Abstract
Few studies have considered the effects of environmental variables at different spatial scales on Neotropical stream biodiversity. Furthermore, scale-related studies mostly include only one facet of biodiversity. To determine the contribution of local and landscape variables to the variation in the taxonomic, functional and phylogenetic α-diversity of stream fish assemblages, we sampled 85 streams in the Upper Paraná River basin, Brazil. Local variables explained a substantial fraction of the variance in almost all biodiversity facets. Landscape variables (i.e., land-use and spatial variables) contributed little to the variation in the α-component of biodiversity. Our results thus highlight the importance of local features for maintaining stream fish biodiversity in agroecosystems. Probably, land-use were not significant because the study area was in a relatively homogeneous landscape severely impacted by anthropogenic activities. It is possible that insignificant effects of spatial structuring occurred because the ichthyofauna has already gone through a homogenization process and/or due to the spatial scale of our study. We suggest that even though local-scale restoration actions would influence biodiversity, we should not neglect landscape restoration because substantial improvements in the ecological integrity of streams are more likely to be accomplished with large-scale actions (e.g., re-establishment of the native riparian forest).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ecological systems typically show considerable environmental and biological heterogeneity. Streams are particularly heterogeneous systems at multiple spatial scales (Frissell et al., 1986), and this heterogeneity is mirrored in the organization of their biological communities (Heino et al., 2015a). Much of the stream research has traditionally been conducted at small spatial scales (Allan, 2004; Johnson et al., 2007), often within stream reaches of a few hundred meters and in their immediate surroundings (Allan, 2004). However, it has been recognized that local habitat and stream biodiversity are strongly influenced by landforms and land-use in the surrounding valley (Hynes, 1975; Allen & Starr, 1982; Johnson et al., 2007). In fact, fish assemblage structure is driven by small-scale (e.g., reach) and large-scale (e.g., catchment) alterations in environmental characteristics (Fitzpatrick et al., 2001; Strayer et al., 2003), but their relative importance is debated. For instance, local-scale factors (e.g., substratum composition) explained a higher amount of the variation in the structure of stream fish assemblages among sites than large-scale variables (e.g., catchment land-use) (Lammert & Allan, 1999; Diana et al., 2006; Johnson et al., 2007). In contrast, other studies have emphasized the high importance of catchment features as drivers of fish assemblage structure (Roth et al., 1996; Leitão et al., 2018). Although scientists have increasingly adopted a catchment-scale view of streams (Allan et al., 1997; Johnson et al., 2007), the understanding of the relative influence of local-scale versus catchment-level factors on stream biota remains elusive (Angermeier & Winston, 1998; Strayer et al., 2003; Cianfrani et al., 2012; Li et al., 2019).
In Neotropical streams, few studies have considered the effects of environmental variables at different scales on fish assemblages (Roa-Fuentes et al., 2019; Benone et al., 2020). For some studies, catchment and local predictors together explained most of the variation (Bordignon et al., 2015; Leitão et al., 2018), while authors have found no effects of catchment features (Casatti et al., 2015; Gerhard & Verdade, 2016; Roa-Fuentes & Casatti, 2017). A better understanding of the influence of small-scale and large-scale environmental factors on fish assemblages in Neotropical streams could be useful to design conservation strategies and more robust monitoring and restoration programs (Johnson et al., 2007; Feld, 2013; Wahl et al., 2013; Leitão et al., 2018). Moreover, this knowledge can subsidize models for regional stream management, since catchment-scale features may be manipulated to influence factors at local scale, which ultimately would influence the structure of aquatic assemblages (Allan et al., 1997; Wang et al., 2003; Cruz et al., 2013; Feld, 2013). This is also in accordance with recent considerations on the stream restoration effectiveness, where catchment actions are more efficient than interventions focused exclusively on instream habitat restoration at the local scale (Palmer et al., 2010).
Beyond catchment and local-scale factors, assessing the relative contribution of spatial structure (a proxy for dispersal-related processes) to stream fish assemblages may suggest guidelines for biodiversity management, especially in human-dominated landscapes (Bengtsson, 2010; Heino, 2013). For instance, when there are low dispersal rates between sites and local processes determine diversity (i.e., species sorting), the management of local features and local habitat heterogeneity is fundamental to maintain diversity (Bengtsson, 2010). On the other hand, if dispersal has major effects on local biodiversity, it will be necessary to identify and manage source sites and promote landscape heterogeneity to maintain colonization sources for different species (Bengtsson, 2010).
Recently, functional, phylogenetic and taxonomic approaches have been used in (meta)community studies (Roa-Fuentes et al., 2019; Li et al., 2020), because they can improve our understanding on how species interact with each other and with the environment (García-Girón et al., 2020). Functional diversity may reflect the ability of a given assemblage to effectively respond to environmental changes (Díaz et al., 2007), or is linked to biological changes driven by modification of land-use and their consequences for ecosystem functioning (Luck et al., 2013; Leitão et al., 2018). In Neotropical streams, the functional diversity of fish assemblages has been addressed through characterization of traits associated with body size, fish trophic guilds, vertical and horizontal habitat preference, and foraging period, i.e., traits exhibiting response to local and landscape environmental variation (Teresa & Casatti, 2012; Casatti et al., 2015; Dala-Corte et al., 2016; Leitão et al., 2018; Zeni et al., 2019). Phylogenetic diversity is a proxy for the accumulated evolutionary history of a given assemblage and, therefore, might be associated with its ability to generate new evolutionary solutions in the face of disturbance and/or to species persist despite those disturbances (Forest et al., 2007; Faith, 2008; Safi et al., 2011). Phylogenetic diversity has been proposed as a means to prioritize species and areas for conservation (Faith, 2008; Safi et al., 2011), but its use in the context of stream fish assemblages is still rare (but see Aquino & Colli, 2017; Roa-Fuentes et al., 2019). Taxonomic diversity (e.g., species richness) is the most widely used measure for an approximation of biodiversity and to assess the effects of land-use on the structure of stream fish assemblages (e.g., Lammert & Allan, 1999). It has been used for many years in ecological studies, however, there is a general consensus that taxonomic diversity alone cannot adequately describe the processes involved in species coexistence and ecosystem functioning, and the differences in assemblage structure among sites (Safi et al., 2011). From a stream biomonitoring perspective, there is evidence that functional and phylogenetic facets are more sensitive to environmental disturbance, being able to discriminate impacted from preserved streams (Menezes et al., 2010; Saito et al., 2015a). Therefore, multiple and complementary facets should be included in studies describing not only stream biodiversity and their response to multiple-scale environmental changes, but also stream assessment or biomonitoring (Heino et al., 2008; Saito et al., 2015a).
In this study, we assessed the relative contribution of local features, catchment land-use and spatial structure to explain the variation in the taxonomic, functional and phylogenetic α-diversity of stream fish assemblages. Thus, we answered the following questions: (i) Are taxonomic, functional, and phylogenetic facets correlated with local and/or catchment environmental features? And, (ii) are these facets spatially structured? We hypothesized that: (i) both local and catchment features would explain the variation in the three biodiversity facets, since catchment land-use would affect local features, which in turn would influence stream fish assemblages. Moreover, (ii) biodiversity facets will be weakly spatially structured, as dispersal limitation will not be important at this spatial extent. In fact, Heino et al. (2015b) found that environmental control prevails over spatial constraints within single small drainage basins. Furthermore, the taxonomic facet will be more strongly affected by spatial factors, whereas the functional facet often distinguishes stream fish assemblages along habitat gradients, irrespective of spatial position of a site (Hoeinghaus et al., 2007).
Methods
Study area
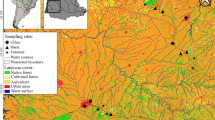
This study was carried out in the Turvo-Grande and São José dos Dourados basins, Upper Paraná River basin, in the northwestern part of São Paulo State, Brazil (Fig. 1). These two basins belong to the same biogeographic province and, consequently, fish assemblages have a common evolutionary history (Géry, 1969). The region is part of the Serra Geral geological formation and shows a relatively flat slope and plains of quaternary fluvial sedimentary nature (IPT, 1999). The soil has a high erosive potential since it is composed of unconsolidated sediments (e.g., sand and clay) (Silva et al., 2007). The climate is tropical and hot, with one dry season with lower rainfall and cooler temperatures between June and September, and a wet season between December and February with higher rainfall and warmer temperatures (IPT, 1999). The region was formerly covered by semi-deciduous seasonal Atlantic forest (Silva et al., 2007), but the landscape has been changed since the beginning of the last century (1900) through the development of coffee crops, followed by the establishment of cattle (Victor et al., 2005), and more recently sugarcane (Rudorff et al., 2010). Nowadays, the native forest is limited to less than 4% of its original area, distributed in small and unconnected fragments embedded in agricultural matrices (Nalon et al., 2008). Similarly to other São Paulo river basins (e.g., Corumbataí basin; Gerhard & Verdade, 2016), the stream fish fauna of the study area is presumed to have already been homogenized, probably due to habitat simplification (Casatti et al., 2009), species introductions (Rahel, 2002) and an extensive, dynamic and long-lasting history of land-use change (Victor et al., 2005; Rudorff et al., 2010).
