Abstract
The University of São Paulo Gracilariaceae Germplasm Bank has 50 strains collected mostly in Brazil, but also elsewhere in the world. This bank has been used as a source of material for research developed locally and abroad. With over 200 species, some of which have high economic value, the family Gracilariaceae has been extensively studied. Nonetheless, taxonomic problems still persist by the existence of cryptic species, phenotypic plasticity, and broad geographic distribution. In the case of algae kept in culture for long periods of time, the identification is even more problematic as a consequence of considerable morphological modification. Thus, the use of molecular markers has been shown to be an efficient tool to elucidate taxonomic issues in the group. In this work, we sequenced the 5′-end of the cox1 gene for 41 strains and the universal plastid amplicon (UPA) plastid region for 45 strains, covering all 50 strains in the bank. In addition, the rbcL for representatives of the cox1/UPA clusters was sequenced for 14 strains. The original species identification based on morphology was compared with the molecular data obtained in this work, resulting in the identification of 13 different species. Our analyses indicate that cox1 and UPA are suitable markers for the delineation of species of Gracilariales in the germplasm bank. The addition of DNA barcode tags to the samples in the Gracilariaceae germplasm bank and the molecular identification of the species will make this bank even more useful for future research as the species can be easily traced and confirmed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The family Gracilariaceae is widely distributed on tropical and temperate marine coasts of the world. The main genera in the family are Gracilaria Greville with around 167 species and Gracilariopsis E.Y. Dawson with 20 species (Algaebase, Guiry and Guiry 2010). These seaweeds have considerable economic importance as the main global source of agar (Oliveira et al. 2000), which is largely used by the pharmaceutical and food industries (Oliveira and Plastino 1994). The quantity and quality of agar vary among species of Gracilariales; therefore, a precise identification may be very important (Macchiavello et al. 1999; Skriptsova and Nabivailo 2009).
In spite of the great effort applied to understand the biology of the group, precise taxonomic identification is limited by phenotypic plasticity, the occurrence of cryptic species, and the absence of male and cystocarpic reproductive structures (e.g., Fredericq and Hommersand 1989; Gurgel et al. 2003, 2004; Oliveira 1984). Many alternative approaches to conventional morphological analysis have been attempted (cf. Oliveira and Plastino 1994; Plastino and Oliveira 1996), including, for example, hybridization (Plastino and Oliveira 1988, 1990). To accomplish that, E.C. Oliveira and E.M. Plastino established the Germplasm Bank of the Laboratory of Marine Algae of the University of São Paulo (LAM-USP) for crossing experiments (Plastino et al. 1995; Plastino and Oliveira 1988, 1990, 1996, 1997, 2000). The germplasm bank currently has 50 strains of Gracilariaceae in culture, some of which have been kept in vitro for more than 30 years. These strains were mostly collected in Brazil, but also elsewhere in the world (Lourenço and Vieira 2004).
This bank has been used as a source of material for several investigations, contributing to the knowledge of different aspects of this economically important group of algae. The life history of some species has been completed in vitro: G racilaria birdiae (Costa and Plastino 2001); Gracilaria caudata and Gracilaria cornea (Oliveira and Plastino 1984); Gracilaria chilensis (Plastino and Oliveira 1984); Gracilaria domingensis (Guimarães et al. 1999); and Gracilaria tenuistipitata (Barufi et al. 2010). Physiological aspects related to growth rates (Ferreira et al. 2006; Plastino et al. 1998, 2004; Ursi et al. 2008; Ursi and Plastino 2001; Yokoya and Oliveira 1992a, b, 1993), pigment characterization (Barufi et al. 2010; Costa and Plastino 2011; Guimarães et al. 2003; Plastino et al. 2004), photosynthetic and respiratory characterization (Ursi et al. 2003), enzymatic activity (Chow et al. 2004, 2007; Chow and Oliveira 2008; Collén et al. 2003; Lopes et al. 1997, 2002; Rossa et al. 2002), and polysaccharide content (Guimarães et al. 2007) have been studied using strains from the germplasm bank. Furthermore, some strains have been used in color inheritance studies (Costa and Plastino 2001, 2011; Guimarães et al. 2003; Plastino et al. 1999, 2004), ultrastructure characterization (Bouzon et al. 2000, 2011; Guimarães and Plastino 1999; Plastino and Costa 1999, 2001), axenic tissue cultures (Ramlov et al. 2009; Yokoya 2000), phylogeny and systematic studies (Bellorin et al. 2002; Bird and Oliveira 1986), and gene sequencing and expression studies (Falcão et al. 2008, 2010; Hagopian et al. 2002, 2004; Nyvall et al. 2011).
However, once in culture, strains of Gracilaria and Gracilariopsis may change their morphology, usually remaining infertile, thus making species identification very difficult, if not impossible. Therefore, it is necessary to implement a more direct approach, based on the use of molecular markers, for the identification and tracking of species in the bank.
