Abstract
Preservation, storage, and safe transport of samples are key factors affecting brown algal phlorotannin (phenolics) quantification, their extraction yield, and antioxidant activity. This is especially relevant when field sampling is carried out in remote areas. The objective of the present study was to evaluate and compare five preservation (frozen control, freeze-dried, silica-dried, oven-dried, and air-dried) and two extraction methods (“rapid” and “traditional” extraction) of phlorotannins in the kelp Lessonia spicata (former Lessonia nigrescens). The antioxidant power of the samples treated with the different drying methods was compared through three different in vitro antioxidant assays: 2,2-diphenyl-1-picrylhydrazyl (DPPH) and oxygen radical absorbance capacity (ORAC) methods, employing fluorescein (ORAC-FL) and pyrogallol red (ORAC-PGR) as target molecules. The results of the testing methods indicated that freeze-drying afforded the best extraction yields of phlorotannins (18–30 % higher than control using frozen material) whereas concentrations from samples dried in silica and oven averaged between 17 and 20 % lower than control. The antioxidant activity of phlorotannins measured using the DPPH test decreased significantly in samples kept dried compared to that in control, with activities varying from 65 (freeze-dried) to 21 % (air-dried). Accordingly, with ORAC-PGR and ORAC-FL indexes, sample preservation employing silica-drying or air-drying caused a high decrease of the antioxidant activity of polyphenols. No differences in both total phlorotannin concentration and antioxidant activity between the two extraction methods were found.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phlorotannins are phenolic compounds, which are exclusively found in brown algae. Their synthesis represents a multifunctional strategy in algae under stress because of the ability of these compounds to absorb in the range of UV radiation, acts as herbivore deterrents, and also has a repair role in wounds (Schoenwaelder 2002). In recent years, the antioxidant properties of phlorotannins have been emphasized, especially in response to environmental stressors (Connan et al. 2007; Wang et al. 2012). However, analyzing these compounds for ecophysiological purposes still has a series of fundamental limitations, especially the preservation of samples before to the laboratory analysis. These limitations are related to the fact that phlorotannins are highly reactive substances, have a short-term induction (<2 h), and also have a high variability in their concentrations as a consequence of external and endogenous factors (Pavia and Toth 2000; Connan et al. 2004; Gómez and Huovinen 2010).
To determine total phlorotannins, the algal samples are usually “killed” by freezing them in liquid nitrogen (Pavia et al.1997; Blanc et al. 2011; Steinhoff et al. 2011). After this procedure, samples are commonly stored in a freezer at −80 °C (Toth and Pavia 2001), freeze-dried (Wang et al. 2009; Zubia et al. 2009), dried in silica gel (Pansch et al. 2008; Gómez and Huovinen 2010), or simply dried in an oven for a certain period of time (Targett et al. 1992; Van Alstyne et al. 1999). In some cases, the maintenance of samples at room temperature in low light or darkness has been also reported (Shibata et al. 2008). However, in many remote areas, the facilities required for a correct preservation of samples (e.g., ultra freezer or lyophilization equipment) are usually not available. This generates measurable variability in both the quantity and quality of the phlorotannins after extraction as well as in their antioxidant capacity. Therefore, protocols of preservation and drying of macroalgal samples need to be optimized to minimize the effects on phlorotannin concentration.
Following storage, a critical factor in extracting phlorotannins is the choice of a highly polar solvent. It has been demonstrated that the extraction efficiency decreases using solvent with moderated polarity, such as methanol or ethanol (Wang et al. 2009). Koivikko et al. (2005) and Wang et al. (2012) reported that polar solvents were more efficient reaching highest extraction values when 70 % aqueous acetone was used. Moreover, the use of extraction techniques such as sonication, which allows disruption of cells and reduced particle size, does not only improve the yield but also reduces extraction time and solvent volume (Chemat et al. 2008; Azmir et al. 2013).
