Abstract
The effect of air drying temperature on physical properties of dried Fucus vesiculosus seaweed and the antiradical capacity and composition of its aqueous extracts were studied. Air drying was performed in a tray dryer employing different temperatures (35, 40, 60 and 75 °C). Dried seaweed (12.2 g water (100 g)−1 dry solid) was milled and particle size characterization and colour analysis of obtained powder were performed. Seaweed powder dried at different temperatures showed significant differences regarding colour properties. Lower brightness and yellowness values were determined in samples dried at 50 and 60 °C in comparison to those dried at 35 °C; greenness at 50 °C was enhanced. Nevertheless, particle size distributions of powders were invariant with drying temperature. Dried seaweed powders (<500 μm) were subjected to ultrasound-assisted aqueous extraction (for 4 min and liquid/solid ratio of 30). Total polyphenol content and antioxidant activity (using DPPH• radical scavenging activity) decreased with increasing drying temperature. A linear relationship between both properties was found. Extracts obtained from seaweed dried at 35 °C and sieved to obtain several particle size fractions showed that the maximum polyphenol content was achieved with the intermediate size fractions (80–200 μm). High drying temperatures had a positive effect on alginate extraction yield, but carbohydrate content was not affected (both content referred to raw seaweed powder).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, there has been an increased interest in natural antioxidants to replace synthetic additives in foods or nutraceuticals. Natural antioxidants not only have the capability to improve oxidative stability, but they can also provide a wide variety of additional health benefits (Wang et al. 2012). Although marine algae are exposed to light and oxygen, causing the formation of free radicals, there is no presence of oxidative damage in the structural components of seaweeds. This suggests that their cells have protective antioxidative defence systems (Jiménez‐Escrig et al. 2001). In fact, there are several substances (mainly polysaccharides and polyphenols) present in marine algae that are strongly related to the antioxidant activity (Keyrouz et al. 2011; Hahn et al. 2012).
South Asian countries were the first to introduce seaweeds for their utilization for medicinal and food purposes. Conventionally, the Western world has used marine algae for the production of colloids (agar, carrageenan and alginates). Marine algae are abundant in Europe and have the potential to become an excellent source of bioactive compounds (Kadam et al. 2013). Brown seaweeds represent a suitable supplement and additive for food due to their high nutritional value and the health benefits they can provide. Brown algae have high polyphenol content, with genera like Ascophyllum and Fucus reaching up to 14 g (100 g)−1 dry solid (d. s.) (Holdt & Kraan 2011); particularly, phlorotannins (polyphenols) have been reported to show anti-inflammatory properties and high antioxidant activities (Balboa et al. 2013). Several species such as Ecklonia cava, Ecklonia kurome, Fucus vesiculosus, Hizika fusiformis and Sargassum ringgoldianum have high phlorotannin content, which is correlated with the antioxidant activity (Wang et al. 2012).
Fucus vesiculosus is a dominant species of macroalgae in the northern Atlantic Ocean. Hahn et al. (2012) reported the average composition (d. s.) of Fucus vesiculosus: 47.8 % carbohydrates (mainly 14.4 % alginate, 12.4 % fucoidan and 12.3 % mannitol), 17.5 % minerals, 10.5 % polyphenols, 10 % proteins, 4.8 % lipids and 9.4 % other components. Other authors determined that this seaweed contains up to 65 % d. s. as polysaccharides (Rioux et al. 2007). Several compounds present in F. vesiculosus such as fucoidans (sulfated fucose oligomers), phlorotannins and carotenoids have been reported to show antioxidant activity (Ngo et al. 2011). The current main use of this seaweed in Europe is related to weight loss applications (using raw seaweed or extracts with organic solvents). Besides these uses, further research is needed to increase F. vesiculosus applications. This fact makes necessary to study how processing (collection, drying, storage, and extraction, among others) affects these properties.
Drying is one of the most employed industrial operations worldwide as it accounts for about 10–25 % of the total energy consumption in manufacturing processes (Mujumdar 2006). Many bioproducts, as seaweeds, are generally sundried for long periods of time. The current increase of production rates of marine algae requires the application of faster and controlled industrial methods. Air drying conditions are restricted mainly by air temperature and material characteristics. During drying, the solid material can undergo several processes that modify the physical (rehydration, colour loss), chemical (browning reaction, lipid oxidation) and also nutritional (vitamin and protein loss) properties (Bonazzi & Dumoulin 2011). Particularly, colour is the main attribute with respect to the quality of dried materials and can change during drying due to chemical and biochemical reactions. Consequently, colour characteristics, as a measure of the processes promoted during drying, could be related to the properties of the extracts. Some researchers have studied the convective air drying effect on antioxidant activity of different marine algae species (Tello-Ireland et al. 2011; Jiménez‐Escrig et al. 2001; Kuda et al. 2005a; Kuda et al. 2005b; Le Lann et al. 2008) but no studies on F. vesiculosus were found.
