Abstract
In this study, we investigated the influence of environmental variables (predictor variables) on the species richness, species diversity, functional diversity, and functional redundancy (response variables) of stream fish assemblages in an agroecosystem that harbor a gradient of degradation. We hypothesized that, despite presenting high richness or diversity in some occasions, fish communities will be more functionally redundant with stream degradation. Species richness, species diversity, and functional redundancy were predicted by the percentage of grass on the banks, which is a characteristic that indicates degraded conditions, whereas the percentage of coarse substrate in the stream bottom was an important predictor of all response variables and indicates more preserved conditions. Despite being more numerous and diverse, the groups of species living in streams with an abundance of grass on the banks perform similar functions in the ecosystem. We found that riparian and watershed land use had low predictive power in comparison to the instream habitat. If there is any interest in promoting ecosystem functions and fish diversity, conservation strategies should seek to restore forests in watersheds and riparian buffers, protect instream habitats from siltation, provide wood debris, and mitigate the proliferation of grass on stream banks. Such actions will work better if they are planned together with good farming practices because these basins will continue to be used for agriculture and livestock in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Agroecosystems are ecological and socioeconomic systems where biological communities interact with human-modified environments that are used to produce food and other products for human use (Conway 1987).
Currently, these systems represent more than 38 % of the planet’s surface (Ramankutty et al. 2008), and they are the main factor associated with global biodiversity losses (Tscharntke et al. 2005; Dolédec and Statzner 2010). Agricultural impacts on the environment include the replacement of naturally forested areas by the expansion of crops and pasture lands, as well as the intensification of increased production through irrigation, fertilizers, biocides, and mechanization on areas that have previously been altered (Foley et al. 2012). When compared to natural ecosystems, agroecosystems demonstrate high fluidity, high vulnerability, spatiotemporal differences, poor stability, and low biodiversity (Zhu et al. 2012). In this scenario, both agricultural expansion and intensification can affect aquatic ecosystems in different ways and levels (Harding et al. 1998; Feld 2013), such as by causing the loss of biodiversity and ecosystem services (Foley et al. 2007), by generating excessive nutrient inputs (Canfield et al. 2010; Woodward et al. 2012), by shifting the main energy sources (from C3 to C4) for aquatic food webs (Bunn et al. 1997), and by increasing pesticide runoff (Bareswill et al. 2013).
The expansion of agribusiness in many tropical regions has been conducted at the cost of natural resource degradation. An example is the recent approval of the new Brazilian forest code, which provided amnesty for illegal deforestation and relaxed restoration requirements in riparian preservation areas (Soares-Filho et al. 2014). In this context of land use and exploitation, a more accurate picture of stream habitat quality can be provided by the functional approach, which is a way to express the functional differences between species, differently than species richness or diversity that assume that all species contribute in the same manner to the ecosystem functioning (Díaz and Cabido 2001). This approach may be particularly useful in cases in which simplified, poor quality habitats result in habitat constraints that filter out fish species that rely on habitat types that are no longer represented or fish species that are sensitive to harsh environmental conditions, such as grass-dominated, deforested, and heavily silted sites (e.g., Casatti et al. 2009).
Descriptions of communities using functional perspectives have recently been receiving increased attention (Cadotte et al. 2011). The increased attention is partly due to the high predictive power of functional diversity descriptors regarding ecosystem processes (Tilman et al. 1997; Díaz and Cabido 2001), such as the robust habitat-trait relationship observed for different taxa in different environmental gradients (Flynn et al. 2009; Villéger et al. 2008; Mouchet et al. 2010). Measuring functional diversity can provide information that is complementary to that traditionally obtained through taxonomic diversity metrics. In estuarine systems, for example, Villéger et al. (2010) verified that intensified degradation led to increased species richness but decreased functional divergence and specialization, which was explained by the decrease in specialist species and by the fact that the added species were redundant in relation to those that were previously established. In fact, the relationship between species richness and functional diversity can vary (Mayfield et al. 2010), thus revealing the actions of distinct ecological processes (Pavoine and Bonsall 2011).
Agroecosystems can lead to environmental alterations that limit the occurrence of species that have functional traits incompatible with local conditions (Devictor et al. 2008). According to the niche-filtering hypothesis, coexisting species are functionally more similar to one another than would be expected by chance (redundancy) because environmental conditions act as a filter, allowing only species with traits compatible with local conditions to survive (Zobel 1997). In this context, different environmental variables at local and landscape scales would represent environmental filters in agroecosystems (see Pease et al. 2012 for an environmental filtering support for trait-environment relationships in stream fish assemblages). Burcher et al. (2008) found that functional diversity was lower in streams that were surrounded by pasture in the past or streams with scarce riparian plant cover when compared to streams with forested riparian zones. Such findings can be more complex, however, because the presence/absence of forests can interact with other factors at finer scales (Strayer et al. 2003) to determine assemblage’s diversity patterns (Teresa and Casatti 2012; Cruz et al. 2013).