Colorimetry-based methodologies such as the Folin-Ciocalteu assay have improved the quantification of phlorotannins in algae in recent years (Iken et al. 2007; Rautenberger et al. 2015). However, the “Folin-Ciocalteu index” does not always reflect the biological activity or the reactivity of a phlorotannin-rich sample. Different studies have shown that, in several species of brown algae, the concentration of phlorotannins is directly related to their antioxidant capacity (Shibata et al. 2008; Chowdhury et al. 2011; Cruces et al. 2013). For example, an increase of phlorotannins induced by UV radiation was correlated with an enhanced free radical scavenging activity (r = 0.80) (Cruces et al. 2012). Nevertheless, the putative mechanisms involved in the antioxidant activity of phlorotannins are not well understood yet.
Considering the limitations and constraints mentioned above, the goal of the present study was to compare different methods to preserve, extract, and measure the antioxidant capacity of phlorotannins from the South Pacific kelp Lessonia spicata Santelices (former Lessonia nigrescens). This species is a key structuring organism at intertidal rocky shores in the central/southern Chile (Santelices 1990; Westermeier et al. 1994) and is commercially exploited mainly as source of cell wall polysaccharides (alginates) (Vásquez 2008). Recently, studies on the ecophysiological role of phlorotannins from L. spicata have been carried out in the context of UV photoprotection and temperature (Gómez and Huovinen 2010; Cruces et al. 2012, 2013; Rautenberger et al. 2015). The suitability of the preservation methods was evaluated in five types of algal material: frozen (control), freeze-dried, dried in silica gel, dried in an oven, and dried in the air. Two methods of phlorotannin extraction were compared: the traditional extraction based on incubation for 24 h at 4 °C under shaking and a rapid extraction where extracts were sonicated for 2 h at 25 °C. Finally, three different in vitro assays, bleaching of the DPPH radical, ORAC using pyrogallol red (ORAC-PGR) and fluorescein (ORAC-FL) as target molecules, were used to test the antioxidant activity.
Materials and methods
Sampling and storing of algal material
Juvenile fronds were collected in the late austral winter (September) from the intertidal rocky shores of Calfuco (39° 51′ S, 73° 23′ W) near Valdivia, Chile, and immediately transferred to a dark and cool box to the Marine Laboratory of Calfuco. All fronds were carefully cleaned and acclimated for 24 h in tanks with circulating seawater, continuous aeration, salinity of 30 PSU, at a temperature corresponding to that of the natural surface water at the sampling site (12 ± 1 °C), and under dim light (40 μmol photons m−2 s−1, Daylight, TL Philips, Netherlands). Afterwards, the fronds were frozen in liquid nitrogen to stop all running biochemical processes, and five different preservation and storage protocols were employed (Fig. 1):
-
A.
Frozen (used as control): Fronds of L. spicata were immediately stored at −80 °C in a freezer.
-
B.
Freeze-dried: Following freezing in liquid nitrogen, fronds were lyophilized (VirTis Benchtop 4K Freeze Dryer model 4KBTXL-75, USA) for 3 days and then stored avoiding any light and humidity until extraction.
-
C.
Silica-dried: After freezing in liquid nitrogen, samples were dried for 3 days in silica gel (indicating blue, nSIO2*nH2O, VETEC), in the dark until extraction.
-
D.
Oven-dried: After samples were frozen in liquid nitrogen, they were dried in a hot air-drying oven (LABTECH, model LDO-060E) at 60 °C for 24 h. Afterwards, they were stored at room temperature in the dark.
-
E.
Air-dried: After fronds were collected and transferred to the laboratory, they were dried on absorbent paper at room temperature in the dark for 72 h. Samples were stored under identical conditions until analysis.
The loss of water for each drying treatment (B to E) was monitored using an analytical balance and expressed as percentage of weight loss.
Preparation of extracts and extraction methods
Approximately 75 mg of macroalgal tissue from the different storage methods was ground to a fine powder in liquid nitrogen using a mortar and pestle. The powder was mixed with 1.5 mL of 70 % (v/v) aqueous acetone, and phlorotannins were extracted by two different methods:
-
1.
Rapid extraction: The samples were sonicated at 50–60 Hz for 2 h at 25 °C according to the method of Rostagno et al. (2003),
-
2.
Traditional extraction: The samples were shaken (200 rpm) for 24 h at 4 °C in the dark (Pavia et al. 1997).
