenging activity could be obtained in oven-dried (40 °C) sample extract (IC50 12.80 mg mL−1), followed by oven-dried (80 °C) sample extract (IC50 13.33 mg mL−1), shade-dried sample extract (IC50 15.07 mg mL−1), hang-dried sample extract (IC50 22.93 mg mL−1), freeze-dried sample extract (IC50 37.33 mg mL−1), sauna-dried sample extract (IC50 48.73 mg mL−1) and sun-dried sample extract (IC50 56.93 mg mL−1), respectively. The lowest value of IC50 indicates strongest ability of the extract as DPPH scavengers.
Table 2 shows the values of the total phenolic, flavonoid, anthocyanin and carotenoid content for each dried samples extracts. Higher values of these phytochemical content were detected in the samples dried by oven drying (40 °C), followed by oven drying (80 °C). Lower values were detected in the samples dried by the sun-drying and the sauna-drying techniques. The sequence of total phenolic content of the dried samples in decreasing order was as follows: oven-dried (40 °C) > oven-dried (80 °C) > shade-dried > hang-dried > freeze-dried > sauna-dried > sun-dried. The sequence of total flavonoid content of the dried samples in decreasing order was as follows: oven-dried (40 °C) > oven-dried (80 °C) > shade-dried > hang-dried > freeze-dried > sun-dried > sauna-dried. The sequence of total anthocyanin content of the dried samples in decreasing order was as follows: oven-dried (40 °C) > oven-dried (80 °C) > shade-dried > hang-dried > freeze-dried > sun-dried > sauna-dried. The sequence of total carotenoid content of the dried samples in decreasing order was as follows: oven-dried (40 °C) > oven-dried (80 °C) > freeze-dried > shade-dried > hang-dried > sun-dried > sauna-dried.
Discussion
Previous studies show that various solvent extracts of K. alvarezii exhibited excellent scavenging effect (%) by DPPH assay, reducing power, ferrous ion-chelating activity and antioxidant property in the linoleic acid system (Kumar et al. 2008; Matanjun et al. 2008). The cell wall of K. alvarezii is composed of carrageenan, sulfated polysaccharides (Yuan et al. 2005), which may contribute to its antioxidant potential in addition to the presence of ascorbic acid, vitamin A and various phenolics (Ragan and Glombitza 1986; Kumar et al. 2008; Matanjun et al. 2008). Processing of any kind will affect content, activity and bioavailability of bioactive compounds. According to Tomaino et al. (2004), the drying process would generally result in a depletion of naturally occurring antioxidants in raw materials from plants. The phytochemical content and antioxidant activity in seaweeds could be affected by high temperature, long drying time, UVA-UVB and dehydration during drying process. According to Li et al. (2006), a combination of high drying temperatures and long drying times might destroy some of the phenol compounds. In addition, all the plant cell components adhere to one another in the absence of water, possibly making the extraction with solvent more difficult, and as a result, the overall recoveries might be lower than expected (Li et al. 2006). Garau et al. (2007) also reported that long drying times resulted in a reduction of total phenolic content for orange by-product.
In this study, the oven-dried (40 °C) sample showed higher values of total phenolic, flavonoid, anthocyanin and carotenoid content and displayed stronger scavenging activity and reducing ability followed by the oven-dried (80 °C), shade-dried, hang-dried, freeze-dried, sun-dried and sauna-dried samples, respectively. The phytochemical content and antioxidant activity in the oven-dried (40 °C) sample are less affected because the moderate temperature (temperature set at 40 °C) and short drying time (24 h) were applied in this drying technique. According to Gupta et al. (2011), drying at lower temperatures (25 and 30 °C) resulted in a continuous reduction of total phenolic content; however, for higher temperatures (35 and 40 °C), an increase of total phenolic content was seen for the first 2 h, after which it started to decrease. They also reported that maximum increase of 41 % in the total phenolic content was seen after drying at 40 °C for 2 h. This increase could be related to the developmental changes and wound-like response due to drying. Dixon and Paiva (1995) reported that plants respond to wounding with increase in phenolic compounds involved in the repair of wound damage.