In this study, we investigated the influence of environmental variables on species richness, species diversity, functional diversity, and functional redundancy of stream fish assemblages in agroecosystems. It is known that environmental variables are related to the gradient of structural habitat quality (Wang et al. 1997; Diana et al. 2006). However, severe degradation can represent limiting conditions to the occurrence of species with more specialized niches, even though they can support high species diversity under certain circumstances (Villéger et al. 2010). We conducted our study in river basins that harbors streams that vary from relatively preserved to extremely degraded (Casatti et al. 2009) and we hypothesized that, despite presenting high richness or diversity in some occasions, fish communities will be more functionally redundant in the most degraded conditions. With the aim of exploring this hypothesis, we also determined the functional similarity among species, identified the species-habitat relationships, and tested the relationship of the selected variables with the response variables. Through this approach, we also expected to identify the habitat variables and species traits that are of interest to watershed management and stream restoration efforts in the basin and comparable locations.
Materials and Methods
Study Area and Site Selection
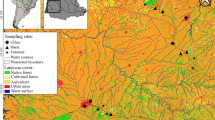
We conducted this study in non-urban areas of the hydrographic basins of the São José dos Dourados and Turvo-Grande Rivers in the northwest region of the State of São Paulo, located in southeastern Brazil (Fig. 1). This region was originally covered by semi-deciduous seasonal forest (Silva et al. 2007). Since the second half of the nineteenth century, this region has experienced high rates of deforestation: first for the development of coffee cultivation and second, from 1929 on, for the creation of pasture for livestock grazing that replaced the coffee crops (Monbeig 1998). Currently, only 4 % of the original vegetation coverage remains (Nalon et al. 2008) and is distributed in small and isolated fragments (Silva et al. 2007). For this study, we selected only streams that were embedded in agricultural lands, which were mostly used for livestock grazing or sugarcane plantations, and avoided direct urban influence. The soil of the region is characterized by unconsolidated sand and clay sediments, which have a high erosive potential (IPT 1999). The climate is hot and tropical, with maximum average temperatures between 31 and 32 °C, minimum average temperatures between 13 and 14 °C, and an annual rainfall between 1300 and 1800 mm (Silva et al. 2007). There are two well-defined climatic periods: a dry period with lower rainfall between June and August and a wet period with higher rainfall between December and January (IPT 1999).
We selected the sampling sites using a randomized approach (Kasyak 2001). We selected one site for each 100 km of a specific length order (from first to third order determined in a 1:50,000 map scale, sensu Sthraler 1957), with one sampled reach in each stream. Overall, we sampled 95 stream reaches on one occasion during the dry seasons from 2003 to 2005 to limit the effects of seasonal differences.
Environmental Variables
At each 75 m long sampling reach, we obtained 21 environmental variables, which represented the local riparian, and watershed scales (Table 1). At local scale we measured dissolved oxygen, conductivity, pH, temperature, and turbidity with a portable multiparameter probe. Nitrate, orthophosphate, and ammonia were dosed at a specialized laboratory; ammoniacal nitrogen and orthophosphate were determined by a spectrophotometry technique using MERCK® reagents and nitrate by following the APHA Standard Methods (method 4500-NO3). We used the average width and depth of each reach to calculate habitat volume (=average width × reach length × average depth). We calculated the average current from three measurements with a mechanical flowmeter taken side by side across the mesohabitat (pools, runs, riffles) present in the reach. Using visual inspection, we obtained the percent of silt, sand and coarse substrate (particle size based on Krumbein and Sloss’s 1963 classification), the percentage of wetted area covered by woody debris, and the percentage of stream bank length occupied by grass.
The riparian corridor (Table 1) was represented by the percentage of the stream corridor covered by shrubs and trees, obtained by visual inspection, and the riparian canopy cover, which was based on the degree of coverage at both margins of the sampled reach (0, without coverage; 1, from 1 to 25 % of coverage; 2, from 26 to 50 %; 3, from 51 to 75 %; 4, more than 75 %). The watershed scale (Table 1) included the land use and coverage, obtained through the analysis of Landsat 5 TM (2010, 30 × 30 m2 resolution) satellite images, made available by the National Institute for Space Research (INPE). We conducted the classification according to the supervised classification using the maximum likelihood algorithm (Jensen 2000) in the software Erdas Imagine 9.2. The watershed limits and the drainage network were generated using the hydrological model ArcSWAT (Soil and Water Assessment Tool) and satellite images from the Digital Elevation Model (DEM) SRTM (90 × 90 m2 resolution) generated by the National Aeronautics and Space Administration (NASA), made available by the United States Geological Survey (USGS). We generated the watersheds using georeferenced points taken during the sampling period considering a minimum contribution area of 150 ha, using the ArcMap module of the software ArcGIS version 9.3 (ESRI 2008). We calculated the percentage of forest in the riparian buffer zone (30-m wide on each side), the percentage of the watershed covered by forest, the distance of each sampled reach to its headwaters, and the number of dams upstream of the reach for each watershed. We calculated the road density through the extension of paved roads (km) divided by the watershed area (km2), according to Kautza and Sullivan (cited by them as Regional Development Index, 2012).