Because acetone reacts better with hydrolysable tannins than other solvents like water or methanol (Mueller-Harvey 2001), it is regarded as the best solvent for the extraction of high-molecular-weight polyphenols such as phlorotannins from brown algae.
Quantification of the total phlorotannins
Phlorotannins were determined using the Folin-Ciocalteu method (Koivikko et al. 2005) with adjustments of volumes to match 96-well microplates (Cruces et al. 2012). The extract was centrifuged at 4000 rpm for 10 min at 4 °C, and the supernatant was filtered (0.22 μm) to remove any algal impurities. Then, 100 μL of the filtered extract was mixed with 100 μL of ultrapure H2O (18.2 MΩ cm at 20 °C), 100 μL of 1 N Folin-Ciocalteu reagent, 200 μL of 20 % NaCO3, and 100 μL of 2 N Folin-Ciocalteu reagent. The samples were incubated for 45 min at room temperature in the dark and centrifuged at 5000 rpm for 3 min. Absorbance was measured photometrically at 730 nm (Multiskan Spectrum microplate reader, Thermo Fisher, USA). The concentration of phlorotannins in the extracts was calculated using a standard curve of phloroglucinol (Sigma-Aldrich, USA). The phlorotannin concentration was expressed as milligram per gram of dry weight (DW).
Determination of the DPPH-based antioxidant activity (bleaching of DPPH)
The antioxidant activity of the extracts was determined by the DPPH free radical scavenging method described by Brand-Williams et al. (1995) and modified by Fukumoto and Mazza (2000) for 96-well microplates. DPPH (150 μM) was prepared freshly in 80 % (v/v) aqueous methanol and mixed with 22 μL of the sample extract. The absorbance was measured at 520 nm in a microplate reader using gallic acid as a standard. Distilled water was used as a negative control. The antioxidant activity was defined as milligram of gallic acid equivalent (GAE) per gram of DW.
ORAC determinations
The ORAC test is based on the transfer reaction of a hydrogen atom in which the antioxidant and substrate compete for peroxyl radicals thermally generated through the decomposition of azo compounds (Huang et al. 2005). In particular, depending on the selected probe, it is possible to obtain an ORAC index more related to the reactivity (rate of the reaction between antioxidants with peroxyl radicals) or to the stoichiometry of the reaction (number of peroxyl radicals trapped per antioxidant molecule) (Poblete et al. 2009). Stock solutions of pyrogallol red (PGR, 41.6 μM) or fluorescein (FL, 0.583 μM) were prepared freshly in 75 mM phosphate buffer at pH 7.4. A reaction mixture containing 10 mM of 2,2′-azo-bis(2-amidinopropane) dihydrochloride (AAPH), 5 mM of PGR either with or without sample extract, was incubated in phosphate buffer (75 mM, pH 7.4) at 37 °C. PGR consumption was evaluated from the progressive decrease in the absorbance (A) measured at 540 nm. A similar procedure was carried out by using FL disodium salt (FL, 70 nM). Its consumption was measured by the decrease in the fluorescence intensity (FL, exCitation at 493 nm; emission at 515 nm) using the fluorescence mode of the Varioskan Flash Multimode Reader (Thermo Fisher, USA).
Values of (F/F0) or (A/A0) were plotted as a function of time. Integration of the area under the curve (AUC) was performed up to a time such that (F/F0) or (A/A0) reached a value of 0.2. These areas were employed to obtain ORAC values, according to Eq. (1). All experiments were carried out in triplicate.
where, AUC is the area under the curve in the presence of the tested extracts, integrated between time 0 and that corresponding to 80 % of the probe consumption, AUC 0 is the area under the curve for the control (target molecule and AAPH solution), AUC Gallic acid is the area under the curve for gallic acid, f is the dilution factor, equal to the ratio between the total volume of the AAPH-pyrogallol red or AAPH-fluorescein solution and the added extract volume, and [Gallic acid] is the gallic acid molar concentration. ORAC values were expressed as milligram of GAE per gram of DW.
