Abstract
The ability to develop reversible dermal extensions on the lower jaw in some South American characiform fishes has been proposed as a way to optimize the performance of aquatic surface respiration (ASR) during hypoxic conditions. These structures are formed by edema of the hypodermal tissues and can develop in a large proportion of individuals inhabiting a lake within a few hours following daily changes in dissolved oxygen. Our study report the development of dermal lip protuberances in eleven species of characiform fishes associated with periods of strong environmental hypoxia in floodplain lakes of Salado River, Argentina. Protuberances occurred in different basic forms in fishes with divergent head morphology (genera Astyanax, Ctenobrycon, Aphyocharax, Brycon, Mylossoma, Triportheus, Oligosarcus and Acestrorhynchus). The discovery of dermal projections on the anterior border of maxillary bone extends the known range of structures affected by lip protuberances. Dermal structures began to develop simultaneously in both jaws below dissolved oxygen (DO) concentrations of 1.20–1.75 mgl−1 and rose in a steep manner as oxygen level decreased. The degree of morphological plasticity differed among traits and species. The shape of response of morphology to DO was similar to that previously reported on ASR, providing additional evidence about the functional link between these traits. Our results suggests that dermal lip protuberances are widely spread among characiform fishes, affecting several mouth structures. The different types of protuberances would make up for the limitations imposed by body size and mouth shape and position on the performance of ASR in fishes with divergent morphology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Floodplain lakes of large tropical and subtropical rivers experience wide environmental fluctuations driven by the recurrent seasonal increase in water level, known as flood pulse (Junk et al. 1989; Junk 1997; Welcomme 2001). In these environments, dissolved oxygen (DO) shows strong spatial and temporal variation, from supersaturation to complete anoxia (Saint-Paul and Soares 1987; Furch and Junk 1997; Almeida-Val et al. 1999). To survive during periods of extreme hypoxia fish must possess the capacity to function without oxygen or to obtain oxygen from the thin surface layer of water or directly from atmosphere. Some species are able to perform anaerobic respiration (Hockachka and Somero 1971; Dickson and Graham 1986), whereas others exhibit highly vascularized internal structures for aerial respiration (Graham 1997). However, most of the species inhabiting hypoxic environments possess neither of these adaptations (Kramer et al. 1978; Junk et al. 1983; Val and Almeida-Val 1995). These species can survive by obtaining oxygen from the upper layers of water saturated by continuous diffusion from the atmosphere, a phenomenon known as aquatic surface respiration (ASR) (Kramer and Mehegan 1981; Kramer and McClure 1982; Soares and Junk 2000; Melnychuk and Chapman 2002; Soares et al. 2006). This behavior is generally accompanied by biochemical and physiological adaptations that improve oxygen uptake and hypoxia tolerance (Rantin et al. 1998; Val and Almeida-Val 1999).
The performance of ASR is favored by specific ecomorphological characteristics related to head shape and mouth position and size (Lewis 1970). In this context, some South American characid fishes complement ASR by developing reversible dermal extensions of the lower lip that improve the hydrodynamic flow of oxygenated surface water into the mouth (Braum and Junk 1982; Winemiller 1989). These structures are formed by increased accumulation of lymphatic fluids in the intercellular spaces of dermal tissues (edema) and can appear and disappear within a few hours following the rapid daily changes in DO in floodplain lakes (Braum and Junk 1982; Saint-Paul and Bernardinho 1988; Saint-Paul and Soares 1988; Val and Almeida-Val 1995).
Previous studies have quantified the development of dermal lip protuberances over time when fish are kept in hypoxia (Braum and Junk 1982; Saint-Paul and Soares 1988), or they have observed the occurrence of qualitative categories of lip development (typical lip, intermediate and fully developed protuberance) over a gradient of environmental DO (Braum and Junk 1982; Winemiller 1989). However, the dermal lip development has never been analyzed quantitatively across a wide range of DO concentrations to determine the relationship between the extent of lip enlargement and DO. Although dermal lip protuberances have been described in many species, they appeared to be restricted to surface-feeding herbivorous and omnivorous characids (Winemiller 1989). Nevertheless, we have recently observed dermal extensions in a largely piscivorous characid (Scarabotti et al. 2009). In addition, in all above cited studies, dermal lip protuberances were reported to be limited to the anterior border of lower jaw, although comparative measurements on the upper jaw have not been addressed.
During 2004 and 2005, we collected fish samples across a wide range of environmental oxygen conditions in floodplain lakes of Salado River in Argentina. On several days, when DO dropped to hypoxic values (<1.5 mg·l−1), many fishes were observed performing ASR. This motivated us to examine the occurrence of dermal lip protuberances in a group of 29 fish species that were present during periods of extreme hypoxia. Additionally, we describe the relationship between DO and the magnitude of lip protuberance, providing measurements for both upper and lower jaws. Finally, we discuss the distribution of this adaptation among characiform fishes.