Fish Sampling and Functional Trait Measurement
We sampled fish with standardized sampling efforts at all reaches. We started the collection 15 min after blocking the upstream and downstream limits of each reach with block nets (5 mm mesh) by using two electrofishing passes for 60 min combined. Three collectors conducted the electrofishing; the first handled an electrified dip net (220 V, 50–60 Hz, 3.4–4.1 A, 1000 W), the second a grill-shaped electrode, and the third a regular dip net. We fixed fish in a 10 % formalin solution and transferred them to a 70 % ethanol solution after 48 h. They were identified, counted, weighed, and deposited at the Fish Collection of the Department of Zoology and Botany of São Paulo State University (DZSJRP 5833–6190, 7264–7443), São José do Rio Preto, São Paulo, Brazil.
We measured nine functional traits (see Table 2 for details and Appendix Table 5 for the matrix of traits) in 36 species that were represented by a sufficient number of specimens (≥10) to obtain quantitative traits. Traits were associated with vertical habitat use, body size, trophic ecology, velocity preference, hard substrate preference, and feeding tactics. We obtained fish measurements with a digital caliper and pectoral fin areas with a Zeiss® SteREO Discovery V12 stereomicroscope and AxioVision Zeiss® image software. For specimens larger than 80 mm, we obtained fin areas by contouring the fins over graph paper (according to Beaumord and Petrere 1994) due to the view limitation of the optic equipment field in such cases. For species with accentuated sexual dimorphism, such as Poecilia reticulata and Phalloceros harpagos, we measured only females for procedure standardization. Despite being observed in the sampled streams, we excluded Synbranchus marmoratus from the functional analysis because the absence of fins precludes the application of the proposed indexes. We obtained the body size of each species from the literature to code this trait so that it could represent the predominant size class of adult individuals. We adopted this procedure to avoid underestimating the species’ characteristic size as an adult if there were a large number of juveniles present in the samples. We obtained the functional traits related to feeding through stomach content analysis and treated them as fuzzy variables. We determined velocity, substrate preference, and feeding tactic traits according to previous studies (e.g., Teresa and Casatti 2013) and our own underwater observations.
Selection of Environmental Variables
We selected environmental variables according to their contribution to the variability among streams using principal component analysis (PCA), performed with the SAM 4.0 software (Rangel et al. 2010). We normalized variables to present the same weight (mean = 0, standard deviation = 1), and the axes that contributed the most to explain the observed variation were selected according to the Broken-Stick model, one of the methods with the most consistent results in ecological studies (Jackson 1993). This model constructs a null distribution of eigenvalues and compares it with observed ones; a principal component is interpretable if it exceeds the eigenvalue randomly generated by the Broken-Stick model (Legendre and Legendre 1998). Because some variables were correlated, we considered the most important variables to be those that presented the highest loadings (loadings <−0.7 or >0.7 at least in one axis).
Data Analysis
We represented the functional similarity among species in a graph resulting from PCoA according to Pavoine et al. (2009), in which a matrix of functional distance among species is generated through the generalization of Gower´s Distance (Pavoine et al. 2009). We conducted this analysis with the function “dist.ktab” in the “vegan” and “ade4” packages of R software (R Development Core Team 2011), according to Pavoine et al. (2009). To aid in the interpretation of the results, we added the main functional characteristics of each species group to the graph.
To evaluate the relationship between the fish abundance and environmental variables, we used the redundancy analysis (RDA), a constrained ordination technique, which directly analyzes the relationships between multivariate ecological datasets (ter Braak and Smilauer 2012). Based on the results of the PCA, we included the (normalized) variables with the highest contribution to the between-stream variability. To test the significance of the first axis, we performed a Monte Carlo permutation, under full model (4999 permutations, P < 0.05). To test the significance of each variable, we used stepwise model selection of the Generalized Linear Models to obtain F statistics, again using a 0.05 significance level. We conducted all these procedures in the software CANOCO 5 (ter Braak and Smilauer 2012).
We followed the procedures of Pillar et al. (2013) to calculate species diversity (D, Gini-Simpson index for species diversity), functional diversity (Q, Rao’s quadratic entropy), and functional redundancy (FR). FR is defined as the difference between species diversity and functional diversity (de Bello et al. 2007), given by the formula FR = D − Q (Pillar et al. 2013). We calculated D and Q in the “vegan” and “ade4” packages of R software (R Development Core Team 2011). If FR = 0, the species have completely different traits; if FR = 1, all the species have identical traits; this second condition should be expected in redundant assemblages (Pillar et al. 2013).
To investigate the determinants of the diversity patterns on assemblages, we generated multiple regression models. The response variables were species richness, species diversity, functional diversity, and FR and the explanatory variables were the set of selected environmental. Overall, 31 models were generated for each response variable and they were compared according to the Akaike information criterion (AIC) corrected by the sample size and the number of parameters in the model (AICc according to Burnham and Anderson 2002). The relative importance of each variable was estimated based on by averaging summing the AIC weights across all the models, using AIC weights in the set where the given variable occurs. These analyses were conducted with the software SAM 4.0 (Rangel et al. 2010).