The phytochemical content and antioxidant activity in the oven-dried (80 °C) and shade-dried samples are affected because the high temperature was applied in these drying techniques. Oven heating at high temperature rapidly inactivates polyphenol oxidases present in plant materials by absorbing the water molecule through microwave energy (Ponmari et al. 2011). Some reports suggested that freeze drying is the most appropriate drying technique in retaining the nutritional composition (Chan et al. 1997) and anti-flammatory activities of polysaccharides fraction (Hammed et al. 2013) of seaweeds. However, in this study, the values of phytochemical content and antioxidant activity of freeze-dried sample were lower than oven-dried (40 °C), oven-dried (80 °C) and shade-dried samples. Wong and Cheung (2001) reported that oven drying was better than freeze drying for the extractability and quality of proteins isolated from three subtropical brown seaweeds.
The lowest values of phytochemical content and antioxidant activity were detected in the hang-dried sample followed by sun-dried and sauna-dried samples, respectively. These techniques are strongly dependent on the weather and the length of the day. The phytochemical content and antioxidant activity in these drying techniques were affected because of the long drying time (3 to 4 days under direct sunlight) and dehydration during the drying process. Its slower drying rate likely increased the leaching effect and prolonged the exposure time of seaweed to air. According to Klein and Kurilich (2000), dehydration, whether by sun or artificial heat sources will affect not only ascorbate but also tocopherols and carotenoids, as a result of exposure to UVA-UVB light, air and heat.
The fresh seaweeds used in this study were cut into smaller pieces (1 to 2 cm size) before drying. This cutting process was done so that the seaweeds samples were evenly dried. However, cutting or slicing of food during processing will also cause significant loss of bioactive compounds. According to Ponmari et al. (2011), depletion of antioxidants could be due to operations such as peeling, cutting and slicing as they induce a rapid enzymatic oxidation of natural antioxidant. Vitamins and bioactive compounds are naturally protected in plant tissues. Cutting or slicing of the sample destroys this protection, increase exposure to oxygen and release enzymes that catalyse their degradation (Amarowicz et al. 2009). Sun drying is considered as the cheapest and most accessible means of food preservation. However, it has been reported to cause a reduction in the levels of labile antioxidants such as L-ascorbate and GSH (Burritt et al. 2002; Jiménez-Escrig et al. 2001).
In conclusion, this study showed that temperature, long drying time, UVA-UVB light, dehydration and cutting/slicing of samples during drying process could cause significant losses of phytochemical components and lead to the reduction in the phytochemical content and antioxidant potential. Among seven drying techniques used in this study, the oven drying at 40 °C showed the highest values of phytochemical content and displayed better scavenging and reducing ability. Drying is an important step in seaweed production (semi-dried seaweed product), but it can negatively impact the phytochemical constituents in the seaweed. Thus, new research into protecting phytochemical components and antioxidant properties of seaweeds upon processing would be needed. As a robust natural source of important bioactive compounds, having knowledge of the optimum post-harvest drying treatment for K. alvarezii would be commercially advantageous.