Methods
The study was performed in seven small lakes (median surface area 2.15 ha. Range 0.28–16.39 ha) located in alluvial zones on both banks of the Salado River, 2 km upstream from its confluence with Middle Paraná River (S 31º 39′ 30′′, W 60º 45′ 10′′). In this area, the Salado River has a 1.5 km wide floodplain with a main channel about 100 m wide. The water level is strongly influenced by Parana River and shows an average seasonal variation of 2 m. The region receives an annual rainfall of 950 mm and the mean daily air temperature varies from 10°C in winter to 25°C in summer. Floodplain lakes exhibit a belt of aquatic macrophytes mainly composed of rooted (Ludwigia peploides and Oplismenopsis najada) and floating plants (Azolla filiculoides, Pistia stratiotes and Eichhornia crassipes).
Sampling was carried out by seine (10 m, 5 mm mesh), every month from September 2004 to September 2005. Each haul were performed in deeper areas of lakes and concluded over the shore, in areas with few or no vegetation. Sampling was standardized covering an area of approximately 100 m2 and controlling the number of seine hauls, two or three per sampling date, each performed on different sites. Fish were immediately anesthetized in 1:5000 benzocaine as stated by Parma (1990), preserved in 10% formalin and transferred to 70% ethanol within 1 year. Collected specimens were deposited at the fish collection of the Instituto Nacional de Limnología (CONICET-UNL), Santa Fe, Argentina. Measurements of DO and temperature were performed near the center of each lake between 900 and 1200 hs, at a depth of 20 cm with an YSI oxygen meter model 57 and a mercury thermometer, respectively. DO concentrations considered in this study ranged from supersaturation (10 mg·l−1) to extremely hypoxic values (1.2 mg·l−1) that are commonly encountered in floodplain environments of the neotropics (Kramer et al. 1978; Saint-Paul and Soares 1987; Almeida-Val et al. 1999).
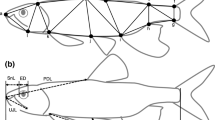
Morphological measurements were performed on 520 individuals belonging to 11 species (Table 1). For all species we measured both adult and juvenile fish except for B. orbignyanus and M. duriventre for which only juveniles were collected. The number of individuals available for O. jenynsii (n = 3; Table 1), was insufficient to perform statistical tests. Each specimen was measured with a digital caliper (nearest 0.01 mm) for standard body length (SL) and for three measurements in the dermal structures around the mouth: (1) median lip width (MLW), from the outer face of dentary tooth row to the rostral tip of lower lip on the median plane; (2) maximum lip with (MxLW) measured over a line perpendicular to the tooth row in the point of the lower jaw where the lip exhibit their maximum width; and (3) maxillary width (MW), (including dermal tissues attached to it) just below the junction with premaxillary bone, over a line perpendicular to the axis of maxillary bone (Fig. 1). The four species of Astyanax, C. alleni and A. anisitsi, showed values of MxLW identical to MLW in 97.77% of the individuals, then, only MLW data are shown for lower jaw for these species. Because lip protuberances can become folded after preservation, the lower jaw was positioned below a glass plate to deploy the structures during measurements.
All morphmetric variables were divided by SL to remove effect of size before analysis. The use of ratios for scaling mophometric data has been criticized by several authors due to allometric biases and spurious effects on correlation patterns among ratio and raw variables (Atchley and Anderson 1978; Albrecht et al. 1993). However, traditional methods for body size corrections are especially problematic in studies dealing with phenotypic plasticity (McCoy et al. 2006). For example, the technique of regression residuals uses pooled data and would confound the effects of the independent variable (DO) and the covariable (body size) if samples taken at different DO concentrations differ also in body size as was the case for some species. Other methods proposed to remove the effect of body size in morphometric data (Lleonart et al. 2000) are equivalent to the calculation of ratios in the case of isometric growth. We do not suspect strong allometric effects in the growth of lip width within the range of body size included in our analysis. In spite of the mentioned limitations, to our particular case, the use of ratios provided a straightforward and reasonably reliable method to remove body size effect.
The relationship between environmental temperature and DO was observed by mean of a Pearson correlation. Scatterplots were used to depict changes in protuberance development along the environmental gradients of DO. Since protuberances develop rapidly when fish are kept in hypoxic conditions (Saint-Paul and Soares 1988) we expected a non-linear relationship between morphology and DO. For this reason, we applied locally weighted regression (LOWESS: Cleveland 1985) that makes few assumptions about the kind of relationship, to describe the shape of response curve. We estimated critical DO concentrations for the initiation of lip protuberance development in each species. This point could be graphically determined as an inflection point in the LOWESS curve on the morphology/DO scatterplot. To assess the significance of critical concentrations, we compared the degree of protuberance development above and below (equal or less than) these values for each trait in each species using t-tests. As lip measurements in hypoxia and normoxia were inherently heterosedastic, P values were obtained by the method of Cochran-Cox for unequal variances. Additionally, we compared the variance of the data above and below critical values using Fisher’s F-test. Normality of both residuals and original variables were evaluated by Shapiro-Wilk’s tests. Non normal variables were transformed as Ln(X).