Results
Of all variables, only six, which were related to physical characteristics at local and riparian corridor scales, contributed the most to among-stream variation (Table 3). However, some of these varied in the same way (Table 3). The percentage of coarse substrate, the percentage of woody debris, the percentage of shrubs and trees, and canopy cover showed negative component loadings and demonstrated correspondence at local and riparian corridor scales; in turn, the percentage of sand and the percentage of grass showed positive component loadings (Table 3).
Overall, 13,245 individuals belonging to 60 fish species were sampled (Appendix Table 6). The first PCoA axis ordered the 36 selected species according to their swimming ability in faster or slower running waters, preference for coarse or sand substrate, and the use of vertical strata (Fig. 2). The feeding traits influenced the similarity/dissimilarity among species according to the predominant consumption of the different prey items. Allochthonous invertebrates and detritus are common for species that live in slow water and sandy bottom and that explore the water column (nektonic), occasionally exploring the stream bed (nektobenthics). In this left portion of the PCoA bliplot are most of the tetras, knifefishes, livebearers, and cichlids. Autochthonous invertebrates, algae, and periphyton are important items for benthic species that are commonly found in fast waters and bottom covered by coarse substrate. These fish species are represented by catfishes, armored catfishes, and South American darters. The second PCoA axis ordered species according to body size (Fig. 2).
Bidimensional graph from Principal Coordinates Analysis that represents the global functional distance among species. Some characteristics have been added to the graph to aid interpretation. Characteristics that represent habitat use are on the horizontal axis, and body size is represented on the vertical axis. The diet items that influenced the most the fish fauna were added a posteriori to the biplot, following their importance for each species (see Table 5). For species codes see Table 6
Among the ordination axes extracted from RDA, the first one was significant and accounted for 44 % of the variance in species-environment relationships (eigenvalue of first axis = 0.032, pseudo F = 2.3, P = 0.043). All variables explained the assemblage’s structure (sand, F = 107.42, P < 0.0001; coarse substrate, F = 212.20, P < 0.0001; woody debris, F = 44.2, P < 0.0001; grass, F = 336.04, P < 0.0001; shrubs and trees, F = 60.15, P < 0.0001). According to axis 1 in the RDA representation, a greater number of species was associated with grassy and sandy conditions (see left side of the biplot in Fig. 3) than to the opposite, which is coarse substrate, woody debris, or shrubs and trees.
Bidimensional graph from redundancy analysis that represents the relationship between species and environmental variables. For species codes see Table 6. Only the 20 species that best fit the environmental variables are displayed
All the response variables were predicted by the percentage of coarse substrate on the streambed; species richness, species diversity, and functional redundancy were also predicted by the percentage of grass on the banks (Table 4). Such results indicate that, despite presenting higher richness or diversity in some instances, species occurring in this type of stream (occupied by grasses in the banks) play similar functional roles in the community.
Discussion
The environmental variables predicted fish species richness, species diversity, functional diversity, and functional redundancy in a similar way. Our initial hypothesis was confirmed because functional redundancy was high, even in conditions of high species richness or diversity. The amount of grass on the stream banks was the main predictor of functional redundancy, which indicates that despite being more numerous and diverse, the groups of species living in this condition perform similar functions in the ecosystem. This vegetation comes from adjacent pastures in areas of limited shade from the riparian zone, and it is a good indicator of low habitat integrity (Bunn et al. 1997, Casatti et al. 2009) and habitat homogenization (Zeni and Casatti 2014). The presence of grass at the border and in the instream habitat, as observed herein, can provide additional microhabitats that favor the colonization, reproduction, and feeding of aquatic insects, playing a similar role to that of aquatic macrophytes, which increase macroinvertebrate abundance in streams (Marques et al. 2013) and lakes (Walker et al. 2013). High macroinvertebrate availability can in turn favor the persistence of fish populations, particularly those that are characterized by opportunistic feeding. Among the submerged roots of invasive grass, large amounts of sediment can be trapped with detritus that, together with high respiration rates, may reduce dissolved oxygen (Bunn et al. 1997). In this type of environment, midge larvae are abundant and represent, along with detritus, the most consumed resources by local fish (Zeni and Casatti 2014). Among the species able to live in such conditions, we can cite, for example, the electric knifefish Gymnotus sylvius, the tetras Knodus moenkhausii and Serrapinnus notomelas, the cichlid Laetacara araguaiensis, and the guppy Poecilia reticulata (see Fig. 3).
Habitat degradation in its multiple forms can represent an important environmental filter, as documented by studies in other ecosystems, such as reefs (Bellwood et al. 2006) and estuaries (Villéger et al. 2010), in which fish assemblages were considered to be functionally clustered with functionally redundant coexisting species. In a similar way, based on the present findings, we can presume that the physical degradation of the stream caused by the establishment of agroecosystems can also represent an important environmental filter, in which the nektonic/nektobenthic individuals, with large body sizes that use slow waters, sandy substrate, and marginal portions occupied by grass and that feed mainly on detritus and aquatic invertebrates, predominate (see Fig. 2). From the results obtained herein, it is also possible to conclude that such assemblages are redundant and include many species with functional traits that indicate a generalist strategy.
