Abstract
This study aimed to analyze the cold stress effects (in terms of hematology, energy reserves, and oxidative stress) in Piaractus mesopotamicus (pacú) and their mitigation by a Pyropia columbina red seaweed-supplemented diet. For this purpose, juvenile fish were fed with a control (CD) or a red seaweed-supplemented diet (RD) for 60 days, and then, the animals were exposed to a low temperature (14 °C) and a control temperature (24 °C) for 24 h. The cold shock generated an increase of hemoglobin levels in fish fed with both diets. In CD-fed fish, plasmatic triglycerides, cholesterol, and hepatic glycogen decreased after the thermal shock; meanwhile, the animals fed with RD showed decreased hepatic proteins, but increased cholesterol and hepatic glycogen. Regarding oxidative stress, antioxidant enzymes augmented their activity in the liver, intestine, and gills; meanwhile, lipid oxidative damage was observed in the liver and intestine of fish exposed to 14 °C and fed with both diets. Pacú was sensitive to cold shock, but no mitigation effects were observed in fish fed with the supplemented diet. Further research should target higher concentrations of P. columbina in supplemented diets to take advantage of this valuable resource.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Temperature is an important stress factor in fish aquaculture since its changes could disrupt biochemical reactions and physiological functions (Wen et al. 2017). However, most of the studies took into account biological aspects of fish after long periods of exposure to constant temperatures, and there is a lack of available information considering short-term variations and stress biomarker responses like metabolic parameters (Pinto et al. 2019). Particularly, cold shock (natural or anthropogenic) in fish is a common stress situation which occurs when fish have been acclimated to a specific water temperature or range of temperatures and are subsequently exposed to a rapid decrease in temperature, resulting in a cascade of physiological and behavioral responses and, in some cases, death (Donaldson et al. 2008).
Temperature under the optimum limits of a species could negatively influence the health status and increase susceptibility to diseases (Ahmad et al. 2011). Changes in hematological parameters have been proposed as easy detectors and excellent biomarkers of the health status of fish to represent early diagnosis of pathological disorders generated by variable temperatures (Pinto et al. 2019). Moreover, thermal stress causes homeostasis modifications which can be evaluated via fish energy reserves (Wang et al. 2012; Nobrega et al. 2019). Lastly, it is widely reported that low water temperature increases endogenous reactive oxygen species (ROS) and trigger DNA, protein, and lipid damage (Cheng et al. 2018).
In order to cope with the prevalence and appearance of new diseases and overcome obstacles to sustainable aquaculture, fish nutrition is recognized to be one of the most important topics in management of fish farming. Thus, increasing attention has been given to the development of nutritional strategies that could mitigate the deleterious effects of stressors associated with aquaculture practices (Gasco et al. 2018). Functional diets have become a suitable alternative for the inclusion of natural ingredients which tend to be more biodegradable than synthetic ones and are less likely to generate resistance (Muñoz et al. 2018).
There are several reports which tested different kinds of supplemented diets. In a recent study, mitigation effects by β-carotene-supplemented diet on physiological and antioxidant biomarkers were analyzed in Piaractus mesopotamicus exposed to 14 °C (Bacchetta et al. 2020). In Cyprinus carpio fish, a diet supplemented with myrcene and menthol reduced the adverse effects of ammonia in terms of tissue damage and anemia (Hoseini et al. 2019). Another study showed that a diet supplemented with cottonseed meal and exogenous protease improves growth, nutrient assimilation, and hematology parameters in Nile tilapia (Hassaan et al. 2019). Other authors found that synthetic astaxanthin supplementation improved antioxidant activity and resistance to thermal stress in fish (Goda et al. 2018; Cheng et al. 2018).
Dietary algae as feed additive for fish has proved to have many benefits like acceleration of ascorbic acid and improvement of physiological conditions in relation to vitamin C nutrition and lipid metabolism (Nakagawa 1997). Besides, seaweeds are considered a valuable food source that contains high levels of proteins, dietary fibers, well-balanced amino acid profile, and significant amounts of vitamins, omega-3 fatty acids, pigments, and minerals (Burtin 2003; Ngo et al. 2011). In this way, there have been growing commercial interests regarding seaweed diet supplementations in most aquatic species and in many land-farmed animals (Sotoudeh and Mardani 2017). However, up to date, there is still lack of studies that have focused on the evaluation of micro- or macroalgae as dietary supplements for fish despite their abundance and easy availability in nature (Teimouri et al. 2019).
Pyropia columbina is a red seaweed with high economic interest, usually found on hard substrates in Patagonia Argentina coasts. It was demonstrated that P. columbina has bioactive compounds with antioxidant properties (Cian et al. 2014, 2016). Moreover, Cian et al. (2019) found that a supplemented diet with this seaweed improved antioxidant status, promoted higher iron bioavailability, and had a lipid-lowering effect in juvenile fish (P. mesopotamicus). Another study showed that Atlantic salmon (Salmo salar) fed with a P. columbina-based feed improved their immune system based on the expression of immune-relevant genes and white blood cell lysozyme expression (Muñoz et al. 2018).