Site selection
For site selection, we mapped the land-use in the São José dos Dourados and Turvo-Grande basins through the digital processing of LANDSAT-5/TM satellite images of 2011 year (221–74, 221–75 and 222–74 scenes; 30 m spatial resolution). We used 2011 data because it was the most recent year available at the time of the analysis. We defined four land-use classes through visual estimate using Google Earth™ program: (i) native forest, (ii) pasture, (iii) sugarcane and (iv) other land-use, which included any other land-use different from native forest, pasture and sugarcane (i.e., towns and villages, rural installations, temporary cultures, highways, exposed soil and others). As the product of this processing, a land-use map was obtained for the study area (unpublished data). Using land-use information, we conducted a catchment pre-selection of catchments with area between 400 and 1,400 ha (corresponding to first-order streams to third-order streams according to the Strahler system, L. Casatti personal observation). Based on this pre-selection, we selected 85 catchments considering the environmental gradient in the region (i.e., pasture-sugarcane transition), accessibility and owners’ consent (Table S1). Finally, to increase the reliability of our land-use data, we refined land-use maps for each selected catchment using orthorectified aerial photographs (‘orthophotos’) with a 1 m spatial resolution (years 2010/2011 and 2012 only for sugarcane; most recent years available at the time of the analysis). We identified eight land-use classes: native forest, herbaceous and shrub vegetation, pasture, sugarcane, perennial crops, forest plantation, urban area and other land-use (see details in Table S2). For the sugarcane land-use class, the CANASAT project (sugarcane crop monitoring in Brazil; Rudorff et al., 2010) provided data about sugarcane area and location of in the São Paulo State.
For the digital preparation, processing, and classification of LANDSAT-5/TM satellite images and orthophotos, we used ERDAS IMAGINE 9.2 and ArcGis 9.3 softwares. The LANDSAT-5/TM satellite images were downloaded from the repository of the Instituto Nacional de Pesquisas Espaciais (INPE, http://www.inpe.br/). Orthophotos were supplied by Empresa Paulista de Planejamento Metropolitano SA – EMPLASA (CLU No. 060/14).
Fish sampling
Stream surveys and fish sampling were carried out in the dry season, between July and September in 2013. In each of 85 selected catchments, we used 5-mm-mesh stop nets to block upstream and downstream of a 75 m-long reach, following the standard method to sample fish in the region (see Casatti et al., 2009). We used two different methods of electrofishing: a stationary generator (AC, 220V, 50–60Hz, 3.4–4.1 A, 1000 W) to sample 42 stream reaches and a Smith Root Model LR-24 backpack electrofishing (pulsed DC, 50-990V, 1–120 Hz, 40 A peak max, 400 W) to sample the remaining 43 reaches. We used the stationary generator method because it was the sampling method of a long-term research project (Zeni et al., 2017). To conduct backpack electrofishing, the settings were adjusted in situ based on environmental conditions (i.e., with the quick set up feature activated, which automatically sets output voltage, frequency and duty cycle) and on the observation of fish behavior and recovery times. In each reach, a two-pass electrofishing technique was conducted during 45 min from downstream to upstream direction, covering from bank to bank to sample all available microhabitats. Moreover, to identify whether the observed pattern in fish assemblage structure was a product of the electrofishing method, we conducted a test for each of the biodiversity facet (i.e., binary variable; 1 = stationary generator, 0 = backpack). We did not observe significant effect of the electrofishing method on taxonomic, functional and phylogenetic diversity (Table S5).
Fish specimens were anaesthetized using a 100 mg/L clove oil solution, fixed in 10% formalin and transferred to a 70% alcohol. Fish were identified to species and voucher specimens were deposited at the fish collection of the Department of Zoology and Botany of São Paulo State University (DZSJRP 19264-19326), São José do Rio Preto, São Paulo, Brazil.
Predictor variables
Catchment-scale variables
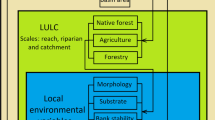
For each catchment (i.e., catchment area delimited upstream from the sampled stream reach), we measured 30 descriptors related with land-use composition and heterogeneity quantified through (i) the number of the land-use classes (i.e., richness), (ii) diversity of land-use classes (i.e., Shannon index), and (iii) the relative proportion of each land-use class (Gustafson, 1998) (Tables S3 and S4). Considering that riparian land-use and stream fish assemblages are associated (Pusey & Arthington, 2003; Cruz et al., 2013; Santos et al., 2015), we grouped the catchment descriptors into three different sub-sets: catchment, drainage network and local (according to Strayer et al., 2003). The “catchment” sub-set included land-use in the entire catchment area; the “drainage network” sub-set comprised land-use within 60-m buffer zone (30 m on each stream margin) around the river network (minimum width established by the current Brazilian Forest Code for streams less than 10 m wide); and the “local” sub-set included land-use within a 150-m radius circle from the center of our sampled reach (Roa-Fuentes & Casatti, 2017).
Local scale variables
We quantified for each stream reach 31 local descriptors related with marginal vegetation (e.g., marginal grasses, roots, shrubs), water properties (e.g., water temperature, pH), instream structures (e.g., litter, wood debris), stream channel morphology (e.g., width, depth, flow), habitat composition and heterogeneity (e.g., mesohabitat, substrate composition) (Tables S3 and S4), following standard protocols previously used in the study area (Casatti et al., 2009). These descriptors are commonly used in stream fish ecology studies (Cruz et al., 2013; Carvalho & Tejerina-Garro, 2015a).
Spatial variables
We considered the distance between sites as network distance (i.e., the distance between sites following the riverine dendritic network, sensu Brown & Swan, 2010; Altermatt, 2013). Network distance is able to capture such spatial patterns that overland distance does not account for, and it can also better describe spatial patterns generated by fish dispersal along a riverine dendritic network (Altermatt, 2013; Heino et al., 2017). We calculated the network distance using Hawth’s Analysis Tool (Beyer, 2004) for ArcGIS 9.3. From the network distance matrix, we generated Principal Coordinates of Neighborhood Matrix (PCNM) and retained only PCNM eigenvectors with positive spatial correlation because we were concerned with patterns produced by spatially contagious processes (Borcard et al., 2011). To do this, we used the ‘PCNM’ function from the PCNM package (Legendre et al., 2013). The threshold value used in the PCNM analysis was the minimum distance giving connected network. All analyses were carried out in the R environment (R Development Core Team 2020).
Response variables
Taxonomic facet
For taxonomic facet, we considered species richness in each stream reach as response variable.
Functional traits
We obtained 12 functional traits for each fish species (S = 63; Table S6). Seven traits were ecomorphological indexes associated with fish functional specializations to water flow, to swimming ability and to their position in the water column (i.e., compression index; relative area of pectoral fin; pectoral fin aspect ratio; relative eye position; relative depth; index of ventral flattening; and fineness coefficient). For details about ecological interpretations of ecomorphological indices, see Casatti & Castro (2006) and Ribeiro et al. (2016).
The remaining five traits were related with trophic ecology (i.e., species were grouped in trophic guilds: algivores, detritivores, aquatic insectivores, terrestrial insectivores, lepidophagous, periphytivores, piscivores, and omnivores); size (i.e., three categories of standard length); preference for substrate (i.e., unconsolidated, consolidated); preference for water velocity (i.e., fast, medium, low); and adaptation to anoxic conditions (i.e., unadapted, adapted; Table S6). We used literature to obtain trophic guild (Zeni & Casatti, 2014); size categories (Teresa & Casatti, 2012; Casatti et al., 2015); preference for substrate and water velocity (Casatti et al., 2015); and adaptation to anoxic conditions (Chapman et al., 1995; Graham, 1997; Casatti et al., 2009; Boswell et al., 2009; Scarabotti et al., 2011; Teresa & Casatti, 2012). When information was not available in the literature, we measured at least five adult individuals of each species using fish sampled in 2013 or individuals from study area available in the fish collection of the Department of Zoology and Botany of São Paulo State University (DZSJRP).
The traits used in this study provide important ecological information about species and have been widely used to examine the functional structure in Neotropical stream fish (Teresa & Casatti, 2012; Carvalho & Tejerina-Garro, 2015a, b; Dala-Corte et al., 2016). Because we considered both quantitative and qualitative traits, we used the mixed-variables coefficient of distance, a generalization of Gower’s distance, to extract a between species functional distance matrix and to use it in subsequent analyses (Pavoine et al., 2009). The functional distance matrix was calculated using ‘ktab.list.df’ and ‘dist.ktab’ functions of ‘ade4’ package (Dray & Dufour, 2007) in the R environment (R Development Core Team 2020).