Different molecular markers have been used for Gracilariales, such as the nuclear gene coding for the small subunit of ribosomal RNA, the internal transcribed spacers of ribosomal genes, and the rbcL gene coding for RUBICO large subunit (Bellorin et al. 2002, 2008; Bhattacharya et al. 1990; Goff et al. 1994; Gurgel et al. 1999). These markers proved to be suitable for species identification and phylogenetic analysis within the group, but they are of relatively large size, requiring some effort for amplification and sequencing with the need for several internal primers, which results in additional cost in both time and resources.
On the other hand, the technique of DNA barcoding is a fast, practical, and uniform system based on polymerase chain reaction (PCR) amplification of relatively short (∼400–700 bp) DNA fragments that can be fully sequenced with the same two primers used in PCR (Savolainen et al. 2005). Hebert et al. (2003) proposed the use of the 5′-end of the mitochondrial gene cox1 coding for cytochrome oxidase 1 to facilitate the rapid identification of specimens and as a powerful ally in understanding biodiversity. Given the difficulties that exist in species identification in several red algae, Saunders (2005) proposed and developed primers for the use of cox1 for DNA barcoding in this group of organisms. Another region that has been proposed by Sherwood and Presting (2007) as a DNA barcode for photosynthetic organisms is the universal plastid amplicon (UPA), which is part of the chloroplast gene coding for the large ribosomal RNA (23SRNAr).
In this work, we first sequenced the 5′-end of cox1 and the UPA region of Gracilariaceae kept in culture in the LAM-USP Germplasm Bank. These sequences were compared and grouped. Moreover, chloroplast DNA sequences for rbcL were obtained for each of the different groups. In this way, the original species identification based on morphology was compared with the molecular data obtained in this work, leading to the identification of 13 different species in the bank.
Materials and methods
Samples were collected from several locations (Table 1) and transported to the laboratory. The unialgal cultures were established from apical segments or spores. As soon as the algae were brought to the lab, a careful process for the removal of contaminants using brushes under stereoscopic microscope was performed. Successive cleanups were performed at 2–4 days, with the algae kept in sterile seawater without nutrients (Plastino and Oliveira 1990). Once isolated, cultures were maintained in modified von Stosch (Ursi and Plastino 2001) enriched seawater, diluted to 50 % with sterile seawater (32 psu). The cultures were kept at 25 ± 1°C under 30 μmol photons m−2 s−1 photosynthetically active radiation (PAR) provided by 40-W daylight fluorescent tubes on a 14-h light/10-h dark cycle. The medium was renewed monthly.
Before DNA extraction, the apical fragments of each sample were transferred to Erlenmeyer flasks with 50 mL of enriched seawater for 2 weeks. Cultures were maintained under 150 μmol photons m−2 s−1 PAR and aerated for 30 min h−1. The medium was renewed weekly. After this period, the algae were rapidly rinsed in fresh water, blotted dry, frozen in liquid nitrogen, and stored at −70°C.
DNA extraction, PCR amplification, and sequencing
DNA was extracted from approximately 30 mg of frozen samples by grinding in liquid nitrogen and using the method described by Bellorin et al. (2002). The mitochondrial cox1 was amplified and sequenced using the synthetic primers GazF1 and GazR1 described by Saunders (2005). The plastidial UPA was amplified and sequenced using the primers p23Sv_f1 and p23Sv_r1 described by Sherwood and Presting (2007). The plastidial rbcL was amplified with the primers FrbcL and RbcS and sequenced with the addition of internal primers described by Freshwater et al. (1994). PCR amplification, purification, and sequencing are described in Milstein et al. (2011).
Molecular analyses
The consensus Gracilaria and Gracilariopsis cox1, UPA, and rbcL sequences were each aligned using ClustalW within BioEdit (Hall 1999) together with sequences of the same markers available from the GenBank. For rbcL, the sequences of Curdiea racovitzia Hariot and Melanthalia abscissa (Turner) J.D. Hooker and Harvey were used as outgroups. The following matrices were assembled: 57 sequences (41 sequences generated in this work and 16 obtained from databanks) and 664 positions for cox1; 56 sequences (45 sequences generated in this work and 11 obtained from databanks) and 370 positions for UPA; and 78 sequences (14 sequences generated in this work and 64 obtained from databanks) and 1,393 positions for rbcL. For all three markers, positions corresponding to amplification primers were excluded.