The use of ultrasound technology is widely extended in the food industry. It has been implemented in several large-scale commercial applications such as emulsification, homogenization, extraction, crystallization, etc. It has attracted the attention for its application for the extraction of natural products in a short time. The use of ultrasound improves solvent penetration and disrupts cell walls, releasing its content. Ultrasound-assisted extraction usually increases yields and the quality of the extract and can replace efficiently the traditional technologies to extract bioactive compounds from biomaterials (Picó 2013). Yields and extraction rates increase with smaller particle size, but milling is an energy-intensive operation and must operate without denaturing the material to be extracted and commensurate with separation from the solvent post extraction (Balachandran et al. 2006). Ultrasound-assisted extraction has been used, for example, for the extraction of lycopene from tomatoes (Lianfu & Zelong 2008), anthocyanins from raspberries (Chen et al. 2007), phenolic compounds from citrus peel (Ma et al. 2009) and Ascophyllum nodosum and Laminaria hyperborea seaweeds (Kadam et al. 2015).
The objectives of the present study are to investigate the effect of air drying temperatures on the physical properties of dried seaweed and its influence on the antioxidant activity and the phytochemical constituents of the aqueous extracts of F. vesiculosus seaweed obtained by ultrasound-assisted extraction.
Materials and methods
Raw material and chemicals
Fresh Fucus vesiculosus (84.4 ± 2.9 g water (100 g)−1 wet solid, w. s.) were collected in the western coast of Galicia, Spain (42.782255 N, −8.929705 W), in October and November of 2014. The seaweed was washed with tap water to remove sand, epiphytes and bugs, and stored (for a maximum of 1 week) at 5 °C until further processing. The analytical grade chemicals used for chemical characterizations were acetone, sodium carbonate, Folin-Ciocalteau reagent, sulphuric acid, phenol, sodium tetraborate, sulfamic acid and 3-hydroxybiphenyl (Panreac, Barcelona, Spain).
Drying
Drying experiments were carried out in a hot air convective tray dryer (Angelantoni, Challenge 250, Italy) at different temperatures (35, 50, 60 and 75 °C) keeping relative humidity (30 %), air velocity (2 m s−1) and initial loading density (14.9 ± 0.1 kg m−2, with a tray area of 0.2 m2) constant in all experiments. Drying was performed until moisture content of dried seaweed achieved 12.2 ± 0.9 % d. s. The whole seaweed with the exception of the holdfast, which was cut with a thin blade, was employed in the drying experiments. All experiments were carried out at least in duplicate. Samples were briefly withdrawn from the dryer and weighed (Cobos D-6000-CS, ±0.1 g, Spain) every 15 min in the first stages of drying and every hour towards the end. More experimental details were previously reported (Moreira et al. 2015).
Milling
After drying, F. vesiculosus was left aerating for 1–2 days to obtain uniform moisture content for dried seaweed. In order to facilitate milling, dried seaweed was previously ground to a size lower than ∼0.5 cm in a Waring laboratory blender (Waring, HGBTWT, USA). Then, it was milled in an ultracentrifugal mill (Retsch GmbH, ZM200, Germany) using a 500 μm internal sieve. Finally, the milled seaweed with moisture content of 10.5 ± 1.4 % d. s. was stored in polyethylene plastic bags under vacuum with a vacuum packer (Sammic V201, Spain) and stored at 4 °C for further utilization.
Particle size characterization
The analysis of milled seaweed particle size distribution was carried out using sieves (Cisa Cedaceria Industrial, Spain) with different standardized meshes (500, 250, 125, 80, 63 and 40 μm). From particle size distributions, the mass mean diameter D w , (Eq. (1)), volume mean diameter D v (Eq. (2)) and surface mean diameter D s (Eq. (3)) were evaluated for powdered seaweed previously dried at different temperatures.
where D pi (μm) is the mean diameter for each fraction and x i (−) is the weight fraction.
Colorimetric characterization
The surface colour of F. vesiculosus seaweed powder and the corresponding particle size fractions were measured using a colorimeter (CR 400, Konica Minolta, Japan) previously calibrated measuring the colour parameters of a standardized white glossy ceramic tile. Colour was evaluated by means of CIELAB coordinates (L*, a* and b*) (CIE 1976). Total colour difference (∆E*) was calculated taking as reference the milled seaweed previous to sieving (mixture of all fractions), corresponding to the tested temperatures (35, 50, 60 and 75 °C), (Eq. (4)):
where L* is whiteness (L* = 0) or brightness (L* = 100), a* is redness (a* > 0) or greenness (a* < 0) and b* is yellowness (b* > 0) or blueness (b* < 0) and r is the reference value. At least ten colour measures were carried during powder surface scanning.