Phylogenetic hypothesis
We constructed a composite phylogenetic hypothesis for all fish species sampled in 2013 (Fig. S1) based in the following studies: Montoya-Burgos (2003), Genner et al. (2007), Near et al. (2012), Betancur-R et al. (2013), Chen et al (2013), Mariguela et al. (2013) and Sullivan et al (2013). The phylogeny was built manually using Mesquite software v.2.75 (Maddison & Maddison, 2011). Because there is no consensus on the timing of divergence among the major actinopterygian and teleostean lineages (Near et al., 2012), the phylogenetic distance between species was estimated using the ‘bladj’ utility in Phylocom software (Webb et al., 2011). Even if only some nodes are dated, the resulting phylogenetic distances are an improvement compared to using only the number of intervening nodes (Webb et al., 2011). We used the constructed phylogeny to extract a phylogenetic distance matrix used in the further analysis. The phylogenetic distance matrix was calculated using the ‘cophenetic’ function in R environment (R Development Core Team, 2020).
Functional and phylogenetic diversity metrics
We used metrics based on functional and phylogenetic distances among species because they provide the same mathematical basis for comparing both facets (Swenson, 2014). We used the standardized effect size of mean pairwise distance (i.e., SES.MPD; Webb, 2000) and standardized effect size of the mean nearest taxon distance (i.e., SES.MNTD; Webb, 2000), because the observed functional and phylogenetic metrics could be correlated with species richness, which can create problems to identify what additional information is actually gained using functional and phylogenetic facets (Swenson, 2014). The SES.MPD and SES.MNTD represent the average functional or phylogenetic differences among taxa in each assemblage (Tucker et al., 2016), indicating functional or phylogenetic clustering when lower than zero or functional or phylogenetic overdispersion when values are greater than zero (Webb et al., 2002). Because SES.MPD calculates all pairwise distances in a sample, it is often considered to be a “basal” metric, i.e., it captures the overall functional or phylogenetic dissimilarity of the taxa in a sample (Swenson, 2014; Geheber & Geheber, 2016). Conversely, SES.MNTD is considered as “terminal” relatedness measure because it detects finer-scale functional or phylogenetic patterns (Webb, 2000; Webb et al., 2002; Swenson, 2014). For these reasons, comparing both metrics is useful (Swenson, 2014).
To calculate standardized effect size (SES), we used the independent swap null model (i.e., maintain the observed species richness and occurrence frequency in the null assemblages; Gotelli & Entsminger, 2003) with the mean value obtained from 999 randomly generated assemblages. We established our species pool as all fish species (S = 63) found in the 85 stream reaches because (i) we assumed that species sampled in each site were not dispersal limited; thus, it was feasible that all species had similar potential to occur in each site (Geheber & Geheber, 2016), and (ii) stream reaches are located in the same biogeographical province (Géry, 1969). All functional and phylogenetic metrics were calculated using incidence-based assemblage data (i.e., presence-absence). Functional and phylogenetic metrics were estimated using ‘ses.mpd’ and ‘ses.mntd’ functions of ‘picante’ package (Kembel et al., 2010) in R environment (R Development Core Team 2020).
Data analysis
Exploratory data analysis (i.e., box-plots and quantile-quantile plots) were used to identify the normality of our predictor variables and the presence of outliers (Legendre & Legendre, 2012; see Fig. S2 for a schematic representation of the analysis procedure, and Roa-Fuentes et al., 2020 for results of exploratory data analysis). We used the logit transformation to the variables representing proportions (Warton & Hui, 2011), while for variables in other units, we applied square root or loge (X) transformations (Roa-Fuentes et al., 2020). After that, all predictor variables were standardized to zero mean and unit variance since these were measured in different units. To reduce strong linear dependencies among our predictor variables, we used two approaches (Fig. S2). First, we conducted Spearman correlations and removed predictor variables with correlations ≥ 0.7 (Dormann et al., 2013). Second, we performed a forward selection procedure with two stopping rules (sensu Blanchet et al., 2008; Table S5; Fig. S2).
To describe the main environmental gradients in the study area, we performed a principal component analysis (PCA) based on local and landscape variables with Spearman correlations < 0.7 (Dormann et al., 2013; Fig. S2). To aid the interpretation, we tested the significance of the correlation coefficient between a component and each variable (P ≤ 0.001) (Lê et al., 2008).
Finally, we performed a series of multiple regression analysis to explain the taxonomic, functional, and phylogenetic facets of biodiversity as a function of local, catchment or spatial variables previous retained by the forward selection (Fig. S2). Additionally, to use the beta coefficients to interpret multiple regression, we considered the structure and commonality coefficients to gain a broader and fuller perspective on the contributions that predictor variables made to the regression equation (Nathans et al., 2012). To do this, we used commonality analysis that decomposes the variance of R2 into unique (the amount of variance in the response variable that is uniquely accounted by a single predictor variable) and common (the amount of variance in the response variable that can be explained by two or more predictors together) effects of predictors (Ray-Mukherjee et al., 2014). Total (Total = Unique effects + Common effects) represents the total contribution of a predictor to the response variable regardless of collinearity with other variables (Prunier et al., 2015). In addition, commonality analysis helps to identify predictor variables with a suppression effect (i.e., variables that indirectly enhances the prediction, by improving the prediction of others, Nathans et al., 2012; Ray-Mukherjee et al., 2014). For forward selection, we used the functions ‘forward.sel’ (‘packfor’ package; Blanchet et al., 2008) and ‘rda’ (‘vegan’ package; Oksanen et al., 2015); for PCA we used the functions ‘PCA’ and ‘dimdesc’ (‘FactoMineR’ package; Lê et al., 2008). Moreover, we used ‘lm’ function for multiple regression analysis, and ‘regr’ function ‘(yhat’ package; Nimon et al., 2008) to calculate structure and commonality coefficients. All analyses and graphical displays were carried out in the R environment (R Development Core Team 2020). The datasets generated and/or analyzed during the current study are available from the authors CARF and LC on a reasonable request.
Results
The first two PCA axes accounted for 34% of the variation in the environmental variables across the study sites (Fig. 2). The first principal component accounted for 22% of variation and differentiated streams with high values for Physical Habitat Index, a high proportion of runs, high mesohabitat heterogeneity and marginal vegetation dominated by large roots, bryophytes and pteridophytes, from streams dominated by unconsolidated substrate (mainly sand) and a high proportion of grasses (mostly Brachiaria spp.) in the marginal vegetation. The second principal component accounted for 12% of variation and differentiated streams with high proportions of pasture in the catchment, higher pH and water temperatures, from streams with a high proportion of sugarcane in the catchment. In general, the PCA differentiated two main environmental gradients, one related to local environmental variation and the other associated with variation in land use in the catchment (Fig. 2).
Principal component analysis representing the main environmental gradients in the study area. Only variables with significant correlation with the PC1 and/or PC2 are shown (P ≤ 0.001). Sampling units represented by points. Codes: bry, bryophytes and pteridophytes; Coth, other land-use in the catchment; Cpas, pasture in the catchment; Cper, perennial crops in the catchment; Csug, sugarcane in the catchment; csu, consolidated substrate; gra, grasses (mostly Brachiaria spp.); lit, leaf litter; Lro, large roots; pH, pH; phi, physical habitat index; pool, pool; run, run; shr, shrubs; sub_H, ecotone diversity; TDS, total dissolved solids; tem, water temperature; usu, unconsolidated substrate; velSD, standard deviation of water velocity
We sampled 63 fish species belonging to 18 families and six orders in the 85 stream reaches (Table S7). In general, local-scale environmental variables explained taxonomic, functional and phylogenetic facets better than catchment-scale and/or spatial variables (Table 1). Catchment-scale variables accounted only for a small portion of variation in the phylogenetic SES.MNTD, whereas network distance was not related to any facet (Tables 1 and S5).
For the taxonomic facet, local-scale variables accounted for 33% of the variation (P < 0.001; Table 1). The squared structure coefficients (r 2s ) showed that mean depth, standard deviation of width, the proportion of pools and large roots were able to account for 33%, 32%, 25% and 18% of the regression effect given by the R2. The proportion of large roots and the mean depth were the predictor variables that had the higher unique effects (11% and 10%) to predict variation in species richness (Table 1). The β coefficients for the taxonomic facet multiple regression indicated that an increase in the mean depth, standard deviation of width and the proportion of pools led to an increase in the number of species (β = 1.236; β = 0.959; β = 0.993), while an inverse effect was observed with the increase in the proportion of large roots (β = − 1.336; Table 1).
Functional and phylogenetic SES.MPD were influenced only by local-scale variables, which accounted for ≥19% of the variation (Table 1). Functional SES.MPD was affected by proportion of unconsolidated substrate, mean water velocity and mean depth (R 2Adj = 0.30; Table 1); with squared structure coefficients (r 2s ) indicating that these variables were able to account for 65%, 35% and 17% of the regression effect given by the R2 (Table 1). The proportion of unconsolidated substrate had the higher unique effect (16%) to predict functional SES.MPD variance (Table 1). The β coefficients for SES.MPD multiple regression indicated that an increase of the proportion of unconsolidated substrate and of the depth led to functional clustering of stream fish assemblages (β = -0.454; β = -0.242), while an increase in water velocity led to a functional overdispersion (β = 0.280; Table 1).