For cox1 and UPA only, a neighbor-joining (NJ) analysis using PAUP* 4.0b10 (Swofford 2002) with 2,000 replicates of bootstrap was performed to visualize the species groups. For rbcL, an evolution model was selected using MrModeltest 2.2 (Posada and Crandall 1998), and phylogenetic analyses were inferred by: (1) NJ with 2,000 replicates of bootstrap; (2) maximum parsimony heuristic search, using starting trees obtained by stepwise addition, with random sequence addition (ten replicates) using tree bisection–reconnection branch-swapping algorithm and with 2,000 replicates of bootstrap, using the PAUP* 4.0b10 (Swofford 2002); and (3) Bayesian analysis with two runs of four chains and with 4,000,000 generations sampled every 100 (initial 100,000 generations were discarded as burn in) using MrBayes (v3.1.2) (Huelsenbeck and Roanquist 2001).
Results
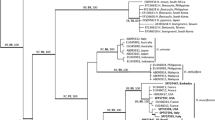
The germplasm bank has a total of 50 samples of Gracilariaceae; out of which 33 originated from Brazil and 17 from abroad (Table 1). Sequences for the 5′-end of cox1 were obtained for 41 samples and presented 16 unique sequences that grouped into nine clusters representing different species (Fig. 1). Sequences for the UPA plastid region were obtained for 45 samples and presented 13 unique sequences that grouped into clusters representing different species (Fig. 2).
Neighbor-joining phylogram for the cox1 region showing the grouping of the Gracilariaceae sequenced in this study (in bold) and available from databanks (GenBank and BOLD). Strain numbers are in brackets (see Table 1 for information on each strain). Bootstrap support values for 2,000 replicates are indicated on branches
Neighbor-joining phylogram for the UPA region showing the grouping of the Gracilariaceae sequenced in this study (in bold) and available from the Hawaiian Algal Database. Strain numbers are in brackets (see Table 1 for information on each strain). Bootstrap support values for 2,000 replicates are indicated on branches
In our experience, the UPA region was easier to amplify and sequence compared to cox1 based on the number of PCR and sequencing reactions needed to obtain each consensus sequence. For 45 UPA sequences, 47 PCR and 106 sequencing reactions were performed, whereas to obtain 41 cox1 sequences, 76 PCR and 284 sequencing reactions were performed. As a consequence, around 2.5-fold more reagents were needed to obtain cox1 sequences than to obtain a similar number of UPA sequences.
The cox1 and UPA sequences obtained for the germplasm bank samples, plus the few available sequences in the GenBank, were clustered using NJ (Figs. 1 and 2). In both analyses, the genera Gracilaria and Gracilariopsis were segregated. For the cox1 analysis, four groups were formed for Gracilariopsis: one containing all the Gp. tenuifrons from several locations of Brazil and Venezuela with an intraspecific variation of 0–0.3 %; one for Gp. longissima from the eastern North Atlantic; one for Gp. lemaneiformis from Ecuador; and one for Gp. andersonii from Canada (Fig. 1). For the UPA, the same clusters were formed, with the exception of Gp. andersonii, which was not included in the analyses by the lack of UPA sequences, and with the addition of the only UPA sequence of Gp. mclachlanii (Fig. 2).
Analyses of cox1 and UPA sequences of Gracilaria sequences from the germplasm bank grouped in clusters or isolated branches represent six and nine lineages, respectively (Figs. 1 and 2). G. caudata samples from northeastern Brazil (CE/BR) formed a sister cluster to a sample from southeastern Brazil (SP/BR), albeit at a distance of some 3,000 km from each other, with a cox1 intraspecific divergence of 6 bp (0.8 %) between them (Fig. 1). For UPA, a G. caudata cluster was also formed, but without intraspecific variation (Fig. 2). Samples of Gracilaria cornea cultivated in Israel (originally collected in the Caribbean), Brazil, and Mexico formed a cluster in both the cox1 and UPA analyses with a cox1 intraspecific variation of 0–5 bp (0–0.8 %). For G. birdiae, only a UPA sequence was obtained, showing the close relationship of this species to G. cornea (only 0.3 % divergence). Samples of Gracilaria gracilis from Argentina, Portugal, Namibia, Brazil, and Norway grouped in both the cox1analysis, with an intraspecific variation of 0–2 bp (0–0.5 %), and the UPA analysis without intraspecific variation. Samples of G. isabellana from Brazil grouped both in the cox1 and UPA analyses without intraspecific variation. The other samples, G. domingensis, G. tenuistipitata, G. sp. BG0057, and G. rangiferina, did not group with significant support to other species in either the cox1 or UPA analyses.
The rbcL was sequenced for 14 samples selected from one or more representatives of each of the cox1 and/or UPA clusters (Table 1): G. caudata, G. cornea, G. domingensis, G. gracilis, Gp. isabellana, G. rangiferina, Gp. tenuifrons, Gp. lemaneiformis, Gp. longissima, and Gp. mclachlanii. The rbcL for G. tenuistipitata was previously sequenced for the same strain used in this work and was available from GenBank. The phylogenetic analyses for these rbcL sequences with others from the Genbank are shown in Fig. 3. By using two other Gracilariaceae genera, Curdiea and Melanthalia, as outgroups, the species of the genus Gracilariopsis formed a monophyletic assemblage highly supported in all analyses, but Gracilaria was monophyletic only in the Bayesian analysis (0.89 a posteriori probability). The sequences obtained from the samples in the germplasm bank clustered with other available sequences from the same species obtained in the GenBank. The germplasm bank strains of Gp. tenuifrons from Brazil, Mexico, and Venezuela grouped with a Gp. tenuifrons from Guadaloupe in the Caribbean. The sample of Gp. longissima from England grouped with another sample from the same species from Italy. The sample of Gp. lemaneiformis from Ecuador grouped with another one from Peru. The sample of Gp. mclachanii grouped with a previously sequenced sample from Tanzania.