Statistical analysis
Concentrations of phlorotannins and antioxidant status were compared between different preservation methods and extraction times (regarded as the main factors) using two-way analysis of variance (ANOVA), followed by Tukey’s HSD post hoc test when differences were detected. Mathematical transformations were used to meet the ANOVA requirements. ANOVA assumptions (homogeneity of variances and normal distribution) were examined using the Levene and Shapiro-Wilk W tests. To determine the correlation between DPPH and phlorotannins, Pearson’s test was performed. Statistical significance was set to p < 0.05.
Results
Concentration of total phlorotannins and extraction methods
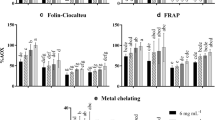
In each preservative treatment, no significant differences were observed between the two extraction methods used (factor A × B in Table 1), both in the concentration of phlorotannins as well as in their antioxidant capacity (Fig. 2, Table 1). The total phlorotannin concentration according to the Folin-Ciocalteu assay showed differences between preservation treatments (p < 0.001, Fig. 2), with values ranging between 6.9 mg g−1 DW in freeze-dried to 2.7 mg g−1 DW in air-dried samples. Additionally, the loss of water with respect to each drying treatment was 85 % in freeze-dried, 81 % to oven and silica gel, and 70 % in air-dried. The concentration of phlorotannins indicated that there was a tendency towards higher concentration associated with decrease in the percentage of moisture from the sample (Table 2).
Effect of five preservation treatments and two extraction methods on the total phlorotannin concentration in Lessonia spicata. Data are mean ± SD, n = 5. Different lower case letters indicate statistically significant differences (p < 0.05) among the different treatments after Tukey’s HSD post hoc test. The content of phlorotannins in the extracts is expressed as milligrams of phloroglucinol per gram of dry weight (DW)
Effect of the preservation procedures on the antioxidant capacity of the samples
The antioxidant activity determined by the DPPH assay showed a direct correlation with phenolic concentration (r = 0.81, p < 0.01, Table 2). There were statistical differences between the drying methods (ANOVA, p < 0.001) (Fig. 3). Samples of frozen control treatment showed the highest DPPH scavenging activity with a maximum of 34.6 mg GAE g−1 DW, a value which gradually decreased, accordingly with the drying methods, down to 7.1 mg GAE g−1 DW (air-dried samples). According to the DPPH-based antioxidant activity, the following order was observed: frozen control > freeze-dried > oven-dried > silica-dried > air-dried. The ORAC-PGR index showed the following order: frozen control > freeze-dried, oven-dried > silica-dried > air-dried, where both freeze-dried and oven-dried samples were similar (p > 0.05, Fig. 4). The frozen control value was 119.9 mg GAE g−1 DW, and the lowest value was in air-dried samples showing 10.9 mg GAE g−1 DW (p < 0001). The ORAC-FL results are shown in Fig. 5. As can be seen in this figure, the frozen control samples showed the highest ORAC-FL value (339.8 mg GAE g−1 DW) with differences between treatments in both extraction methods (p < 0.001). In employing this assay, the following order was established: frozen control > freeze-dried > oven-dried, silica-dried > air-dried.
DPPH radical scavenging activity in samples of Lessonia spicata subjected to five preservation treatments and two extraction methods. Data are mean ± SD, n = 5. Different lower case letters indicate statistically significant differences (p < 0.05) among the different treatments after Tukey’s HSD post hoc test
Discussion
This study shows that the method chosen to preserve macroalgal tissue and extract phlorotannins can have significant consequences not only for the extraction yield of phlorotannins but also for their in vitro antioxidant activity.