Because the analysis included three morphological traits measured in a high number of species, it represented an opportunity to compare the morphological plasticity induced by DO among traits and among species. We used the coefficient of variation (Zar 1999) to obtain a standardized measurement of the morphological plasticity as suggested by Scheiner and Goodnight (1984). This index has the advantage of allowing plasticity comparisons among traits with different magnitudes. We applied the test of Feltz and Miller (1996) to seek for differences in coefficients of variation among the species within each trait and among traits within each species. This test permits the comparison of the coefficients of variation of more than two samples calculating a statistic that approximates the chi-square distribution (Zar 1999).
Results
Occurrence of dermal lip protuberances
The rise of river water level during the summer (December, January and February) stimulated extensive proliferation and decomposition of aquatic macrophytes (mainly L. peploides, A. filiculoides, O. najada and E. crassipes) generating conditions of aquatic hypoxia in all lakes (probably due to bacterial respiration). Lowest DO concentrations occurred in coincidence with highest temperatures (mean±SD; DO: 1.79 ± 1.01 mg·l−1; T: 28.1 ± 1.9°C) and these variables were negatively correlated (Pearson correlation; r =−0.650, P=0.0001). For this reason, we could not clearly distinguish between the effect of temperature and DO on protuberance development.
Twenty-nine of 87 fish species registered in the area were common in periods with strong oxygen depletion (DO range: 1.20–1.35 mg·l−1) during December 2004 and January 2005. Thirteen of these species showed signs of dermal lip protuberances. One individual of Hyphessobrycon luetkenii (Characidae), collected at a DO level of 1.20 mg·l−1, showed an intermediate lip development in comparison with voucher specimens, but this single example is insufficient to confirm lip protuberance occurrence in this species. Data referring to Salminus brasiliensis were presented in a previous work (Scarabotti et al. 2009). The eleven remaining species belonging to the genera Astyanax, Aphyocharax, Ctenobrycon, Mylossoma, Brycon, Triportheus, Oligosarcus and Acestrorhynchus (Table 1), were observed with fully developed lip protuberances. These species exhibit a rather divergent morphology and present different feeding behaviors: B. orbignyanus and M. duriventre are herbivorous, T. paranensis, Astyanax spp., C. alleni and A. anisitsi are omnivorous and O. jenynsii and A. pantaneiro are largely piscivorous (Drago et al. 2003).
No evidence of lip swellings was detected in the following sixteen fish species present in hypoxic conditions: the characiform species Apareiodon affinis (Parodontidae), Steindachnerina brevipinna, Cyphocharax platanus, Potamorhina squamoralevis (Curimatidae), Prochilodus lineatus (Prochilodontidae), Leporinus obtusidens, Schizodon borrelli (Anostomidae), Roeboides microlepis (Characidae) and Hoplias malabaricus (Erythrinidae); the siluriform species Corydoras paleatus (Callichthyidae), Hypostomus commersoni, Pterygoplichthys anisitsi, Loricariichthys platymetopon (Loricariidae), Pimelodus maculatus, Pseudoplatystoma corruscans (Pimelodidae); and the perciform Cichlasoma dimerus (Cichlidae). Roeboides microlepis (Characinae) was the only characid species that was common under extreme hypoxic conditions and did not exhibit any sign of lip enlargement.
Morphological description of the protuberances
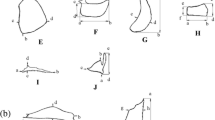
The external morphology of protuberances varied among the species, occurring in five general types (Fig. 2). Astyanax spp., C. alleni, and A. anisitsi (Figs. 2a and 3a) exhibited a uniform enlargement of the border of the lower jaw approximately rounded when viewed dorsally. Brycon orbignyanus and M. duriventre (Figs. 2b and 3b), showed rostral broadenings of the lower lip with two large rounded antero-lateral lobes. Triportheus paranensis presented a more elaborated protuberance, consisting of a rounded swelling at the base of the lip and two flattened anterolateral barbells with membranous flaps on the posterior border (Figs. 2c and 3c). In individuals with normal lips, barbells were very short and folded backward along the edge of the lower jaw, whereas in individuals with fully developed lips they increased 3–4 fold in length and were projected forward. Oligosarcus jenynsii exhibited distal projections along the border of lower lip which covered the sides of the mouth laterally (Figs. 2d and 3d). Finally, A. pantaneiro showed lateral lobes dorsally directed that did not continue on the rostral portion of lower jaw (Fig. 3e). As a consequence of the dermal swelling of the lower jaw, the mouth was generally positioned in a more upturned position in individuals whit lip protuberances. All species, except for A. anisitsi, exhibited thin dermal extensions in the upper jaw from the anterior border of the maxillary bone that developed in deferent degrees among species (Fig. 3e–f). These structures covered the mouth sides and limited the mouth opening in a more terminal position.