P. mesopotamicus (commonly named pacú) represents a worldwide important resource for fisheries and aquaculture because of its potential of rustic management, good growth rates, and acceptance in the consumer market. Besides, its herbivorous/omnivorous habits represent an excellent attribute to deal with feed restrictions (Barbieri and Vigliar Bondioli 2013; Claudiano et al. 2019). Optimal range for pacú culture is 20–28 °C, as such, it has been a challenge for farmers to deal with cold temperatures in winter like poor growth performance and low survival rates (Bacchetta et al. 2020). As aquaculture systems are commonly challenged to deal with cold stress, this study aimed to evaluate hematological, energetic, and oxidative stress parameters in P. mesopotamicus after a cold-shock exposure and the mitigation effects in fish fed with a Pyropia columbina red seaweed-supplemented diet.
Materials and methods
Diets
The formulation and chemical composition of both control (CD) and red seaweed (Pyropia columbina)-supplemented (RD) diets are detailed in Table 1. The CD ingredients consisted of commercial cornmeal (613 g kg−1), soybean meal (200 g kg−1), bovine plasma protein concentrate (130 g kg−1), cornstarch (20 g kg−1), vitamin-mineral mix (7 g kg−1), and canola oil (30 g kg−1). In the case of RD, the ingredients were the same as CD but 35 g kg−1 of cornmeal was replaced with P. columbina. The level of red seaweed inclusion was selected according to a previous study of Cian et al. (2019), where the production of the RD-based diet is also described. From the raw P. columbina material, the samples were washed in distilled water, dried at 100 ± 4 °C, and ground up into a particle size lower than 1 mm using a laboratory hammer mill (Retsch, Haan, Germany). The powder obtained was passed through a 20-mesh sieve (0.85 mm) and stored at 4 °C in plastic bags until analysis or diet formulation.
Fish and feeding assays
All assays were conducted in the Aquaculture Laboratory at the Instituto Nacional de Limnología (CONICET-UNL, Argentina). Juvenile Piaractus mesopotamicus fish were purchased from a local fish farm (Pez Campero, Argentina) (N = 210; 7.0 ± 0.5-cm standard length; 12.2 ± 2.5 g). Firstly, fish were acclimated to laboratory conditions for 2 weeks at controlled temperature (24 ± 1 °C). Then, the animals were divided in 35 individual groups per 300-L tanks and fed with CD or RD twice a day at a rate consisted of 5% biomass weight per day. The feeding assay lasted 60 days. All treatments were replicated three times with the consent of the national and institutional guidelines for the protection animal welfare (CONICET 2005).
Low temperature exposure
The thermal-stress exposure was defined at 14 °C, as 7–7.5 °C is considered the lethal temperature for P. mesopotamicus according to Milstein et al. (2000). Artificial climate chambers were employed for exposing fish fed with CD or RD (n = 18 per diet) to control (24 ± 0.1 °C) or cold temperature (14 ± 0.1 °C) in 10-L aquaria (n = 3 per aquarium, in triplicate). After 24 h, animals were anesthetized with 100 mg L−1 of benzocaine (Parma de Croux 1990). Then, blood was taken from the caudal peduncle according to Reichenbach-Klinke (1980) to measure hematological parameters. Lastly, the fish dissection was performed, and the liver, intestine, gills, and muscle were extracted and stored at −80 °C.
Hematological parameters
The following parameters were measured from blood samples of fish (n = 9): red blood cell count (RBC), hematocrit (Ht) through the micro-method, and hemoglobin concentration (Hb) employing the cyanmethemoglobin method (Houston 1990). From those values, the hematimetric indexes were calculated as proposed by Cazenave et al. (2005): mean cell volume (MCV = Ht × 10/RBC), mean cell hemoglobin (MCH = Hb × 10/RBC), and mean cell hemoglobin concentration (MCHC = Hb × 100/Ht).
Blood metabolites and energy reserves in the liver and muscle
Plasmatic total protein, triglycerides, cholesterol, and glucose levels (n = 9) were measured employing colorimetric commercial kits tested in dish (Wiener Lab®). Total plasma protein concentration was measured by a kit reagent containing EDTA/Cu complex in an alkaline medium that reacts with peptide bonds to yield a purple-blue complex. Plasma levels of total cholesterol and triglycerides were analyzed by using standard enzymatic-colorimetric test, and finally, plasma glucose was assayed by a colorimetric test based on the glucose oxidase method (Rossi et al. 2017).
In the liver and muscle, glycogen, total proteins, and lipid contents (n = 6) were quantified. Glycogen was measured according to Seifter et al. (1950). Briefly, 20 mg of hepatic and 60 mg of muscle tissues were treated with 1 ml KOH 30% and 0.5 ml KOH 60% at 10 °C. After alkaline tissue disruption, glycogen was precipitated by ethanol, and glucose was determined using the anthrone reagent method. Lipid content was extracted using chloroform: methanol (2:1) by the method described by Folch et al. (1957), and total protein concentration was determined in tissue homogenates according to Lowry et al. (1951) using bovine serum albumin as standard. All biochemical analyses were measured in triplicate.