Ultrasound extraction
Samples of powdered seaweed that were dried at different temperatures (and samples with different particle sizes from seaweed previously dried at 35 °C) were processed with an ultrasound processor (Hielscher, UIP-1000 hdT, Germany) to enhance the extraction of polyphenols and carbohydrates. All experiments were carried out in batch, the procedure starting with a 15-min rehydration step before extraction. Then, extraction operation took place using a 200-mL beaker at controlled temperature (<35 °C) employing a cold water bath to avoid that temperatures increased could affect antioxidant activity. All extractions were performed using water as solvent, excepting in the cases that acetone/water (70:30 v/v) was used as solvent to obtain reference extracts according to Koivikko et al. (2007). The equipment operated with a frequency of 20 kHz and the irradiation power (<1000 W) was regulated in the ultrasound generator at 80 % amplitude. Preliminary tests (data no shown) were carried out, using F. vesiculosus powder formerly dried at 35 °C, varying liquid/solid ratio (20, 30 and 40 w/w) and contacting time (4, 12, and 20 min) and analyzing polyphenol, carbohydrate and alginate contents, to establish the most adequate extraction conditions for further studies. The selected conditions were 4 min of contact time and 30 g g−1 liquid/solid ratio. These conditions provide the highest content in polyphenols, carbohydrates and alginates in the extracts.
Finally, obtained extracts were centrifuged at 12,400 rpm for 15 min using a benchtop centrifuge (SciQuip, Sigma 2 15, UK) and the supernatant obtained was then filtered (0.25 μm) and used for characterization analysis.
Extract characterization
All experimental determinations were carried out at least in triplicate and the corresponding mean values and standard deviations were evaluated.
Total solids content
The total solids content in the extracts was determined after sample drying at 104 ± 1 °C. Samples were weighed daily until constant weight was reached after two consecutive measurements (Symons & Morey 1941).
Polyphenol content
The quantitative determination of total polyphenol content (TP) was measured as phloroglucinol (PHL) equivalents following a colorimetric method (Singleton & Rossi 1965). This method is based on the absorbance changes of the Folin-Ciocalteu reagent when reacting whit the hydroxyl groups of the polyphenolic substances. TP was evaluated in reference to raw seaweed powder sample (mg PHL (100 g)−1 dry sample, TPw) and also to total solids content in the extract (mg PHL (100 g)−1 g dry solids, TPs).
DPPH scavenging activity
The DPPH scavenging activity assay measures the capacity of a system to react with a free radical agent (2, 2-diphenyl-1-picrylhydrazyl, DPPH). It was employed a method previously proposed (Brand-Williams et al. 1995). In its radical form, DPPH• shows an absorption peak at 515 nm, but upon reduction by an antioxidant (AH) or a radical species (R•), the absorption disappears. As the reaction takes time to fully develop, for the determination of the DPPH scavenging activity, absorbance is measured every 5 min until it reaches the stationary state. Scavenging activity is evaluated by means of (Eq. 5):
where A 0 (−) is absorbance at time 0 and A f (−) is the absorbance after one hour.
Carbohydrate content
Carbohydrate content of the extracts was determined using the Dubois et al. (1956) method that employs sulfuric acid and phenol as reagents. In the presence of strong acids and heat, carbohydrates form furan derivatives such as furanaldehyde and hydroxymethyl furaldehyde. These compounds react with phenol, leading to the formation of orange-coloured compounds. Furan derivatives from pentoses and hexoses exhibit peaks of light absorbance in the range of 480–490 nm (Brummer & Cui 2005). Samples were evaluated measuring the absorbance read at 485 nm, and glucose was used for the calibration curve. Hence, carbohydrate content was expressed as glucose equivalents (GL) referred to raw seaweed powder sample (mg GL (100 g)−1 g dry sample, CHOw) and also to total solids content in the extract (mg GL (100 g)−1 dry solids, CHOs).
Alginate content
Alginate content determination was carried out by means of Blumenkrantz & Asboe-Hansen (1973) method and a further modification by Filisetti-Cozzi & Carpita (1991). The assay consists on the measurement of absorbance at 520 nm of the extracts in the presence of sodium tetraborate in sulphuric acid and m-hydroxydiphenil as colour reagent. The Filisetti-Cozzi and Carpita modification introduces the use of sulfamate to reduce the interference of neutral sugars in the measurement (Wrolstad et al. 2005). Glucuronic acid was used as reference for the calibration curve. Therefore, all measures were expressed as glucuronic acid equivalents (GLU) referred to raw seaweed powder sample (mg GLU (100 g)−1 dry sample, GLUw) and also to total solids content in the extract (mg GLU (100 g)−1 dry solids, GLUs).
Statistical analysis
Statistical analyses were performed with IBM SPSS Statistics 20.0.0 software. Levene’s test (P ≤ 0.05) was applied to determine homogeneity of variances of data. For data with non-homogeneity of variances, the Kruskal-Wallis non-parametric test was performed. In the case of homogeneity of variances, when groups of values (k) > 2, differences among means were identified by one-factor analysis of variance (ANOVA), followed by the Scheffe’s test and considering significant P values ≤ 0.05; when k = 2, Student’s t test was applied.