Consensus tree derived from Bayesian analyses of rbcL sequences obtained in this study (in bold) and available from Genbank (accession numbers in brackets). Thickness of the branches indicates Bayesian a posteriori probabilities. Bootstrap supports (2,000) replicates which are shown on the branches as follows: maximum parsimony/neighbor-joining
The Gracilaria species formed different clades with varying support. A basal clade was formed only in the Bayesian analyses joining G. vermiculophylla, G. chilensis, and G. tenuistipitata. The following groupings were observed: (1) Strains of G. caudata from Brazil grouped with G. caudata from FL, USA; (2) G. cornea from Brazil grouped with one strain from Venezuela; (3) G. domingensis from Brazil formed a clade; (4) G. isabellana from Brazil grouped with a sample from Venezuela (as G. lacinulata in GenBank); and (5) G. tenuistipitata from China grouped with a sample attributed to the same species from India.
Discussion
The LAM-USP Germplasm Bank has been a very useful resource for several studies on Gracilariales. Successful unialgal isolation is a key step in setting up cultures. Once isolated, samples of Gracilaria and Gracilariopsis can be maintained in vitro for a long time at relatively low cost and with little labor, as the medium only needs to be replaced once a month by the requirement of low irradiance. For experimental purposes, apices are progressively cultivated in higher irradiance and nutrients. Depending on the treatment, the species can be successfully propagated in 1 month.
Only rarely is it possible to identify Gracilaria species without the presence of cystocarps and male reproductive structures. Identification based on gross morphology and vegetative anatomy is generally subjective and cumbersome because of high morphological plasticity, which explains the frequent misidentifications and extensive synonymy in this group. Most species have no economic value, but for the few that do, this confusion in nomenclature has practical consequences (Bellorin et al. 2002, 2004; Saunders 2009).
Although Gracilariopsis has a much smaller biodiversity, species identification is even more difficult in the genus. With the sole exception of Gracilariopsis silvana Gurgel, which is flattened, all Gracilariopsis taxa are terete and stringy, looking very much alike. Therefore, in this genus, species are separated mostly based on geographical distribution, rather than on morphology and anatomy. This seemed adequate until some papers showed that some species of Gracilariales have a broad distribution and may be invasive (Bellorin et al. 2004; Saunders 2009).
Consequently, in addition to its inherent academic value, the use of short molecular tags for species identification is also demanded by industry, as a matter of economic exigency. Furthermore, molecular tags can be quite useful for field studies and to pinpoint the occurrence of invasive species. For instance, Saunders (2009) identified the invasive species G. vermiculophylla in Canadian waters using the 5′ region of cox1 in routine DNA barcoding of Gracilariales.
Based on the work of several investigators, the data obtained so far indicate that a significant amount of intraspecific variation (∼1 %) for cox1 may occur in some in Gracilaria species. For example, intraspecific variation for cox1 found for the Gracilaria and Gracilariopsis species in this work was, in some cases, higher (up to 0.8 %) than that found by Saunders (2005)(1 or 2 bp ∼0.2 %) for several genera of Rhodophyta. Yang et al. (2007) used cox1 (1,245 bp) to evaluate intraspecific variation in G. vermiculophylla from Asia and found a pairwise divergence up to 11 bp (0.9 %). Similar to the results of Yang et al. (2007), G. caudata from the southeast coast diverged by 0.8 % from strains of the same species from the northeastern coast of Brazil, and G. cornea cultivated in Israel (but originally collected in the Caribbean region, Alvaro Israel personal com.) diverged by 0.8 % from strains of the same species from Brazil.
The interspecific variation for cox1 found for Gracilariopsis (5.8–6.5 %; 38–43 bp) was similar to the one found by Saunders (2005), but for Gracilaria (9.1–13.7 %; 60–91 bp), the values were higher. For this region, Saunders (2005) found that interspecific variation in several genera of Rhodophyta was around 30–40 bp, with some exceptions. For cox1, Yang et al. (2007) found that the interspecific nucleotide difference was also high among different species of Gracilariales (>41 bp, 3.2–16.1 % of 1,245 bp). Thus, the use of cox1 seems to be adequate for DNA barcoding of species in the Gracilariales, as previously demonstrated in various red algae (Robba et al. 2006; Saunders 2005, 2009).