Oxidative stress
Enzyme extracts from the liver, intestine, gills, and muscle of fish (n = 6) were made as proposed by Bacchetta et al. (2014). Briefly, tissues were homogenized using 0.1 M sodium phosphate buffer, pH 6.5 containing 20% (v/v) glycerol, 1 mM EDTA, and 1.4 mM dithioerythritol (DTE). The homogenate was centrifuged at 20,000g at 4 °C for 30 min, and the supernatant was collected and stored at −80 °C for enzyme measurement.
The activity of the enzyme superoxide dismutase (SOD, EC 1.15.1.1) was determined by its ability to inhibit the epinephrine autoxidation (Misra and Fridovich 1972). Catalase activity (CAT, EC 1.11.1.6) was measured according to the method of Beutler (1982) following the decomposition of H2O2. The assay mixture consisted of 1 M Tris-HCl, 5 mM EDTA (pH 8.0), 10 mM H2O2, and enzyme extract. The activity of glutathione-S-transferase (GST, EC 2.5.1.18) was determined following the conjugation of reduced glutathione with 1-chloro-2,4-dinitrobenzene (CDNB) that produces a dinitrophenyl thioether as described by Habig et al. (1974). Glutathione reductase activity (GR, EC 1.6.4.2) was determined as described by Tanaka et al. (1994) by measuring the oxidation of NADPH. The reaction mixture contained 100 mM sodium phosphate buffer (pH 7.5), 20 mM oxidized glutathione, 2 mM NADPH, and enzyme extract. Lastly, lipid peroxidation levels (n = 6) were analyzed by measuring the formation of thiobarbituric reactive substances (TBARS) (Yagi 1976). Enzymatic activities and TBARS levels were calculated in terms of the protein content according to Bradford (1976) using serum bovine albumin as standard.
Statistical analyses
All results were reported as mean ± standard error (SE). To corroborate normality and homogeneity of variance, Shapiro-Wilk’s and Levene’s tests were carried out, respectively. When normal distribution was not accomplished, variables were transformed to log10. A two-way ANOVA was used to analyze the effects of temperature and diet and the interaction between them for the parametric variables. After two-way ANOVA, we performed simple main effects analysis to test the effects of temperature separately for each diet treatment. This test was based in the lineally independent pair-wise comparisons between marginal estimated means using the error terms and the degree of freedom of the whole design (Logan 2010). We did not perform post hoc tests because there are only two temperatures for each diet treatment. Significant differences were considered when p<0.05. Variables that remained non-parametric after transformation were analyzed using Mann-Whitney tests. For these variables, we performed one test to evaluate the effects of temperature and another test to evaluate the effects of the diet. Then, we performed another test to evaluate the effects of temperature stress separately for each diet. All tests were carried out using the SPSS software (SPSS Inc., Chicago, USA).
Results
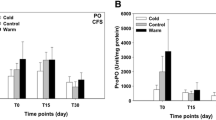
No mortality was evidenced during the feeding or low temperature-exposure assays. Hematological measurements are shown in Table 2. After the thermal stress, both hemoglobin levels and MCHC increased significantly. There were no differences in hematological measurements between the diets. No interactions were evidenced through the two-way ANOVA.
Blood metabolites and energy reserves in the liver and muscle are summarized in Table 3. After the low temperature stress, fish fed with CD showed diminished plasmatic triglycerides and cholesterol levels. In the case of RD, cholesterol increased in low temperature-exposed fish. Two-way ANOVA showed a significant interaction between diet and temperature for glycogen in the liver. Hepatic glycogen content decreased after the low temperature exposure in CD-fed fish but increased in RD-fed fish. The hepatic protein levels decreased in fish fed with RD exposed to thermal stress.
Activities of antioxidant enzymes and TBARS levels are presented in Table 4. The two-way ANOVA showed significant interactions for SOD in the liver and GST in the intestine. Activities of SOD and GR in the liver and GST in the intestine augmented in fish fed with CD and exposed to 14 °C. In the case of RD-fed fish, the SOD activity increased in gills of fish exposed to 14 °C. TBARS levels significantly increased in the liver and intestine of fish exposed to thermal stress but did not show differences between diets in any tissue.
Discussion
Cold-suboptimal temperatures have been widely reported to cause negative impacts in aquaculture production and generate economic loss (Nobrega et al. 2019). This problem is particularly important for fish since, as ectothermic animals, they are challenged to adapt their physiological functions and overcome thermal stress (Turchini et al. 2010). Moreover, when temperature radically changes, the effects on organisms are quite different from those exposed to low but constant temperature (Barbieri 2009a, b). As such, research regarding how fish nutrition could improve the health status of animals and mitigate the negative effects of environmental stressors has gained importance in order to achieve a sustainable aquaculture and an optimal management of fish farming (Gasco et al. 2018).
In the present study, a 24-h exposure to low temperature (or cold shock) increased the hemoglobin level in juvenile Piaractus mesopotamicus fed with CD and RD, which could be a compensatory response and an adaptation to temperature-imposed increases in oxygen requirements. A study carried out by Panase et al. (2018), who found an increase in hemoglobin content (among other hematological parameters) in Nile tilapia exposed to 13 °C during 24–72 h, reinforces this statement. The authors explained that fish exposure to low temperatures increases the oxygen demand to enhance the ATP synthesis for maintaining the body temperature.