The percentage of coarse substrate was a good predictor for all the response variables, but does not necessarily indicate a degraded condition. In our sites, coarse substrate is a good predictor for riffle mesohabitat (pers. obs.). Although presenting high fish diversity, riffles are characterized by hydraulic conditions that select species able to live in such a harsh habitat (Teresa and Casatti 2012). Fish able to live and persist in fast-flowing waters normally present morphological adaptations (Martin-Smith 1998), such as periphytivorous benthic grazers like the armored catfishes (Hypostomus ancistroides and H. nigromaculatus), which attach to the bottom substrate or wood debris, and the scrapetooth (Parodon nasus), which has a hydrodynamic body. Other fish in riffles include invertivorous species, which feed on the streambed using sit-and-wait predation (e.g., the South American darter Characidum zebra), and crepuscular to nocturnal predator species (e.g., the catfishes Imparfinis schubarti, Rhamdia quelen, and Pimelodella avanhandavae), which explore the water layer close to the bottom where they are minimally affected by the current. This filtering process selects groups of species with similar functions and therefore explains the high functional redundancy in such conditions, with this process occurring even in preserved sites (Teresa and Casatti 2012).
Our results indicate that in the homogeneous agroecosystem landscape, the functional patterns of fish assemblages are greatly influenced by the local variables, such as substrate composition and bank condition. In such basins, which were deforested in the distant past, riparian and watershed forests are not well represented on the landscape and hence these variables do not account for the environmental gradient. Conservation strategies for aquatic biodiversity in these systems should seek to restore and conserve riparian and landscape diversity (but see a context dependence evaluation by Nislow 2005). Through this way, it is possible to protect instream habitats from siltation (Sweeney and Newbold 2014), provide woody debris for instream habitat (Paula et al. 2011, 2013), and mitigate the proliferation of grass on stream banks (Bunn et al. 1997). This last goal is the main factor associated with functional redundancy in the studied region. Notably, riparian restoration in small tributaries can introduce additional benefits, since riparian restoration in small tributaries is most likely to result in improved environmental conditions that may extend downstream and consequently improved the quality of larger rivers (Pracheil et al. 2013), thus securing benefits for terrestrial ecosystems in several ways (e.g., Fukui et al. 2006; Chan et al. 2008; Lorion and Kennedy 2009; Gonçalves et al. 2012).
It is noteworthy that according to several authors (e.g., Harding et al. 1998; Teels et al. 2006; Lévêque et al. 2008), the maintenance of aquatic biodiversity and ecological processes depends on the protection of a large percentage of the watershed area. This implies that restoration of the riparian forest alone is not sufficient to improve the integrity of the entire system, although it can maintain the ecological integrity of streams (Lorion and Kennedy 2009) and mitigate impacts on the remaining catchment area (Sweeney and Newbold 2014). The studied basins are located in one of the most productive areas in the most developed state of Brazil. For this reason, it is highly unlikely that these areas will be entirely recovered. Thus, to promote ecosystem functioning and fish diversity, reforestation and rural land management need to be planned together and include sustainable farming practices (Tilman et al. 2002). All of these benefits have great ecological value, particularly in landscapes fragmented by agriculture and livestock expansion.
References
Bareswill R, Golla B, Streloke M, Schulz R (2013) Entry and toxicity of organic pesticides and copper in vineyard streams: erosion rills jeopardise the efficiency of riparian buffer strips. Agric Ecosyst Environ 146:81–92. doi:10.1016/j.agee.2013.05.007
Beaumord AC, Petrere M (1994) Comunidades de peces del rio Manso, Chapada dos Guimarães, MT, Brasil. Acta Biol Venez 15:21–35
Bellwood DR, Hoey AS, Ackerman JL, Depczynski M (2006) Coral bleaching, reef fish community phase shifts and the resilience of coral reefs. Glob Change Biol 12:1587–1594. doi:10.1111/j.1365-2486.2006.01204.x
Bunn SE, Davies PM, Kellaway DM (1997) Contributions of sugar cane and invasive pasture grass to the aquatic food web of a tropical lowland stream. Mar Freshw Res 48:173–179. doi:10.1071/MF96055