Results and discussion
Drying
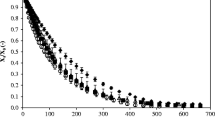
Fucus vesiculosus seaweed was dried from 84.4 ± 2.9 up to 11.4 ± 0.8 % (w. s.) employing different air drying temperatures (35, 50, 60 and 75 °C). Figure 1 shows the experimental drying kinetics. It can be observed that drying time decreased with increasing drying temperature. In fact, drying at 35 °C exhibited the longest drying time with 25.5 ± 0.5 h, followed by 50 °C with 23.5 ± 0.5 h, and 60 °C and 75 °C required almost the shortest same drying time with 20.0 ± 0.5 h.
Particle size characterization
After drying, algae was milled and sieved in fractions with different particle sizes (from 40 to 500 μm). Table 1 shows the particle size characterization of powders from seaweed dried at different temperatures. In the tested powders assayed, the highest fractions are present at a particle size of 375 μm (38.1–46.7 %). The particle size with the second highest fraction was 162.5 μm (14.1–19.3 %). The minimum yield was obtained for fines or size lower than 40 μm (1.3–4.3 %), and the particle size higher than 500 μm is considered residual, being lower than 1.6 %. Regarding mean diameter values, none of the diameters showed significant differences between temperatures employed during drying. This result seems to indicate that no notorious textural differences are developed during drying at different conditions. All powders showed similar mean size and surface area; consequently, this last variable should not have any influence on the extraction yield of F. vesiculosus compounds.
Colorimetric characterization
The colour of seaweed powders and the corresponding particle size fractions obtained after sieving were measured. Parameter ∆E* of each size fractions was estimated using as reference the colour coordinates of the algae powder prior to sieving. Table 2 shows colour parameter values of powders from seaweeds dried at 35, 50, 60 and 75 °C. Seaweed powders were exhibited in all cases greenness (a* < 0) and yellowness (b* > 0) predominance. The main change in colour characteristics is found for mixture powders formerly dried at 50 °C due to the attenuation in yellowness (b*) in comparison with those treated at 35 °C. These results may be linked to the reactions of carotenoids or other pigments, which could result in their degradation, or in the formation of alternative coloured substances or volatile compounds (Landrum 2009). This fact was also noticeable in powders from seaweed dried at 60 °C, which present also low b* values.
Regarding parameter a*, powders from seaweed dried at 50 °C revealed a relevant decrease. During dehydration, the tonoplast, the plasmalemma and the chloroplast membrane may suffer structural damage, and as result, a solute loss of chlorophyll and carotenoids, among others components, can occur (Burritt et al. 2002; Oliver et al. 1998). This damage of cell integrity could be related to a loss of antioxidant capacity due to membrane damage which could be enhanced by an increased reactive oxygen species (ROS) production induced by stress conditions (Burritt et al. 2002). Fucoxanthin is an important component of brown algae colouration, and in raw seaweed, it covers the pigmentation of chlorophyll. However, the chlorophyll leaching during drying may expose its colour, and consequently, the parameter a* drastically decreases. During drying at higher temperatures (>60 °C), the released chlorophyll undergoes degradation reactions. Chlorophylls are easily degraded in the presence of dilute acids, heat, light and oxygen. Along with degradation produced by external agents, chlorophyll is also degraded by chlorophyllase enzyme (Erge et al. 2008). Degradation of the chlorophyll is manifested as yellowing, as it allows the preponderance of carotenoid colouration (Drążkiewicz & Krupa 1991). At room temperature, this enzyme only acts in the presence of high concentrations of organic solvents. However, its optimum activity is found to be within the range of 60–82 °C (Erge et al. 2008). It is difficult to distinguish if chlorophyll breakdown is produced by enzymatic or non-enzymatic reactions but, eventually, they both lead to the formation of non-colourant species (Delgado-Vargas & Paredes-Lopez 2002). Finally, the additional increase of b* coordinate (yellowness) after drying at 75 °C may be induced by Maillard reactions.
Colour parameter values of size fractions were analysed by means of ANOVA due to homogeneity of variances (Levene’s test; P ≥ 0.082), Table 2. Regarding colour properties of size fractions, parameters L* and b* decreased significantly for all systems as mean size increased. Both trends might be related to the presence of still structurally undamaged parts of the alga in the biggest particles. Parameter a* slightly decreased its absolute value with particle size with exception of powder from seaweed dried at 50 °C, in which very low values were found for fine fractions (D p < 63 μm), and above this size, a* increased with diameter. This behavior has a noticeable influence on the ∆E* parameter for the fractions with the lowest size. Particle size fraction of seaweed powder formerly dried at 60 °C showed brightness, greenness and yellowness loss in all fractions, this effect being likely related with colourant degradation. Finally, the fractions of F. vesiculosus powder from seaweed dried at 75 °C displays similar loss in greenness than seaweed dried at 60 °C along with the raise in brightness and yellowness-related parameters (L*, b*), which were regarded with browning reactions.