Stress responses and preservation of homeostasis increase energetic costs (Jager et al. 2014). Cortisol is the principal glucocorticoid secreted under stress condition by the interrenal tissue located in the head-kidney of teleost fish (Geslin and Benoit Auperin 2004; Castillo et al. 2008). It activates different processes to produce energy according to the increased demand during the stressful event (Donaldson et al. 2008). Rotllant et al. (2001) reported that a drop in water temperature affects the pituitary-interrenal axis in gilthead seabream, triggering cortisol release. As cholesterol is the precursor of cortisol, these two parameters are expected to show a similar trend (Miller 1988; Castillo et al. 2008). However, Panase et al. (2018) observed decreased cholesterol and increased cortisol levels in plasma of Oreochromis niloticus exposed to cold shock. In the present study, fish fed with CD showed decreased plasmatic levels of cholesterol after the cold stress event, while fish fed with RD showed an increment of this biochemical parameter. This difference in fish response could be attributed to the presence of the seaweed in the diet, as Wang et al. (2019) observed that the concentrations of serum cholesterol, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol all gradually increased with increasing levels of dietary Sargassum horneri algae to juvenile turbot (Scophthalmus maximus).
Plasmatic triglycerides were diminished in juvenile fish fed with CD and exposed to 14 °C. Decreased triglyceride levels were also found by Lermen et al. (2004) in Rhamdia quelen exposed to 15 °C for 21 days. In fasted fish, plasma triglyceride levels represent the result between the rate of hepatic secretion of very-low-density lipoproteins and the rate of their clearance by peripheral tissues (Greene and Selivonchick 1987). In addition, low water temperature could modify the rate of lipolysis or fatty acid reesterification. Then, the decrease in plasmatic triglyceride levels observed in P. mesopotamicus was probably due to the utilization of fat stores to face thermal stress.
Hepatic glycogen levels decreased in CD-fed fish. This result was also observed in H. littorale and R. quelen exposed to 10–15 °C after acute or chronic conditions (Lermen et al. 2004; Rossi et al. 2017). Additionally, Ibarz et al. (2010) analyzed the liver proteome of gilthead sea bream (Sparus aurata) exposed to a cold stress challenge and found a rise in glycogen-phosphorylase levels in agreement with their observation of liver glycogen depletion. These results indicate that energy substrates, such as glycogen, are broken down into glucose to provide the extra energy needed (Viant et al. 2003; Lermen et al. 2004; Chatzifotis et al. 2010; Sun et al. 2019). On the other hand, rats fed with the seaweed Undaria pinnatifida showed that gluconeogenesis was upregulated and, by contrast, glycolysis-related genes were downregulated (Yoshinaga et al. 2018). These mechanisms could be operating in fish as increased liver glycogen reserves were observed in RD-fed fish exposed to 14 °C. Additionally, this group of fish showed lower hepatic protein levels. This issue requires further research to elucidate the mechanism involved in seaweed effects on this macromolecule.
Temperature oscillations lead to changes in fish metabolic rate and, consequently, in the generation of reactive oxygen species (ROS) (Pavlović et al. 2010). Then, farmers are challenged to overcome this situation and keep the animals under optimal welfare and developmental conditions; thus, early detection of damage in macromolecules like proteins and lipids has gained importance (Pinto et al. 2019). Bioactive compounds have been identified in P. columbina red seaweed which are good electron donors and could act as antioxidants (Cian et al. 2014); however, no consistent differences were found in comparison with the CD. In the present study, P. mesopotamicus fed with both diets and exposed to a 24-h thermal shock showed lipid oxidative damage in the liver and intestine although augmented antioxidant activities were observed in these tissues. Particularly, SOD and GR were increased in fish liver of CD-fed fish exposed to 14 °C, meanwhile GST augmented in the intestine. In fish fed with RD, only SOD activity was increased in gills. Joy et al. (2017) found increased activities of SOD and CAT in several tissues (liver, gills, brain, and muscle) of Etroplus suratensis exposed to cold water for 24–48 h. SOD and CAT enzymes are considered as the first line of antioxidant defense, as SOD catalyzes the dismutation of superoxide anion, and CAT breaks down the hydrogen peroxide in water and oxygen (Halliwell and Gutteridge 1999). Since GST enzyme conjugates aldehyde products of lipid peroxidation process (Schlenk et al. 2008), its activation seems important to prevent oxidative damage. However, in the intestine of P. mesopotamicus exposed to 14 °C and CD, it was not enough probably due to the lack of activation of other antioxidant enzymes.
The high activity of antioxidant enzymes did not prevent oxidative damage generated by cold stress, since increased levels of TBARS were observed in the liver and intestine of fish fed with both diets. Increased TBARS levels were also found in the liver of Solea senegalensis and Danio rerio exposed to low temperatures for 24 and 1–12 h, respectively (Castro et al. 2012; Wu et al. 2015). Joy et al. (2017) explained that low temperatures could enhance the formation of ROS and proton leakage, and then, favor peroxidation of lipids due to increased polyunsaturation in the mitochondrial membranes and the respiration rates. Oxidative stress seems increased because low temperature enhances oxygen solubility; meanwhile, the transfer of electrons could be disrupted, and the ROS production enhanced because the mitochondrial membrane may diminish its fluidity (Weiss 1970; Hazel 1995).