On the other hand, phylogenetic SES.MPD was significantly affected by mean depth and proportion of grasses (R 2Adj = 0.19; Table 1). Squared structure coefficients (r 2s ) indicated that mean depth and proportion of grasses accounted for 43% and 30% of the regression effect given by the R2. Mean depth had the higher unique effect (10%) to predict variance in phylogenetic SES.MPD (Table 1). The β coefficients for SES.MPD multiple regression indicated that an increase in mean depth led to phylogenetic clustering of stream fish assemblages (β = − 0.385), while an increase of the proportion of grass led to phylogenetic overdispersion (β = 0.288; Table 1).
Phylogenetic SES.MNTD was the only metric influenced by one catchment-scale variable. Although weak, catchment land-use diversity accounted for 7% of the variation in the SES.MNTD and its unique effect was 6% (Table 1). The β coefficients indicated that high catchment land-use diversity led to phylogenetic overdispersion between closely related species (β = 0.263; Table 1). Functional SES.MNTD was not explained by any of the variables (Table S5).
Commonality analysis identified the proportion of leaf litter as a suppressor variable of the phylogenetic SES.MPD regression, since its unique effect (U = 0.065) was offset by its common effect (C = − 0.064; Table S8). The same was observed for the catchment land-use richness variable on phylogenetic SES.MNTD (U = 0.094; C = -0.094; Table S8). For this reason, these variables were omitted from the multiple regression analysis of phylogenetic SES.MPD and SES.MNTD, respectively.
Discussion
We found that local environmental factors explained a substantial fraction of variance of taxonomic, functional and phylogenetic α-diversity in a highly impacted landscape. Catchment land-use and dispersal-related factors (i.e., network distance) contributed little to the variation in these three biodiversity facets or were not significant at all. Furthermore, each facet was affected by different environmental variables, and functional and phylogenic facets responded differently than the taxonomic facet. For instance, an increase of depth, pools and width variation through stream reach led to an increase in the species richness. However, for the functional facet, we observed that unconsolidated substrate and increase of depth led to more redundant assemblages, while increase of water velocity led to more complementary assemblages. For the phylogenetic facet, an increase of depth resulted in assemblages with species with similar phylogenetic history, while marginal grasses had the opposite effect. Therefore, we would like to highlight that channel depth showed an opposite effect, since it led to an increase in species richness, but resulted in functional and phylogenetic homogenization of fish assemblages. Interestingly, the phylogenetic facet was the only one that responded significantly to landscape variables.
In general, studies addressing the importance of scale-related factors for stream fish have reported that both local and catchment-scale features influence assemblage structure; however, these studies also emphasized that local habitat variables are more important than landscape variables (Lammert & Allan, 1999; Wang et al., 2003; Diana et al., 2006; Barbosa et al., 2019; but see Fitzpatrick et al., 2001). Thus, the effect of local-scale environmental factors on the structure of stream fish assemblages has been recognized widely. For instance, changes in the taxonomic and functional facets of fish assemblages have been associated with channel depth (Sheldon, 1968; Schlosser, 1982; Carvalho & Tejerina-Garro, 2015a, b; Leitão et al., 2018), marginal vegetation propagation (Brachiaria spp. grasses; Casatti et al., 2009; Casatti et al., 2015), substrate composition (Casatti et al., 2015; Leitão et al., 2018), proportion of pool habitats (Schlosser, 1982), stream width (Angermeier & Karr, 1983; Lammert & Allan, 1999) and instream habitat structures (Dala-Corte et al., 2016; Leitão et al., 2018). Our results are consistent with these previous findings. In particular, variables such as mean channel depth, mean and standard deviation of channel width and mean water velocity were important predictors of the three facets, suggesting that species’ niche differences, in terms of environmental characteristics related with stream channel morphology, contribute to the variability in stream fish assemblage structure at the spatial scale evaluated by us. This outcome is also in accordance with species sorting, which is often considered the main mechanism structuring stream communities within single small drainage basins (Heino & Mykrä 2008; Heino et al., 2015b; but see Saito et al., 2015b).
We hypothesized that landscape structure can affect the way fish assemblages respond to the landscape itself. As noted by Allan et al. (1997), contrasting results regarding the importance of environmental variables at different scales could be a consequence of the study design or indicate that mechanisms operating at local and catchment scales are in fact different and uncorrelated. Considering that local and catchment variables were obtained in a standardized way for all streams, we assumed that our sampling design was not biased to detect local-scale effects and, therefore, catchment influence is really weak in this region. In agricultural areas under a long history of land-use change, the past (i.e., decades ago) land-use in the catchment and riparian zone can be a better predictor of present day taxonomic facet of stream biota than the current land-use (Harding et al., 1998; Surasinghe & Baldwin, 2014). In this regard, Zeni et al., (2017) found that current instream habitat and fish assemblages in our study area are also related to past catchment land-use; thus, our streams seem to show evidence of legacy effects and time-lag response. This finding could indicate that stream fish assemblages in heavily modified landscapes already overpassed the threshold of response to catchment modification. It is possible that, in these assemblages, the initial disturbance (e.g., deforestation) filtered the most sensitive species and nowadays they display a weak response to agricultural intensification (Balmford, 1996; Fitzpatrick et al., 2001; Balmford & Bond, 2005). In any case, it should be highlighted that high catchment land-use diversity led to phylogenetic overdispersion assemblages at finer scale (i.e., between closely related species). In other words, catchments with a greater number and evenness of land-uses, even in this long-term agroecosystem, were able to harbor fish assemblages that are phylogenetically complementary and comprising closely related species. This could be explained by the species turnover in an impoverished regional species pool, where most of the taxa belong to a few functionally similar clades (e.g., Characidae; Table S8). However, this pattern should be further explored considering that more than 93% of the variation in phylogenetic structure at finer scale was left unexplained.
Another possible explanation for the weak relationship between assemblage diversity and landscape variables is that homogeneous landscapes can display lesser variability to overwhelm the influence of local environmental variables (Heino et al., 2007; Casatti et al., 2015). Due to the long deforestation process and agricultural land-use development (Victor et al., 2005), our study region is an agroecosystem with high dominance of agriculture, especially pasture and sugarcane, representing 70% of all area. In this context, local environment (i.e., instream habitat) could represent the last ‘shield’ against the effects of extensive agricultural systems for fishes. Thus, local-scale environmental features may become even more important for the maintenance of stream fish biodiversity, and this may be a common phenomenon in heavily modified tropical landscapes (Casatti et al., 2015; Gerhard & Verdade, 2016; but see Wang et al., 2003 for opposite conclusion for temperate streams).
We also found that different local variables were important for the variation in the three diversity facets, and the effect of a common environmental variable could be different depending on the diversity facet analyzed. For example, depth had contrasting effects on taxonomic (i.e., increased species richness) and functional or phylogenetic facets (i.e., decreased diversity). The different effects of local environmental features highlight the importance of addressing complementary facets of biodiversity, since each one can point out different patterns in the structure of stream fish assemblages under the influence of agricultural land-use. According to Lyashevska & Farnsworth (2012), species richness, the most commonly used taxonomic measure to describe biodiversity, may result in the loss of a significant portion of the information (≈ 89%). Thus, taxonomic facet could be a poor substitute for other diversity facets. For instance, by considering taxonomic facet alone, we may underestimate the importance of some environmental variables that affect complementary facets (i.e., water velocity and substrate for functional diversity and proportion of grasses for phylogenetic diversity). In the same way, we may conclude that the increase in stream depth is a good strategy to stream restoration because it is followed by an increase of species richness. However, from functional and phylogenetic perspectives, it is possible to notice that the increase in species richness, mediated by the increase in depth, is due to an addition of functionally and phylogenetically similar species (i.e., functional and phylogenetic clustering).
An increase of species richness mediated by an increase of depth is predicted by the species-area hypothesis. According to this, a large area, or volume in this case, can support more species by increasing habitat heterogeneity (i.e., habitat diversity hypothesis) and/or by increasing colonization probability and decreasing extinction risk (for review, see Connor & McCoy, 2001). However, contrary to what is found in the literature (Tilman, 2001; Petchey & Gaston, 2002), an increase of species richness in our study did not lead to an increase of functional and phylogenetic diversity. It is possible that, in this region impacted by anthropogenic influences for a long time, past environmental filtering excluded most of the distinct species, leading to functional and phylogenetic homogenization of the regional species pool (Zeni et al., 2019). Thus, even if a large stream volume (i.e., increasing depth) is available, only redundant species can colonize the study streams. Although the increase of depth did not lead to complementary assemblages, redundancy in agricultural landscapes can act as a buffer to further functional diversity loss, since one redundant species can be replaced by another one without jeopardizing ecosystem function. In this context, siltation (i.e., decrease of depth) due to watershed and riparian degradation (Allan, 2004) can gradually eliminate redundant species through time, leading to the functional diversity loss of stream fish assemblages in tropical agroecosystems (Dala-Corte et al., 2016). Consequently, watershed management and riparian restoration, even in long-term agroecosystems, are also necessary to protect streams from siltation and further ecosystem function loss.