The colour difference (ΔE*) trend showed minimum values at intermediate particle size fractions, with exception of powder formerly dried at 50 °C in which a continuous decrease was observed. The global ΔE* analysis with the particle size fractions for all tested powders indicated that the fractions corresponding to sizes from 63 up to 200 μm showed the lowest average values (ΔE* = 7.47 ± 0.31) and for the powders that showed a minimum the lowest average value (ΔE* = 5.78 ± 1.78) corresponded to the fractions between 63 and 125 μm. On the other hand, it is noticeable that evaluated volume mean diameters, D v (74–91 μm), are into the previous interval. This result indicates that this characteristic diameter is the most adequate to estimate approximately the colour properties of the mixtures of seaweed powder formerly dried at 35, 60, and 75 °C. In the case of drying at 50 °C, this relationship was not met, by the reasons previously explained, and the largest size fractions showed the minimum colour differences. Tello-Ireland et al. (2011) also reported that drying Gracilaria chilensis at 50 °C resulted in the highest ∆E* value, and drying at higher temperatures showed similar colorimetric coordinates to sample dried at low temperature (35 °C).
Extract characterization
Antioxidant activity
Antioxidant activity of the extracts was evaluated by means of total polyphenol (TP) content and total DPPH• radical scavenging activity. TP values showed homogeneity of variance (Levene’s test, P = 0.47 for TPw and P = 0.29 for TPs), and ANOVA analysis was performed. Table 3 shows the influence of drying temperature of algae on the total polyphenol content of the extracts. TP decreased when drying temperature was raised. The significantly highest TP was achieved for the extract made with seaweed dried at 35 °C (1571 ± 76 TPw, 2940 ± 141 TPs). TPrel defined as the ratio of TPw content of the current extract and TPw from seaweed formerly dried at 35 °C indicated that increasing drying temperature to 50 °C, a reduction of TPw of 37 % is produced and diminishes up to 54 % when drying temperature of 75 °C is employed. TPS in the extract showed the same trend. This result implies that differences in TP can be attributed to the effect of drying temperature, and not to a difference in extraction yield.
Extractions were also carried out with acetone/water mixture (70/30 v/v) to achieve the highest extraction yield (Koivikko et al. 2007), replicating the remaining operation conditions. The TP attained was 11,428 ± 1124 TPw, which is within the interval of 8–13 % of dry matter reported by Ragan & Jensen (1978) and is comparable to TPw achieved by Díaz-Rubio et al. (2009) in extractions with acetone/water and methanol/water mixtures using F. vesiculosus. The maximum TP obtained employing only water as solvent in the ultrasound extraction accounted for 14.4 ± 1 % of TPw achieved with an acetone/water mixture. Acetone may contribute to a higher degradation of seaweed structure and, therefore, a higher release of these compounds. In addition, polyphenols exhibit a wide difference among their composition and structure and, as a result, in polarity. The use of water as the only solvent allows the extraction of water-soluble polyphenols, while the addition of acetone promotes the extraction of the non-polar fraction as well (López et al. 2011).
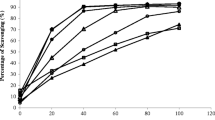
On the other hand, the extracts obtained from seaweed dried at 35 °C exhibited the highest radical scavenging activity (57.7 ± 3.4 %), Table 3. As in the case of polyphenols, an increase in drying temperature gave as result a reduction of radical scavenging activity up to the lowest value (26.0 ± 1.7 %) in extracts from the powder of seaweed dried at 75 °C. A linear correlation (R 2 > 0.998) could be established between TPs and radical scavenging activity, Eq. (6):
This positive linear relationship between total phenolic content and antioxidant capacity was also found by other authors in fruits (Igual et al. 2010; Bahorun et al. 2004), hulls (Rubilar et al. 2007), leaf extracts (Rubilar et al. 2006) and brown (Connan et al. 2007), green and red seaweeds (Matanjun et al. 2008).
The reduction in polyphenol content and antioxidant activity at high drying temperatures may be due to several factors: release of phenolic compounds bound to the cell wall during drying, thermal degradation by oxidative enzymes, phenolic compounds may rapidly degrade at drying temperatures above 40 °C, binding of polyphenols to other substances (proteins) or alterations in their chemical structure (Gupta et al. 2011; Tello-Ireland et al. 2011; Le Lann et al. 2008). Tello-Ireland et al. (2011) reported the loss of antioxidant activity when drying Gracilaria chilensis at high temperatures (70 °C). Gupta et al. (2011) observed a 30 % decrease in TP of Himanthalia elongata when dried at 40 °C for 24 h in comparison with fresh seaweed.
Total polyphenol content of aqueous extracts obtained from different particle size fractions of F. vesiculosus powder dried at 35 °C are indicated in Table 4. These data showed heterogeneity of variance (Levene’s test; P = 0.02 for TPw and P = 0.03 for TPs) so the Kruskal-Wallis test was applied. The size fraction between 80 and 125 μm exhibited the significantly highest total polyphenol content (TPw of 1672 ± 13 mg PHL (100 g)−1 dry sample and TPs of 3260 ± 20 mg PHL (100 g)−1 dry solids). The fraction with the significantly lowest TPw was that with the highest particle diameter (>200 μm). No significant differences were found among the remaining size fractions. The smallest and the biggest fractions exhibited a reduction in total polyphenol content. The first observation may be attributed to the accumulation of inorganic substances, like salts in the smallest fractions. In the largest fractions, seaweed cells could not be totally degraded, and polyphenols, along with other substances, might not have been released indicating that higher extraction times would be necessary for particles this size. Hence, polyphenols are better extracted when intermediate fractions are employed.