Conclusions
The cultured fish Piaractus mesopotamicus was found to be sensitive to cold shock based on several biomarkers. Our results contribute to the current knowledge regarding cold shock in fish, a situation which could occur either in aquaculture or during animal transport. We hypothesized that the thermal stress effects would be mitigated in fish fed with the Pyropia columbina seaweed based-diet, but no significant differences were observed between fish fed with the algae-based diet and the ones fed with the control diet. Although it is widely known that seaweeds contain several bioactive substances with potential health properties (Sørensen et al. 2019), fish responses to dietary seaweed inclusion are dependent on seaweed species and dose, besides being species dependent. We suggest that further research needs to consider different powder algae concentrations in supplemented diets, to take advantage of this valuable and abundant natural resource. Last, we consider this report as relevant to complement the baseline information regarding nutritional requirements of this important farmed species P. mesopotamicus.
Data availability
It is not applicable.
References
Ahmad SM, Shah FA, Bhat FA, Bhat JIA, Balkhi MH (2011) Thermal adaptability and disease association in common carp (Cyprinus carpio communis) acclimated to different (four) temperatures. J Therm Biol 36(8):492–497. https://doi.org/10.1016/j.jtherbio.2011.08.007
Bacchetta C, Rossi A, Ale A, Campana M, Parma MJ, Cazenave J (2014) Combined toxicological effects of pesticides: a fish multi-biomarker approach. Ecol Indic 36:532–538. https://doi.org/10.1016/j.ecolind.2013.09.016
Bacchetta C, Ale A, Rossi A, Karakachoff M, Cazenave J (2020) Effects of cold stress on juvenile Piaractus mesopotamicus and the mitigation by β-carotene. J Therm Biol 88:102497. https://doi.org/10.1016/j.jtherbio.2019.102497
Barbieri E (2009a) Effects of zinc and cadmium on oxygen consumption and ammonium excretion in pink shrimp (Farfantepenaeus paulensis, Pérez-Farfante, 1967, Crustacea). Ecotoxicology 18:312–318. https://doi.org/10.1007/s10646-008-0285-y
Barbieri E (2009b) Effect of 2, 4-D herbicide (2, 4-dichlorophenoxyacetic acid) on oxygen consumption and ammonium excretion of juveniles of Geophagus brasiliensis (Quoy & Gaimard, 1824) (Osteichthyes, Cichlidae). Ecotoxicology 18:55–60. https://doi.org/10.1007/s10646-008-0256-3
Barbieri E, Vigliar Bondioli AC (2013) Acute toxicity of ammonia in Pacu fish (Piaractus mesopotamicus, Holmberg, 1887) at different temperature levels. Aquac Res 46:1–7. https://doi.org/10.1111/are.12203
Beutler E (1982) Catalase. In: Beutler E (ed) Red cell metabolism, a manual of biochemical methods. Grune and Stratton Inc., New York, pp 105–106
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of proteins utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Burtin P (2003) Nutritional value of seaweeds. Elec J Env Agricult Food Chem Title 2:498–503
Castillo J, Castellana B, Acerete L, Planas JV, Goetz FW, Mackenzie S, Tort L (2008) Stress-induced regulation of steroidogenic acute regulatory protein expression in head kidney of Gilthead seabream (Sparus aurata). J Endocrinol 196:313–322. https://doi.org/10.1677/JOE-07-0440
Castro C, Pérez-Jiménez A, Guerreiro I, Peres H, Castro-Cunha M, Oliva-Teles A (2012) Effects of temperature and dietary protein level on hepatic oxidative status of Senegalese sole juveniles (Solea senegalensis). Comp Biochem Physiol B 163:372–378. https://doi.org/10.1016/j.cbpa.2012.07.003
Cazenave J, Wunderlin DA, Hued A, Bistoni MA (2005) Hematological characterization of a Neotropical fish, Corydoras paleatus (Pisces, Callichthyidae), captured from pristine and polluted water. Hydrobiologia 537:25–33
Chatzifotis S, Panagiotidou M, Papaioannou N (2010) Effect of dietary lipid levels on growth, feed utilization, body composition and serum metabolites of meager (Argyrosomus regius) juveniles. Aquaculture 307:65–70. https://doi.org/10.1016/j.aquaculture.2010.07.002
Cheng C-H, Guo Z-X, Wang A-L (2018) The protective effects of taurine on oxidative stress, cytoplasmic free-Ca2+ and apoptosis of pufferfish (Takifugu obscurus) under low temperature stress. Fish Shellfish Immunol 77:457–464. https://doi.org/10.1016/j.fsi.2018.04.022
Cian RE, Fajardo MA, Alaiz M, Vioque J, González RJ, Silvina R, Drago SR (2014) Chemical composition, nutritional and antioxidant properties of the red edible seaweed Porphyra columbina. Int J Food Sci Nutr 65(3):299–305. https://doi.org/10.3109/09637486.2013.854746