References
Amarowicz R, Carle R, Dongowski G, Durazzo A, Galensa R, Kammerer D, Maiani G, Piskula MK (2009) Influence of postharvest processing and storage on the content of phenolic acids and flavonoids in foods. Mol Nutr Food Res 53:S151–S183
Benzie IF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239:70–76
Burritt DJ, Larkindale J, Hurd CL (2002) Antioxidant metabolism in the intertidal red seaweed Stictosiphonia arbuscula following desiccation. Planta 215:829–838
Carrillo S, Castro MI, Perez-gil F, Rosales E, Manzano RE (1992) The seaweed (Sargassum sinicola Setchel & Gardner) as an alternative for animal feeding. Agric Sci 26:177–181
Chan JCC, Cheung PCK Jr, POA (1997) Comparative studies on the effect of three drying methods on the nutritional composition of seaweed Sargassum hemiphyllum (Turn.) C. Ag. J Agric Food Chem 45:3056–3059
Chatterji A, Dhargalkar VK, Sreekumar PK, Parameswaran PS, Rodrigues R, Kotnala S (2004) Anti-influenza activity in the Indian seaweeds—a preliminary investigation. National Institute of Oceanography, Goa
Dixon RA, Paiva NL (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7:1085–1097
FAO (1976) Production, trade and utilization of seaweeds and seaweed products. In FAO Fisheries Technical Paper No. 159; Food and Agriculture Organization of the United Nations: Rome 8
Garau MC, Simal S, Rosselló C, Femenia A (2007) Effect of air-drying temperature on physico-chemical properties of dietary fibre and antioxidant capacity of orange (Citrus aurantium v. canoneta) by-products. Food Chem 104:1014–1024
Giusti MM, Wrolstad RE (2001) Characterization and measurement of anthocyanins by UV-visible spectroscopy. In: Wrolstad RE (ed) Current protocols in food analytical chemistry. Wiley, NY, pp 1–13
Gupta S, Cox S, Abu-Ghannam N (2011) Effect of different drying temperatures on the moisture and phytochemical constituents of edible Irish brown seaweed. LWT Food Sci Technol 1–7
Hamdy AEA, Dawes CJ (1988) Proximate constituents and lipid chemistry in two species of Sargassum from the west coast of Florida. Bot Mar 31:79–81
Hammed AM, Asiyanbi-Hammed TT, Jaswir I, Amid A, Alam MZ (2013) Effects of drying methods on nitric oxide inhibition potential of water soluble extracts of Turbinaria turbinata: a brown seaweed species of Malaysian origin. Am J Drug Discov Dev 3:279–285
Hess D, Keller HE, Oberlin B, Bonfanti R, Schuep W (1991) Simultaneous determination of retinol, tocopherols, carotenes and lycopene in plasma by means of high performance liquid chromatography on reversed phase. Vitam Nutr Res 61:232–238
Hurtado AQ, Gerung GS, Yasir S, Critchley AT (2014) Cultivation of tropical red seaweeds in the BIMP-EAGA region. J Appl Phycol 26:707–718
Ismail A, Tan SH (2002) Antioxidant of selected commercial seaweeds. Malays J Nutr 8(2):167–177
Jiménez-Escrig A, Jiménez- Jiménez I, Pulido R, Saura-Calixto F (2001) Antioxidant activity of fresh and processed edible seaweeds. J Sci Food Agric 81:530–534
Klein BP, Kurilich AC (2000) Processing effects of dietary antioxidants from plant food. Hortscience 35:580–584
Kuda T, Tsunekawa M, Goto H, Araki Y (2005) Antioxidant properties of four edible algae harvested in the Noto Peninsula, Japan. J Food Compos Anal 18:625–633
Kumar SK, Ganesan K, Subba Rao PV (2008) Antioxidant potential of solvent extracts of Kappaphycus alvarezii (Doty) Doty—an edible seaweed. Food Chem 107:289–295
Li BB, Smith B, Hossain MM (2006) Extraction of phenolics from citrus peels: I. Solvent extraction method. Sep Purif Technol 48:182–188
Mabeau S, Fleurence J (1995) Seaweeds in food products: biochemical and nutritional aspects. Trends Food Sci Technol 6:103–107
Mabeau S, Cavaloc E, Fleurence J, Lahaye M (1992) New seaweed based ingredients for the food industry. Int Food Ingred 3:38–45
Martins CDL, Ramlov F, Nocchi Carneiro NP, Gestinari LM, dos Santos BF, Bento LM, Lhullier C, Gouvea L, Bastos E, Horta PA, Soares AR (2013) Antioxidant properties and total phenolic contents of some tropical seaweeds of the Brazilian coast. J Appl Phycol 25:1179–1187