Photographs of individuals exhibiting dermal lip protuberances (left) and comparative individuals with typical lip morphology (right): (a) Brycon orbignyanus (dorsal view), (b) Astyanax abramis (dorsal view) (c) Triportheus paranensis (dorsal view) and (d) Oligosarcus jenynsii (lateral view). (e–f) Dermal expansions on the rostral border of maxillary bone in (e) Brycon orbignyanus and (f) Astyanax abramis. Scale bar = 5 mm
Relationship between lip morphology and dissolved oxygen
Morphological variables exhibited similar response curves to DO varying slightly among species with different morphological types of protuberances. The values of lip size showed little variation at DO concentrations between 2 and 10 mg·l−1, but increased sharply when DO diminished below critical values, visualized as inflection points in the LOWESS curves (Fig. 4). The increment in MxLW was larger in species exhibiting barbells or lateral lobes in its protuberances. LOWESS curves showed similar patterns of response to DO among traits within each species indicating a synchronized development of the different structures. Triportheus paranensis exhibited a particular shape of response in the variable MxLW, showing a first increment in barbell length at DO concentrations of 2.50 mg·l−1 compared with samples at more than 6.50 mg·l−1, followed by a second increment in samples with DO below 1.75 mg·l−1.
Scatterplots showing the relationships between dissolved oxygen (DO) and three lip measurements in four species of Characiform fishes. Solid lines represent LOWESS curves (tension = 0.5). Vertical dotted lines indicate critical DO concentration for protuberance development (DOc) whose values are shown in the upper right corner of each chart
Coefficients of variation indicating the plasticity of each morphometric variable of lip development in ten species of characiform fishes. A abr = Astyanax abramis, A asu = Astyanax asuncionensis, A fas = Astyanax fasciatus, A cor = Astyanax correntinus, C all = Ctenobrycon alleni, A ani = Aphyocharax anisitsi, B orb = Brycon orbignyanus, M dur = Mylossma duriventre, T par = Triportheus paranensis, A pan = Acestrorhynchus pantaneiro
All species showed significant increments of mean values for at least one of the three morphomentric variables when DO concentrations dropped blow critical values between 1.2 and 1.75 mg·l−1 (Table 2). In A. pantaneiro the response to dissolved oxygen was limited to de variable MxLW. Though in O. jenynsii we do not performed statistical tests, one individual collected at 1.35 mg·l−1 showed a MxLW 71% and 42% greater than the other two individuals collected at 5.75 and 6.5 mg·l−1 DO concentrations, respectively. In five out of ten species, the variance of morphometric variables increased significantly below critical DO concentrations. This fact was caused by the presence of individuals with both fully developed and typical lips within a sample. Triportheus paranensis was the only species with a lesser variation in hypoxic respect to normoxic conditions.
The degree of plasticity, represented by the coefficient of variation, differed significantly among the species and within each of the three morphological traits (Feltz and Miller’s test; MLW: χ2 = 49.25, P < 0.001; MxLW: χ2 = 69.22, P < 0.001; MW: χ2 = 82.31, P < 0.001). The highest morphological plasticity in MxLW was observed in B. orbignyanus and T. paranensis, whereas A. correntinus and A. anisitsi exhibited the highest coefficients of variation for MLW and B. orbignyanus and M. duriventre for MW (Fig. 5). Within each species morphological traits presented different degrees of plasticity (A. abramis: χ2 = 71.52, P < 0.001; A. asuncionensis: χ2 = 20.57, P < 0.001; A. fasciatus: χ2 = 70.92, P < 0.001; A. correntinus: χ2 = 7.30, P = 0,025; C. alleni: χ2 = 10.28, P < 0.001; A. anisitsi: χ2 = 9.53, P < 0.001; B. orbignyanus: χ2 = 78.51, P < 0.001; T. paranensis: χ2 = 252.31, P < 0.001; A. pantaneiro: χ2 = 24.54, P < 0.001), being lower jaw variables generally more plastic than the upper jaw variable, except for M. duriventre where plasticity was similar for all variables (χ2 = 3.29, P = 0,25).