Sample drying and extraction method
The freeze-drying of the samples resulted in the best phlorotannins yields and antioxidant activity. Probably, this type of sample preservation minimizes the degradation of heat-sensitive molecules in a liquid state (Le Lann et al. 2008). However, the difficulty in accessing lyophilization equipment in remote sites such as Polar regions makes the other three methods (oven-dried, silica-dried, or even air-dried) more accessible (Huovinen and Gómez 2013; Rautenberger et al. 2015). Drying samples in an oven could be a reliable method for preserving phlorotannins. When macroalgal samples are incubated at temperatures ≤65 °C, the water into algal cells is quickly removed by evaporation and adverse thermal effects can be avoided (Wong and Cheung 2001; Gupta-Elera et al. 2011). The use of either silica gel or air, which is common in many laboratories and often the only option in remote regions, resulted in a significant loss of antioxidant activity of phenols. Under these conditions, during the long time required to completely dehydrate macroalgal samples, processes of oxidation or degradation of phlorotannins could be responsible for the loss in the antioxidant capacity (Mueller-Harvey 2001; Georgetti et al. 2008; Esteban et al. 2009). Therefore, it is crucial to keep the time required for dehydration to a minimum, avoiding the complete loss of the antioxidant capacity of the extracts. On the other hand, while the samples are more dehydrated, higher concentrations of phlorotannin should be expected in the extracts (Table 2). This effect seems to be associated with the fact that vesicles known as physodes, where the phlorotannins are contained in brown algal, increase their concentrations per area unit in dehydrated tissue, increasing, therefore, the concentration of phenolic compounds by dry weight material (Schoenwaelder and Clayton 1999; Shibata et al. 2004; Gómez and Huovinen 2010).
In contrast with the noticeable effect of drying on the antioxidant activity, the two extraction methods (rapid and traditional) did not result in marked differences in terms of concentration and antioxidant capacity of phlorotannins. This suggests the use of standardized protocols for ultrasonic baths in conjunction with microplates in order to reduce the volume of reagents and the extraction time (Cruces et al. 2012; Ortiz et al. 2012; Lee et al. 2013). In addition, traditional methods of phlorotannin analysis require longer incubation times and amount of reagents, increasing the economic costs and the release of chemical products to the environment (Wang et al. 2009).
Use of DPPH, ORAC-PGR, and ORAC-FL methods for evaluating antioxidant capacity
Qualitative studies on phlorotannins using purification techniques revealed that the removal of other interfering compounds in the extracts (e.g., mannitol) increases the antioxidant activity of the samples (Tierney et al. 2013). In agreement with our results, such studies support the assumption that phlorotannins are probably largely responsible for these properties in brown algae. The decreased ability to bleach DPPH observed with the use of different preservation methods could be related to the oxidation, decomposition, or loss by volatilization that phlorotannins suffer due to the heat or the long drying time during the treatments (Moure et al. 2001; Georgetti et al. 2008). In a general context, drying of samples may affect the phenolic compounds stability, either by chemical and enzymatic degradation (Larrauri et al. 1997; Moure et al. 2001).
The use of FL and PGR as target molecules in the ORAC assay is based on the presumption that the ORAC-FL index gives values more related to stoichiometric factors of the antioxidant-peroxyl radical reaction (López-Alarcón and Lissi 2006). In contrast, the ORAC-PGR methodology gives values associated with the reactivity of antioxidants towards AAPH-derived peroxyl radicals (Table 2) (López-Alarcón and Lissi 2005). Thus, changes in the antioxidant quality of the sample with respect to frozen samples would be related with the drying times and/or the disruption of the native conformation of the compounds influencing in the reactivity and stoichiometric of the acetone extracts (Franks 1998; Le Lann et al. 2008).
The ORAC and DPPH antioxidant assays, which allowed us to evaluate distinct in vitro antioxidant mechanisms of the phlorotannins (Frankel and Meyer 2000; Prior et al. 2005), showed consistent results with the different preservation methods. Comparatively, the values of antioxidant activity measured are in the range of values reported for various brown algae (Chandini et al. 2008; Ganesan et al. 2008; Wang et al. 2009). However, it is necessary to consider that, due to high molecular weights and the complex molecular skeletons that can reach some of these polymers (126 Da–650 kDa), the selection of the assays to evaluate antioxidant activity should also consider the drawbacks regarding steric molecular accessibility which could limit or underestimate the reaction capacity of phlorotannins towards free radicals (Grosse-Damhues et al. 1983; Ahn et al. 2007; Xie and Schaich 2014).
In conclusion, our results indicate that the drying methods are suitable to guarantee the extraction of phlorotannins, but in comparison with frozen samples, they decrease the antioxidant activity of the samples. Thus, dry material could not be suitable when the potential antioxidant capacity phlorotannins is examined but may perfectly serve to estimate the relative radical scavenging activity in samples of the same cohort exposed to different experimental treatments. Both, the phlorotannin contents and antioxidant capacity, were not affected by either the traditional or by the rapid extraction method, and thus, for time-saving purposes, the second option is preferable.