Finally, it is noticeable that no relationship was found between the colorimetric properties of dried seaweed powder and antioxidant properties of the extracts. The antioxidant activity decreased continuously with increasing drying temperature, and the colour showed a different trend due to different chemical processes promoted thermally.
Carbohydrate content
The carbohydrate content values (CHO) showed heterogeneity of variances (Levene’s test; P = 0.002 for CHOw and P = 0.001 for CHOs) so the Kruskal-Wallis test was applied. For CHOw, the Kruskal-Wallis test showed no significant differences (P = 0.188, mean value of 5204 mg eq. glucose (100 g)−1 seaweed) as the effect of drying temperature, Fig. 2. The content of carbohydrates referred to total solids content (CHOs) was significantly lowest for samples dried at 35 °C (P = 0.034) (8217 ± 414 mg eq. glucose (100 g)−1). The significantly highest value corresponded to samples dried at 60 °C and 75 °C (12,588 ± 1269 and 12,895 ± 784 mg eq. glucose (100 g)−1, respectively). No significant differences in CHOs values between samples dried at 60 and 75 °C were observed. Although extraction yields referred to raw seaweed powder are similar, there might be compounds already extracted or degraded during drying at 60 and 75 °C. If these substances are not removed at lower drying temperatures, they are extracted during ultrasound-assisted extraction thus increasing the total solids content of the extract. The extraction with acetone/water (70/30 v/v) of seaweed dried at 35 °C gave CHOw of 11,388 ± 156 mg eq. glucose (100 g)−1. The aqueous extraction of F. vesiculosus seaweed dried at 75 °C exhibited the highest yield in relation to the acetone/water extract (∼52 %). As most polysaccharides present in the seaweed are structural substances, a higher drying temperature (75 °C) might have contributed to a better subsequent extraction of carbohydrates.
Effect of drying temperature on carbohydrate content (CHO) of aqueous F. vesiculosus extracts (contact time of 4 min and L/S ratio = 30 g g−1): referred to raw powder (mg GL (100 g)−1 dry sample, CHOw (white square)), and to total solids content in the extract (mg GL(100 g)−1 dry solids, CHOs (grey square))
Alginate content
The alginate content of the extracts were only determined for powders from seaweed dried at 35 and 75 °C, Fig. 3. The value of alginate content of the extracts showed homogeneity of variances (Levene’s test; P = 0.065 for GLUw and P = 0.056 for GLUs). In this case due to number of groups, k = 2, the Student’s test was performed. Alginate content significantly increased with increasing drying temperature from 2070 ± 107 mg. eq. glucuronic acid (100 g)-1 powdered seaweed (GLUw) to 3240 ± 266 GLUw (P = 0.018) and from 3301 ± 170 mg eq. glucuronic acid (100 g)−1 solid (GLUs) to 7102 ± 583 GLUs (P = 0.006). Tello-Ireland et al. (2011) observed a similar behaviour with red seaweed G. chilensis due to, in this case, high temperatures decreased the antioxidant activity of the seaweed but also increased the extraction yield of agar (a structural polysaccharide of red seaweeds). This result seems to indicate that high drying temperature may allow an easier extraction of structural algae polysaccharides. Although alginates can contribute to the scavenging activity, in this case, higher alginate content is associated with lower antioxidant activity. This fact, together with the found relationship between TP and scavenging activity, indicates that the thermal degradation of polyphenols is critical for extract quality.
Conclusions
Particle size distributions of F. vesiculosus seaweed after milling were not modified by the drying temperature. Consequently, no significant textural differences were found between seaweeds dried at different temperatures. Seaweed powders exhibited significant differences in colour as the function of drying temperature. Temperatures close to 50 °C causes a greenish colouration of powder. Ground seaweeds previously dried at 35, 60 and 75 °C showed a similar yellow tone. Aqueous ultrasound-assisted extraction is a feasible method to obtain antioxidant compounds from F. vesiculosus. Seaweed drying at low temperatures (35 °C) resulted in aqueous extracts with higher phenolic contents and higher DPPH• radical scavenging activities. Higher drying temperatures negatively affected the total phenolic content and the antioxidant activity of extracts. A linear correlation between the phenolic content of extracts dried at different temperatures and their DPPH• radical scavenging activity was found. Nevertheless, no relationship was found between the colour of seaweed powders and antioxidant activity of the corresponding aqueous extracts. Extracts obtained from 80–200 μm particle size fractions of powder obtained from seaweed previously dried at 35 °C showed the highest polyphenol content. Alginate content in the aqueous extracts increased with drying temperature of seaweed, but no significant differences were observed in carbohydrate content, in both cases referred to raw seaweed. As conclusion, F. vesiculosus is a suitable raw material to acquire bioactive compounds, which may have applications in the food, cosmetic and pharmaceutical industries. Drying conditions must be controlled to preserve the bioactivity characteristics of the corresponding extracts and to control characteristics as the colour of the meal.