As expected, the UPA sequences were more conserved and showed less interspecific and no intraspecific variation, and as in cox1 (Sherwood et al. 2010), interspecific variation for UPA was relatively higher for Gracilaria species (2.2–5.2 %; 8–19 bp) than that observed for Gracilariopsis (0.8–3.7 %; 3–14 bp). Nonetheless, the interspecific variation found for UPA was enough to separate the species in the germplasm bank. Considering that UPA is easier to amplify and sequence than cox1, UPA is a reliable molecular marker that can be used as a routine tag for the addition and tracking of strains in culture collections.
Relatively few sequences of cox1 and UPA for Gracilariales species are to be found in the databanks. Therefore, to help in species identification, rbcL was sequenced for one or more representatives of each of the cox1 and/or UPA clusters, as there are rbcL sequences for several species of Gracilariales available in the GenBank. The use of rbcL confirmed the previous identification of most samples or helped in the identification of those that were not given a species name when first included in the germplasm bank (Table 1).
In a few cases, the molecular marker results did not corroborate the original morphological identification. For example, based on the molecular markers, BG0007, originally identified as G. cervicornis, and BG0005, originally identified as G. mammillaris, both correspond to G. domingensis, while BG0050, originally identified as G. caudata (collected in Mexico), was identified as Gp. tenuifrons based on molecular markers. These discrepancies indicate a possible mislabeling during the manipulation of the isolates along the 20 years of media and vial changes. This is further supported by the fact that G. domingensis is not found on the coast of São Paulo State (both BG0007 and BG0005 were originally collected from the São Paulo coast). Gp. tenuifrons has not been cited to Mexico, thus reinforcing the idea that some mistake was made with the labeling of specimens in the laboratory. Besides, the original algae have a verrucosa-type spermatangia distribution, which is different from Gp. tenuifrons that presents a chorda-type. These results highlight the importance of routinely using molecular markers to identify species kept in the germplasm bank.
Conclusions
Implementing the use of molecular markers for strains contained in the germplasm bank allowed us to define the existence of 13 different species in the bank. Unpublished sequences for cox1 and UPA were generated for 7 and 12 species of Gracilariales, respectively. Both cox1 and UPA were suitable DNA barcode markers to help track species of Gracilariaceae kept in culture in the germplasm bank, although UPA demanded considerably less effort and material for amplification. On the other hand, cox1 presents, in some cases, a low level of intraspecific variation and could be used to track individual strains of different populations of the same species, which can be also useful for the purpose of germplasm bank management. The addition of the DNA barcode tag to the samples in the Gracilariaceae germplasm bank and the molecular identification of the species will make this bank even more useful for future research as the species can be easily traced and confirmed.
References
Barufi JB, Oliveira EC, Plastino EM, Oliveira MC (2010) Life history, morphological variability and growth rates of the life phases of Gracilaria tenuistipitata (Rhodophyta: Gracilariales) in vitro. Sci Mar 74:297–303
Bellorin AM, Oliveira MC, Oliveira EC (2002) Phylogeny and systematics of the marine algal family Gracilariaceae (Gracilariales, Rhodophyta) based on SSU DNAr and ITS sequences of Atlantic and Pacific species. J Phycol 38:551–563
Bellorin AM, Oliveira MC, Oliveira EC (2004) Gracilaria vermiculophylla: a western Pacific species of Gracilariaceae (Rhodophyta) first recorded from the eastern Pacific. Phycol Res 52:69–79
Bellorin AM, Buriyo A, Sohrabipour J, Oliveira MC, Oliveira EC (2008) Gracilariopsis mclachlanii sp. Nov. and Gracilariopsis persica sp. Nov. of the Gracilariaceae (Gracilariales, Rhodophyceae) from the Indian Ocean. J Phycol 44:1022–1032
Bhattacharya D, Elwood HJ, Goff LJ, Sogin ML (1990) Phylogeny of Gracilaria lemaneiformis (Rhodophyta) based on sequence analysis of its small ribosomal RNA coding region. J Phycol 26:181–186