References
Ahn GN, Kim KN, Cha SH, Song CB, Lee J, Heo MS, Yeo IK, Lee NH, Jee YH, Kim JS, Heu MS, Jeon YJ (2007) Antioxidant activities of phlorotannins purified from Ecklonia cava on free radical scavenging using ESR and H2O2-mediated DNA damage. Eur Food Res Technol 226:71–79
Azmir J, Zaidul ISM, Rahman MM, Sharif KM, Mohamed A, Sahena F, Jahurul MHA, Ghafoor K, Norulaini NAN, Omar AKM (2013) Techniques for extraction of bioactive compounds from plant materials: a review. J Food Eng 117:426–436
Blanc N, Hauchard D, Audibert L, Gall EA (2011) Radical-scavenging capacity of phenol fractions in the brown seaweed Ascophyllum nodosum: an electrochemical approach. Talanta 84:513–518
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of free radical method to evaluate antioxidant activity. LWT-Food Sci Technol
Chandini SK, Ganesan P, Bhaskar N (2008) In vitro antioxidant activities of three selected brown seaweeds of India. Food Chem 107:707–713
Chemat F, Tomao V, Virot M (2008) Ultrasound-assisted extraction in food analysis. In: Ötleş S (ed) Handbook of food analysis instruments. CRC Press, Boca Raton, pp 85–104
Chowdhury M, Bangoura I, Kang JY, Park NG, Ahn DH, Hong YK (2011) Distribution of phlorotannins in the brown alga Ecklonia cava and comparison of pretreatments for extraction. Fish Aquat Sci 14:198–204
Connan S, Goulard F, Stiger V, Deslandes E, Gall EA (2004) Interspecific and temporal variation in phlorotannin levels in an assemblage of brown algae. Bot Mar 47:410–416
Connan S, Deslandes E, Gall EA (2007) Influence of day-night and tidal cycles on phenol content and antioxidant capacity in three temperate intertidal brown seaweeds. J Exp Mar Biol Ecol 349:359–39
Cruces E, Huovinen P, Gómez I (2012) Phlorotannin and antioxidant responses upon short-term exposure to UV radiation and elevated temperature in three South Pacific kelps. Photochem Photobiol 88:58–66
Cruces E, Huovinen P, Gómez I (2013) Interactive effects of UV radiation and enhanced temperature on photosynthesis, phlorotannin induction and antioxidant activities of two sub-Antarctic brown algae. Mar Biol 160:1–13
Esteban R, Balaguer L, Manrique E, Rubio de Casas R, Ochoa R, Fleck I, Pintó-Marijuan M, Casals I, Morales D, Jiménez MS, Lorenzo R, Artetxe U, Becerril JM, García-Plazaola JI (2009) Alternative methods for sampling and preservation of photosynthetic pigments and tocopherols in plant material from remote locations. Photosynth Res 101:77–88
Frankel EN, Meyer AS (2000) The problems of using one dimensional methods to evaluate multifunctional food and biological antioxidants. J Sci Food Agric 80:1925–1941
Franks F (1998) Freeze-drying of bioproducts: putting principles into practice. Eur J Pharm Biopharm 45:221–229
Fukumoto LR, Mazza G (2000) Assessing antioxidant and prooxidant activities of phenolic compounds. J Agric Food Chem 48:3597–3604
Ganesan P, Kumar CS, Bhaskar N (2008) Antioxidant properties of methanol extract and its solvent fractions obtained from selected Indian red seaweeds. Bioresour Technol 99:2717–2723
Georgetti SR, Casagrande R, Souza CRF, Oliveira WP, Fonseca MJV (2008) Spray drying of the soybean extract: effects on chemical properties and antioxidant activity. LWT- Food Sci Technol 41:1521–1527
Gómez I, Huovinen P (2010) Induction of phlorotannins during UV exposure mitigates inhibition of photosynthesis and DNA damage in the kelp Lessonia nigrescens. Photochem Photobiol 86:1056–1063
Grosse-Damhues J, Glombitza KW, Schulten H (1983) An eight-ring phlorotannin from the brown alga Himanthalia elongata. Phytochemistry 22:2043–2046