Through complementary facets of biodiversity, we can also identify environmental variables that are responsible for functional or phylogenetic overdispersion, such as water velocity and proportion of grasses, respectively. The increase of water velocity in our region usually indicates streams that are physically more structured with a higher proportion of riffles (i.e., shallow areas with consolidated substrate and high flow). Riffles could mediate the establishment of functionally distinct species (e.g., species with large pectoral fins, flattened bodies, dorsally situated eyes and ventral mouths), which are usually absent in less structured streams (Ribeiro et al., 2016). Marginal grasses can act similarly to macrophytes by proving shelter and foraging sites to fish species from different linages (i.e., phylogenetically distinct), as gymnotids (Gymnotus spp.) and cichlids (Crenicichla spp.). According to Roa-Fuentes et al. (2015), because some functional traits (e.g., ecomorphological traits) can exhibit strong phylogenetic signal, then the concordance between functional and phylogenetic facets could be expected. Although not observed in our study, marginal grasses have been associated with functional homogenization (Casatti et al., 2015). For this reason, we strongly recommend further studies about the influence of marginal grasses on all stream fish biodiversity facets.
In general, we found no effects of dispersal-related processes (i.e., network distance) on all three biodiversity facets. In a certain way, this is not surprising, since environmental control usually prevails over spatial constraints within small drainage basins (Mykrä et al., 2007; Heino & Mykrä 2008; Heino et al., 2015b). Among the possible reasons for absence of spatial structuring in stream assemblages is that, given enough time, stream biota can readily disperse between sites (Heino & Mykrä 2008). Another possibility that does not necessary exclude the first one is that the fish fauna here has already gone through a homogenization process, through extensive habitat modifications, introductions of non-native species, and extirpation of native ones. Therefore, fish assemblages are probably dominated by widespread habitat generalist species (McKinney & Lockwood, 1999; Devictor et al., 2008), which are closely related phylogenetically. It is also known that the importance of dispersal-related processes (e.g., colonization history or mass effects) is less expected to occur when the regional species pool is small or if it has been degraded by an intense disturbance (Chase, 2003; Goldenberg Vilar et al., 2014), as has probably happened in our present study region.
Stream restoration in human-altered landscapes
One critical factor for the success of stream restoration is the spatial scale of intervention (Lake et al., 2007). Despite that, habitat restoration projects are usually implemented without an understanding of the spatial scale necessary to produce positive ecological effects (Alexander & Allan, 2007; Sheldon et al., 2012). These scale-related effects remain an important and poorly understood question, and they might be one of the reasons for why a part of restoration projects have provided little evidence of ecological success (Alexander & Allan, 2007). One of the most used approaches to restore the ecological integrity of streams is the re-establishment of the native riparian forest (Harding et al., 1998; Sheldon et al., 2012). It has been demonstrated that one conserved riparian forest can regulate water temperature, diminish sediment inputs, stabilize stream banks (Osborne & Kovacic, 1993), provide large wood debris to stream channels in tropical agricultural landscapes (Paula et al., 2013), and maintain biotic integrity of fish assemblages in agricultural streams (Fitzpatrick et al., 2001).
Our results indicated that riparian forest, measured as a 30 m buffer zone around the river network (i.e., the minimum width established by the current Brazilian Forest Code) had weak or no effects on the three analyzed biodiversity facets. We would like to highlight, though, that this does not mean that riparian forest is not important for fish assemblages (for a review, see Pusey & Arthington, 2003). Conversely, since the riparian forest in our study area has been heavily altered over past decades (Silva et al., 2007), this result could indicate a weak riparian buffering effect and, consequently, that ecosystem functions and processes mediated by this adjacent area were already lost. In fact, in the 85 studied catchments only, 21% of the buffer zone along drainage network is composed of forests, whereas 60% is covered by herbaceous and shrub vegetation and 12% by pasture for livestock (unpublished data). However, the areas occupied by forest, herbaceous and shrub vegetation are not pristine because cattle usually can graze there. According to Fitzpatrick et al. (2001), even minor alterations of stream network buffer (e.g., 10% agriculture within a 50-m buffer) may inflict harmful effects on fish fauna in agricultural catchments. Consequently, actions directed toward riparian forest restoration in the drainage network should be a priority in the studied area (Casatti et al., 2012; Casatti et al., 2015).
Our findings also suggest that, in highly altered tropical streams, actions on a local-scale habitat features could produce effects on the three facets of biodiversity. Although this is an interesting finding, we should not neglect restoration actions at the landscape scale; in contrast, landscape restoration should be a priority in heavily modified tropical agroecosystems. Substantial improvements in the ecological integrity of streams are more likely to be accomplished with large-scale actions (Lake et al., 2007; Palmer et al., 2010; Wahl et al., 2013), as suggested by the hierarchy theory (Hynes, 1975; Allen & Starr, 1982; Johnson et al., 2007). For instance, water quality, disturbance regime, regional species pools and hydrological regimes respond to actions on large spatial scales (Palmer et al., 2010).
Finally, it is worth to mention that biodiversity responses to any local environmental variable must be thoroughly investigated, since changes in one environmental feature (e.g., depth) could generate contrasting effects on the different facets of biodiversity (Li et al., 2020). Additionally, the same spatial scale may not be important in every catchment and, therefore, successful restoration is context dependent (Sheldon et al., 2012). For this reason, we strongly recommend that any restoration actions should be correctly monitored over time (Palmer et al., 2010; Sheldon et al., 2012). This is because intensive agricultural practices may severely alter stream biota, and the influence of this disturbance may be long lasting (Harding et al., 1998).
Conclusions
In conclusion, our results indicated that local environmental factors are the most important predictors for variation in taxonomic, functional and phylogenetic facets of stream fish assemblages in a heavily modified tropical landscape. In contrast, catchment features and spatial structuring contributed little to the variation in the biodiversity facets or were not significant at all. It is possible that the long deforestation history in the study region homogenized the landscape and the regional species pool, thereby decreasing the potential importance of factors acting on large spatial scales over fish assemblages. Our results suggest that restoration actions focused on local-scale factors could produce significant effects on the three biodiversity facets. However, landscape restoration should also be a priority in heavily modified tropical agroecosystems, and restoration actions should thus carefully examine the effects of environmental variables, since each factor could show contrasting effects on different biodiversity facets.
References
Alexander, G. G. & J. D. Allan, 2007. Ecological success in stream restoration: case studies from the Midwestern United States. Environmental Management 40: 245–255.
Allan, J. D., 2004. Landscapes and riverscapes: the influence of land use on stream ecosystems. Annual Review of Ecology, Evolution, and Systematics 35: 257–284.
Allan, J. D., D. L. Erickson & J. Fay, 1997. The influence of catchment land use on stream integrity across multiple spatial scales. Freshwater Biology 37: 149–161.
Allen, T. H. F. & T. B. Starr, 1982. Hierarchy: Perspectives for Ecological Complexity. Chicago University Press, Chicago, IL.
Altermatt, F., 2013. Diversity in riverine metacommunities: a network perspective. Aquatic Ecology 47: 365–377.
Angermeier, P. L. & J. R. Karr, 1983. Fish communities along environmental gradients in a system of tropical streams. Environmental Biology of Fishes 9: 117–135.
Angermeier, P. L. & M. R. Winston, 1998. Local vs. regional influences on local diversity in stream fish communities of Virginia. Ecology 79: 911–927.
Aquino, P. P. U. & R. G. Colli, 2017. Headwater captures and the phylogenetic structure of freshwater fish assemblages: a case study in central Brazil. Journal of Biogeography 44: 207–216.
Balmford, A., 1996. Extinction filters and current resilience: the significance of past selection pressures for conservation biology. Trends in Ecology & Evolution 11: 193–196.
Balmford, A. & W. Bond, 2005. Trends in the state of nature and their implications for human well-being. Ecology Letters 8: 1218–1234.
Barbosa, H. D. O., P. P. Borges, R. B. Dala-Corte, P. T. D. A. Martins & F. B. Teresa, 2019. Relative importance of local and landscape variables on fish assemblages in streams of Brazilian savanna. Fisheries Management and Ecology 26: 119–130.
Bengtsson, J., 2010. Applied (meta)community ecology: diversity and ecosystem services at the intersection of local and regional processes. In Verhoef, H. A. & P. J. Morin (eds.), Community ecology. Oxford University Press, Oxford: 115–130.
Benone, N. L., C. G. Leal, L. L. de Santos, T. P. Mendes, J. Heino & L. F. A. Montag, 2020. Unravelling patterns of taxonomic and functional diversity of Amazon stream fish. Aquatic Sciences 82, Article number: 75.
Betancur-R, R., R. E. Broughton, E. O. Wiley, K. Carpenter, J. A. López, C. Li, N. I. Holcroft, D. Arcila, M. Sanciangco, J. C. Cureton II, F. Zhang, T. Buser, M. A. Campbell, J. A. Ballesteros, A. Roa-Varon, S. Willis, W. C. Borden, T. Rowley, P. C. Reneau, D. J. Hough, G. Lu, T. Grande, G. Arratia & G. Ortí, 2013. The Tree of Life and a New Classification of Bony Fishes. PLOS Currents Tree of Life. Apr 18. Edition 1.
Beyer, H. L., 2004. Hawth’s Analysis Tools for ArcGIS. http://www.spatialecology.com/htools.