References
Bahorun T, Luximon-Ramma A, Crozier A, Aruoma O (2004) Total phenol, flavanoid, proanthocyanidin and vitamin C levels and antioxidant activities of Mauritian vegetables. J Sci Food Agric 84:1553–1561
Balachandran S, Kentish E, Mawson R, Ashokkumar M (2006) Ultrasonic enhancement of the supercritical extraction from ginger. Ultrason Sonochem 13:471–479
Balboa EM, Conde E, Moure A, Falqué E, Domínguez H (2013) In vitro antioxidant properties of crude extracts and compounds from brown algae. Food Chem 138:1764–1785
Blumenkrantz N, Asboe-Hansen G (1973) New method for quantitative determination of uronic acids. Anal Biochem 489:484–489
Bonazzi C, Dumoulin E (2011) Quality changes in food materials as influenced by drying processes. In: Tsotsas E, Mujumdar A (eds) Modern drying technology Volume 3: product quality and formulation. Wiley, Weinheim, pp 1–20
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol 28:25–30
Brummer Y, Cui SW (2005) Understanding carbohydrate analysis. In: Cui SW (ed) Food carbohydrates: chemistry, physical properties and applications. CRC Press, Boca Raton, pp 68–104
Burritt DJ, Larkindale J, Hurd CL (2002) Antioxidant metabolism in the intertidal red seaweed Stictosiphonia arbuscula following desiccation. Planta 215:829–838
Chen F, Sun Y, Zhao G, Liao X, Hu X, Wu J, Wang Z (2007) Optimization of ultrasound-assisted extraction of anthocyanins in red raspberries and identification of anthocyanins in extract using high-performance liquid chromatography-mass spectrometry. Ultrason Sonochem 14:767–778
CIE (1976) CIE Colorimetry - Part 4: 1976 L* a* b* Color space. International Commission of Illumination (CIE), Vienna
Connan S, Deslandes E, Gall EA (2007) Influence of day–night and tidal cycles on phenol content and antioxidant capacity in three temperate intertidal brown seaweeds. J Exp Mar Biol Ecol 349:359–369
Delgado-Vargas F, Paredes-Lopez O (2002) Natural colorants for food and nutraceutical uses. CRC Press
Díaz-Rubio ME, Pérez-Jiménez J, Saura-Calixto F (2009) Dietary fiber and antioxidant capacity in Fucus vesiculosus products. Int J Food Sci Nutr 60:23–34
Drążkiewicz M, Krupa Z (1991) The participation of chlorophyllase in chlorophyll metabolism. Acta Soc Bot Pol 60:139–154
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Erge HS, Karaden Z, Koca N, Soyer Y (2008) Effect of heat treatment on chlorophyll degradation and color loss in green peas. Gida 33:225–33
Filisetti-Cozzi TM, Carpita NC (1991) Measurement of uronic acids without interference from neutral sugars. Anal Biochem 197:157–62
Gupta S, Cox S, Abu-Ghannam N (2011) Effect of different drying temperatures on the moisture and phytochemical constituents of edible Irish brown seaweed. LWT-Food Sci Technol 44:1266–1272
Hahn T, Lang S, Ulber R, Muffler K (2012) Novel procedures for the extraction of fucoidan from brown algae. Process Biochem 47:1691–1698
Holdt SL, Kraan S (2011) Bioactive compounds in seaweed: functional food applications and legislation. J Appl Phycol 23:543–597
Igual M, Garcia-Martinez E, Camacho MM, Martinez-Navarrete N (2010) Effect of thermal treatment and storage on the stability of organic acids on the functional value of grapefruit juice. Food Chem 118:291–299
Jiménez‐Escrig A, Jiménez-Jiménez I, Pulido R, Saura-Calixto F (2001) Antioxidant activity of fresh and processed edible seaweeds. J Sci Food Agric 81:530–534
Kadam SU, Tiwari BK, O’Donnell CP (2013) Application of novel extraction technologies for bioactives from marine algae. J Agric Food Chem 61:4667–4675
Kadam SU, O’Donnell CP, Rai DK, Hossain MB, Burgess CM, Walsh D, Tiwari BK (2015) Laminarin from Irish brown seaweeds Ascophyllum nodosum and Laminaria hyperborea: ultrasound assisted extraction, characterization and bioactivity. Mar Drugs 13:4270–4280
Keyrouz R, Abasq ML, Le Bourvellec C, Blanc N, Audibert L, ArGall E, Hauchard D (2011) Total phenolic contents, radical scavenging and cyclic voltammetry of seaweeds from Brittany. Food Chem 126:831–836
Koivikko R, Loponen J, Pihlaja K, Jormalainen V (2007) High-performance liquid chromatographic analysis of phlorotannins from the brown alga Fucus vesiculosus. Phytochem Anal 18:326–332
Kuda T, Tsunekawa M, Goto H, Araki Y (2005a) Antioxidant properties of four edible algae harvested in the Noto Peninsula, Japan. J Food Compos Anal 18:625–633