Gupta-Elera G, Garrett AR, Martinez A, Robison RA, O’Neill KL (2011) The antioxidant properties of the cherimoya (Annona cherimola) fruit. Food Res Int 44:2205–2209
Huang D, Ou B, Prior RL (2005) The chemistry behind antioxidant capacity assays. J Agric Food Chem 53:1841–1856
Huovinen P, Gómez I (2013) Photosynthetic characteristics and UV stress tolerance of Antarctic seaweeds along the depth gradient. Polar Biol 36:1319–1332
Iken K, Amsler CD, Hubbard JM, McClintock JB, Baker BJ (2007) Allocation patterns of phlorotannins in Antarctic brown algae. Phycologia 46:386–395
Koivikko R, Loponen J, Honkanen T, Jormalainen V (2005) Contents of soluble, cell-wall bound and exuded phlorotannins in the brown alga Fucus vesiculosus with implications on their ecological functions. J Chem Ecol 31:195–212
Larrauri JA, Rupérez P, Saura-Calixto F (1997) Effect of drying temperature on the stability of polyphenols and antioxidant activity of red grape pomace peels. J Agric Food Chem 45:1390–1393
Le Lann K, Jégou C, Stiger-Pouvreau V (2008) Effect of different conditioning treatments on total phenolic content and antioxidant activities in two Sargassacean species: comparison of the frondose Sargassum muticum (Yendo) Fensholt and the cylindrical Bifurcaria bifurcata R. Ross. Phycol Res 56:238–245
Lee LS, Lee N, Kim YH, Lee CH, Hong SP, Jeon YW, Kim YE (2013) Optimization of ultrasonic extraction of phenolic antioxidants from green tea using response surface methodology. Molecules 18:13530–13545
López-Alarcón C, Lissi E (2005) Interaction of pyrogallol red with peroxyl radicals. A basis for a simple methodology for the evaluation of antioxidant capabilities. Free Radic Res 39:729–736
López-Alarcón C, Lissi E (2006) A novel and simple ORAC methodology based on the interaction of pyrogallol red with peroxyl radicals. Free Radic Res 40:979–985
Moure A, Cruz JM, Franco D, Domı́nguez JM, Sineiro J, Domı́nguez H, Núñez MJ, Parajó JC (2001) Natural antioxidants from residual sources. Food Chem 72:145–171
Mueller-Harvey I (2001) Analysis of hydrolysable tannins. Anim Feed Sci Technol 91:3–20
Ortiz R, Antilén M, Speisky H, Aliaga ME, López-Alarcón C, Baugh S (2012) Application of a microplate-based ORAC-pyrogallol red assay for the estimation of antioxidant capacity: first action 2012.03. J AOAC Int 95:1558–1561
Pansch C, Gómez I, Rothäusler E, Veliz K, Thiel M (2008) Defense of vegetative vs. reproductive blades of the Pacific kelps Lessonia nigrescens and Macrocystis integrifolia: a test on species-specific defense strategies. Mar Biol 155:51–62
Pavia H, Cervin G, Lindgren A, Åberg P (1997) Effects of UV-B radiation and simulated herbivory on phlorotannins in the brown alga Ascophyllum nodosum. Mar Ecol Prog Ser 157:139–146
Pavia H, Toth GB (2000) Influence of light and nitrogen on the phlorotannin content of the brown seaweeds Ascophyllum nodosum and Fucus vesiculosus. Hydrobiologia 440:299–305
Poblete A, López-Alarcón C, Lissi E, Campos AM (2009) Oxygen radical antioxidant capacity (ORAC) values of herbal teas obtained employing different methodologies can provide complementary data. J Chil Chem Soc 54:154–157
Prior RL, Wu X, Schaich K (2005) Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem 53:4290–4302
Rautenberger R, Huovinen P, Gómez I (2015) Effects of increased seawater temperature on UV tolerance of Antarctic marine macroalgae. Mar Biol 162:1087–1097
Rostagno MA, Palma M, Barroso CG (2003) Ultrasound-assisted extraction of soy isoflavones. J Chromatogr 1012:119–128