Burcher CL, McTammany ME, Benfield EF, Helfman GS (2008) Fish assemblage responses to forest cover. Environ Manag 41:336–346. doi:10.1007/s00267-007-9049-3
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York
Cadotte MW, Carscadden K, Mirotchnick N (2011) Beyond species: functional diversity and the maintenance of ecological processes and services. J Appl Ecol 48:1079–1087. doi:10.1111/j.1365-2664.2011.02048.x
Canfield DE, Glazer AN, Falkowski PG (2010) The evolution and future of earth’s nitrogen cycle. Science 330:192–196. doi:10.1126/science.1186120
Casatti L (2002) Alimentação dos peixes em um riacho do Parque Estadual Morro do Diabo, bacia do Alto Rio Paraná, sudeste do Brasil. Biota Neotrop 2:1–15. doi:10.1590/S1676-06032002000200012
Casatti L, Ferreira CP, Carvalho FR (2009) Grass-dominated stream sites exhibit low fish species diversity and dominance by guppies: an assessment of two tropical pasture river basins. Hydrobiologia 632:273–283. doi:10.1007/s10750-009-9849-y
Chan EKW, Zhang Y, Dudgeon D (2008) Arthropod ‘rain’ into tropical streams: the importance of intact riparian forest and influences of fish diets. Mar Freshw Res 59:653–660. doi:10.1071/MF07191
Conway GR (1987) The properties of agroecosystems. Agric Syst 24:95–117. doi:10.1016/0308-521X(87)90056-4
Cruz BB, Miranda LE, Cetra M (2013) Links between riparian landcover, instream environment and fish assemblages in headwater streams of south-eastern Brazil. Ecol Fresh Fish 22:607–616. doi:10.1111/eff.12065
de Bello F, Lepš J, Lavorel S, Moretti M (2007) Importance of species abundance for assessment of trait composition: an example based on pollinator communities. Community Ecol 8:163–170. doi:10.1556/ComEc.8.2007.2.3
Development Core Team R (2011) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Austria
Devictor V, Julliard R, Jiguet F (2008) Distribution of specialist and generalist species along spatial gradients of habitat disturbance and fragmentation. Oikos 117:507–514. doi:10.1111/j.2008.0030-1299.16215.x
Diana M, Allan JD, Infante D (2006) The influence of physical habitat and land use on stream fish assemblages in southeastern Michigan. Am Fish Soc Symp 48:359–374
Díaz S, Cabido M (2001) Vive la différence: plant functional diversity matters to ecosystem processes. Trends Ecol Evol 16:646–655. doi:10.1016/S0169-5347(01)02283-2
Dolédec S, Statzner B (2010) Responses of freshwater biota to human disturbances: contribution of J-NABS to developments in ecological integrity assessments. J N Am Benth Soc 29:286–311. doi:10.1899/08-090.1
ESRI (2008) ArcGIS Professional GIS for the desktop, version 9.3. Redlands
Feld CK (2013) Response of three lotic assemblages to riparian and catchment-scale land use: implications for designing catchment monitoring programs. Freshwater Biol 58:715–729. doi:10.1111/fwb.12077
Flynn DFB, Gogol-Prokurat M, Nogeire T, Molinari N, Richers BT, Lin BB, Simpson N, Mayfield MM, DeClerck F (2009) Loss of functional diversity under land use intensification across multiple taxa. Ecol Lett 12:22–33. doi:10.1111/j.1461-0248.2008.01255.x
Foley JA et al (2007) Amazonia revealed: forest degradation and loss of ecosystem goods and services in the Amazon Basin. Front Ecol Environ 5:25–32. doi:10.1890/1540-9295(2007)5[25:ARFDAL]2.0.CO;2
Foley JA et al (2012) Solutions for a cultivated planet. Nature 478:337–342. doi:10.1038/nature10452
Froese R, Pauly D (2013) FishBase. Available from http://www.fishbase.org. Accessed December 2014
Fukui D, Murakami M, Nakano S, Aoi T (2006) Effect of emergent aquatic insects on bat foraging in a riparian forest. J Anim Ecol 75:1252–1258. doi:10.1111/j.1365-2656.2006.01146.x
Gonçalves P, Alcobia S, Simões L, Santos-Reis M (2012) Effects of management options on mammal richness in a Mediterranean agro-silvo-pastoral system. Agric Syst 85:383–395. doi:10.1007/s10457-011-9439-7
Graça WJ, Pavanelli CS (2007) Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Editora da Universidade de Maringá, Maringá
Harding JS, Benfield EF, Bolstad PV, Helfman GS, Jones EBD III (1998) Stream biodiversity: the ghost of land use past. Proc Natl Acad Sci USA 95:14843–14847. doi:10.1073/pnas.95.25.14843
IPT (1999) Diagnóstico da situação atual dos recursos hídricos e estabelecimento de diretrizes técnicas para a elaboração do Plano da Bacia Hidrográfica do São José dos Dourados—minuta. Comitê da Bacia Hidrográfica do São José dos Dourados e Fundo Estadual de Recursos Hídricos
Jackson DA (1993) Stopping rules in principal components analysis: a comparison of heuristical and statistical approaches. Ecology 74:2204–2214. doi:10.2307/1939574
Jensen JR (2000) Remote sensing of the environment: an earth resource perspective. Prentice-Hall, Upper Saddle River