Blanchet, F. G., P. Legendre & D. Borcard, 2008. Forward selection of explanatory variables. Ecology 89: 2623–2632.
Borcard, D., F. Gillet & P. Legendre, 2011. Numerical Ecology with R. Springer, New York.
Bordignon, C. R., L. Casatti, M. A. Pérez-Mayorga, F. B. Teresa & G. L. Brejão, 2015. Fish complementarity is associated to forests in Amazonian streams. Neotropical Ichthyology 13: 579–590.
Boswell, M. G., M. C. Wells, L. M. Kirk, Z. Ju, Z. Zhang, R. E. Booth & R. B. Walter, 2009. Comparison of gene expression responses to hypoxia in viviparous (Xiphophorus) and oviparous (Oryzias) fishes using a medaka microarray. Comparative Biochemistry and Physiology, Part C 149: 258–265.
Brown, B. L. & C. M. Swan, 2010. Dendritic network structure constrains metacommunity properties in riverine ecosystems. Journal of Animal Ecology 79: 571–580.
Carvalho, R. A. & F. L. Tejerina-Garro, 2015a. Environmental and spatial processes: what controls the functional structure of fish assemblages in tropical rivers and headwater streams? Ecology of Freshwater Fish 24: 317–328.
Carvalho, R. A. & F. L. Tejerina-Garro, 2015b. The influence of environmental variables on the functional structure of headwater stream fish assemblages: a study of two tropical basins in Central Brazil. Neotropical Ichthyology 13: 349–360.
Casatti, L. & R. M. C. Castro, 2006. Testing the ecomorphological hypothesis in a headwater riffles fish assemblage of the rio São Francisco, southeastern Brazil. Neotropical Ichthyology 4: 203–214.
Casatti, L., C. P. Ferreira & F. R. Carvalho, 2009. Grass-dominated stream sites exhibit low fish species diversity and dominance by guppies: an assessment of two tropical pasture river basins. Hydrobiologia 632: 273–283.
Casatti, L., F. B. Teresa, T. Gonçalves-Souza, E. Bessa, A. R. Manzotti, C. S. Gonçalves & J. O. Zeni, 2012. From forests to cattail: how does the riparian zone influence stream fish? Neotropical Ichthyology 10: 205–214.
Casatti, L., F. B. Teresa, J. O. Zeni, M. D. Ribeiro, G. L. Brejão & M. Ceneviva-Bastos, 2015. More of the same: high functional redundancy in stream fish assemblages from tropical agroecosystems. Environmental Management 55: 1300–1314.
Chapman, L. J., L. S. Kaufman, C. A. Chapman & F. E. McKenzie, 1995. Hypoxia tolerance in twelve species of east African cichlids: potential for low oxygen refugia in Lake Victoria. Conservation Biology 9: 1274–1287.
Chase, J. M., 2003. Community assembly: when should history matter? Oecologia 136: 489–498.
Chen, W. J., S. Lavoué & R. L. Mayden, 2013. Evolutionary origin and early biogeography of otophysan fishes (Ostariophysi: Teleostei). Evolution 67: 2218–2239.
Cianfrani, C. M., S. M. Sullivan, W. C. Hession & M. C. Watzin, 2012. A multitaxonomic approach to understanding local- versus watershed-scale influences on stream biota in the Lake Champlain basin, Vermont, USA. River Research and Applications 28: 973–988.
Connor, E. F. & E. D. McCoy, 2001. Species area relationships. In Encyclopedia of Biodiversity, vol. 5. Academic Press, San. Diego: 397–411.
Cruz, B. B., L. E. Miranda & M. Cetra, 2013. Links between riparian landcover, instream environment and fish assemblages in headwater. Ecology of Freshwater Fish 22: 607–616.
Dala-Corte, R. B., X. Giam, J. D. Olden, F. G. Becker, T. F. Guimarães & A. S. Melo, 2016. Revealing the pathways by which agricultural land-use affects stream fish communities in South Brazilian grasslands. Freshwater Biology 61: 1921–1934.
Devictor, V., R. Julliard & F. Jiguet, 2008. Distribution of specialist and generalist species along spatial gradients of habitat disturbance and fragmentation. Oikos 117: 507–514.
Diana, M., J. D. Allan & D. Infante, 2006. The influence of physical habitat and land use on stream fish assemblages in Southeastern Michigan. American Fisheries Society Symposium 48: 359–374.
Díaz, S., S. Lavorel, F. de Bello, F. Quétier, K. Grigulis & T. M. Robson, 2007. Incorporating plant functional diversity effects in ecosystem service assessments. Proceedings of the National Academy of Sciences of the United States of America 104: 20684–20689.
Dormann, C. F., J. Elith, S. Bacher, C. Buchmann, G. Carl, G. Carré, J. R. G. Marquéz, B. Gruber, B. Lafourcade, P. J. Leitão, T. Münkemüller, C. McClean, P. E. Osborne, B. Reineking, B. Schröder, A. K. Skidmore, D. Zurell & S. Lautenbach, 2013. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36: 27–46.
Dray, S. & A. B. Dufour, 2007. The ade4 package: implementing the duality diagram for ecologists. Journal of Statistical Software 22: 1–20.
Faith, D. P., 2008. Threatened species and the potential loss of phylogenetic diversity: conservation scenarios based on estimated extinction probabilities and phylogenetic risk analysis. Conservation Biology 22: 1461–1470.
Feld, C. K., 2013. Response of three lotic assemblages to riparian and catchment-scale land use: implications for designing catchment monitoring programmes. Freshwater Biology 58: 715–729.
Fitzpatrick, F. A., B. C. Scudder, B. N. Lenz & D. J. Sullivan, 2001. Effects of multi-scale environmental characteristics on agricultural stream biota in Eastern Wisconsin. Journal of the American Water Resources Association 37: 1489–1507.
Forest, F., R. Grenyer, M. Rouget, T. J. Davies, R. M. Cowling, D. P. Faith, A. Balmford, J. C. Manning, Ş. Procheş, M. van der Bank, G. Reeves, T. A. J. Hedderson & V. Savolainen, 2007. Preserving the evolutionary potential of floras in biodiversity hotspots. Nature 445: 757–760.
Frissell, C. A., W. J. Liss, C. E. Wareen & M. D. Hurley, 1986. A hierarchical framework for stream habitat classification: viewing streams in a watershed context. Environmental Management 10: 199–214.
García-Girón, J., J. Heino, F. García-Criado, C. Fernández-Aláez & J. Alahuhta, 2020. Biotic interactions hold the key to understanding metacommunity organization. Ecography. https://doi.org/10.1111/ecog.05032.
Geheber, A. D. & P. K. Geheber, 2016. The effect of spatial scale on relative influences of assembly processes in temperate stream fish assemblages. Ecology 97: 2691–2704.
Genner, M. J., O. Seehausen, D. H. Lunt, D. A. Joyce, P. W. Shaw, G. R. Carvalho & G. F. Turner, 2007. Age of cichlids: new dates for ancient lake fish radiations. Molecular Biology and Evolution 24: 1269–1282.
Gerhard, P. & L. M. Verdade, 2016. Stream fish diversity in an agricultural landscape of Southeastern Brazil. In Gheler-Costa, C., M. C. Lyra-Jorge & L. M. Verdade (eds.), Biodiversity in Agricultural Landscapes of Southeastern Brazil. De Gruyter Open, Berlin: 206–224.
Géry, J., 1969. The fresh-water fishes of South America. In Fitkau, E. J. (ed.), Biogeography and Ecology in South America. Dr. W. Junk, The Hague: 828–848.
Goldenberg Vilar, A., H. van Dam, E. E. van Loon, J. A. Vonk, H. van Der Geest & W. Admiraal, 2014. Eutrophication decreases distance decay of similarity in diatom communities. Freshwater Biology 59: 1522–1531.
Gotelli, N. & G. Entsminger, 2003. Swap algorithms in null model analysis. Ecology 84: 532–535.
Graham, J. B., 1997. Air-Breathing Fishes. Academic Press, San Diego.
Gustafson, E. J., 1998. Quantifying landscape spatial pattern: what is the state of the art? Ecosystems 1: 143–156.
Harding, J. S., E. F. Benfield, P. V. Bolstad, G. S. Helfman & E. B. D. Jones III, 1998. Stream biodiversity: The ghost of land use past. Proceedings of the National Academy of Sciences of the United States of America 95: 14843–14847.
Heino, J., 2013. The importance of metacommunity ecology for environmental assessment research in the freshwater realm. Biological Reviews 88: 166–178.
Heino, J. & H. Mykrä, 2008. Control of stream insect assemblages: roles of spatial configuration and local environmental factors. Ecological Entomology 33: 614–622.
Heino, J., H. Mykrä, J. Kotanen & T. Muotka, 2007. Ecological filters and variability in stream macroinvertebrate communities: do taxonomic and functional structure follow the same path? Ecography 30: 217–230.
Heino, J., H. Mykrä & J. Kotanen, 2008. Weak relationships between landscape characteristics and multiple facets of stream macroinvertebrate biodiversity in a boreal drainage basin. Landscape Ecology 23: 417–426.