Cian RE, Garzón AG, Betancur Ancona D, Chel Guerrero L, Drago SR (2016) Chelating properties of peptides from red seaweed Pyropia columbina and its effect on iron bio-accessibility. Plant Foods Hum Nutr 71:96–101. https://doi.org/10.1007/s11130-016-0533-x
Cian RE, Bacchetta C, Rossi A, Cazenave J, Drago SR (2019) Red seaweed Pyropia columbina as antioxidant supplement in feed for cultured juvenile Pacú (Piaractus mesopotamicus). J Appl Phycol 31:1455–1465. https://doi.org/10.1007/s10811-018-1648-2
Claudiano GS, Yunis-Aguinaga J, Marinho-Neto FA, Miranda RL, Martins IM, Otani FS, Mundim AV, Marzocchi-Machado CM, Moraes JRE (2019) Hematological and immune changes in Piaractus mesopotamicus in the sepsis induced by Aeromonas hydrophila. Fish Shellfish Immunol 88:259–265. https://doi.org/10.1016/j.fsi.2019.01.044
CONICET (Consejo Nacional de Investigaciones Científicas y Técnicas) (2005) Marco Ético de Referencia para las Investigaciones Biomédicas en Animales de laboratorio, de granja y obtenidos de la naturaleza, Buenos Aires, Argentina. Retrieved from www.conicet.gov.ar/wp-content/uploads/OCR-RD-20050701-1047.pdf
Donaldson MR, Cooke SJ, Patterson DA, MacDonald JS (2008) Cold shock and fish. J Fish Biol 73:1491–1530
Folch J, Sloane L, Stanley G (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Gasco L, Gai F, Maricchiolo G, Genovese L, Ragonese S, Bottari T, Caruso G (2018) Chapter 4: supplementation of vitamins, minerals, enzymes and antioxidant in fish feeds. In: Feeds for the aquaculture sector. Springer Briefs in Molecular Science. Springer, Cham. doi: https://doi.org/10.1007/978-3-319-77941-6_4
Geslin M, Benoit Auperin B (2004) Relationship between changes in mRNAs of the genes encoding steroidogenic acute regulatory protein and P450 cholesterol side chain cleavage in head kidney and plasma levels of cortisol in response to different kinds of acute stress in the rainbow trout (Oncorhynchus mykiss). Gen Comp Endocrinol 135:70–80
Goda AA, Sallam AE, Srour TM (2018) Evaluation of natural and synthetic carotenoid supplementation on growth, survival, total carotenoid content, fatty acids profile and stress resistance of European seabass, Dicentrarchus labrax, Fry. Aquac Stud 18(1):27–39. https://doi.org/10.4194/2618-6381-v18_1_04
Greene D, Selivonchick D (1987) Lipid metabolism in fish. Prog Lipid Res 26:53–85
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first step in mercapturic acid formation. J Biol Chem 249:7130–7139
Halliwell B, Gutteridge JMC (1999) Free radicals in biology and medicine, 3rd ed., Oxford. University Press, Oxford, p. 936. In: The toxicology of fishes (Eds. Di Giulio RT y Hinton DE). 2008. CRC Press, Boca Raton, 273-326
Hassaan MS, El-Sayed AIM, Soltan MA, Iraqi MM, Goda AM, Davies SJ, El-Haroun ER, Ramadan HA (2019) Partial dietary fish meal replacement with cotton seed meal and supplementation with exogenous protease alters growth, feed performance, hematological indices and associated gene expression markers (GH, IGF-I) for Nile tilapia, Oreochromis niloticus. Aquaculture 503:282–292. https://doi.org/10.1016/j.aquaculture.2019.01.009
Hazel JR (1995) Thermal adaptation in biological membranes: is homeoviscous adaptation the explanation? Annu Rev Physiol 57:19–42
Hoseini SM, Yousefi M, Seyed Hoseinifar SH, Van Doan H (2019) Antioxidant, enzymatic and hematological responses of common carp (Cyprinus carpio) fed with myrcene- or menthol supplemented diets and exposed to ambient ammonia. Aquaculture 506:246–255. https://doi.org/10.1016/j.aquaculture.2019.03.048
Houston AH (1990) Blood and circulation. In: Schreck CB, Moyle PB (eds) Methods for fish biology. American Fisheries Society, Bethesda
Ibarz A, Martín-Pérez M, Blasco J, Bellido D, de Oliveira E, Jaume Fernández-Borras J (2010) Gilthead sea bream liver proteome altered at low temperatures by oxidative stress. Proteomics 10:963–975. https://doi.org/10.1002/pmic.200900528
Jager T, Barsi A, Hamda NT, Martin BT, Zimmer EI, Ducrot V (2014) Dynamic energy budgets in population ecotoxicology: applications and outlook. Ecol Model 280:140–147. https://doi.org/10.1016/j.ecolmodel.2013.06.024
Joy S, Alikunju AP, Jose J, Sudha HSH, Parambath PM, Puthiyedathu ST, Philip B (2017) Oxidative stress and antioxidant defense responses of Etroplus suratensis to acute temperature fluctuations. J Therm Biol 70:20–26. https://doi.org/10.1016/j.jtherbio.2017.10.010