Discussion
Occurrence and morphological diversity of dermal lip protuberances
Our results report the development of dermal lip protuberances in eleven fish species during periods of strong environmental hypoxia. We described this adaptation for the first time in nine species and confirmed such adaptation for the Paraná River in two species previously described for other South American rivers (M. duriventre and A. fasciatus) (Saint-Paul and Soares 1988; Casciotta 1993). Oligosarcus. jenynsii and A. pantaneiro, two largely piscivorous species, represent genera formerly unknown to develop protuberances. With this report, 33 South American characiform species of the families Characidae (within which protuberances are present in at least six subfamilies), Gasteropelecidae and Acestrorhynchidae are known to develop dermal lip protuberances (Braum and Junk 1982; Saint-Paul and Bernardinho 1988; Saint-Paul and Soares 1988; Winemiller 1989; Schmitter-Soto et al. 2008; Scarabotti et al. 2009). Although the phylogenetic relationships between characiform families and subfamilies have not been completely elucidated (Ortí and Meyer 1997; Malabarba et al. 1998; Calcagnotto et al. 2005), the development of dermal lip protuberances have been reported in evolutionary distant taxa of characiforms. The genus Ctenolucius has flattened flaps of tissue on the lateral surface of the dentary (Vari 1995) that seem to expand during hypoxia (J. Lundberg, pers. comm.). If these structures are homologous, the ability to modify the morphology in response to hypoxia may have appeared relatively early in the evolutionary history of characiforms and may occur in hundreds of species within this group.
The structural design of protuberances was different among the eleven species studied. Species attaining larger body sizes as B. orbignyanus, M. duriventre and T. paranensis showed larger and more complex protuberances with anterolateral lobes and barbells, whereas small sized species like Astyanax spp., C. alleni and A. anisitsi exhibited simple disc-shaped protuberances. Within this group of species, the complexity of the protuberances seems to increase in relation to the maximum body size attained by fishes. This relationship can be explained by the higher oxygen demands of larger fishes. During ASR, a larger fish have to cover a disproportionally greater surface of water in search of oxygen and is comparatively less efficient to incorporate selectively the thin layer of oxygenated water because of increased mouth size and turbulence generated during swimming. Barbells and lateral lobes could function as a funnel increasing the volume of oxygenated water directed into the mouth and could provide a larger surface of oxygenation as the water pass over the protuberance during inspiration. In the piscivorous species O. jenynsii and A. pantaneiro, the shape of protuberances could be additionally associated to the inefficacy of having bigger mouth and elongated head for the performance of ASR. In these fishes, the corner of the mouth remains placed relatively far from the surface of the water allowing the ingress of deoxygenated water from the lower layers. As was suggested for Salminus brasiliensis (Scarabotti et al. 2009), lateral lobes covering mouth sides may function limiting the entrance of water to the anterior portion of the lower jaw in contact with the surface water. Then, the same factors known to affect the performance of ASR in several fish species, such as body size, head morphology and mouth size and position (Lewis 1970) could be considered important determinants of the morphological design of lip protuberances.
Unlike the other species, T. paranensis developed protuberances at a wider range of oxygen concentrations and exhibited changes in the disposition of the barbells at lower DO levels. This ability presumably allow the fish to keep this complex structure in an intermediate development without affecting their feeding behavior but allowing an earlier response to the rapid daily fluctuations of DO observed in floodplain habitats (Braum and Junk 1982; Saint-Paul and Soares 1987). Osteoglossum bicirrhosum, a non characiform species, exhibit barbells very similar in form, function and changes in position in response to DO to those of T. paranensis, with the unique difference that they are permanent structures (Braum and Bock 1985). Like O. bicirrhosum the posterior border of the barbels of T. paranensis exhibited a membranous flap that gives the structure a hydrodynamic cross section similar to a foil. This design could assist the suspension of these flexible organs just below water surface, where they channel the uppermost layers of water during ASR. This finding can be considered a new example of convergent morphology in distant related fishes subjected to similar environmental pressures.
In previous studies, development of dermal lip protuberances was reported to be limited to the anterior portion of lower jaw (Braum and Junk 1982; Winemiller 1989). However, we observed several characid species with dermal extensions on the anterior border of the maxillary bone in addition to swellings observed in the lower jaw. These structures may function in a similar way to the lateral lobes of the protuberances of piscivorous fishes described above: as lateral walls at the sides of the mouth limiting the entrance of water to the anterior portion of the lower jaw during ASR. This finding increases the range of structures known to be affected during lip protuberance development, suggesting a larger potential for ecomorphological adjustment of characiform fishes in response to hypoxia.