Matanjun P, Mohamed S, Mustapha NM, Muhammad K, Ming CH (2008) Antioxidant activities and phenolics content of eight species of seaweeds from north Borneo. J Appl Phycol 20:367–373
Mensor LI, Menezes FS, Leitao GG, Reis AS, Dos Santos T, Coube CS, Leitao SG (2001) Screening of Brazillian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res 15:127–130
Mishra VKF, Temelli PF, Shacklock O, Craigie JS (1993) Lipids of the red alga Palmaria palmata. Bot Mar 36:169–174
Msuya FE, Buriyo A, Omar I, Pascal B, Narrain K, Ravina JJM, Mrabu E, Wakibia JG (2014) Cultivation and utilisation of red seaweeds in the Western Indian Ocean (WIO) Region. J Appl Phycol 26:699–705
Murphy C, Hotchkiss S, Worthington J, McKeown S (2014) The potential of seaweed as a source of drugs for use in cancer chemotherapy. J Appl Phycol 26:2211–2264
Ponmari G, Sathishkumar R, Lakshmi PTV (2011) Effect of drying treatment on the contents of antioxidants in Cardiospermum halicacabum Linn. J Pharm Biosci 2:304–313
Prud’homme van Reine WF, Toron GC Jr (2001) Plant resources of south-east Asia 15(1) Cryptogams. Algae. Backhuys Publishers, Leiden, 318
Ragan MA, Glombitza KW (1986) Phlorotannins, brown algal polyphenols. Prog Phycol Res 4:129–241
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorisation assay. Free Radic Biol Med 26:231–237
Sabeena KHF, Jacobsen C (2013) Phenolic compounds and antioxidant activities of selected species of seaweeds from Danish coast. Food Chem 138:1670–1681
Tan J, Lim P-E, Phang S-M (2013) Phylogenetic relationship of Kappaphycus Doty and Eucheuma J. Agardh (Solieriaceae, Rhodophyta) in Malaysia. J Appl Phycol 25:13–29
Tomaino A, Cimino F, Zimbalatti V, Venuti V, Sulfaro V, Pasquale A, Saija A (2004) Influence of heating on antioxidant activity and the chemical composition of some spice essential oils. Food Chem 89:549–554
Valdebenito H, Bittner M, Sammes PG, Silva M, Watson WH (1982) A compound with antimicrobial activity isolated from the red seaweed Laurencia chilensis. Phytochem 21:1456–1457
Velioglu YS, Mazza G, Gao L, Oomah BD (1998) Antioxidant activity and total phenolics in selected fruits, vegetables and grain products. J Agric Food Chem 46:4113–4117
Wong K, Cheung PC (2001) Influence of drying treatment on three Sargassum species 2. Protein extractability, in vitro protein digestibility and amino acid profile of protein concentrates. J Appl Phycol 13:51–58
Wong CK, Ooi VEC, Ang P (2000) Protective effects of seaweeds against liver injury caused by carbon tetrachloride in rats. Chemosphere 41:173–176
Yuan YV, Walsh NA (2006) Antioxidant and antiproliferative activities of extracts from a variety of edible seaweeds. Food Chem Toxicol 44:1144–1150
Yuan H, Zhang W, Li X, Li N, GaO X (2005) preparation and in vitro antioxidant activity of ĸ-carrageenan oligosaccharides and their oversulfated, acetylated and phosphorylated derivatives. Carbohydr Res 340:685–692
Zhishen J, Mengcheng T, Jianming W (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64:555–559
Acknowledgments
The authors would like to acknowledge Institute for Tropical Biology and Conservation and Seaweed Research Unit, Universiti Malaysia Sabah, Malaysia, for the use of the laboratory facilities and technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ling, A.L.M., Yasir, S., Matanjun, P. et al. Effect of different drying techniques on the phytochemical content and antioxidant activity of Kappaphycus alvarezii . J Appl Phycol 27, 1717–1723 (2015). https://doi.org/10.1007/s10811-014-0467-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-014-0467-3




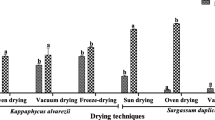

 Oven-dried (40 °C),
Oven-dried (40 °C),  oven-dried (80 °C),
oven-dried (80 °C),  sun-dried,
sun-dried,  hang-dried,
hang-dried,  sauna-dried,
sauna-dried,  shade-dried and
shade-dried and  freeze-dried seaweed samples
freeze-dried seaweed samples