Bird CJ, Oliveira EC (1986) Gracilaria tenuifrons sp. Nov. (Gigartinales, Rhodophyta) a specie from the tropical western Atlantic with superficial spermatangia. Phycologia 25:313–320
Bouzon ZL, Miguens F, Oliveira EC (2000) Male gametogenesis in the red algae Gracilaria and Gracilariopsis (Rhodophyta, Gracilariales). Cryptogam Algol 21:33–47
Bouzon ZL, Schmidt EC, Almeida AC, Yokoya NS, Oliveira MC, Chow FF (2011) Cytochemical characterization and ultrastructural organization in calluses of the agarophyte Gracilariopsis tenuifrons (Gracilariales, Rhodophyta). Micron 42:80–86
Chow FF, Oliveira MC (2008) Rapid and slow modulation of nitrate reductase activity in the red macroalga Gracilaria chilensis (Gracilariales, Rhodophyta): influence of different nitrogen sources. J Appl Phycol 20:325–332
Chow FF, Oliveira MC, Pedérsen M (2004) In vitro assay and light regulation of nitrate reductase in the red alga Gracilaria chilensis. J Plant Physiol 161:769–776
Chow FF, Capociama V, Faria R, Oliveira MC (2007) Characterization of nitrate reductase activity in vitro in the red seaweed Gracilaria caudata J. Agardh (Rhodophyta, Gracilariales). Rev Bras Bot 30:123–129
Collén J, Pinto E, Pedersén M, Colepicolo P (2003) Induction of oxidative stress in the red macroalga Gracilaria tenuistipitata by pollutant metals. Arch Environ Contam Toxicol 45:337–342
Costa VL, Plastino EM (2001) Histórico de vida de espécimes selvagens e variantes cromáticas de Gracilaria sp. (Gracilariales, Rhodophyta) in laboratory. Rev Bras Bot 24(suppl):491–500
Costa VL, Plastino EM (2011) Color inheritance and pigment characterization of red (wild-type), greenish-brown, and green strains of Gracilaria birdiae (Gracilariales, Rhodophyta). J Appl Phycol 23:599–605
Falcão VDR, Tonon AP, Oliveira MC, Colepicolo P (2008) RNA Isolation method for polysaccharide rich algae: agar producing Gracilaria tenuistipitata (Rhodophyta). J Appl Phycol 20:9–12
Falcão VDR, Oliveira MC, Colepicolo P (2010) Molecular characterization of nitrate reductase gene and its expression in the marine red alga Gracilaria tenuistipitata (Rhodophyta). J Appl Phycol 22:613–622
Ferreira LB, Barufi JB, Plastino EM (2006) Growth of red and green strains of the agarophyte tropical Gracilaria cornea (Gracilariales, Rhodophyta) in laboratory. Rev Bras Bot 29:185–190
Fredericq S, Hommersand MH (1989) Proposal of the Gracilariales ord. nov. (Rhodophyta) based on an analysis of the reproductive development of Gracilaria verrucosa. J Phycol 25:213–227
Freshwater DW, Fredericq S, Butler BS, Hommersand MH, Chase MW (1994) A gene phylogeny of the red algae (Rhodophyta) based on plastid rbcL. Proc Natl Acad Sci USA 91:7281–7285
Goff LJ, Moon DA, Coleman AW (1994) Molecular delineation of species and species relationship in the red algal agarophytes Gracilariopsis and Gracilaria (Gracilariales). J Phycol 30:521–537
Guimarães M, Plastino EM (1999) Plastid organization of color variants of the red macroalga Gracilaria domingensis (Gracilariales). Acta Microsc 8(Sup. C):795–796
Guimarães M, Plastino EM, Oliveira EC (1999) Life-history, reproduction, and growth of Gracilaria domingensis (Gracilariales, Rhodophyta) from Brazil. Bot Mar 42:481–486
Guimarães M, Plastino EM, Destombe C (2003) Green mutant frequency in natural populations of Gracilaria domingensis (Gracilariales, Rhodophyta) from Brazil. Eur J Phycol 38:165–169
Guimarães M, Viana AG, Duarte MER, Ascêncio SD, Plastino EM, Noseda MD (2007) Low-molecular-mass carbohydrates and soluble polysaccharides of green and red morphs of Gracilaria domingensis (Gracilariales, Rhodophyta). Bot Mar 50:314–317
Guiry MD, Guiry GM (2010) Algaebase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org. Accessed 24 Aug 2011
Gurgel CFD, Fredericq S, Norris JN (1999) Characterization and biogeographic affinities of the red algal genus, Gracilaria (Gracilariales), in the Gulf of México. J Phycol 35(suppl):13
Gurgel CFD, Liao LM, Fredericq S, Hommersand MH (2003) Systematyics of Gracilariopsis (Gracilariales, Rhodophyta) based on rbcL sequence analyses and mophological evidence. J Phycol 39:154–171
Gurgel CFD, Fredericq S, Norris JN (2004) Gracilaria apiculata and G. flabelliformis (Gracilariaceae, Rhodophyta): restoring old names for common tropical western Atlantic species, including the recognition of three new subspecies, and a replacement name for “G. lacinulata”. Cryptogam Algol 25:367–396