Kasyak PF (2001) Maryland biological stream survey: sampling manual. Maryland Department of Natural Resources, Monitoring and Non-tidal Assessment Division, Annapolis
Kautza A, Sullivan SMP (2012) Relative effects of local- and landscape-scale environmental factors on stream fish assemblages: evidence from Idaho and Ohio, USA. Fundam Appl Limnol 180:259–270. doi:10.1127/1863-9135/2012/0282
Krumbein WC, Sloss LL (1963) Stratigraphy and sedimentation. Freeman, San Francisco
Legendre P, Legendre L (1998) Numerical ecology. Elsevier Science, Amsterdam
Lévêque C, Oberdorff T, Paugy D, Stiassny MLJ, Tedesco PA (2008) Global diversity of fish (Pisces) in freshwater. Hydrobiologia 595:545–567. doi:10.1007/978-1-4020-8259-7_53
Lorion CM, Kennedy BP (2009) Riparian forest buffers mitigate the effects of deforestation on fish assemblage in tropical headwater streams. Ecol Appl 19:468–479. doi:10.1890/08-0050.1
Marques LC, Ceneviva-Bastos M, Casatti L (2013) Progressive recovery of a tropical deforested stream community after a flash flood. Acta Limnol Bras 25:111–123. doi:10.1590/S2179-975X2013000200002
Martin-Smith KM (1998) Relationships between fishes and habitat in rainforest streams in Sabah, Malaysia. J Fish Biol 52:458–482. doi:10.1111/j.1095-8649.1998.tb02010.x
Mayfield MM, Bonser SP, Morgan JW, Aubin I, McNamara S, Vesk PA (2010) What does species richness tell us about functional trait diversity? Predictions and evidence for responses of species and functional trait diversity to land-use change. Global Ecol Biogeogr 19:423–431. doi:10.1111/j.1466-8238.2010.00532.x
Monbeig P (1998) Pioneiros e fazendeiros de São Paulo. Hucitec, São Paulo
Mouchet MA, Villéger S, Mason NWH, Mouillot D (2010) Functional diversity measures: an overview of their redundancy and their ability to discriminate community assembly rules. Funct Ecol 24:867–876. doi:10.1111/j.1365-2435.2010.01695.x
Nalon MA, Matto ISA, Franco GADC (2008) Meio físico e aspectos da vegetação. Rodrigues RR, Bononi VLR (orgs) Diretrizes para conservação e restauração da biodiversidade no Estado de São Paulo. Instituto de Botânica, São Paulo, pp 12–21
Nislow KH (2005) Forest change and stream fish habitat: lessons from ‘Olde’ and New England. J Fish Biol 67B:186–204. doi:10.1111/j.0022-1112.2005.00913.x
Paula FR, Ferraz SFB, Gerhard P, Vettorazzi CA, Ferreira A (2011) Large wood debris input and its influence on channel structure in agricultural lands of southeast Brazil. Environ Manag 48:750–763. doi:10.1007/s00267-011-9730-4
Paula FR, Gerhard P, Wenger SJ, Ferreira A, Vettorazzi CA, Ferraz SFB (2013) Influence of forest cover on in-stream large wood in a agriculture landscape of southeastern Brazil: a multi-scale analysis. Landsc Ecol 28:13–27. doi:10.1007/s10980-012-9809-1
Pavoine S, Bonsall MB (2011) Measuring biodiversity to explain community assembly: a unified approach. Biol Rev 86:702–812. doi:10.1111/j.1469-185X.2010.00171.x
Pavoine S, Vallet J, Dufort A, Gachet S, Daniel H (2009) On the challenge of treating various types of variables: application for improving the measurement of functional diversity. Oikos 118:391–402. doi:10.1111/j.1600-0706.2008.16668.x
Pease AA, González-Díaz AA, Rodiles-Hernández R, Winemiller KO (2012) Functional diversity and trait–environment relationships of stream fish assemblages in a large tropical catchment. Freshw Biol 57:1060–1075. doi:10.1111/j.1365-2427.2012.02768.x
Pillar VD, Blanco CC, Müller SC, Sosinski EE, Joner F, Duarte LDS (2013) Functional redundancy and stability in plant communities. J Veg Sci 24:963–974. doi:10.1111/jvs.12047
Pracheil BM, McIntyre PB, Lyons JD (2013) Enhancing conservation of large-river biodiversity by accounting for tributaries. Front Ecol Environ 11:124–128. doi:10.1890/120179
Ramankutty N, Evan AT, Monfreda C, Foley JA (2008) Farming the planet: 1. Geographic distribution of global agricultural lands in the year 2000. Glob Biogeochem Cycles 22:GB1003. doi:10.1029/2007GB002947
Rangel TF, Diniz-Filho JAF, Bini LM (2010) SAM: a comprehensive application for spatial analysis in macroecology. Ecography 33:46–50. doi:10.1111/j.1600-0587.2009.06299.x
Romero RM, Casatti L (2012) Identification of key microhabitats for fish assemblages in tropical Brazilian savanna streams. Int Rev Hydrobiol 97:526–541. doi:10.1002/iroh.201111513
Sazima I (1986) Similarities in feeding behaviour between some marine and freshwater fishes in two tropical communities. J Fish Biol 29:53–65. doi:10.1111/j.1095-8649.1986.tb04926.x
Silva AM, Casatti L, Álvares CA, Leite AM, Martinelli LA, Durrant SF (2007) Soil loss risk and habitat quality in streams of a meso-scale river basin. Sci Agric 64:336–343. doi:10.1590/S0103-90162007000400004