Relationship between morphology and dissolved oxygen
To our knowledge, this is the first study that reports the pattern of relationship between quantitative morphological variables of lip development and DO. Studied species exhibited a characteristic pattern of response to DO in which protuberance size increased abruptly below critical values between 1.20 and 1.75 mg·l−1. These values are similar to those reported previously for the occurrence of lip protuberances in 14 characiform species from the Venezuelan Llanos (1.40 mg·l−1) (Winemiller 1989) and for the initiation of ASR (Kramer and McClure 1982; Soares et al. 2006) and other cardiorespiratory and biochemical responses in many characiform fish species (Rantin et al. 1998; Almeida-Val et al. 1999). The determination of relationships among short-term morphological, physiological and behavioral (e.g. ASR) responses would contribute largely to the understanding of the strategies used by each species to cope with hypoxia in these environments.
The occurrence of the lowest DO concentrations in coincidence with the highest temperatures in the summer determined an inverse relationship between DO and temperature. This fact made difficult to distinguish clearly between the effect of temperature and DO on lip protuberance development. Temperature can increase metabolic rate of many tropical species increasing the DO demand and reducing subsequently the ability to tolerate hypoxia (Val and Almeida-Val 1999). However, another field study in which temperature and DO were not correlated, failed to indicate a relationship between temperature and lip protuberance occurrence (Winemiller 1989). Protuberances were not observed in other lakes of the Paraná River with high water temperature but normoxic conditions (unpubl. data). Besides, several laboratory experiments (some of which used species included in this study) demonstrated that low DO concentrations are a sufficient factor to trigger protuberance development (Braum and Junk 1982; Saint-Paul and Bernardinho 1988; Saint-Paul and Soares 1988). Presumably, temperature could modify to some extent the critical DO concentrations required to develop lip protuberances but currently there are no controlled laboratory studies separating the influence of these variables.
Morphological traits exhibited higher dispersion as DO diminished below critical values. A similar pattern, in which individuals with different degree of protuberance development coexist within a sample, was also observed qualitatively in other field studies (Braum and Junk 1982; Braum 1983; Winemiller 1989). Other morphological plastic traits showing a similar sigmoid shape of response can exhibit an increment of dispersion around critical values as a consequence of small quantities of measurement error in the independent variable (DelGiudice 2006). As morphology can change greatly in response to little variations in DO around critical values, fish experiencing somewhat different DO conditions within a sample can show large differences in protuberance development. This hypothesis can be supported by the spatial heterogeneity in DO concentrations observed in floodplain environments (Junk 1997; Almeida-Val et al. 1999). Anyway, this issue should be addressed with adequately designed laboratory experiments.
Morphological changes observed in this study in both the lower and upper jaw clearly contribute to orientate the mouth in a more upturned position and reduce the mouth size by covering their sides. These changes occurred in ecomorphologically divergent fish species in adaptive directions similar to those predicted by Lewis (1970) for the optimization of ASR (cyprinodontiform morphology). Winemiller (1987) suggested that the evolutionary convergence to cyprinodontiform morphology is attenuated by selective advantages provided by morphological specializations related to feeding behavior. In this context, the type of phenotypic plasticity described above would be a way to optimize temporarily the morphological design to perform ASR in fishes with highly divergent mouth size and head shape.
Plasticity comparisons indicated differences among traits and among species in the morphological response to DO. These differences were generally related to the distinct structural designs of protuberances. Theoretical models on the evolution of phenotypic plasticity, indicate that the degree of plasticity exhibited by a given trait is favored by environmental heterogeneity (Berrigan and Scheiner 2005 and references therein). Neotropical floodplain environments exhibit wide range temporal and spatial heterogeneity in DO concentrations (Kramer et al. 1978; Almeida-Val et al. 1999) which could explain the widespread development of biochemical (Val and Almeida-Val 1999), behavioral (Saint-Paul and Soares 1987) and morphological plastic traits (Braum and Junk 1982; Winemiller 1989, this study) in response to hypoxia. When environmental fluctuations are very frequent as daily DO variations in floodplains, plastic responses should have very short lag times and high reversibility to be adaptive (Padilla and Adolph 1996). In laboratory studies fish exhibit morphological responses to hypoxia in 2–4 h and show total reversibility in an even shorter period of time (90 min) (Braum and Junk 1982; Saint-Paul and Bernardinho 1988). Additionally, fitness costs of production and maintenance of plastic traits could reduce the probabilities of evolution of phenotypic plasticity (DeWitt et al. 1998; Relyea 2002). Winemiller (1989) mentioned that protuberances could hinder feeding and increase the possibility to suffer injuries. However, in concordance with Winemiller’s results, fish with fully developed protuberances were frequently observed with stomach contents and exhibited no injuries. Finally, we consider that the study of the development of dermal lip protuberances as a particular case of highly reversible short-term morphological plasticity (see Schlichting and Pigliucci 1998, p 53) could allow a deeper understanding of the functioning and evolution of this adaptation.