Lermen CL, Lappe R, Crestani M, Vieira VP, Gioda CR, Schetinger MRC, Baldisserotto B, Moraes G, Morsch VM (2004) Effect of different temperature regimes on metabolic and blood parameters of silver catfish Rhamdia quelen. Aquaculture 239:497–507. https://doi.org/10.1016/j.aquaculture.2004.06.021
Logan M (2010) Biostatistical design and analysis using R: a practical guide. John Wiley and Sons
Lowry OH, Rosebrough MJ, Far AL, Randall RL (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Miller WL (1988) Molecular biology of steroid hormone synthesis. Endocr Rev 9:295–318
Milstein A, Zoran M, Peretz Y, Joseph D (2000) Low temperature tolerance of Pacu, Piaractus mesopotamicus. Environ Biol Fish 58:455–460
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247(10):3170–3175
Muñoz LI, Wacyk J, Claudio Perez C, Carrasco J, Cortez-San Martin M (2018) Diets enriched in red seaweed (Pyropia columbina and Gracilaria chilensis) cryo concentrates modulate the immune-relevant gene encoding the Mx antiviral protein in salmon (Salmo salar) white blood cells. J Appl Phycol 31(2):1415–1424. https://doi.org/10.1007/s10811-018-1595-y
Nakagawa H (1997) Effect of dietary alga on improvement of lipid metabolisms in fish. Biomed Parmacother 51:345–348
Ngo D-H, Wijesekara I, Vo T-S, Ta QV, Kim S-K (2011) Marine food derived functional ingredients as potential antioxidants in the food industry: an overview. Food Res Int 44:523–529. https://doi.org/10.1016/j.foodres.2010.12.030
Nobrega RO, Batista RO, Corrêa CF, Mattioni B, Filer K, Pettigrew JE, Fracalossi DM (2019) Dietary supplementation of Aurantiochytrium sp. meal, a docosahexaenoic-acid source, promotes growth of Nile tilapia at a suboptimal low temperature. Aquaculture 507:500–509. https://doi.org/10.1016/j.aquaculture.2019.04.030
Panase P, Saenphet S, Saenphet K (2018) Biochemical and physiological responses of Nile tilapia Oreochromis niloticus Lin subjected to cold shock of water temperature. Aquac Rep 11:17–23. https://doi.org/10.1016/j.aqrep.2018.05.005
Parma de Croux MJ (1990) Benzocaine (ethyl-p-aminobenzoate) as an anaesthetic for Prochilodus lineatus, Valenciennes (Pisces, Curimatidae). J Appl Ichthyol 6:189–192. https://doi.org/10.1111/j.1439-0426.1990.tb00578.x
Pavlović SZ, Borković Mitić SS, Radovanović TB, Perendija BR, Despotović SG, Gavrić JP, Saičić ZS (2010) Seasonal variations of the activity of antioxidant defense enzymes in the Red mullet (Mullus barbatus l.) from the Adriatic Sea. Mar Drugs 8:413–428. https://doi.org/10.3390/md8030413
Pinto D, Pellegrin L, Fiori Nitz LF, da Costa ST, Monserrat JM, Garcia L (2019) Haematological and oxidative stress responses in Piaractus mesopotamicus under temperature variations in water. Aquac Res 00:1–11. https://doi.org/10.1111/are.14260
Reichenbach-Klinke HH (1980) Enfermedades de los peces. Acribia, Zaragoza
Rossi A, Bacchetta C, Cazenave J (2017) Effect of thermal stress on metabolic and oxidative stress biomarkers of Hoplosternum littorale (Teleostei, Callichthyidae). Ecol Indic 79:361–370. https://doi.org/10.1016/j.ecolind.2017.04.042
Rotllant J, Balm PH, Perez-Sanchez J, Wendelaar-Bonga SE, Tort L (2001) Pituitary and interrenal function in gilthead seabream (Sparus aurata L., Teleostei) after handling and confinement stress. Gen Comp Endocrinol 121:333–342. https://doi.org/10.1111/are.14260
Schlenk D, Handy R, Steinert S, Depledge MH, Benson W (2008) Biomarkers. In: The toxicology of fishes (Ed. Di Giulio RT, Hinton DE). CRC Press, Boca, 683-731 pp
Seifter S, Dayton S, Novic B, Montwyler E (1950) The estimation of glycogen with the anthrone reagent. Arch Biochem 25:191–200
Sørensen LE, Jeppesen PB, Christiansen CB, Hermansen K, Gregersen S (2019) Nordic seaweed and diabetes prevention: exploratory studies in KK-Ay mice. Nutrients 11(6):1435. https://doi.org/10.3390/nu11061435
Sotoudeh E, Mardani F (2017) Antioxidant-related parameters, digestive enzyme activity and intestinal morphology in rainbow trout (Oncorhynchus mykiss) fry fed graded levels of red seaweed, Gracilaria pygmaea. Aquac Nutr 24(2):1–7. https://doi.org/10.1111/anu.12606
Sun Z, Tan X, Liu Q, Ye H, Zou C, Xu M, Zhang Y, Ye C (2019) Physiological, immune responses and liver lipid metabolism of orange-spotted grouper (Epinephelus coioides) under cold stress. Aquaculture 498:545–555. https://doi.org/10.1016/j.aquaculture.2018.08.051