Soares-Filho B, Rajão R, Macedo M, CarneiroA Costa W, Coe M, Rodrigues H, Alencar A (2014) Cracking Brazil’s forest code. Science 344:363–364. doi:10.1126/science.1246663
Sthraler AN (1957) Quantitative analysis of watershed geomorphology. Trans Am Geophys Union 38:913–920
Strayer DL, Beighley RE, Thompson LC, Brooks S, Nilsson DC, Pinay G, Naiman RJ (2003) Effects of land cover on stream ecosystems: roles of empirical models and scaling issues. Ecosystems 6:407–423. doi:10.1007/s10021-002-0170-0
Sweeney BW, Newbold JD (2014) Streamside forest buffer width needed to protect stream water quality, habitat, and organisms: a literature review. JAWRA 50:560–584. doi:10.1111/jawr.12203
Teels BM, Rewa AA, Myers J (2006) Aquatic condition response to riparian buffer establishment. Wildlife Soc B 34:927–935. doi:10.2193/0091-7648(2006)34[927:ACRTRB]2.0.CO;2
ter Braak CJF, Smilauer P (2012) CANOCO reference manual and user’s guide: software for ordination (version 5). Microcomputer Power, New York
Teresa FB, Casatti L (2012) Influence of forest cover and mesohabitat types on functional and taxonomic diversity of fish communities in Neotropical lowland streams. Ecol Freshw Fish 21:433–442. doi:10.1111/j.1600-0633.2012.00562.x
Teresa FB, Casatti L (2013) Development of habitat suitability criteria for Neotropical stream fishes and an assessment of their transferability to streams with different conservation status. Neotrop Ichthyol 11:395–402. doi:10.1590/S1679-62252013005000009
Tilman D, Lehman CL, Thomson KT (1997) Plant diversity and ecosystem productivity: theoretical considerations. Proc Natl Acad Sci USA 94:1857–1861. doi:10.1073/pnas.94.5.1857
Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S (2002) Agricultural sustainability and intensive production practices. Nature 418:671–677. doi:10.1038/nature01014
Tscharntke T, Klein AM, Steffan-Dewenter I, Thies C (2005) Landscape perspectives on agricultural intensification and biodiversity—ecosystem service management. Ecol Lett 8:857–874. doi:10.1111/j.1461-0248.2005.00782.x
Villéger S, Mason NWH, Mouillot D (2008) New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 89:2290–2301. doi:10.1890/07-1206.1
Villéger Miranda JR, Hernández DF, Mouillot D (2010) Contrasting changes in taxonomic vs. functional diversity of tropical fish communities after habitat degradation. Ecol Appl 20:1512–1522. doi:10.1890/09-1310.1
Walker PD, Wijnhoven S, Veld V (2013) Macrophyte presence and growth form influence macroinvertebrate community structure. Aquat Bot 104:80–87. doi:10.1016/j.aquabot.2012.09.003
Wang L, Lyons J, Kanehl P, Gatti R (1997) Influences of watershed land use on habitat quality and biotic integrity in Wisconsin streams. Fisheries 22:6–12. doi:10.1577/1548-8446(1997)022<0006:IOWLUO>2.0.CO;2
Watson DJ, Balon K (1984) Ecomorphological analysis of fish taxocenes in rainforest streams of northern Borneo. J Fish Biol 25:371–384. doi:10.1111/j.1095-8649.1984.tb04885.x
Woodward G et al (2012) Continental-scale effects of nutrient pollution on stream ecosystem functioning. Science 336:1438–1440. doi:10.1126/science.1219534
Zeni JO, Casatti L (2014) The influence of habitat homogenization on the trophic structure of fish fauna in tropical streams. Hydrobiologia 726:259–270. doi:10.1007/s10750-013-1772-6
Zhu W, Wang S, Caldwell CD (2012) Pathways of assessing agroecosystem health and agroecosystem management. Acta Ecol Sin 32:9–17. doi:10.1016/j.chnaes.2011.11.001
Zobel M (1997) The relative role of species pools in determining plant species richness. An alternative explanation of species coexistence? Trends Ecol Evol 12:266–269. doi:10.1016/S0169-5347(97)01096-3
Acknowledgments
We thank our colleagues from the Ichthyology Laboratory for their help during fieldwork, IBILCE-UNESP for the use of their facilities, IBAMA for the collecting license (001/2003), landowners for permission to conduct research on their properties, Francisco Langeani for fish identification, and Thiago G. Souza for help with the statistical analysis. Anonymous reviewers provided several suggestions that improved this manuscript. LC is supported by National Counsel of Technological and Scientific Development (CNPq 301755/2013-2); FBT by University Research and Scientific Production Support Program (PROBIP/UEG); JOZ, MDC, GLB, and MCB by São Paulo Research Foundation (FAPESP).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Casatti, L., Teresa, F.B., Zeni, J. et al. More of the Same: High Functional Redundancy in Stream Fish Assemblages from Tropical Agroecosystems. Environmental Management 55, 1300–1314 (2015). https://doi.org/10.1007/s00267-015-0461-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00267-015-0461-9