References
Albrecht GH, Gelvin BR, Hartman SE (1993) Ratios as a size adjustment in morphometrics. Am J Phys Anthropol 91:441–468
Almeida-Val VMF, Val AL, Walker I (1999) Long and short-term adaptation of Amazon fishes to varying O2 levels: intra-specific phenotypic plasticity and interespecific variation. In: Val AL, Almeida-Val VM (eds) Biology of tropical fishes. INPA, Manaus, pp 185–206
Atchley WR, Anderson D (1978) Ratios and the statistical analysis of biological data. Syst Zool 27:71–78
Berrigan D, Scheiner SM (2005) Modeling the evolution of phenotypic plasticity. In: DeWitt TJ, Scheiner SM (eds) Phenotypic plasticity. Functional and conceptual approaches. Oxford University Press, Oxford, pp 82–97
Braum E (1983) Beobachtungen über eine reversible Lippen-extension und ihre Rolle bei der Notatmung von Brycon spec. (Pisces, Characidae). und Colossoma macropomum (Pisces, Serrasalmidae) Amazoniana 7:355–374
Braum E, Bock R (1985) Funktions-morphologische Untersuchungen uber die Barteln von Osteoglossum bicirrhosum (Pisces, Osteoglossidae) wahren der notatmung. Amazoniana 9:353–370
Braum E, Junk WJ (1982) Morphological adaptation of two Amazonian characoids (Pisces) for surviving in oxygen deficient waters. Int Rev Ges Hydrobiol 67:869–886
Calcagnotto D, Schaefer SA, DeSalle R (2005) Relationships among characiform fishes inferred from analysis of nuclear and mitochondrial gene sequences. Mol Phylogenet Evol 36:135–153
Casciotta JR (1993) New record of the lip protuberance in characiforms from Río de La Plata Basin in Uruguay. Ichthyol Explor Fres 4:79–80
DelGiudice M (2006) Increased residual variance at developmental switch points: statistical artifact or indicator of exposed genotypic influence? Evolution 60:192–195
DeWitt TJ, Sih A, Wilson DS (1998) Costs and limits of phenotypic plasticity. Trends Ecol Evol 13:77–81
Dickson KA, Graham JB (1986) Adaptations to hypoxic environments in the erythrinid fish Holpias microlepis. Environ Biol Fish 15:301–308
Drago EC, de Drago IE, Oliveros OB, Paira AR (2003) Aquatic habitats, fish and invertebrate assemblages of the middle Paraná river. Amazoniana XVII:291–341
Feltz CJ, Miller GE (1996) An asymptotic test for the equality of coefficients of variatios from k populations Statistics in Medicine 15:647–658
Furch K, Junk WJ (1997) Physicochemical conditions in the floodplains. In: Junk WJ (ed) The Central Amazon floodplain: ecology of a pulsing system. Springer, Heidelberg, pp 69–108
Graham JB (1997) Air breathing in fishes: evolution diversity and adaptation. Academic, San Diego
Hockachka PW, Somero GN (1971) Biochemical adaptation to the environment. In: Hoar WS, Randall DJ (eds) Fish physiology. Academic, New York
Junk WJ (ed) (1997) The Central Amazon floodplain. Ecology of a pulsing system. Springer Verlag, Heidelberg, p 525
Junk WJ, Soares GM, Carvalho FM (1983) Distribution of fish species in a lake of the Amazon River floodplain near Manaus (lago Camaleao) with special reference to extreme oxygen conditions. Amazoniana 7:397–431
Junk WJ, Bayley PB, Sparks RE (1989) The flood pulse concept in river-floodplain systems. In: Dodge DP (ed) Proceedings of international large river symposium (LARS). Can J Fish Aquat Sci Spec Publ, pp 110–127
Kramer DL, McClure M (1982) Aquatic surface respiration, a widespread adaptation to hypoxia in tropical freshwater fishes. Environ Biol Fish 7:47–55
Kramer DL, Mehegan JP (1981) Aquatic surface respiration, an adaptive response to hypoxia in the guppy, Poecilia reticulata (Pisces, Poeciliidae). Environ Biol Fish 6:299–313
Kramer DL, Lindsey CC, Moodie GEE, Stevens ED (1978) The fishes and the aquatic environment of the central Amazon basin, with particular reference to respiratory patterns. Can J Zool 56:717–729
Lewis WM Jr (1970) Morphological adaptations of cyprinodontoids for inhabiting oxygen deficient waters. Copeia 1970:319–326
Lleonart J, Salat J, Torres GJ (2000) Removing allometric effects of body size in morphological analysis. J Theor Biol 205:85–93
Malabarba LR, Reis RE, Vari RP, Lucena ZMS, Lucena CAS (eds) (1998) Phylogeny and classification of neotropical fishes. EDIPUCRS, Porto Alegre
McCoy MW, Bolker BM, Osenberg CW, Miner BG, Vonesh JR (2006) Size correction: comparing morphological traits among populations and environments. Oecologia 148:547–554