Hagopian JC, Nyvall P, Oliveira MC (2002) Purification of plastid DNA from an enriched rhodoplast fraction of the red alga Gracilaria tenuistipitata. Plant Mol Biol Rep 20:406
Hagopian JC, Reis M, Kitajima JP, Bhattacharya D, Oliveira MC (2004) Comparative analysis of the complete plastid genome sequence of the red alga Gracilaria tenuistipitata var. liui provides insights on the evolution of rhodoplasts and their relationship to other plastids. J Mol Evol 59:464–477
Hall TA (1999) BioEdit: a user-friendly biological alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hebert PDN, Cywinska A, Ball SL, de Waard JR (2003) Biological identifications through DNA barcodes. Proc R Soc Lond B 270:313–321
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogeny. Bioinformatics 17:754–755
Lopes PF, Oliveira MC, Colepicolo P (1997) Diurnal fluctuation of nitrate reductase activity in the marine red alga Gracilaria tenuistipitata (Rhodophyta). J Phycol 33:225–231
Lopes PF, Oliveira MC, Colepicolo P (2002) Characterization and daily variation of nitrate reductase in Gracilaria tenuistipitata (Rhodophyta). Biochem Biophys Res Commun 295:50–54
Lourenço SO, Vieira AAH (2004) Culture collections of microalgae in Brazil: progress and constraints. Nova Hedwigia 79:149–173
Machiavello J, Saito R, Garófalo G, Oliveira EC (1999) A comparative analysis of agarans from commercial species of Gracilaria (Gracilariales, Rhodophyta) grown in vitro. Hydrobiologia 399:105–108
Milstein D, Medeiros AS, Oliveira EC, Oliveira MC (2011) Will a DNA barcoding approach be useful to identify Porphyra species (Bangiales, Rhodophyta)? A case study with Brazilian taxa. J Appl Phycol. doi:10.1007/s10811-011-9702-3
Nyvall P, Collén J, Reis MS, Pedersén M, Setubal JC, Varani AM, Colepicolo P, Oliveira MC (2011) Analysis of expressed sequence tags from the agarophyte Gracilaria tenuistipitata (Rhodophyta). J Appl Phycol. doi:10.1007/s10811-011-9681-4
Oliveira EC (1984) Taxonomic criteria in the genus Gracilaria Greville (Rhodophyta): an experience with the Western Atlantic species. Hydrobiologia 116/117:55–58
Oliveira EC, Plastino EM (1984) The life-history of Gracilaria (Rhodophyta) from Brazil. J Phycol 32:203–208
Oliveira EC, Plastino EM (1994) Gracilariaceae. In: Akatsuka I (ed) Biology of economic algae. SSB Academic Publishing, The Hague, pp 185–226
Oliveira EC, Alveal K, Anderson RJ (2000) Mariculture of the agar-producing gracilarioid red algae. J Phycol 8:345–377
Plastino EM, Costa VL (1999) Ultrastructure of vegetative branches of the red macroalga Gracilaria sp. (Gracilariales). Acta Microsc 8(suppl):793–794
Plastino EM, Costa VL (2001) Anomalous plastids in a light green strain of the red macroalga Gracilaria sp. (Gracilariales). Acta Microsc 3(Sup. C):315–316
Plastino EM, Oliveira EC (1988) Sterelity barriers among species of Gracilaria (Rhodophyta, Gigartinales) from the São Paulo littoral, Brazil. Brit Phycol J 23:267–271
Plastino EM, Oliveira EC (1990) Crossing experiments as an aid to the taxonomic recognition of the agarophyte Gracilaria. In: Oliveira EC, Kautsky N (eds) Cultivation of seaweeds in Latin America. University of São Paulo, São Paulo, pp 127–133
Plastino EM, Oliveira EC (1996) Approaches to the identification of terete Brazilian Gracilariaceae. Hydrobiologia 326/327:145–148
Plastino EM, Oliveira EC (1997) Gracilaria caudata J. Agardh (Gracilariales, Rhodophyta)—restoring an old name from a common Western Atlantic alga. Phycologia 36:225–232
Plastino EM, Oliveira EC (2000) Gracilaria birdiae (Gracilariales, Rhodophyta) a new specie from the tropical South American Atlantic with terete frond and deep spermatangial conceptacles. Phycologia 41:389–396
Plastino EM, Paula EJ, Oliveira EC (1995) Técnicas de hibridación en macroalgas marinas. In: Alveal K, Ferrario ME, Sar E (eds) Manual de Métodos Ficológicos. Universidad de Concepción, Concepción, pp 479–487
Plastino EM, Ursi S, Heimbecker AMC (1998) Efeito da temperatura e salinidade no crescimento de Gracilariopsis tenuifrons (Gracilariales, Rhodophyta). In: Paula EJ, Cordeiro-Marino M, Pupo Santos D, Plastino EM, Fujii M, Yokoya N (eds) IV Congresso Latino Americano de Ficologia, II Reunião Ibero-Americana de Ficologia e VII Reunião Brasileira de Ficologia. Sociedade Brasileira de Ficologia, São Paulo, pp 359–369