Heino, J., A. S. Melo & L. M. Bini, 2015a. Reconceptualising the beta diversity-environmental heterogeneity relationship in running water systems. Freshwater Biology 60: 223–235.
Heino, J., A. S. Melo, T. Siqueira, J. Soininen, S. Valanko & L. M. Bini, 2015b. Metacommunity organisation, spatial extent and dispersal in aquatic systems: patterns, processes and prospects. Freshwater Biology 60: 845–869.
Heino, J., J. Alahuhta, T. Ala-Hulkko, H. Antikainen, L. M. Bini, N. Bonada, T. Datry, T. Erős, J. Hjort, O. Kotavaara, A. S. Melo & J. Soininen, 2017. Integrating dispersal proxies in ecological and environmental research in the freshwater realm. Environmental Reviews 25: 334–349.
Hoeinghaus, D. J., K. O. Winemiller & J. S. Birnbaum, 2007. Local and regional determinants of stream fish assemblage structure: inferences based on taxonomic vs. functional groups. Journal of Biogeography 34: 324–338.
Hynes, H. B. N., 1975. The stream and its valley. Verhandlungen der Internationalen Vereinigung für Theoretische und Angewandte Limnologie 19: 1–15.
IPT, 1999. Diagnóstico da situação atual dos recursos hídricos e estabelecimento de diretrizes técnicas para a elaboração do Plano da Bacia Hidrográfica do São José dos Dourados—minuta. Comitê da Bacia Hidrográfica do São José dos Dourados e Fundo Estadual de Recursos Hídricos
Johnson, R. K., M. T. Furse, D. Hering & L. Sandin, 2007. Ecological relationships between stream communities and spatial scale: implications for designing catchment-level monitoring programmes. Freshwater Biology 52: 939–958.
Kembel, S. W., P. D. Cowan, M. R. Helmus, W. K. Cornwell, H. Morlon, D. D. Ackerly, S. P. Blomberg & C. O. Webb, 2010. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26: 1463–1464.
Lake, P. S., N. Bond & P. Reich, 2007. Linking ecological theory with stream restoration. Freshwater Biology 52: 597–615.
Lammert, M. & J. D. Allan, 1999. Assessing biotic integrity of streams: effects of scale in measuring the influence of land use/cover and habitat structure on fish and macroinvertebrates. Environmental Management 23: 257–270.
Lê, S., J. Josse & F. Husson, 2008. FactoMineR: an R package for multivariate analysis. Journal of Statistical Software 25: 1–18.
Legendre, P. & L. F. J. Legendre, 2012. Numerical Ecology, 3rd ed. Elsevier, Amsterdam.
Legendre, P., D. Borcard & F. G. Blanchet, 2013. PCNM: MEM spatial eigenfunction and principal coordinate analyses. R package version 2.1-2/r109. http://R-Forge.R-project.org/projects/sedar/.
Leitão, R. P., J. Zuanon, D. Mouillot, C. G. Leal, R. M. Hughes, P. R. Kaufmann, S. Villéger, P. S. Pompeu, D. Kasper, F. R. de Paula, S. F. B. Ferraz & T. A. Gardner, 2018. Disentangling the pathways of land use impacts on the functional structure of fish assemblages in Amazon streams. Ecography 41: 219–232.
Li, Z., J. Wang, Z. Liu, X. Meng, J. Heino, X. Jiang, X. Xiong, X. Jiang & Z. Xie, 2019. Different responses of taxonomic and functional structures of stream macroinvertebrate communities to local stressors and regional factors in a subtropical biodiversity hotspot. Science of the Total Environment 655: 1288–1300.
Li, Z., Z. Liu, J. Heino, X. Jiang, J. Wang, T. Tang & Z. Xie, 2020. Discriminating the effects of local stressors from climatic factors and dispersal processes on multiple biodiversity dimensions of macroinvertebrate communities across subtropical drainage basins. Science of the Total Environment 711: 134750.
Luck, G. W., A. Carter & L. Smallbone, 2013. Changes in bird functional diversity across multiple land uses: interpretations of functional redundancy depend on functional group identity. PLOS ONE 8: e63671.
Lyashevska, O. & K. D. Farnsworth, 2012. How many dimensions of biodiversity do we need? Ecological Indicators 18: 485–492.
Maddison, W. P. & D. R. Maddison, 2011. Mesquite: a modular system for evolutionary analysis. Version 2.75. http://mesquiteproject.org.
Mariguela, T. C., M. A. Alexandrou, F. Foresti & C. Oliveira, 2013. Historical biogeography and cryptic diversity in the Callichthyinae (Siluriformes, Callichthyidae). Journal of Zoological Systematics and Evolutionary Research 51: 308–315.
McKinney, M. L. & J. L. Lockwood, 1999. Biotic homogenization: a few winners replacing many losers in the next mass extinction. Trends in Ecology & Evolution 14: 450–453.
Menezes, S., D. J. Baird & A. M. V. M. Soares, 2010. Beyond taxonomy: a review of macroinvertebrate trait-based community descriptors as tools for freshwater biomonitoring. Journal of Applied Ecology 47: 711–719.
Montoya-Burgos, J. I., 2003. Historical biogeography of the catfish genus Hypostomus (Siluriformes: Loricariidae), with implications on the diversification of Neotropical ichthyofauna. Molecular Ecology 12: 1855–1867.
Mykrä, H., J. Heino & T. Muotka, 2007. Scale-related patterns in the spatial and environmental components of stream macroinvertebrate assemblage variation. Global Ecology and Biogeography 16: 149–159.
Nalon, M. A., I. S. A. Matto & G. A. D. C. Franco, 2008. Meio físico e aspectos da vegetação. In Rodrigues, R. R. & V. L. R. Bononi (orgs) Diretrizes para conservação e restauração da biodiversidade no Estado de São Paulo. Instituto de Botânica, São Paulo: 12–21.
Nathans, L. L., F. L. Oswald & K. Nimon, 2012. Interpreting multiple linear regression: A guidebook of variable importance. Practical Assessment, Research & Evaluation 17: 1–19.
Near, T. J., R. I. Eytan, A. Dornburg, K. L. Kuhn, J. A. Moore, M. P. Davis, P. C. Wainwright, M. Friedman & W. L. Smith, 2012. Resolution of ray-finned fish phylogeny and timing of diversification. Proceedings of the National Academy of Sciences 109: 13698–13703.
Nimon, K., M. Lewis, R. Kane & R. M. Haynes, 2008. An R package to compute commonality coefficients in multiple regression case: an introduction to the package and a practical example. Behavior Research Methods 40: 457–466.
Oksanen, J., F. G. Blanchet, M. Friendly, R. Kindt, P. Legendre, D. McGlinn, P. R. Minchin, R. B. O’Hara, G. L. Simpson, P. Solymos, M. H. H. Stevens, E. Szoecs & H. Wagner, 2015. Vegan: community ecology package. R Package Version. 2.0-10.
Osborne, L. L. & D. A. Kovacic, 1993. Riparian vegetated buffer strips in water-quality restoration and stream management. Freshwater Biology 29: 243–258.
Palmer, M. A., H. L. Menninger & E. Bernhard, 2010. River restoration, habitat heterogeneity and biodiversity: a failure of theory or practice? Freshwater Biology 55: 205–222.
Paula, F. R., P. Gerhard, S. J. Wenger, A. Ferreira, C. A. Vettorazzi & S. F. B. Ferraz, 2013. Influence of forest cover on in-stream large wood in an agricultural landscape of southeastern Brazil: a multi-scale analysis. Landscape Ecology 28: 13–27.
Pavoine, S., J. Vallet, A. B. Dufour, S. Gachet & H. Daniel, 2009. On the challenge of treating various types of variables: application for improving the measurement of functional diversity. Oikos 118: 391–402.
Petchey, O. L. & K. J. Gaston, 2002. Functional diversity (FD), species richness and community composition. Ecology Letters 5: 402–411.
Prunier, J. G., M. Colyn, X. Legendre, K. F. Nimon & M. C. Flamand, 2015. Multicollinearity in spatial genetics: separating the wheat from the chaff using commonality analyses. Molecular Ecology 24: 263–283.
Pusey, B. J. & A. H. Arthington, 2003. Importance of the riparian zone to the conservation and management of freshwater fish: a review. Marine and Freshwater Research 54: 1–16.
R Development Core Team, 2020. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/
Rahel, F. J., 2002. Homogenization of freshwater faunas. Annual Review of Ecology, Evolution, and Systematics 33: 291–315.
Ray-Mukherjee, J., K. Nimon, S. Mukherjee, D. W. Morris, R. Slotow & M. Hamer, 2014. Using commonality analysis in multiple regressions: a tool to decompose regression effects in the face of multicollinearity. Methods in Ecology and Evolution 5: 320–328.
Ribeiro, M. D., F. B. Teresa & L. Casatti, 2016. Use of functional traits to assess changes in stream fish assemblages across a habitat gradient. Neotropical Ichthyology 14: e140185.