Santelices B (1990) Patterns of organizations of intertidal and shallow subtidal vegetation in wave exposed habitats of central Chile. Hydrobiologia 192:35–57
Schoenwaelder MEA (2002) The occurrence and cellular significance of physodes in brown algae. Phycological reviews 21. Phycologia 41:125–139
Schoenwaelder M, Clayton M (1999) The role of the cytoskeleton in brown algal physode movement. Eur J Phycol 34:223–229
Shibata T, Kawaguchi S, Hama Y (2004) Local and chemical distribution of phlorotannins in brown algae. J Appl Phycol 16:291–296
Shibata T, Kawaguchi S, Yoshikawa H, Hama Y (2008) Antioxidant activities of phlorotannins isolated from Japanese Laminariaceae. J Appl Phycol 20:705–711
Steinhoff FS, Wiencke C, Wuttke S, Bischof K (2011) Effects of water temperatures, UV radiation and low vs high PAR on phlorotannin content and germination in zoospores of Saccorhiza dermatodea (Tilopteridales, Phaeophyceae). Phycologia 50:256–263
Targett NM, Coen LD, Boettcher AA (1992) Biogeographic comparisons of marine algal polyphenolics—evidence against a latitudinal trend. Oecologia 89:464–470
Tierney MS, Smyth TJ, Rai DK, Soler-Vila A, Croft AK, Brunton N (2013) Enrichment of polyphenol contents and antioxidant activities of Irish brown macroalgae using food-friendly techniques based on polarity and molecular size. Food Chem 139:753–761
Toth GB, Pavia H (2001) Removal of dissolved brown algal phlorotannins using insoluble polyvinylpolypyrrolidone (PVPP). J Chem Ecol 27:1899–1910
Van Alstyne KL, McCarthy JJ, Hustead CL, Kearns LJ (1999) Phlorotannin allocation among tissues of northeastern pacific kelps and rockweeds. J Phycol 35:483–492
Vásquez JA (2008) Production, use and fate of Chilean brown seaweeds: resources for a sustainable fishery. J Appl Phycol 20:457–467
Wang T, Jónsdóttir R, Ólafsdóttir G (2009) Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem 116:240–248
Wang T, Jonsdottir R, Liu H, Gu L, Kristinsson HG, Raghavan S, Olafsdóttir G (2012) Antioxidant capacities of phlorotannins extracted from the brown algae Fucus vesiculosus. J Agric Food Chem 60:5874–5883
Westermeier R, Muller DG, Gomez I, Rivera P, Wenzel H (1994) Population biology of Durvillaea antarctica and Lessonia nigrescens (Phaeophyta) on the rocky shores of southern Chile. Mar Ecol Prog Ser 110:187–194
Wong K, Cheung PC (2001) Influence of drying treatment on three Sargassum species 2. Protein extractability, in vitro protein digestibility and amino acid profile of protein concentrates. J Appl Phycol 13:51–58
Xie J, Schaich KM (2014) Re-evaluation of the 2,2-Diphenyl-1-picrylhydrazyl free radical (DPPH) assay for antioxidant activity. J Agric Food Chem 62:4251–4260
Zubia M, Fabre MS, Kerjean V, Le Lann K, Stiger-Pouvreau V, Fauchon M, Deslandes E (2009) Antioxidant and antitumoural activities of some Phaeophyta from Brittany coasts. Food Chem 116:693–701
Acknowledgments
This study was supported by the FONDECYT grants no. 3130516 to E.C. and no 1130794 to I.G. We are grateful to R. Rautenberger for valuable comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cruces, E., Rojas-Lillo, Y., Ramirez-Kushel, E. et al. Comparison of different techniques for the preservation and extraction of phlorotannins in the kelp Lessonia spicata (Phaeophyceae): assays of DPPH, ORAC-PGR, and ORAC-FL as testing methods. J Appl Phycol 28, 573–580 (2016). https://doi.org/10.1007/s10811-015-0602-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-015-0602-9