Melnychuk M, Chapman LJ (2002) Hypoxia tolerance of two haplochromine cichlids: swamp leakage and potential for interlacustrine dispersal. Environ Biol Fish 65:99–110
Ortí G, Meyer A (1997) The radiation of Characiform fishes and the limits of resolution of mitocondrial robosomal DNA sequences. Syst Biol 46:75–100
Padilla DK, Adolph SC (1996) Plastic inducible morphologies are not always adaptive: the importance of time delays in a stochastic environment. Evol Ecol 10:105–117
Rantin FT, Guerra CR, Kalinin AL, Glass ML (1998) The influence of aquatic surface respiration (ASR) on cardio-respiratory function of the serrasalmid fish Piaractus mesopotamicus. Comp Biochem Phys A 119:991–997
Relyea RA (2002) Costs of phenotypic plasticity. Am Natst 159:272–282
Saint-Paul U, Bernardinho G (1988) Behavioural and ecomorphological responses of the neotropical pacu Piaractus mesopotamicus (Teleostei, Serrasalmidae) to oxygen-deficient waters. Exp Biol 48:19–26
Saint-Paul U, Soares MGM (1987) Diurnal distribution and behavioral responses of fishes to extreme hypoxia in an Amazon floodplain lake. Environ Biol Fish 20:91–104
Saint-Paul U, Soares MGM (1988) Ecomorphological adaptation to oxygen deficiency in Amazon floodplains by Serrasalmidae fish of the genus Mylossoma. J Fish Biol 32:231–236
Scarabotti PA, Parma MJ, López JA, Ghirardi R (2009) Dermal lip protuberances associated with aquatic surface respiration in juveniles of the piscivorous characid Salminus brasiliensis (Actinopterygii: Characidae). Neotrop Ichthyol 7:459–464
Scheiner SM, Goodnight CJ (1984) The comparison of phenotypic plasticity and genetic variation in populations of the grass Danthonia spicata. Evolution 38:845–855
Schlichting CD, Pigliucci M (1998) Phenotypic evolution: a reaction norm perspective. Sinauer Associates, Inc, Publishers, Massachusetts
Schmitter-Soto JJ, Valdez-Moreno ME, Rodiles-Hernández R, Gonzalez-Díaz AA (2008) Astyanax armandoi, a Junior Synonym of Astyanax aeneus (Teleostei: Characidae). Copeia 2008:409–413
Soares MGM, Junk WJ (2000) Respiratory adaptations of five curimatid species (Teleostei, Curimatidae) to oxygen depletation in an Amazonian floodplain lake. Verh Internat Verein Limnol 27:1063–1069
Soares MGM, Menezes NA, Junk WJ (2006) Adaptations of fish species to oxygen depletion in a central Amazonian floodplain lake. Hydrobiologia 568:353–367
Val AL, Almeida-Val VMF (1995) Fishes of the Amazon and their environment. Physiological and biochemical features. Springer, Heidelberg
Val AL, Almeida-Val VMF (eds) (1999) Biology of tropical fishes. INPA, Manaus
Vari RP (1995) The neotropical fish family Ctenoluciidae (Teleostei: Ostariophysi: Characiformes): supra and intra familial phylogenetic relationships, with a reversionary study. Smithson Contr Zool 564:1–97
Welcomme R (2001) Inland fisheries: ecology and management. Fishing News Books, Blackwell Science, Oxford, 358 pp
Winemiller KO (1987) Tests of ecomorphological and community level convergence among neotropical fish communities. Dissertation, University of Texas
Winemiller KO (1989) Development of dermal lip protuberances for aquatic surface respiration in South American characid fishes. Copeia 1989:382–390
Zar JH (1999) Biological statistics. Prentice Hall, Upper Saddle River, New Jersey, USA
Acknowledgements
We are grateful to E. Creus, R. Regner and E. Lordi for assistance on the field work. L. Rossi offered the first ideas and stimulus for the initiation of this study. U. Saint-Paul, D. Kramer, J. Cazenave and F. Giri provided critical suggestions that greatly improved early versions of the manuscript. We specially thank D. Kramer for the careful and deep revision of all aspects of a first version of the manuscript. J. Lundberg shared unpublished information about cases of dermal lip protuberances in characiform fishes. Financial support was provided by doctoral fellowships of the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) to P.A. Scarabotti, J.A. López and R. Ghirardi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scarabotti, P.A., López, J.A., Ghirardi, R. et al. Morphological plasticity associated with environmental hypoxia in characiform fishes from neotropical floodplain lakes. Environ Biol Fish 92, 391–402 (2011). https://doi.org/10.1007/s10641-011-9850-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-011-9850-y