Roa-Fuentes, C. A. & L. Casatti, 2017. Influence of environmental features at multiple scales and spatial structure on stream fish communities in a tropical agricultural region. Journal of Freshwater Ecology 32: 281–295.
Roa-Fuentes, C. A., L. Casatti & R. M. Romero, 2015. Phylogenetic signal and major ecological shifts in the ecomorphological structure of stream fish in two river basins in Brazil. Neotropical Ichthyology 13: 165–178.
Roa-Fuentes, C. A., J. Heino, M. V. Cianciaruso, S. Ferraz, J. O. Zeni & L. Casatti, 2019. Taxonomic, phylogenetic and functional β-diversity patterns of stream fish in tropical agroecosystems. Freshwater Biology 64: 447–460.
Roa-Fuentes, C. A., L. Casatti & J. O. Zeni, 2020. Local and landscape environmental variables from 86 Neotropical streams, Upper Paraná River basin, Brazil (Version 1.0.0) [Data set]. Zenodo. http://doi.org/10.5281/zenodo.3976342.
Roth, N. E., J. D. Allan & D. L. Erickson, 1996. Landscape influences on stream biotic integrity assessed at multiple spatial scales. Landscape Ecology 11: 141–156.
Rudorff, B. F. T., D. A. Aguiar, W. F. Silva, L. M. Sugawara, M. Adami & M. A. Moreira, 2010. Studies on the rapid expansion of sugarcane for ethanol production in São Paulo State (Brazil) using landsat data. Remote Sensing 2: 1057–1076.
Safi, K., M. V. Cianciaruso, R. D. Loyola, D. Brito, K. Armour-Marshall & J. A. F. Diniz-Filho, 2011. Understanding global patterns of mammalian functional and phylogenetic diversity. Philosophical Transactions of the Royal Society B: Biological Sciences 366: 2536–2544.
Saito, V. S., T. Siqueira & A. A. Fonseca-Gessner, 2015a. Should phylogenetic and functional diversity metrics compose macroinvertebrate multimetric indices for stream biomonitoring? Hydrobiologia 745: 167–179.
Saito, V. S., J. Soininen, A. A. Fonseca-Gessner & T. Siqueira, 2015b. Dispersal traits drive the phylogenetic distance decay of similarity in Neotropical stream metacommunities. Journal of Biogeography 42: 2101–2111.
Santos, F. B., F. C. Ferreira & K. E. Esteves, 2015. Assessing the importance of the riparian zone for stream fish communities in a sugarcane dominated landscape (Piracicaba River Basin, Southeast Brazil). Environmental Biology of Fishes 98: 1895–1912.
Scarabotti, P. A., J. A. López, R. Ghirardi & M. J. Parma, 2011. Morphological plasticity associated with environmental hypoxia in characiform fishes from Neotropical floodplain lakes. Environmental Biology of Fishes 92: 391–402.
Schlosser, I. J., 1982. Fish community structure and function along two habitat gradients in a headwater stream. Ecological Monographs 52: 395–414.
Sheldon, A. L., 1968. Species diversity and longitudinal succession in stream fishes. Ecology 49: 193–198.
Sheldon, F., E. E. Peterson, E. L. Boone, S. Sippel, S. E. Bunn & B. D. Harch, 2012. Identifying the spatial scale of land use that most strongly influences overall river ecosystem health score. Ecological Applications 22: 2188–2203.
Silva, A. M., L. Casatti, C. A. Álvares, A. M. Leite, L. A. Martinelli & S. F. Durrant, 2007. Soil loss risk and habitat quality in streams of a mesoscale river basin. Scientia Agricola 64: 336–343.
Strayer, R., E. Beighley, L. C. Thompson, S. Brooks, C. Nilsson, G. Pinay & R. J. Naiman, 2003. Effects of land cover on stream ecosystems: roles of empirical models and scaling issues. Ecosystems 6: 407–423.
Sullivan, J. P., J. Muriel-Cunha & J. G. Lundberg, 2013. Phylogenetic relationships and molecular dating of the major groups of catfishes of the Neotropical superfamily Pimelodoidea (Teleostei, Siluriformes). Proceedings of the Academy of Natural Sciences of Philadelphia 162: 89–110.
Surasinghe, T. & R. F. Baldwin, 2014. Ghost of land-use past in the context of current land cover: evidence from salamander communities in streams of Blue Ridge and Piedmont ecoregions. Canadian Journal of Zoology 92: 527–536.
Swenson, N. G., 2014. Functional and Phylogenetic Ecology in R. Springer UseR! Series. Springer, New York.
Teresa, F. B. & L. Casatti, 2012. Influence of forest cover and mesohabitat types on functional and taxonomic diversity of fish communities in Neotropical lowland streams. Ecology of Freshwater Fish 21: 433–442.
Tilman, D., 2001. Functional diversity. In Encyclopedia of Biodiversity, vol. 3. Academic Press, San Diego: 109–120.
Tucker, C. M., M. W. Cadotte, S. B. Carvalho, T. J. Davies, S. Ferrier, S. A. Fritz, R. Grenyer, M. R. Helmus, L. S. Jin, A. O. Mooers, S. Pavoine, O. Purschke, D. W. Redding, D. F. Rosauer, M. Winter & F. Mazel, 2016. A guide to phylogenetic metrics for conservation, community ecology and macroecology. Biological Reviews 92: 698–715.
Victor, M. A. M., A. C. Cavalli, J. R. Guillaumon & R. S. Filho, 2005. Cem anos de devastação - Revisitada 30 anos depois. Ministério do Meio Ambiente, Brasília.
Wahl, C. M., A. Neils & D. Hooper, 2013. Impacts of land use at the catchment scale constrain the habitat benefits of stream riparian buffers. Freshwater Biology 58: 2310–2324.
Wang, L., J. Lyons, P. Rasmussen, P. Seelbach, T. Simon, M. Wiley, P. Kanehl, E. Baker, S. Niemela & P. M. Stewart, 2003. Watershed, reach, and riparian influences on stream fish assemblages in the Northern lakes and forest ecoregion, U.S.A. Canadian Journal of Fisheries and Aquatic Sciences 60: 491–505.
Warton, D. I. & F. K. C. Hui, 2011. The arcsine is asinine: the analysis of proportions in ecology. Ecology 92: 3–10.
Webb, C. O., 2000. Exploring the phylogenetic structure of ecological communities: an example for rain forest trees. The American Naturalist 156: 145–155.
Webb, C. O., D. D. Ackerly, M. A. McPeek & M. J. Donoghue, 2002. Phylogenies and community ecology. Annual Review of Ecology, Evolution, and Systematics 33: 475–505.
Webb, C. O., D. D. Ackerly & S. Kembel, 2011. Phylocom: software for the analysis of phylogenetic community structure and character evolution. User’s manual, version 4: 2.
Zeni, J. O. & L. Casatti, 2014. The influence of habitat homogenization on the trophic structure of fish fauna in tropical streams. Hydrobiologia 726: 259–270.
Zeni, J. O., D. J. Hoeinghaus & L. Casatti, 2017. Effects of pasture conversion to sugarcane for biofuels production on stream fish assemblages in tropical agroecosystems. Freshwater Biology 62: 2026–2038.
Zeni, J. O., M. A. Pérez-Mayorga, C. A. Roa-Fuentes, G. L. Brejão & L. Casatti, 2019. How deforestation drives stream habitat changes and the functional structure of fish assemblages in different tropical regions. Aquatic Conservation: Marine and Freshwater Ecosystems 29: 1238–1252.
Acknowledgements
We thank our colleagues from the Ichthyology Laboratory for their help during laboratory and fieldwork; UNESP campus São José do Rio Preto (IBILCE) and Finnish Environment Institute for facilities; ICMBio for the collecting license (SISBIO 5580-1/11435); landowners for permission to conduct research on their properties; Frederico T. S. Miranda and Márcia S. Morinaga for their help with land-use data; Francisco Langeani and Fernando R. Carvalho for their help with fish identification. We also appreciate the critical reading and suggestions from the two anonymous reviewers. This study received financial support from Fundação de Amparo à Pesquisa do Estado de São Paulo – FAPESP (2012/05983-0). CARF received a scholarship from The World Academy of Sciences and Conselho Nacional de Desenvolvimento Científico e Tecnológico (TWAS–CNPq Postgraduate Fellowship Program 190199/2011-3); JOZ is financially supported by FAPESP (2018/06033-1); LC and MVC are financially supported by CNPq (301877/2017-3 and 307796/2015-9, respectively); CARF receives research support from Universidad Pedagógica y Tecnológica de Colombia, UPTC (Research call: VIE 07-2020; Research project code: SGI-2908).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: David J. Hoeinghaus, Jaquelini O. Zeni, Gabriel L. Brejão, Rafael P. Leitão & Renata G. Frederico / Neotropical Stream Fish Ecology in a Changing Landscape
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Roa-Fuentes, C.A., Heino, J., Zeni, J.O. et al. Importance of local and landscape variables on multiple facets of stream fish biodiversity in a Neotropical agroecosystem. Hydrobiologia 849, 4447–4464 (2022). https://doi.org/10.1007/s10750-020-04396-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-020-04396-7