Kuda T, Tsunekawa M, Hishi T, Araki Y (2005b) Antioxidant properties of dried ‘kayamo-Nori’, a brown alga Scytosiphon lomentaria (Scytosiphonales, Phaeophyceae). Food Chem 89:617–622
Landrum JT (2009) Carotenoids: physical, chemical, and biological functions and properties. CRC Press, Boca Raton
Le Lann K, Jégou C, Stiger-Pouvreau V (2008) Effect of different conditioning treatments on total phenolic content and antioxidant activities in two sargassacean species: comparison of the frondose Sargassum muticum (Yendo) Fensholt and the cylindrical Bifurcaria bifurcata R. Ross. Phycol Res 56:238–45
Lianfu Z, Zelong L (2008) Optimization and comparison of ultrasound/microwave assisted extraction (UMAE) and ultrasonic assisted extraction (UAE) of lycopene from tomatoes. Ultrason Sonochem 15:731–737
López A, Rico M, Rivero A, Suárez de Tangil M (2011) The effects of solvents on the phenolic contents and antioxidant activity of Stypocaulon scoparium algae extracts. Food Chem 125:1104–1109
Ma Y, Chen J, Liu D, Ye X (2009) Simultaneous extraction of phenolic compounds of citrus peel extracts: effect of ultrasound. Ultrason Sonochem 16:57–62
Matanjun P, Mohamed S, Mustapha NM, Muhammad K, Ming CH (2008) Antioxidant activities and phenolics content of eight species of seaweeds from north Borneo. J Appl Phycol 20:367–373
Moreira R, Chenlo F, Sineiro J, Sánchez M, Arufe S (2015) Water sorption isotherms and air drying kinetics modelling of brown seaweed Bifurcaria bifurcata. J Appl Phycol. doi:10.1007/s10811-015-0553-1
Mujumdar AS (2006) Handbook of industrial drying. CRC Press, Boca Raton
Ngo D, Wijesekara I, Vo T, Ta QV, Kim S (2011) Marine food-derived functional ingredients as potential antioxidants in the food industry: an overview. Food Res Int 44:523–529
Oliver MJ, O’Mahony P, Wood AJ (1998) “To dryness and beyond”—preparation for the dried state and rehydration in vegetative desiccation-tolerant plants. Plant Growth Regul 24:193–201
Picó Y (2013) Ultrasound-assisted extraction for food and environmental samples. Trends Anal Chem 43:84–99
Ragan MA, Jensen A (1978) Quantitative studies on brown algal phenols. II. Seasonal variation in polyphenol content of Ascophyllum nodosum (L.) and Fucus vesiculosus (L.). J Exp Mar Biol Ecol 34:245–58
Rioux LE, Turgeon SL, Beaulieu M (2007) Characterization of polysaccharides extracted from brown seaweeds. Carbohydr Polym 69:530–537
Rubilar M, Pinelo M, Ihl M, Scheuermann E, Sineiro J, Nuñez MJ (2006) Murta leaves (Ugni molinae turcz) as a source of antioxidant polyphenols. Alcoholics and aqueous extracts. J Agric Food Chem 54:59–64
Rubilar M, Pinelo M, Shene C, Sineiro J, Nuñez MJ (2007) Separation and HPLC-MS identification of phenolic antioxidants from agricultural residues: almond hulls and grape pomace. J Agric Food Chem 55:10101–10109
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Symons GE, Morey B (1941) The effect of drying time on the determination of solids in sewage and sewage sludges. Sewage Works J 13:936–939
Tello-Ireland C, Lemus R, Vega-Gálvez A, López J, Di Scala K (2011) Influence of hot-air temperature on drying kinetics, functional properties, color, phycobiliproteins, antioxidant capacity, texture and agar yield of alga Gracilaria chilensis. LWT-Food Sci Technol 44:2112–2118
Wang T, Jónsdóttir R, Liu H, Gu L, Kristinsson HG, Raghavan S, Olafsdóttir G (2012) Antioxidant capacities of phlorotannins extracted from the brown algae Fucus vesiculosus. J Agric Food Chem 60:5874–5883
Wrolstad RE, Decker EA, Schwartz SJ, Sporns P (2005) Handbook of food analytical chemistry, water, proteins, enzymes, lipids, and carbohydrates. John Wiley & Sons, New York
Acknowledgments
The authors acknowledge the financial support to the Spanish Ministry of Economy and Competitiveness and European Regional Development Fund (ERDF) of European Union by the research project (CTQ 2013-43616/P).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moreira, R., Chenlo, F., Sineiro, J. et al. Drying temperature effect on powder physical properties and aqueous extract characteristics of Fucus vesiculosus . J Appl Phycol 28, 2485–2494 (2016). https://doi.org/10.1007/s10811-015-0744-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-015-0744-9