Tanaka K, Sano T, Ishizuka K, Kitta K, Kawamura Y (1994) Comparison of properties of leaf and root glutathione reductases from spinach. Physiol Plant 91:353–358. https://doi.org/10.1111/j.1399-3054.1994.tb02960.x
Teimouri M, Yeganeh S, Rahimi Mianji G, Najafi M, Mahjoub S (2019) The effect of Spirulina platensis meal on antioxidant gene expression, total antioxidant capacity, and lipid peroxidation of rainbow trout (Oncorhynchus mykiss). Fish Physiol Biochem 45(3):977–986. https://doi.org/10.1007/s10695-019-0608-3
Turchini GM, Ng W-K, Tocher DR (2010) Fish oil replacement and alternative lipid sources in aquaculture feeds. 1st ed. Boca Raton, Taylor & Francis Group, CRC Press
Viant MR, Werner I, Rosenblum ES, Gantner AS, Tjeerdema RS, Johnson ML (2003) Correlation between heat-shock protein induction and reduced metabolic condition in juvenile steelhead trout (Oncorhynchus mykiss) chronically exposed to elevated temperature. Fish Physiol Biochem 29:159–171. https://doi.org/10.1023/B:FISH.0000035938.92027.81
Wang X, Wang L, Yao C, Qiu L, Zhang H, Zhi Z, Song L (2012) Alternation of immune parameters and cellular energy allocation of Chlamys farreri under ammonia-N exposure and Vibrio anguillarum challenge. Fish Shellfish Immunol 32(5):741–749. https://doi.org/10.1016/j.fsi.2012.01.025
Wang C, Hu W, Wang L, Qiao H, Wu H, Xu Z (2019) Effects of dietary supplementation with Sargassum horneri meal on growth performance, body composition, and immune response of juvenile turbot. J Appl Phycol 31(1):771–778. https://doi.org/10.1007/s10811-018-1590-3
Weiss RF (1970) The solubility of nitrogen, oxygen and argon in water and seawater. Deep-Sea Res Oceanogr Abstr 17:721–735
Wen B, Jin S-R, Chen Z-Z, Gao J-Z, Wang L, Liu Y, Liu H-P (2017) Plasticity of energy reserves and metabolic performance of discus fish (Symphysodon aequifasciatus) exposed to low-temperature stress. Aquaculture 481:169–176. https://doi.org/10.1016/j.aquaculture.2017.09.002
Wu SM, Liu J-H, Shu L-H, Chen CH (2015) Anti-oxidative responses of zebrafish (Danio rerio) gill, liver and brain tissues upon acute cold shock. Comp Biochem Phys A 187:202–213. https://doi.org/10.1016/j.cbpa.2015.05.016
Yagi K (1976) A simple fluorometric assay for lipoperoxide in blood plasma. Biochem Med 15(2):212–216. https://doi.org/10.1016/0006-2944(76)90049-1
Yoshinaga K, Nakai Y, Izumi H, Nagaosa K, Ishijima T, Nakano T, Abe K (2018) Oral administration of edible seaweed Undaria pinnatifida (Wakame) modifies glucose and lipid metabolism in rats: a DNA microarray analysis. Mol Nutr Food Res 62(12):e1700828. https://doi.org/10.1002/mnfr.201700828
Acknowledgements
Authors are thankful to the Área de Cereales y Oleaginosas of the Instituto de Tecnología de Alimentos (FIQ-UNL) for providing the control and supplemented extruded feed.
Funding
This work was supported by grants from Agencia Nacional de Promoción Científica y Tecnológica (PICT-2016 1911), Consejo Nacional de Investigaciones Científicas y Técnicas (PIP- 112 201501 00942 CO), and Universidad Nacional del Litoral (CAI+D 2016-50420150100016LI).
Author information
Authors and Affiliations
Contributions
AA: data curation, formal analysis, methodology, visualization, writing—original draft, writing—review and editing.
CB: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, visualization, writing—original draft, writing—review and editing.
AR: data curation, formal analysis, funding acquisition, project administration, investigation, methodology, supervision, validation, visualization.
PS: data curation, formal analysis, methodology, visualization, writing—review and editing.
JC: conceptualization, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, visualization, writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interest.
Ethics approval
All experiments were carried out following the national and institutional guidelines for the protection of animal welfare (CONICET 2005) and approved by the Committee of Ethics and Safety in Experimental Work (Scientific-Technological Center, CONICET Santa Fe, Argentina).
Consent to participate
It is not applicable.
Consent for publication
It is not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ale, A., Bacchetta, C., Rossi, A.S. et al. Low temperature stress in a cultured fish (Piaractus mesopotamicus) fed with Pyropia columbina red seaweed-supplemented diet. Fish Physiol Biochem 47, 829–839 (2021). https://doi.org/10.1007/s10695-021-00944-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-021-00944-7