Plastino EM, Guimarães M, Matioli SR, Oliveira EC (1999) Codominant inheritance of polymorphic color variants of Gracilaria domingensis (Gracilariales, Rhodophyta). Genet Mol Biol 22:105–108
Plastino EM, Ursi S, Fujii MT (2004) Color inheritance, pigment characterization, and growth of a rare light green strain of Gracilaria birdiae (Gracilariales, Rhodophyta). Phycol Res 52:45–52
Posada D, Crandall KA (1998) Modeltest: testing the model of DNA substitution. Bioinformatics 14:817–818
Ramlov F, Plastino EM, Yokoya NS (2009) Efeitos do ágar no crescimento de explantes e na formação de calos em morfos pigmentares de Gracilaria domingensis (Kutzing) Sonder ex Dickie (Gracilariales, Rhodophyta). Rev Bras Bot 32:605–614
Robba L, Russell SJ, Barker GL, Brodie J (2006) Assessing the use of the mitochondrial cox1 marker for use in DNA barcoding of red algae (Rhodophyta). Am J Bot 93:1101–1108
Rossa MM, Oliveira MC, Okamoto OK, Lopes PF, Colepicolo P (2002) The effect of visible light effect on superoxide dismutase (SOD) activity in the red alga Gracilariopsis tenuifrons (Gracilariales, Rhodophyta). J Appl Phycol 14:151–157
Saunders GW (2005) Applying DNA barcoding to red macroalgae: a preliminary appraisal holds promise for future applications. Phil Trans R Soc 360:1879–1888
Saunders GW (2009) Routine DNA barcoding of Canadian Gracilariales (Rhodophyta) reveals the invasive species Gracilaria vermiculophylla in British Columbia. Mol Ecol Resour 9:140–150
Savolainen V, Cowan RS, Vogler AP, Roderick GK, Lane R (2005) Taxonomy, DNA, and the barcode of life. Phil Trans R Soc 360:1805–1811
Sherwood AR, Presting GG (2007) Universal primers amplify a 23S rDNA plastid marker in eukaryotic algae and cyanobacteria. J Phycol 43:605–608
Sherwood AR, Kurihara A, Conklin KY, Sauvage T, Presting GG (2010) The Hawaiian Rhodophyta Biodiversity Survey (2006–2010): a summary of principal findings. Plant Biol 10:258
Skriptsova AV, Nabivailo YV (2009) Comparison of three gracilarioids: growth rate, agar content and quality. J Appl Phycol 21:443–450
Swofford DL (2002) PAUP* phylogenetic analysis using parsimony (* and other methods), version 4. Sinauer, Sunderland
Ursi S, Plastino EM (2001) Crescimento in vitro de linhagens de coloração vermelha e verde clara de Gracilaria sp. (Gracilariales, Rhodophyta) em dois meios de cultura: análise de diferentes estádios reprodutivos. Rev Bras Bot 24:587–594
Ursi S, Pedérsen M, Plastino EM, Snoeijs P (2003) Intraspecific variation of photosynthesis, respiration and photoprotective carotenois in Gracilaria birdiae (Gracilariales: Rhodophyta). Mar Biol 142:997–1007
Ursi S, Guimarães M, Plastino EM (2008) Deleterious effect of TRIS buffer on growth rates and pigment contents of Gracilaria birdiae Plastino & E.C. Oliveira (Gracilariales, Rhodophyta). Acta Bot Bras 22:891–896
Yang EC, Kim MS, Geraldino PJL, Sahoo D, Shin J, Boo SM (2007) Mitochondril cox1 and plastid rbcL genes of Gracilaria vermiculophylla (Gracilariaceae, Rhodophyta). J Appl Phycol 20:161–168
Yokoya NS (2000) Apical callus formation and plant regeneration controlled by plant growth regulators on axenic culture of the red alga Gracilariopsis tenuifrons (Gracilariales, Rhodophyta). Phycol Res 48:133–142
Yokoya NS, Oliveira EC (1992a) Effects of salinity on the growth rate, morphology and water content of some Brazilian red algae of economic importance. Cienc Mar 18:49–64
Yokoya NS, Oliveira EC (1992b) Geographic distribution and growth responses to temperature variation of some South American red algae of economic importance. J Appl Phycol 4:339–345
Yokoya NS, Oliveira EC (1993) Effects of temperature and salinity on spore germination and sporeling development of South American agarophytes. Phycol Res 41:283–293
Acknowledgments
This research had been supported by the State of São Paulo Research Foundation (FAPESP, 2007/51270-7) and the Brazilian National Council for Scientific and Technological Development (CNPq; BrBOL 564945/2010-2). E. Costa acknowledges a scholarship from CAPES. We thank Carolina de Oliveira Franco for technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Costa, E.S., Plastino, E.M., Petti, R. et al. The Gracilariaceae Germplasm Bank of the University of São Paulo, Brazil—a DNA barcoding approach. J Appl Phycol 24, 1643–1653 (2012). https://doi.org/10.1007/s10811-012-9828-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-012-9828-y